Microstructure and Properties of TA2 Titanium Joints Brazed with Ti–Zr–Cu–Ni Filler Metal
Abstract
1. Introduction
2. Experimental
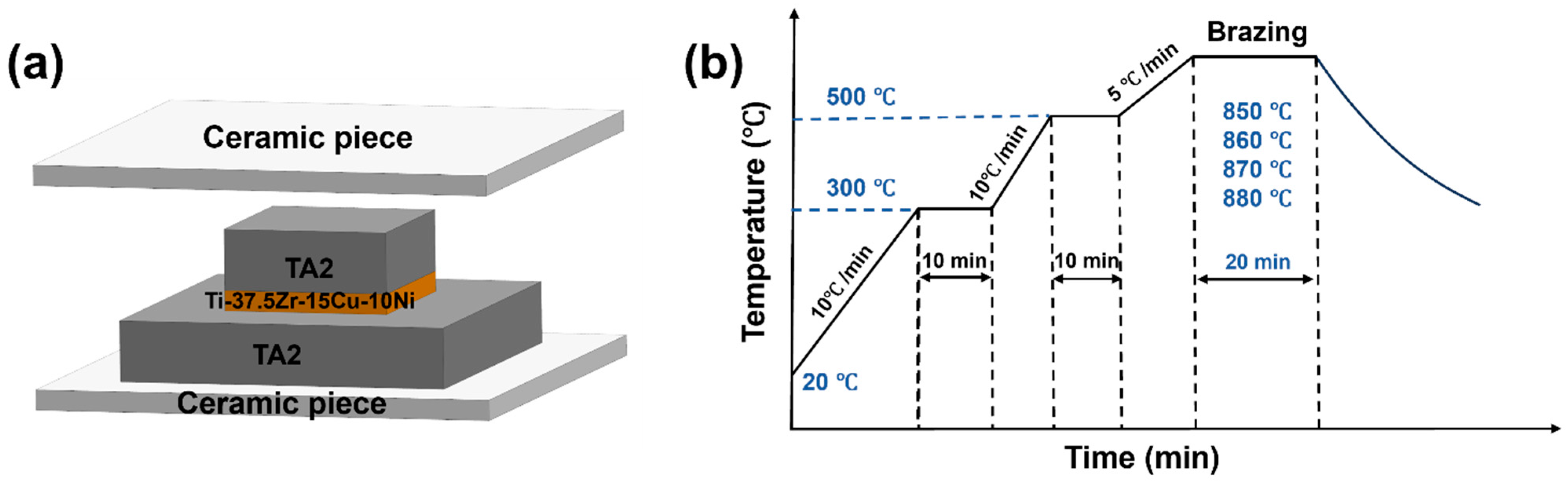
2.1. Brazing and Base Material Treatment
2.2. Microstructural Characterization
2.3. Mechanical Properties
2.4. Corrosion Resistance Testing
3. Results and Discussion
