The Effect of In-Situ-Grown Graphene from Highland Barley Powder on the Properties of Copper Matrix Materials
Abstract
1. Introduction
2. Experiment
2.1. Experimental Material
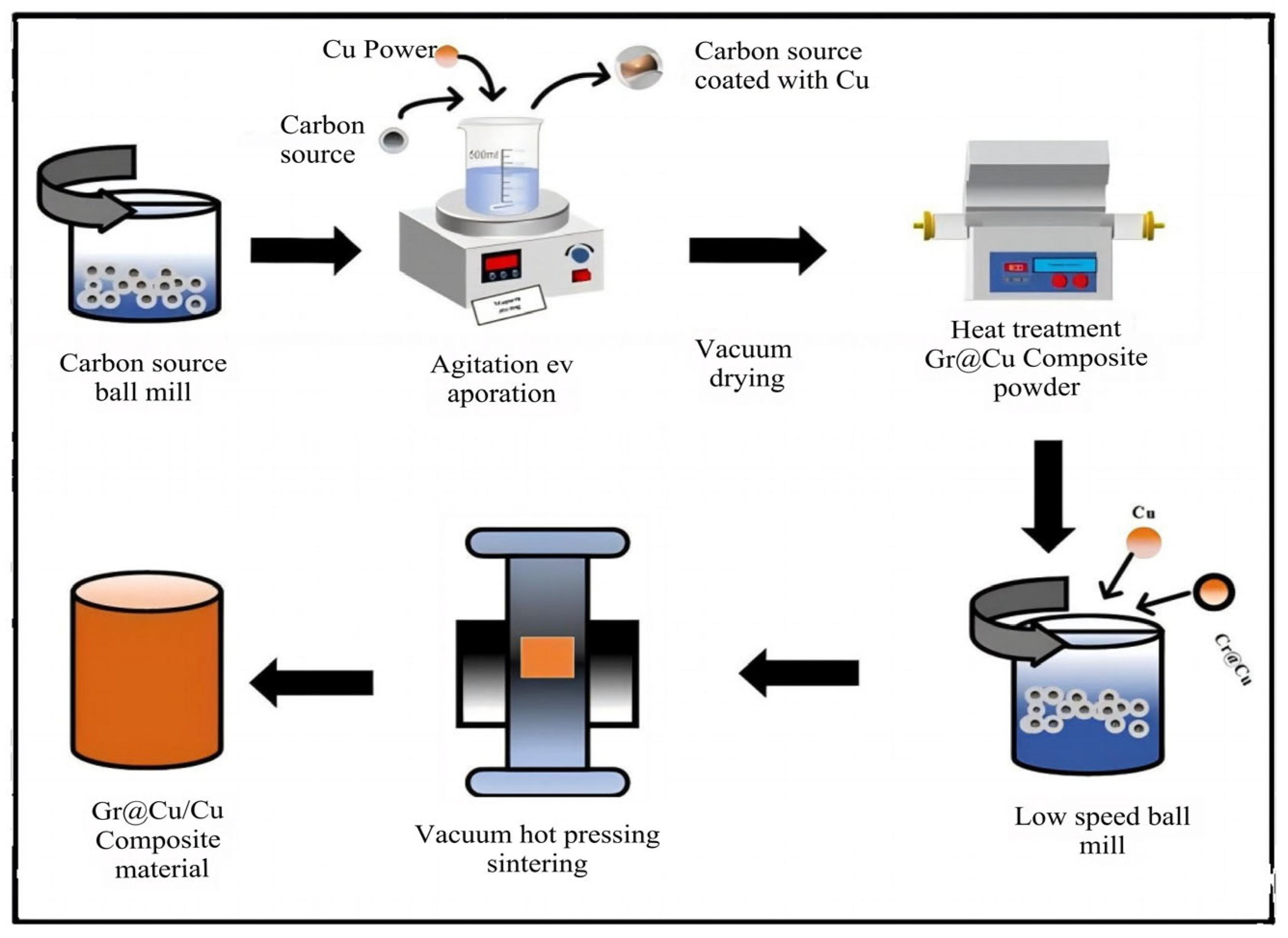
2.2. Gr@Cu Preparation of Composite Powder
- (1)
- Copper-coated barley powder: According to a ball material ratio of 10:1, high-energy ball milling treatment of barley powder was carried out for 1 h, so that it was more soluble in anhydrous ethanol. Mix the ground barley powder with copper powder in a mass ratio of 1:4 by adding anhydrous ethanol. The beaker was heated to 70 °C, and a copper-powder-coated highland barley powder was obtained after stirring until the alcohol was completely evaporated.