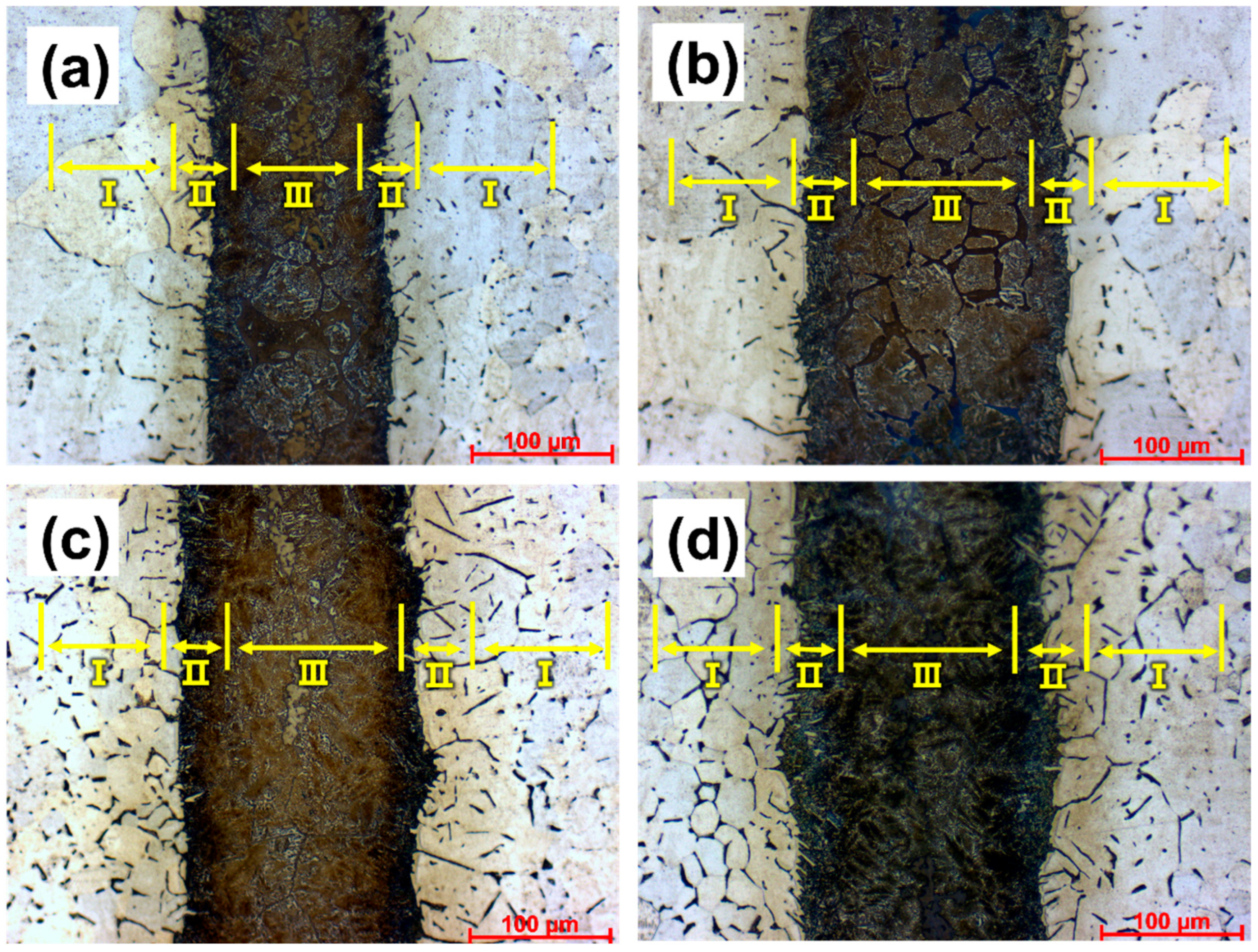
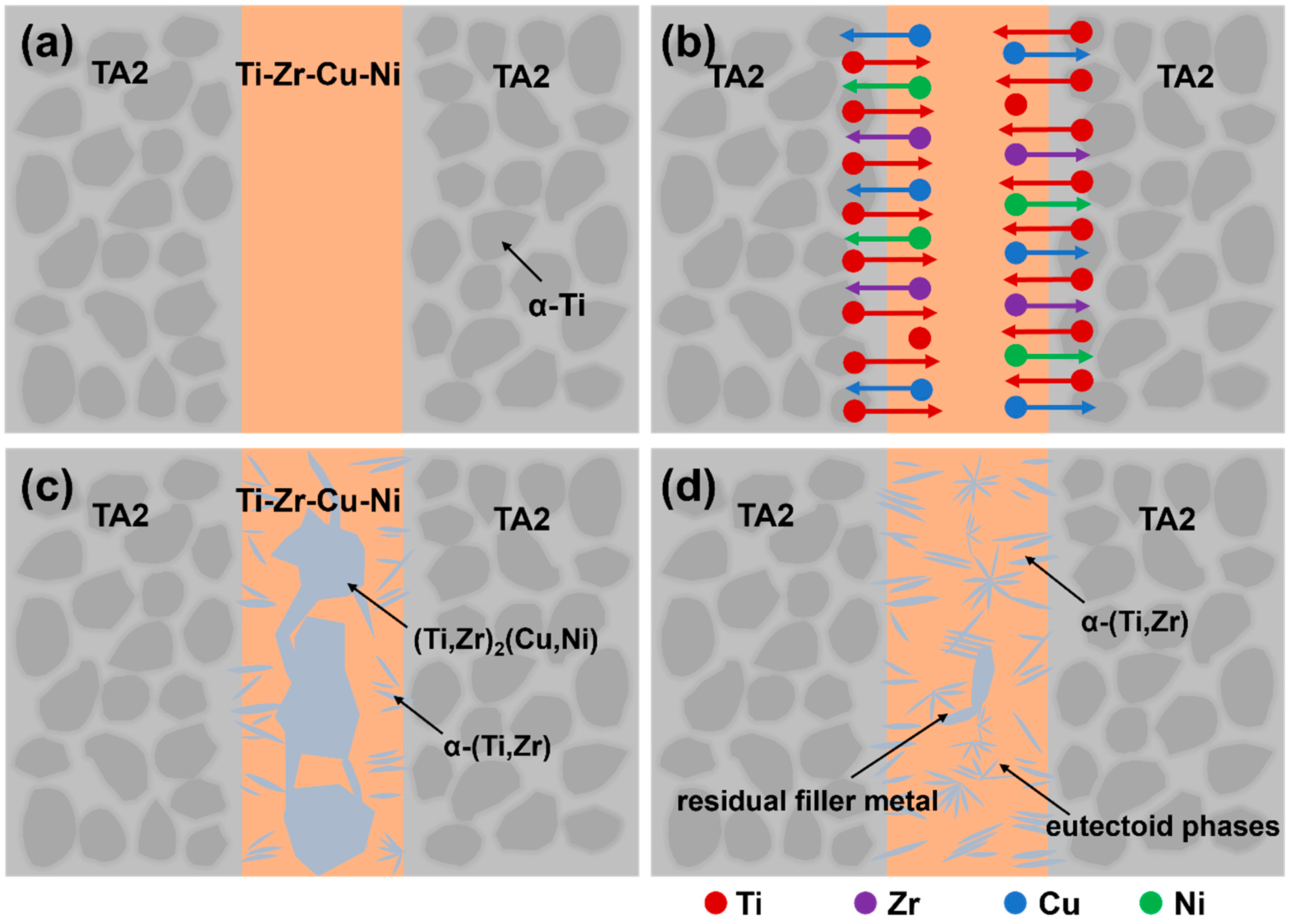
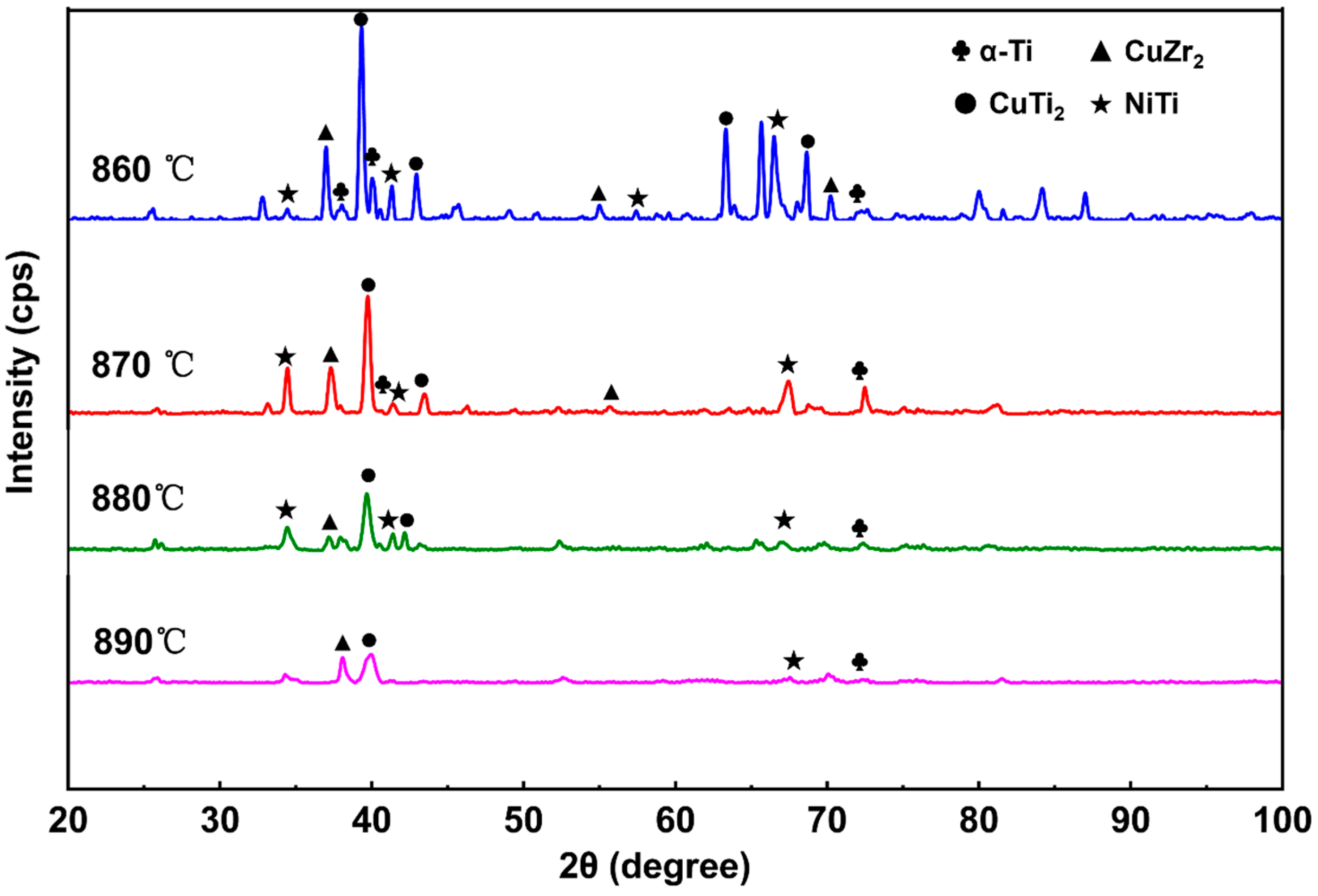
3.1. Microstructural Regions
3.2. Morphology and Elemental Diffusion
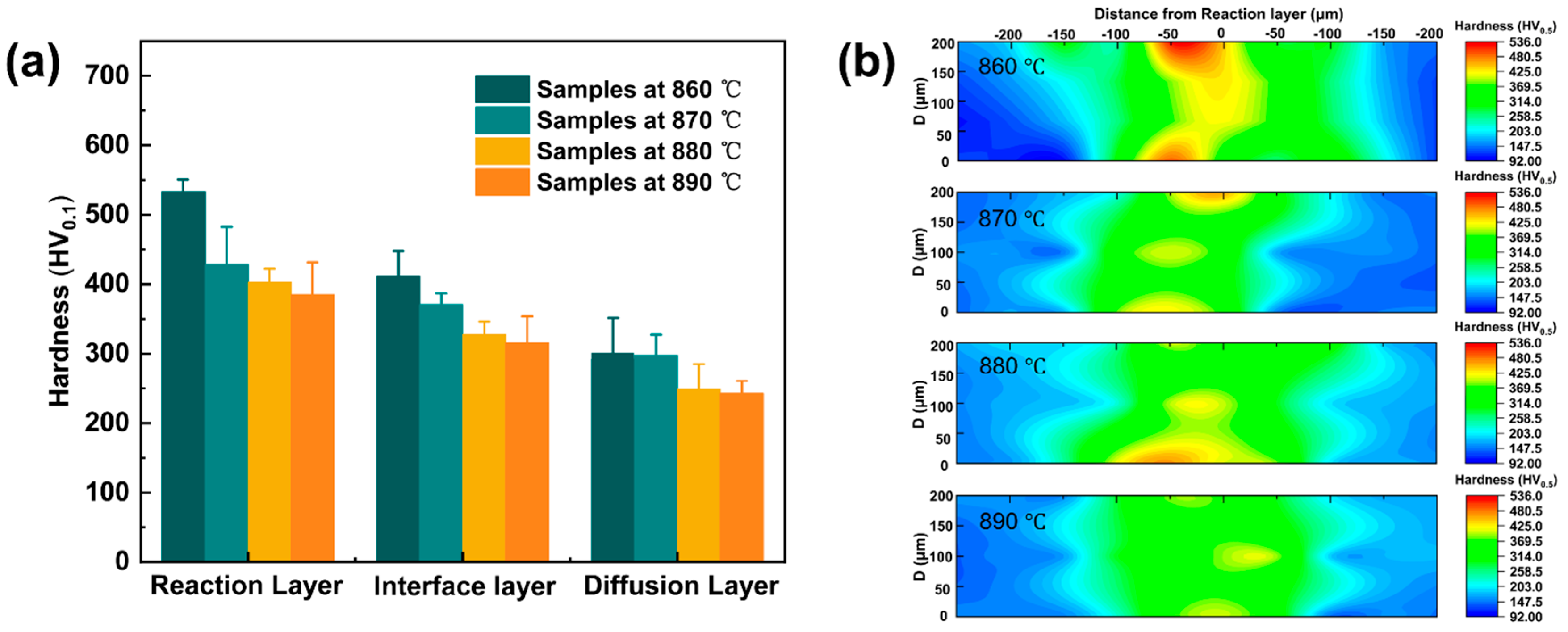
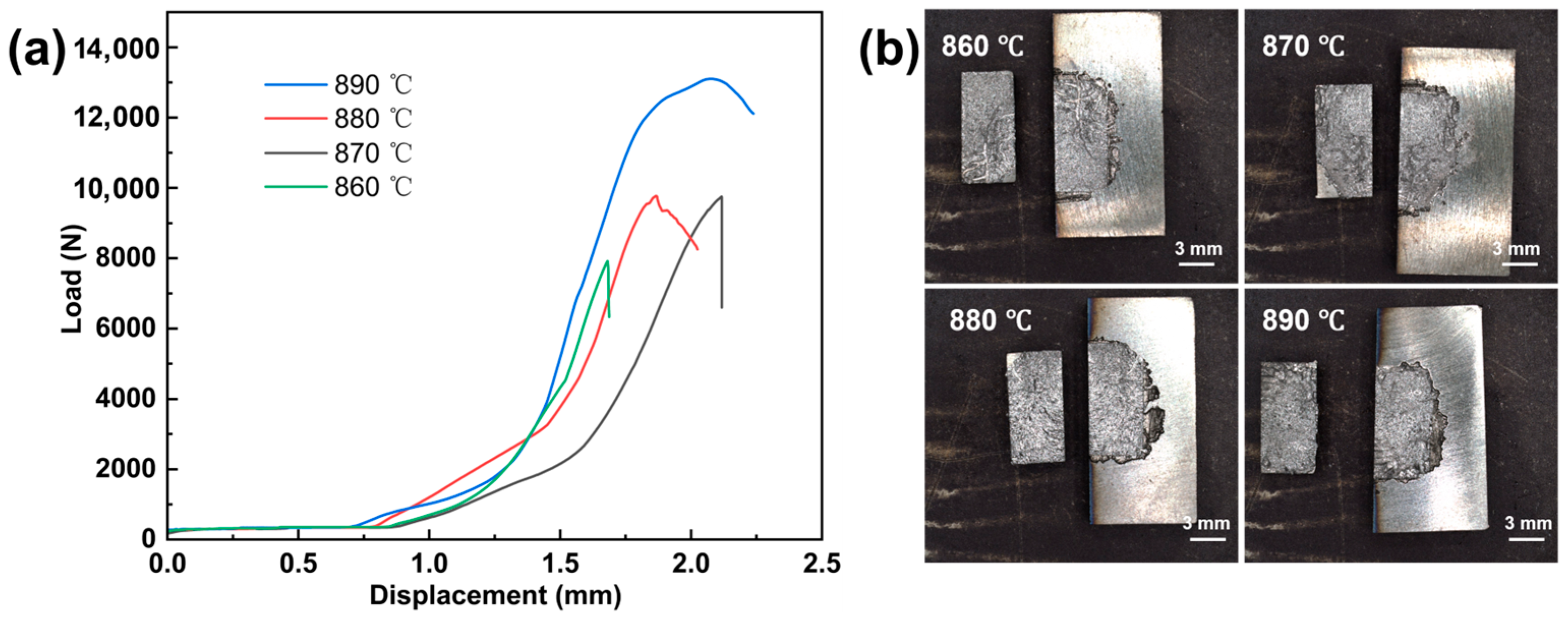
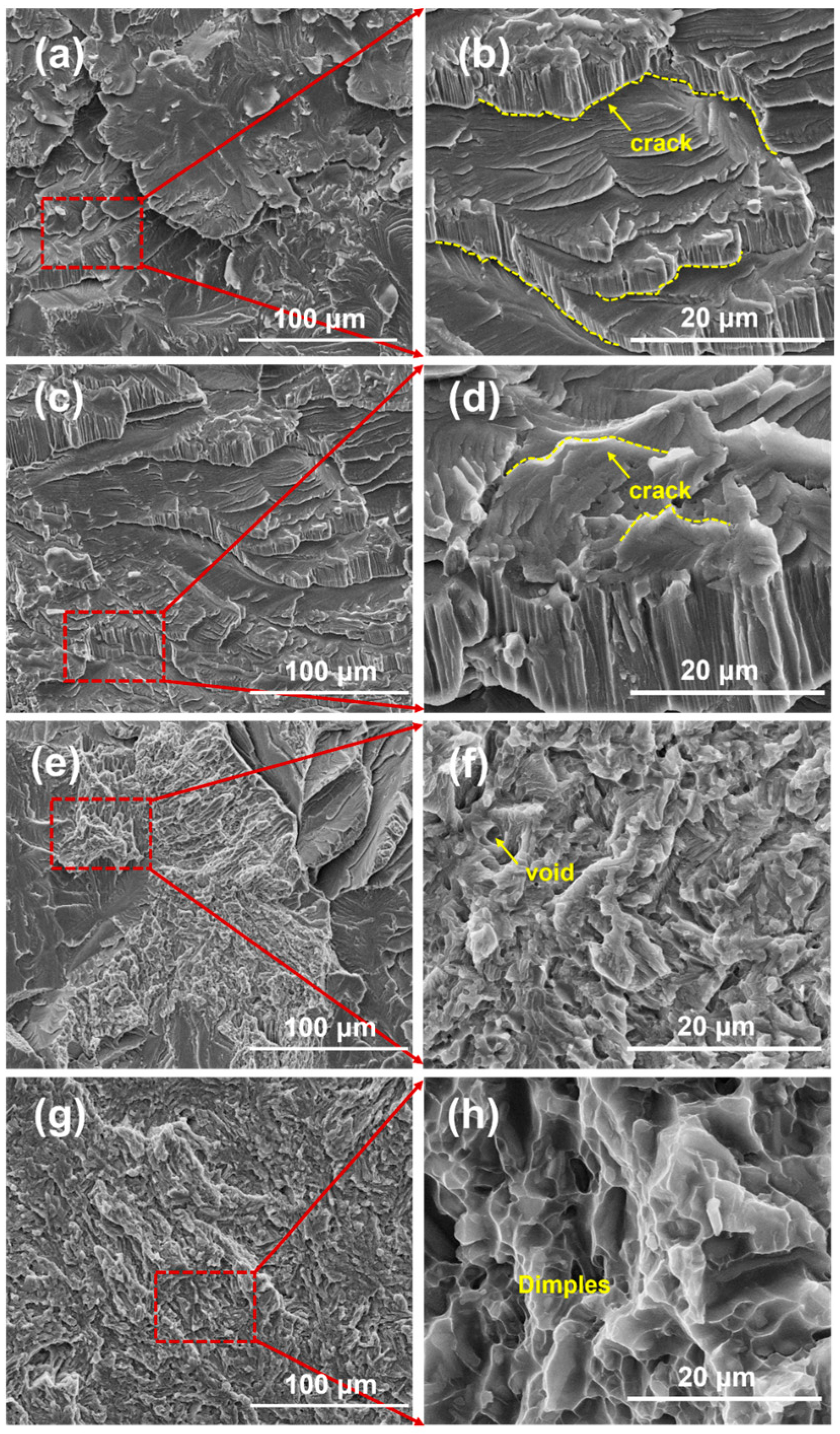
3.3. Mechanical Performance of the Brazed Joints
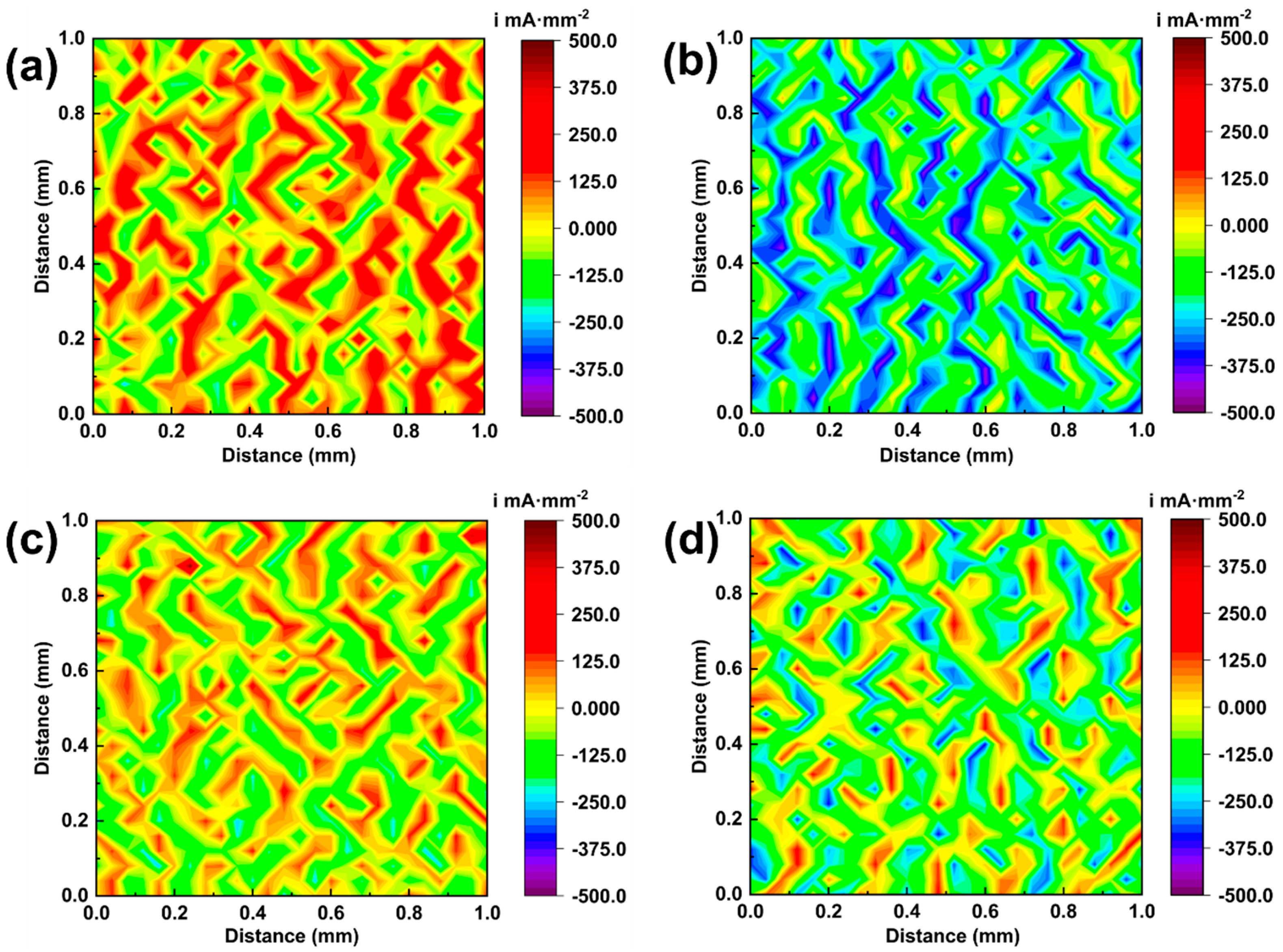
3.4. Corrosion Properties
4. Conclusions
- With increasing brazing temperature, the seam microstructure evolved from residual filler and heterogeneous phases to a uniform structure characterized by smooth elemental distribution and gradual interfacial transition. The seam center was mainly composed of α-(Ti,Zr) solid solution, (Ti,Zr)2(Cu,Ni) intermetallic compounds, and lamellar eutectoid structures. At 890 °C, elemental diffusion was sufficient, and the microstructure transformed into an α-(Ti,Zr)-dominated solid solution with significantly improved homogeneity and stability.