- (2)
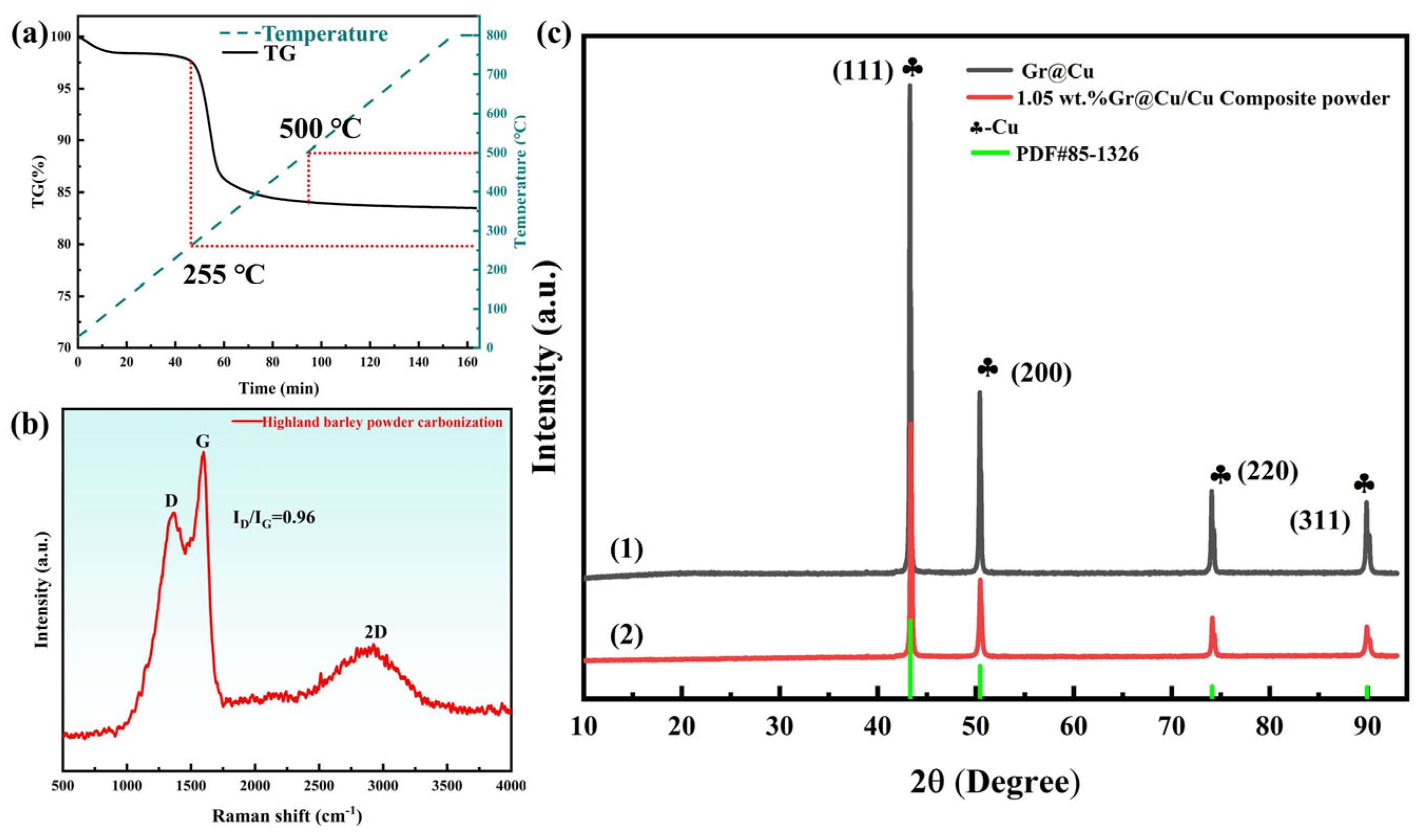
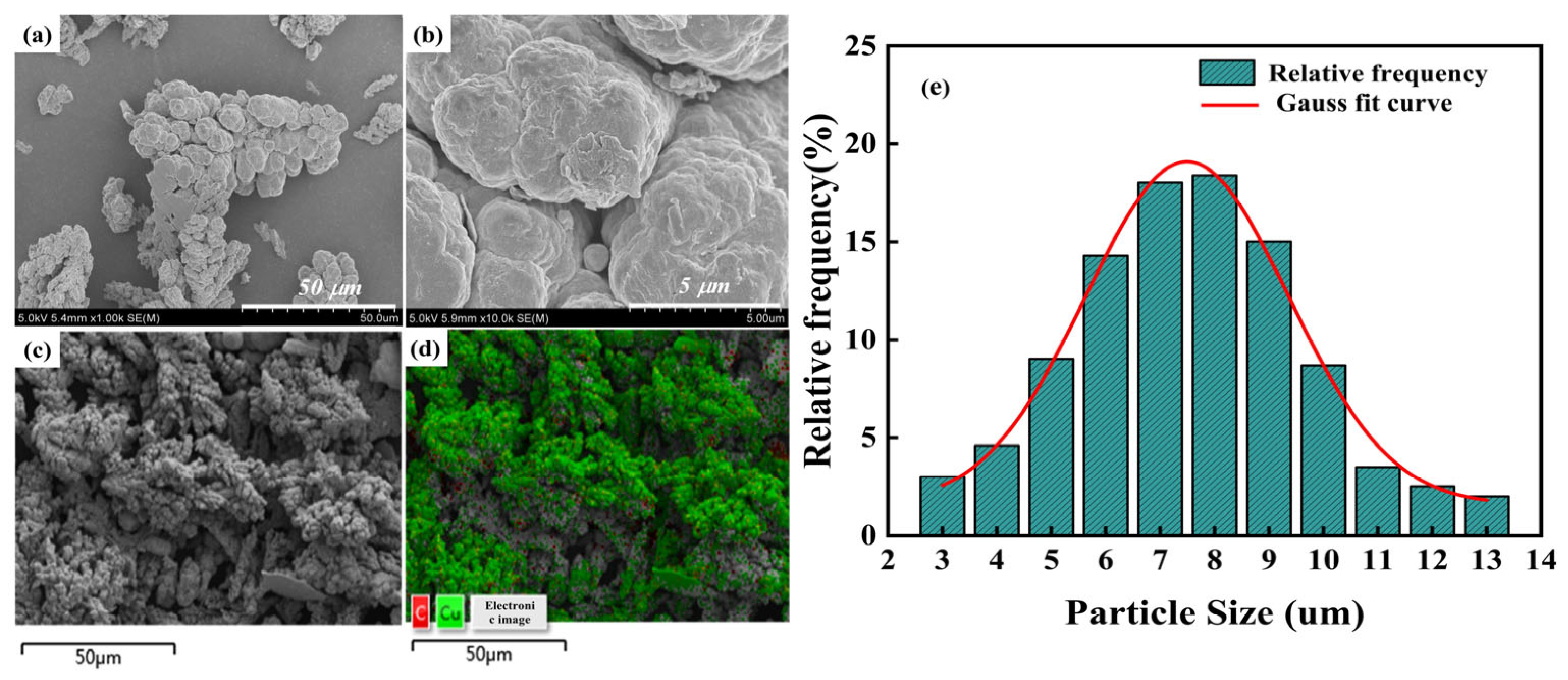
- Graphene growth: A tube furnace was used to heat treat the copper-coated upland barley powder to grow graphene. The rate of temperature increase was 5 °C/min, and nitrogen was introduced as a protective gas; when the temperature was increased to 800 °C, the graphene was held for 10 min with a constant nitrogen flow rate of 200 mL/min. After the holding procedure, the temperature was reduced to room temperature to obtain the Gr@Cu composite powder. A thermogravimetric analysis of carbon-source-coated copper powder was carried out using the same temperature program as that of the tube furnace. It was determined that the Gr content of the Gr@Cu composite powder was about 4.98 wt.%.