- Mechanical tests revealed that low-temperature joints exhibited higher and non-uniform hardness due to the enrichment of hard brittle phases, and the shear strength was only 186 MPa. As the brazing temperature increased, the overall hardness decreased and became more uniform, while the shear strength increased progressively to 302 MPa at 890 °C. The fracture mechanism transitioned from cleavage-dominated brittle fracture to ductile fracture with equiaxed dimples.
- Low-temperature joints exhibited strong anodic hotspots and pronounced micro-galvanic corrosion, mainly concentrated at α-(Ti,Zr)/IMCs phase boundaries. With increasing brazing temperature, both the number of hotspots and current density decreased, and at 890 °C, the current distribution became nearly uniform, demonstrating markedly improved corrosion resistance. The underlying mechanism was attributed to sufficient elemental diffusion and the formation of a protective TiO2–ZrO2 composite film at higher temperatures, which jointly mitigated electrochemical heterogeneity and enhanced interfacial stability.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Q.; Sun, Q.; Xin, S.; Chen, Y.; Wu, C.; Wang, H.; Xu, J.; Wan, M.; Zeng, W.; Zhao, Y. High-strength titanium alloys for aerospace engineering applications: A review on melting-forging process. Mater. Sci. Eng. A 2022, 845, 143260. [Google Scholar] [CrossRef]
- Najafizadeh, M.; Yazdi, S.; Bozorg, M.; Ghasempour-Mouziraji, M.; Hosseinzadeh, M.; Zarrabian, M.; Cavaliere, P. Classification and applications of titanium and its alloys: A review. J. Alloys Compd. Commun. 2024, 3, 100019. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.; Shi, J.; Li, S.; Xiong, J. Microstructure and mechanical properties of diffusion bonded joints of high-entropy alloy Al5(HfNbTiZr)95 and TC4 titanium alloy. J. Mater. Res. Technol. 2021, 11, 1741–1752. [Google Scholar] [CrossRef]
- Li, X.X.; Chen, L.Y.; Hu, W.B.; Wan, S.; Song, L.F.; Wang, Y.P.; Liao, B.K.; Guo, X.P. Corrosion and passive behavior of SLM and wrought TA15 titanium alloys in hydrochloric acid solutions. J. Iron Steel Res. Int. 2024, 325, 1356–1370. [Google Scholar] [CrossRef]
- Wang, T.; Hua, B.J.; Liu, X.J.; Yang, P.H.; Shi, X.X.; Yang, J.C.; Zhou, L.; Yang, C.Q. Mechanism analysis of pitting induced by Al2O3 inclusions: Insight from simulation calculation. J. Iron Steel Res. Int. 2024, 324, 1061–1072. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, X.Q.; Cao, H.W.; Li, X.G. Improving resistance of E690 steel to stress corrosion cracking in high Cl− environments through Sn microalloying. J. Iron Steel Res. Int. 2024, 325, 1396–1412. [Google Scholar] [CrossRef]
- Chen, L.; Liang, M.; Wan, W.; Tang, J.; Lin, B.; Yang, X.; Chen, Y.; Chen, Y. Corrosion of commercial pure titanium and two titanium alloys in extremely high-chloride and high-alkali seawater electrolysis environment. J. Alloys Compd. 2025, 1020, 179431. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Cui, Y.; Liu, R.; Wang, F. Corrosion behavior of pure Ti under continuous NaCl solution spraying at 600 °C. NPJ Mater. Degrad. 2022, 61, 257. [Google Scholar] [CrossRef]
- Baojin, Q.; Li, Z.; Hong, X.; Yan, S. Heat transfer characteristics of titanium/water two-phase closed thermosyphon. Energy Convers. Manag. 2009, 509, 2174–2179. [Google Scholar] [CrossRef]
- Tamimi, A.A.; Thekkuden, D.T.; Mourad, A.I.; Alkhedher, M. Investigation of mechanical and metallurgical properties of commercially pure titanium grade 2 tube-to-tubesheet joints. Sci. Rep. 2025, 151, 21964. [Google Scholar] [CrossRef]
- Estupinan-Lopez, F.; Orquiz-Muela, C.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Bautista-Margulis, R.G.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Almeraya-Calderon, F.; Lopes, A.J. Oxidation Kinetics of Ti-6Al-4V Alloys by Conventional and Electron Beam Additive Manufacturing. Materials 2023, 16, 1187. [Google Scholar] [CrossRef]
- Meng, Y.; Villa, M.; Dahl, K.V.; Wang, B.; Drouet, M.; Dubois, J.-B.; Somers, M.A.J.; Christiansen, T.L. Thermochemical surface hardening of Ti-6Al-4V: On the role of temperature and treatment media. Surf. Coat. Technol. 2021, 422, 127505. [Google Scholar] [CrossRef]
- Vaché, N.; Cadoret, Y.; Dod, B.; Monceau, D. Modeling the oxidation kinetics of titanium alloys: Review, method and application to Ti-64 and Ti-6242s alloys. Corros. Sci. 2021, 178, 109041. [Google Scholar] [CrossRef]
- Hao, P.J.; Liu, Y.M.; Zhang, Y.Y.; Wang, T.; Huang, Q.X.; Wang, Z.G. Effect of heating temperature on microstructure and properties of Ti/steel clad plates with corrugated interfaces. J. Iron Steel Res. Int. 2024, 31, 2997–3006. [Google Scholar] [CrossRef]
- Omoniyi, P.O.; Mahamood, R.M.; Jen, T.C.; Akinlabi, E.T. An Overview of TIG Welding of Ti6Al4V: Recent Developments. Rev. Compos. Matér. Av. 2021, 31, 265–274. [Google Scholar] [CrossRef]
- Yang, J.W.; Liu, X.Y.; Li, T.; Chen, L.C.; Yang, K.; Ding, Z.Y.; Zhang, J.; Qiao, J. Effect of Ni interlayer on microstructures and mechanical properties of 2205 duplex stainless steel joint by laser oscillating welding. J. Iron Steel Res. Int. 2024, 31, 2463–2474. [Google Scholar] [CrossRef]
- Cheng, M.; Yu, B.; Guo, R.; Shi, X.; Xu, L.; Qiao, J.; Yang, R. Electron beam welding of a novel near α high temperature titanium alloy powder compact: Effect of post-welding heat treatment on tensile properties. J. Mater. Res. Technol. 2021, 10, 153–163. [Google Scholar] [CrossRef]
- Mehdi, B.; Badji, R.; Ji, V.; Allili, B.; Bradai, D.; Deschaux-Beaume, F.; Soulié, F. Microstructure and residual stresses in Ti-6Al-4V alloy pulsed and unpulsed TIG welds. J. Mater. Process. Technol. 2016, 231, 441–448. [Google Scholar] [CrossRef]
- Tian, H.; Xiong, J.; Zhao, L.; Mei, J.; Qi, Y.; Wu, J.W.; Li, K.K.; He, J.C.; Zhang, T.Y. Enhanced vacuum brazing joining between Ti–48Al–2Cr–2Nb/Ti–22Al–25Nb intermetallic alloys by Zr-free Ti-based filler. J. Mater. Res. Technol. 2024, 33, 6925–6935. [Google Scholar] [CrossRef]
- Rae, W.; Lomas, Z.; Jackson, M.; Rahimi, S. Measurements of residual stress and microstructural evolution in electron beam welded Ti-6Al-4V using multiple techniques. Mater. Charact. 2017, 132, 10–19. [Google Scholar] [CrossRef]
- Tolvanen, S.; Pederson, R.; Klement, U. Microstructure and Mechanical Properties of Ti-6Al-4V Welds Produced with Different Processes. Materials 2024, 17, 782. [Google Scholar] [CrossRef]
- Yang, Z.; Li, L.; Qiao, Y.X.; Li, C.T.; Zhang, L.M.; Cui, J.; Ren, D.C.; Ji, H.B.; Zheng, Y.G. Cavitation erosion-corrosion properties of as-cast TC4 and LPBF TC4 in 0.6 mol/L NaCl solution: A comparison investigation. Ultrason. Sonochem. 2024, 108, 106947. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wang, F.J.; Qiao, Y.X. Effect of trace Zn addition on electromigration of Cu/Sn–10Bi/Cu solder joints. J. Iron Steel Res. Int. 2024, 31, 2568–2576. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, H.; Wang, B.; Du, R.; Fan, Y.; He, P. Interfacial evolution behavior and mechanical properties of Ti/steel joint via ultrasonic seam assisted resistance spot welding with Cu interlayer. J. Manuf. Process. 2023, 95, 535–550. [Google Scholar] [CrossRef]
- Sulaiman H, A.J.; Aiman, M.H.; Ishak, M.; Quazi, M.M.; Zaharinie, T.; Ariga, T. Vacuum brazing of titanium alloy to stainless steel enhance by fiber laser surface texturing. J. Mech. Eng. Sci. 2021, 154, 8601–8607. [Google Scholar] [CrossRef]
- Laik, A.; Shirzadi, A.A.; Tewari, R.; Kumar, A.; Jayakumar, T.; Dey, G.K. Microstructure and Interfacial Reactions During Active Metal Brazing of Stainless Steel to Titanium. Metall. Mater. Trans. A 2013, 445, 2212–2225. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, B.; Li, D.; Zhu, X.; Chang, Q.; Zhang, B.; Zhang, L.; Long, W.; Zhong, S. Brazing of TC4 Alloy Using Ti-Zr-Ni-Cu-Sn Amorphous Braze Fillers. Materials 2024, 17, 3745. [Google Scholar] [CrossRef]
- Huang, C.-D.; Hwang, J.-R.; Huang, J.-Y. Optimization of Vacuum Brazing Process Parameters in Ti-6Al-4V Alloy. Metals 2022, 12, 974. [Google Scholar] [CrossRef]
- Liu, S.; Miao, J.; Zhang, W.; Wei, R.; Chen, C.; Wang, T.; Zhao, W.; Jiang, Z.; Li, F. Interfacial microstructure and shear strength of TC4 alloy joints vacuum brazed with Ti–Zr–Ni–Cu filler metal. Mater. Sci. Eng. A 2020, 775, 138990. [Google Scholar] [CrossRef]
- Sypien, A. Microstructural and Mechanical Characterization of Interfacial Phenomena Occurring in Brazing Ti-6AL-4V Alloy Using TiZrCuPd Amorphous Ribbons. J. Mater. Eng. Perform. 2023, 32, 5731–5740. [Google Scholar] [CrossRef]
- Anil Kumar, S.V.; Gandhinathan, R. Optimization of process parameters for titanium alloy to itself and stainless steel brazed joints using BAg22 filler metal. Mater. Today Proc. 2021, 46, 9454–9461. [Google Scholar] [CrossRef]
- Huang, L.L.; Qin, J.; Huang, J.L.; Yu, H.; Jiang, C.; Song, L.Y.; Yu, Z.L.; Liao, Z.Q.; Cai, Y.Z.; Ma, L.; et al. Effect of Zr on microstructure and mechanical properties of 304 stainless steel joints brazed by Ag–Cu–Sn–In filler metal. J. Iron Steel Res. Int. 2024, 31, 2448–2462. [Google Scholar] [CrossRef]
- GB/T 11363-2008; Metallic Materials—Shear Test Method for Brazed Joints. Standardization Administration of China: Beijing, China, 2008.