2.3. Gr@Cu/Cu Composite Forming
2.4. Experimental Equipment
3. Results and Discussion
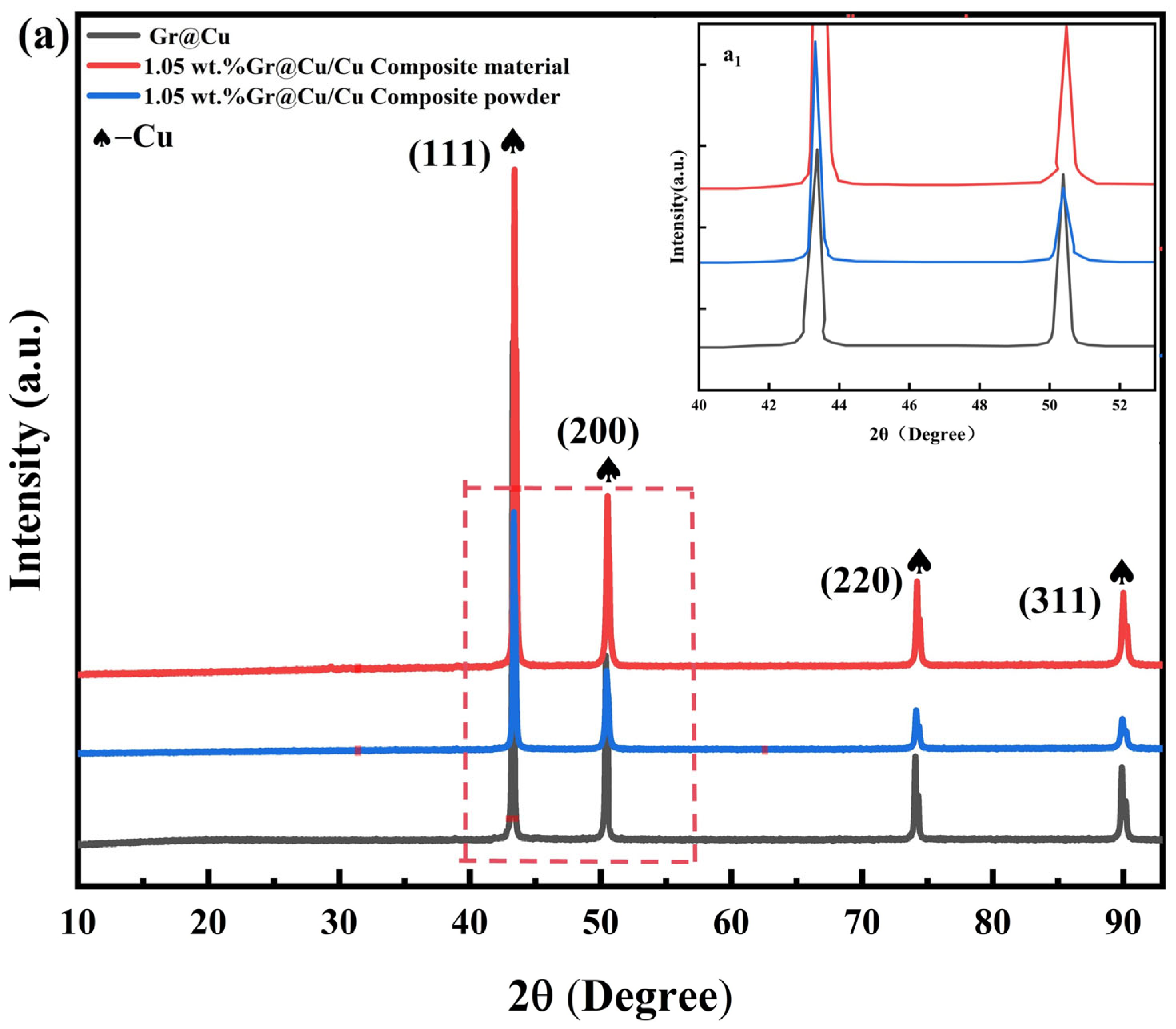
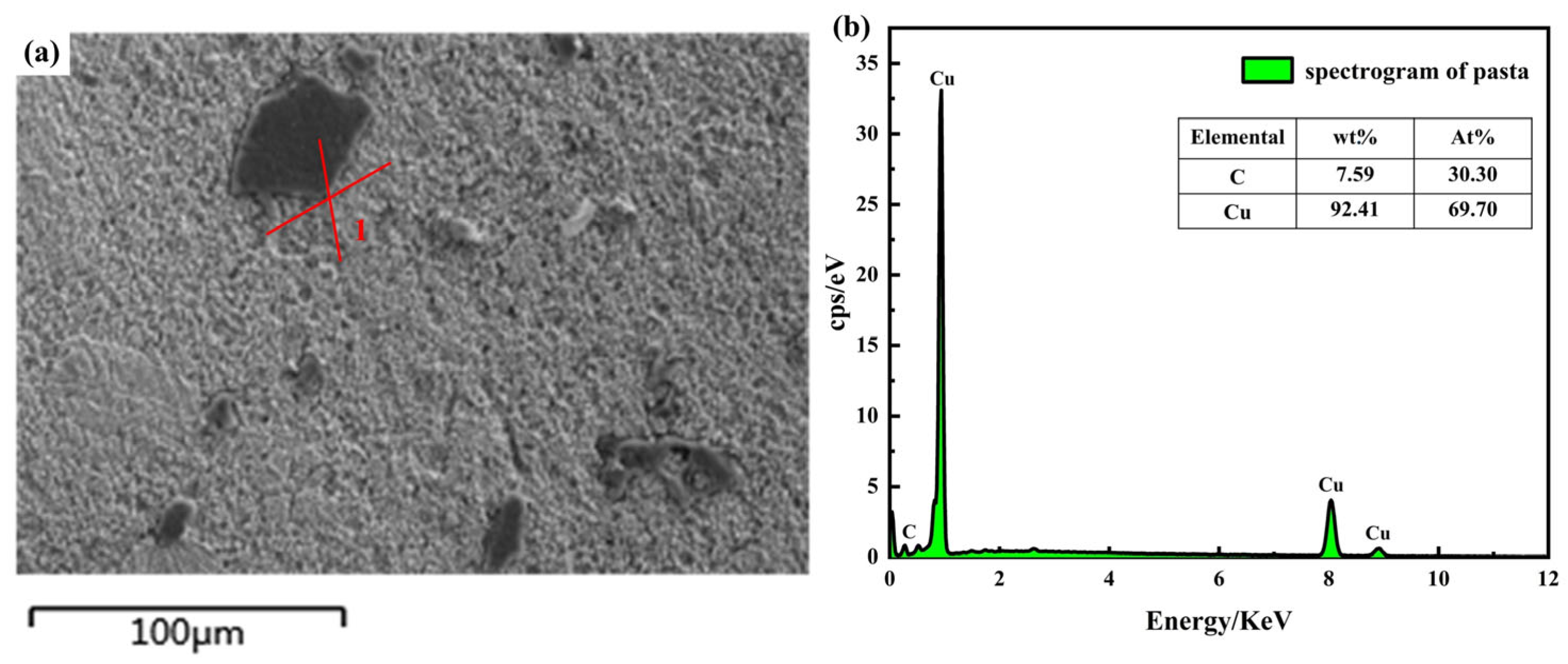
3.1. Test Characterization of Gr@Cu Composite Powders Prepared from Highland Barley Flour
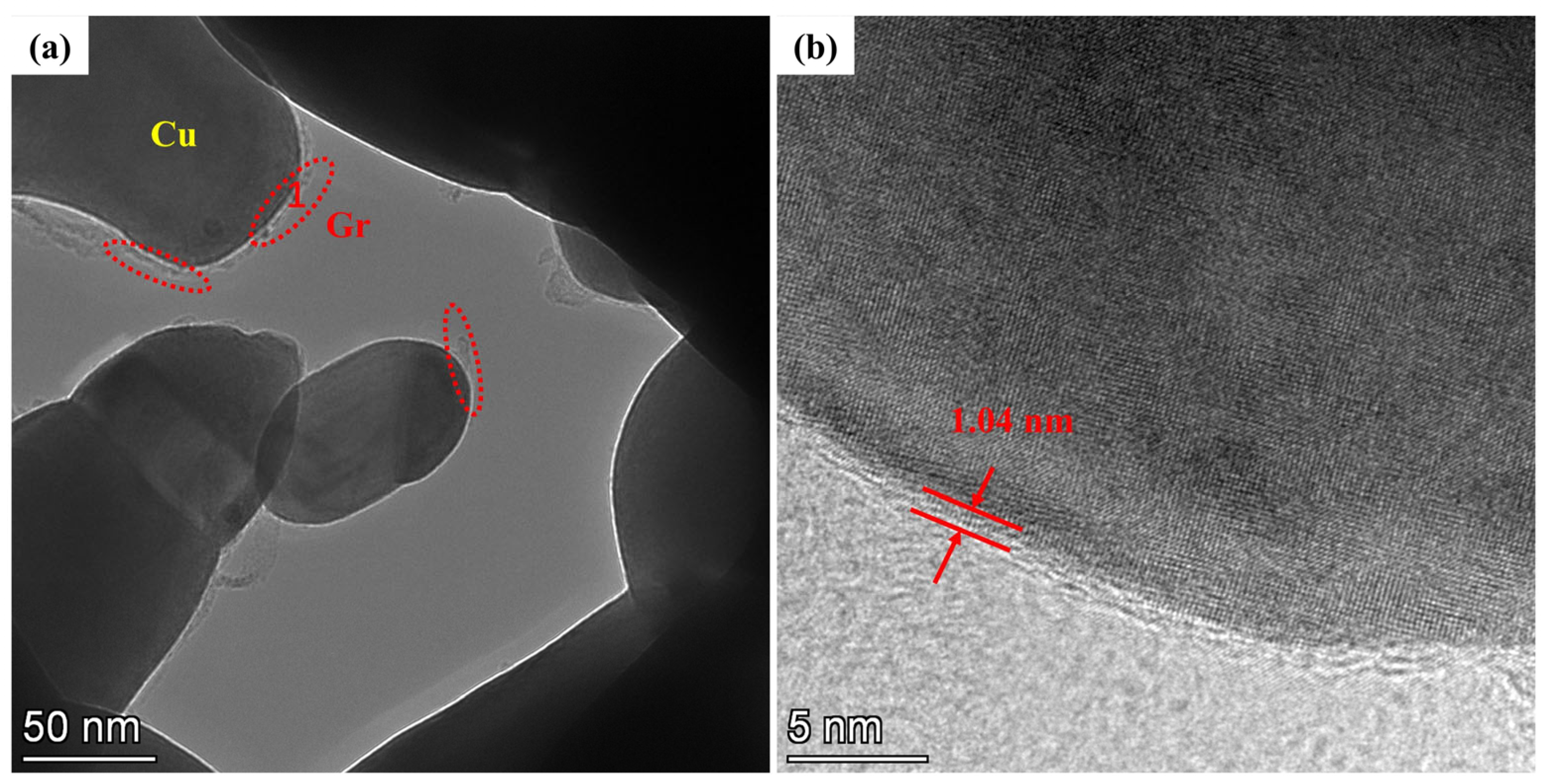
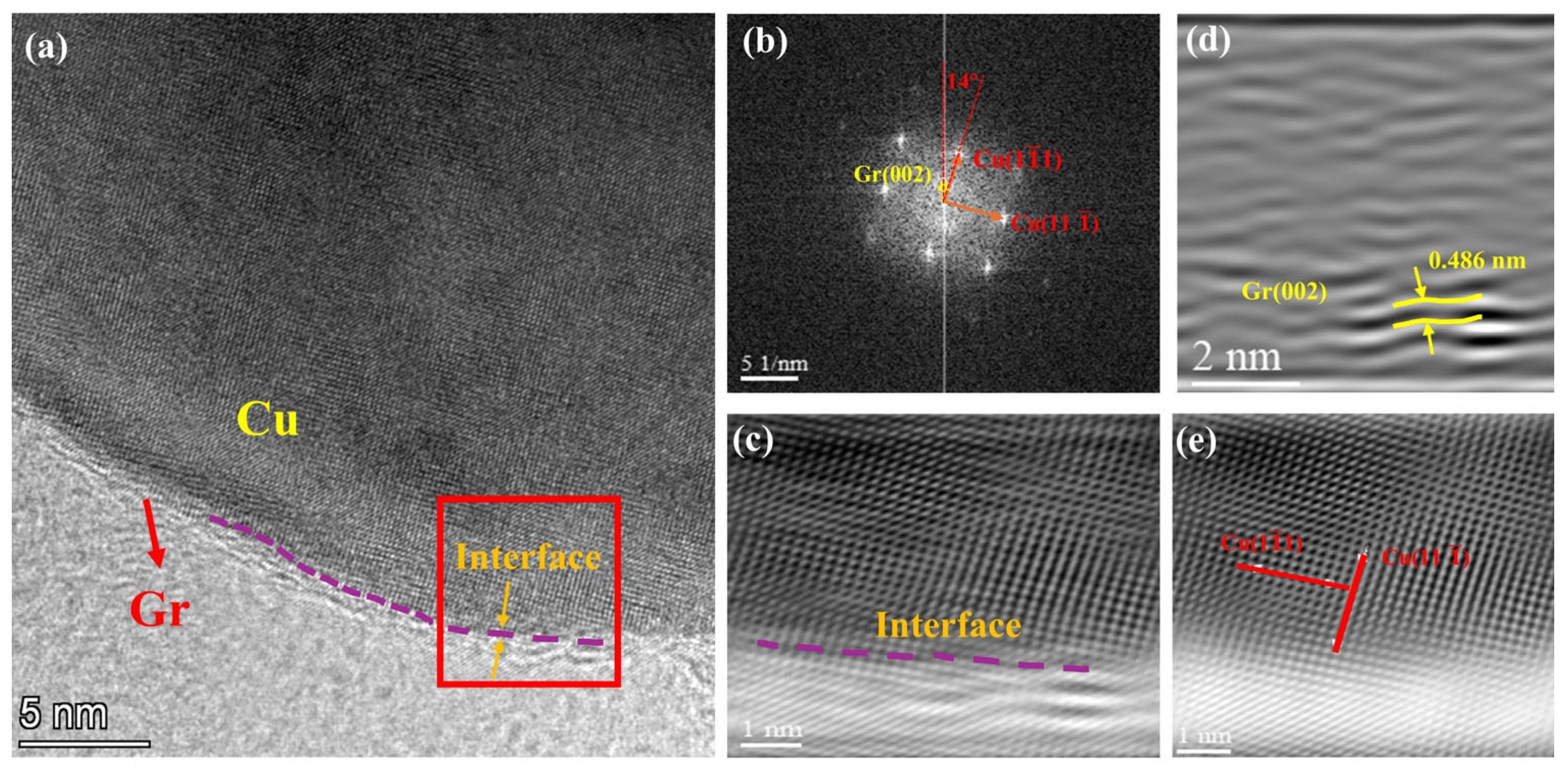
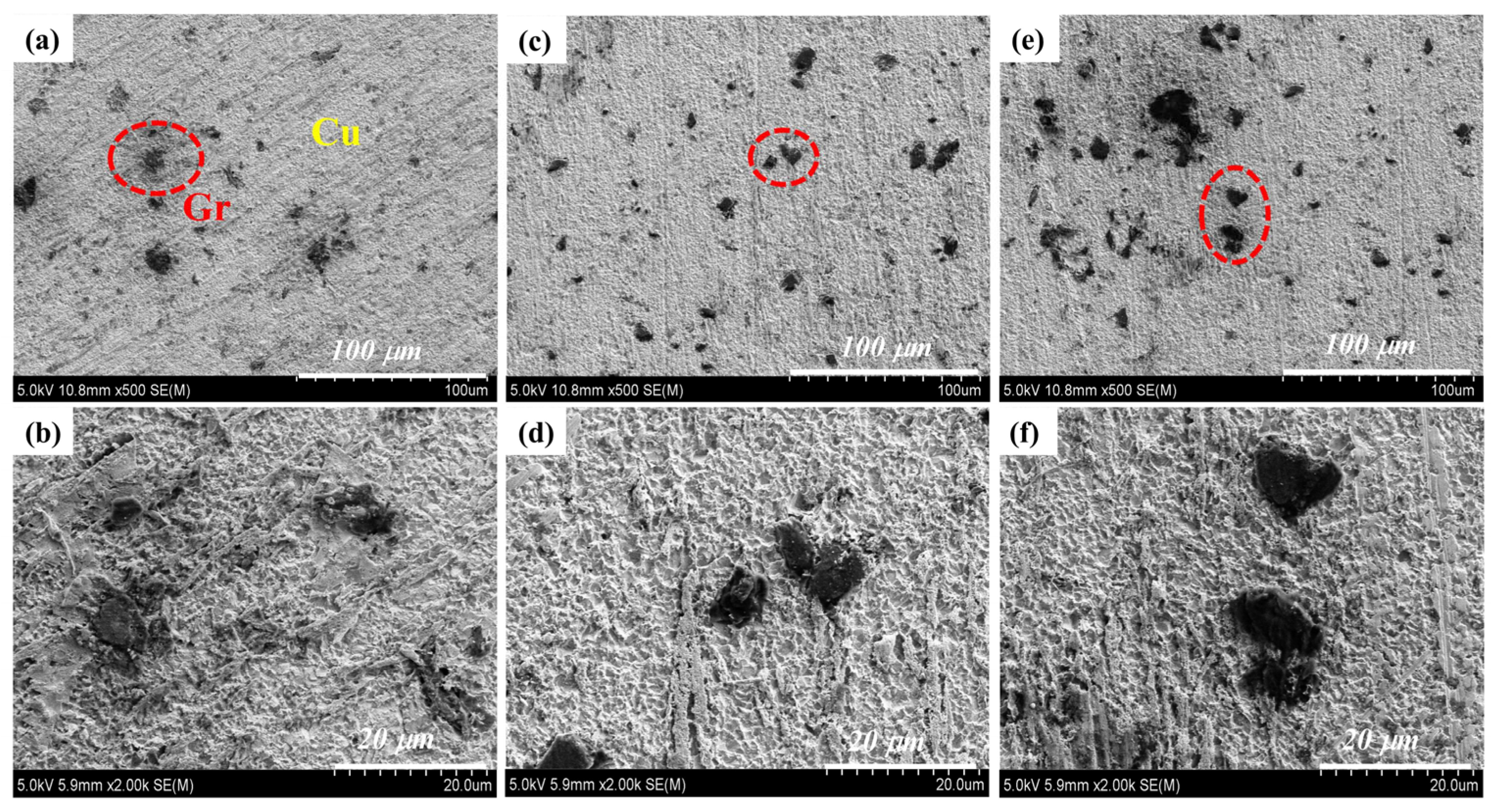
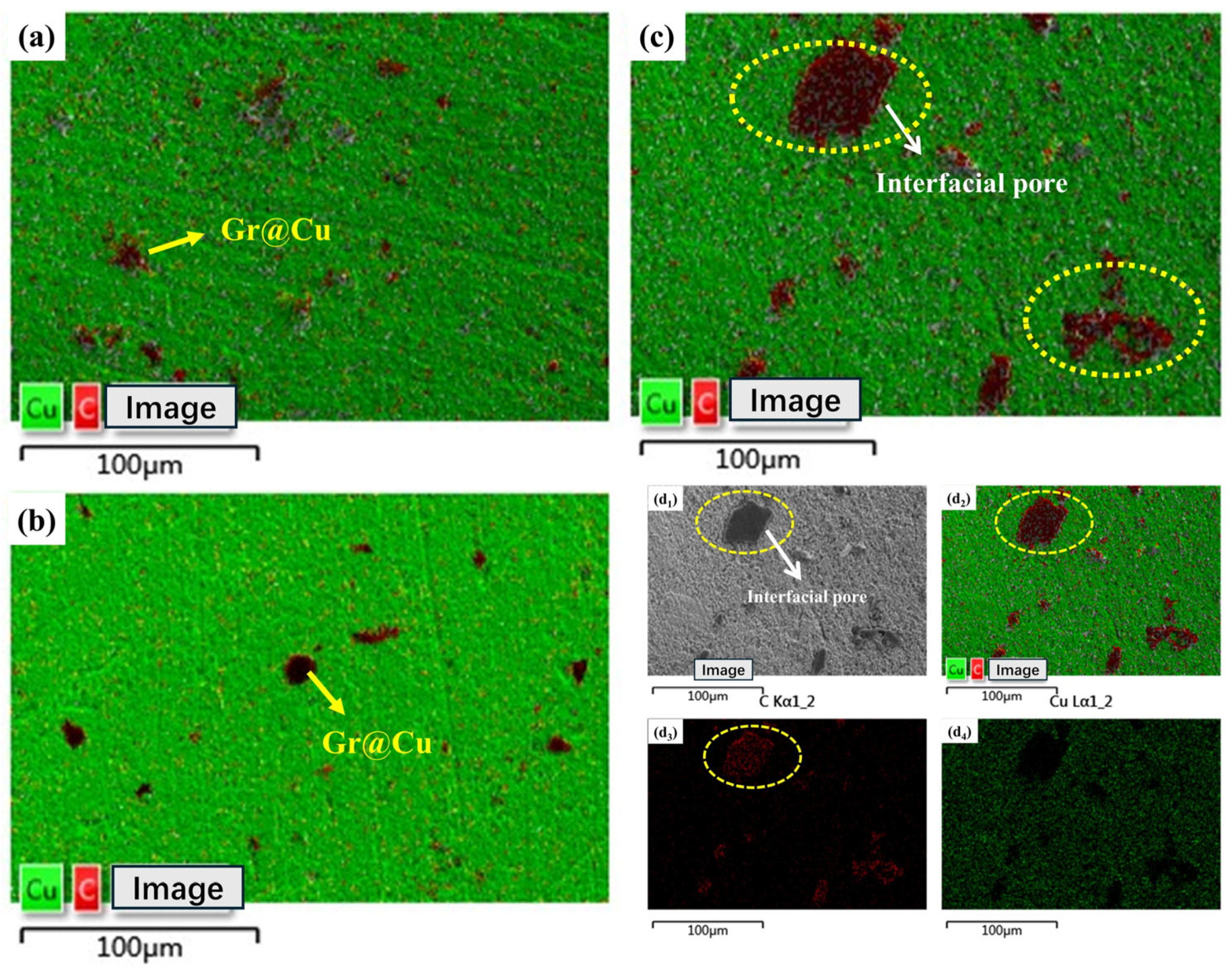
3.2. Microscopic Characterization of Gr@Cu/Cu Composites
3.3. Density and Electrical Properties of Gr@Cu/Cu Composites
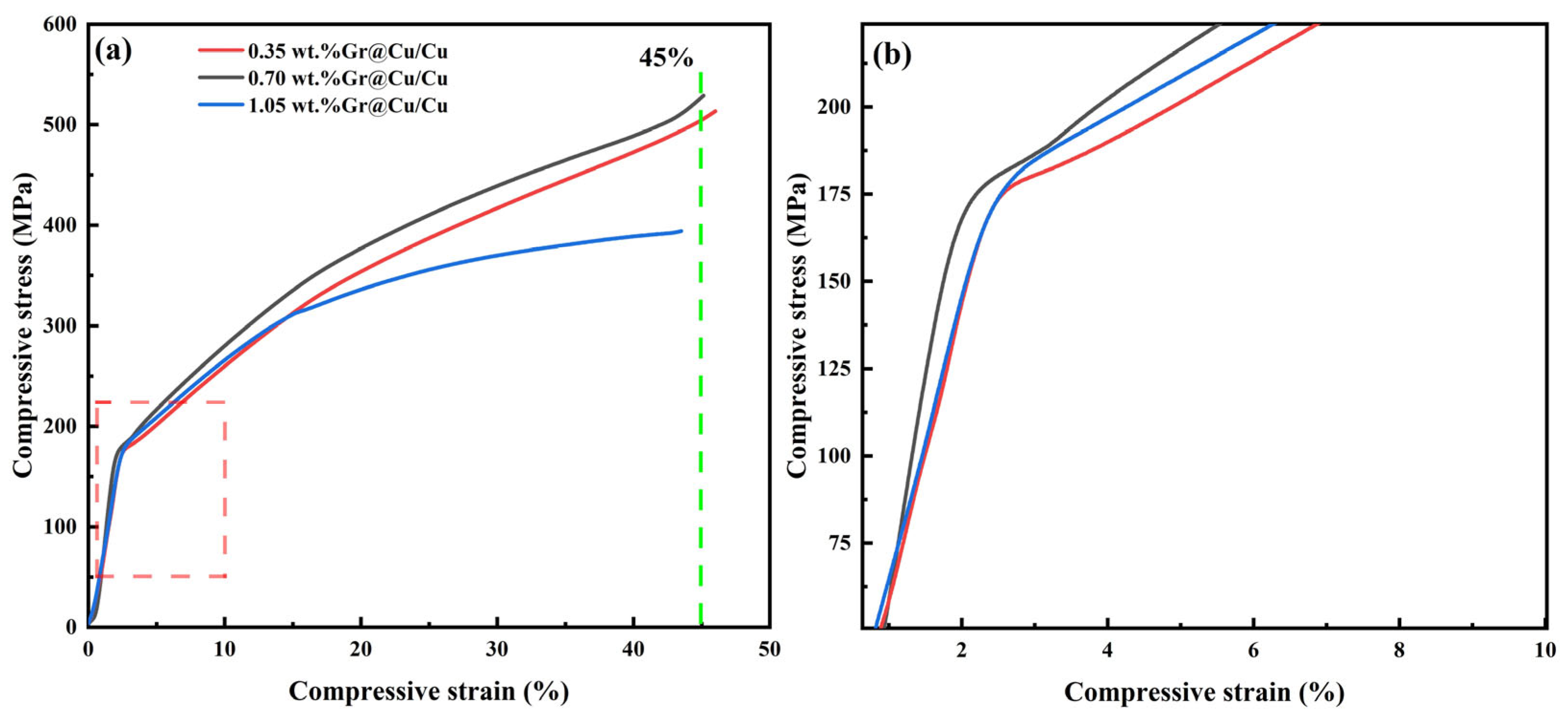