- Lee, J.G.; Kim, G.H.; Lee, M.K.; Rhee, C.K. Intermetallic formation in a Ti–Cu dissimilar joint brazed using a Zr-based amorphous alloy filler. Intermetallics 2010, 184, 529–535. [Google Scholar] [CrossRef]
- Kertscher, R.; Brunatto, S.F. On the kinetics of nitride and diffusion layer growth in niobium plasma nitriding. Surf. Coat. Technol. 2020, 401, 126220. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Yang, W.; Zhang, Q.; Yang, B.; Hu, Y.; Si, X.; Lin, T.; Cao, J.; Qi, J.; et al. Interfacial Microstructure and Mechanical Properties of 1Cr18Ni9Ti/1Cr21Ni5Ti Stainless Steel Joints Brazed with Mn-Based Brazing Filler. Materials 2022, 15, 7021. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, Y.; Tu, Y.; Wang, X.; Ni, Z.; Peng, J.; Chen, X.; Yu, H.; Raza, S.R.A.; Yin, S. Mechanism of liquid film migration and interface reaction in multi-layer aluminum sheets during brazing based on the homogenization annealing. J. Mater. Res. Technol. 2025, 38, 5166–5179. [Google Scholar] [CrossRef]
- Akbari, E.; Kürnsteiner, P.; Steineder, K.; Gruber, M.; Arndt, M.; Letofsky-Papst, I.; Sierlinger, R.; Groiss, H. Insight into grain boundaries with reduced liquid metal embrittlement susceptibility in a boron-added 3rd generation advanced high strength steel. Mater. Des. 2024, 237, 112584. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhou, H.L.; Zhao, W.X.; Qiao, Y.X.; Cai, X.; Xue, Y.; An, Y.L.; Liu, N. High-temperature CMAS corrosion behavior and thermo-physical properties of high-entropy (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Ce2O7 thermal barrier coating. J. Alloys Compd. 2025, 1018, 179216. [Google Scholar] [CrossRef]
- Yue, G.L.; Chen, T.C.; Shiue, R.K.; Tsay, L.W. Dissimilar Brazing of Ti-15Mo-5Zr-3Al and Commercially Pure Titanium Using Ti-Cu-Ni Foil. Materials 2021, 14, 5949. [Google Scholar] [CrossRef]
- Ji, C.; Qin, Y.; Chen, T.; Hu, W.; Zhang, Y. Contact reaction brazing of TC4 alloy with Ni interlayer: Interfacial reaction mechanisms and mechanical enhancement. Intermetallics 2025, 186, 108938. [Google Scholar] [CrossRef]
- Mirabad, H.M.; Akbarpour, M.R.; Gazani, F.; Chadegani, A.A.; Khezri, I.; Mirzamohammad, A.; Damghani, H.; Elahi, O.; Khameneh, M.N.; Kim, H.S. The microstructure-mechanical property relationships in Ti–Cu alloys for biomedical applications: A review. J. Mater. Res. Technol. 2025, 37, 3155–3181. [Google Scholar] [CrossRef]
- Chu, J.; Jiang, X.; Sun, H.; Liu, T.; Wu, Z.; Yang, Y. Effect of Ti and Zr on high temperature mechanical and thermal properties of MoCu composites. Mater. Charact. 2024, 208, 113635. [Google Scholar] [CrossRef]