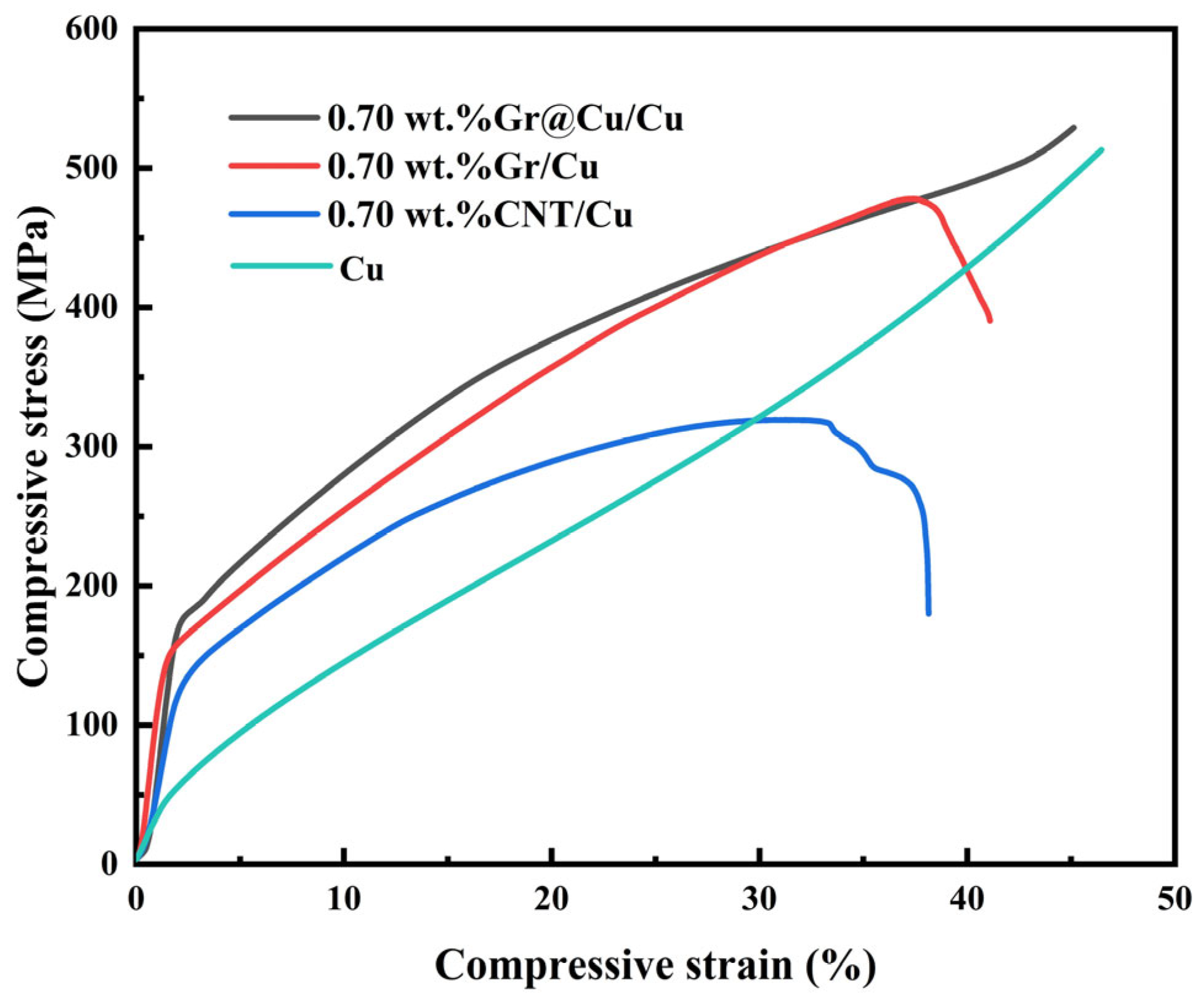
3.4. Mechanical Properties of Gr@Cu/Cu Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.H.; Wang, W.S.; Hu, Y.C.; Tsai, C.H.; Shih, C.J.; Huang, W.C.; Peng, S.M.; Lee, G.H. A study of novel macrocyclic copper complex/graphene–based composite materials for counter electrodes of dye–sensitized solar cells. J. Chin. Chem. Soc. 2019, 66, 996–1007. [Google Scholar] [CrossRef]
- Yan, Y.F.; Kou, S.Q.; Yang, H.Y.; Shu, S.L.; Qiu, F.; Jiang, Q.C.; Zhang, L.C. Ceramic particles reinforced copper matrix composites manufactured