- Ganjeh, E.; Sarkhosh, H. Mechanical and fractographical study of titanium-CP and Ti–6Al–4V similar brazing with Ti-based filler. Mater. Sci. Eng. A 2013, 559, 119–129. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, C.T.; Song, L.J.; Cai, X.; Chen, J.; Chen, Z.L.; Zhang, Q.C.; Jiang, Y.S.; Qiao, Y.X. Effect of temperature on corrosion behaviors of copper in high-level nuclear waste disposal environment. J. Mater. Res. Technol. 2025, 36, 5717–5726. [Google Scholar] [CrossRef]
- Hulka, I.; Uțu, I.-D.; Brito-Garcia, S.; Verdu-Vazquez, A.; Mirza-Rosca, J.C. Electrochemical Study and Mechanical Properties of Ti-Zr Alloy for Biomedical Applications. Crystals 2024, 14, 493. [Google Scholar] [CrossRef]

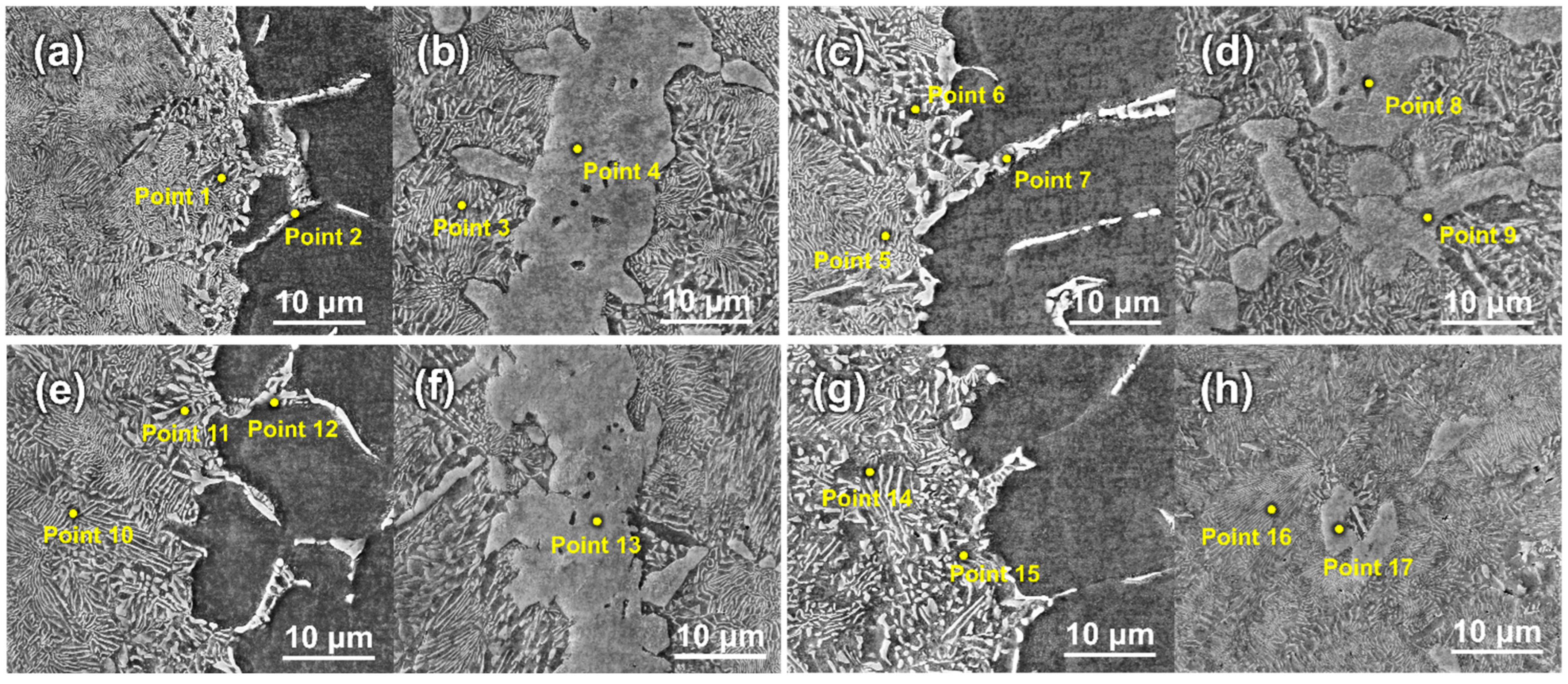
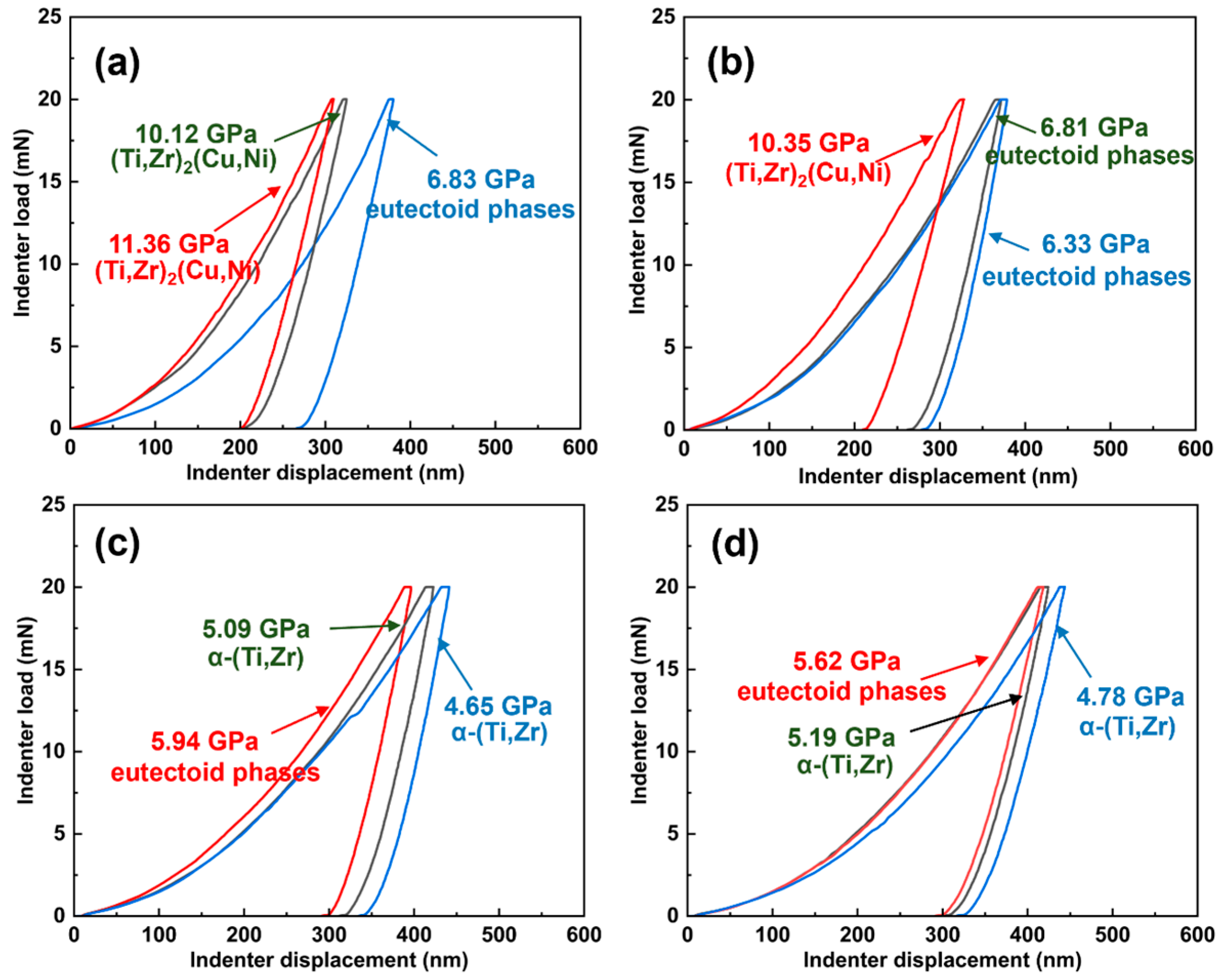
| Points | Ti | Zr | Cu | Ni | Possible Phases |
|---|---|---|---|---|---|
| 1 | 72.18 | 14.77 | 9.76 | 3.29 | α-(Ti,Zr) |
| 2 | 82.27 | 1.95 | 2.02 | 13.76 | eutectoid phases |
| 3 | 68.60 | 21.35 | 6.88 | 3.18 | α-(Ti,Zr) |
| 4 | 32.32 | 41.10 | 15.15 | 11.43 | (Ti,Zr)2(Cu,Ni) |
| 5 | 68.71 | 19.09 | 7.35 | 4.85 | α-(Ti,Zr) |
| 6 | 65.88 | 22.57 | 6.15 | 5.41 | α-(Ti,Zr) |
| 7 | 70.07 | 10.02 | 11.19 | 8.72 | eutectoid phases |
| 8 | 31.42 | 41.51 | 16.29 | 10.77 | (Ti,Zr)2(Cu,Ni) |
| 9 | 30.95 | 42.63 | 14.84 | 11.59 | (Ti,Zr)2(Cu,Ni) |
| 10 | 67.63 | 19.51 | 6.75 | 6.10 | α-(Ti,Zr) |
| 11 | 76.66 | 17.09 | 3.48 | 2.77 | α-(Ti,Zr) |
| 12 | 66.14 | 9.74 | 14.61 | 9.51 | eutectoid phases |
| 13 | 34.82 | 39.29 | 15.44 | 10.45 | (Ti,Zr)2(Cu,Ni) |
| 14 | 76.08 | 12.40 | 8.87 | 2.65 | α-(Ti,Zr) |
| 15 | 68.22 | 8.91 | 13.45 | 9.41 | eutectoid phases |
| 16 | 60.91 | 19.76 | 15.05 | 4.28 | eutectoid phases |
| 17 | 37.87 | 35.54 | 11.63 | 14.96 | (Ti,Zr)2(Cu,Ni) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Zhou, H.; Lu, S.; Wang, Z.; Dobuvyy, O. Microstructure and Properties of TA2 Titanium Joints Brazed with Ti–Zr–Cu–Ni Filler Metal. Metals 2025, 15, 1218. https://doi.org/10.3390/met15111218
Xiao Z, Zhou H, Lu S, Wang Z, Dobuvyy O. Microstructure and Properties of TA2 Titanium Joints Brazed with Ti–Zr–Cu–Ni Filler Metal. Metals. 2025; 15(11):1218. https://doi.org/10.3390/met15111218
Chicago/Turabian StyleXiao, Zimeng, Huiling Zhou, Sheng Lu, Zexin Wang, and Oleksandr Dobuvyy. 2025. "Microstructure and Properties of TA2 Titanium Joints Brazed with Ti–Zr–Cu–Ni Filler Metal" Metals 15, no. 11: 1218. https://doi.org/10.3390/met15111218
APA StyleXiao, Z., Zhou, H., Lu, S., Wang, Z., & Dobuvyy, O. (2025). Microstructure and Properties of TA2 Titanium Joints Brazed with Ti–Zr–Cu–Ni Filler Metal. Metals, 15(11), 1218. https://doi.org/10.3390/met15111218