by advanced powder metallurgy: Preparation, performance, and mechanisms. Int. J. Extrem. Manuf. 2023, 5, 032006. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Mohanavel, V.; Kumar, S.D.; Sathish, T.; Rajkumar, S.; Subbiah, R. Copper Based Powder Metallurgy Composite for Electrical Applications. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2022; Volume 1068, pp. 151–158. [Google Scholar]
- Xu, Z.; Zhao, J.; Liu, M.; Liu, Z.; Cheng, X.; Chang, J.; Yang, X.; Li, B.; Liu, B.; Liu, R. Enhanced strength and toughness of SiC/C composite ceramics via SiC@graphene core–shell nanoparticles. J. Am. Ceram. Soc. 2025, 108, e20151. [Google Scholar] [CrossRef]
- Zhou, W.; Hong, L.; Wang, Y.; Ye, N.; Tang, J. Effect of sintering temperature on microstructure and properties of nano-WC particle reinforced copper matrix composites prepared by hot-pressing sintering. Mater. Res. Express 2020, 7, 126520. [Google Scholar] [CrossRef]
- Balashabadi, P.; Larijani, M.M.; Jafari-Khamse, E.; Seyedi, H. The role of Cu content on the structural properties and hardness of TiN–Cu nanocomposite film. J. Alloys Compd. 2017, 728, 863–871. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Liang, S.; Ren, J.; Du, X.; Liu, F. TiB2 (-TiB)/Cu in-situ composites prepared by hot-press with the sintering temperature just beneath the melting point of copper. Mater. Charact. 2016, 121, 76–81. [Google Scholar] [CrossRef]
- Fathy, A.; Shehata, F.; Abdelhameed, M.; Elmahdy, M. Compressive and wear resistance of nanometric alumina reinforced copper matrix composites. Mater. Des. (1980–2015) 2012, 36, 100–107. [Google Scholar] [CrossRef]
- Dong, Z.; Peng, Y.; Zhang, X.; Xiong, D.B. Plasma assisted milling treatment for improving mechanical and electrical properties of in-situ grown graphene/copper composites. Compos. Commun. 2021, 24, 100619. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, F.; Lin, D.; Nian, Q.; Parandoush, P.; Zhu, X.; Shao, Z.; Cheng, G.J. Laser additive manufacturing bulk graphene–copper nanocomposites. Nanotechnology 2017, 28, 445705. [Google Scholar] [CrossRef]
- Nedumthakady, N.; Bhaskar, P.; Smet, V. Magneto-Assisted Graphene Reinforcement: A New Method to Enhance Nanostructure and Properties of Electrodeposited Copper. In Proceedings of the 2023 IEEE 73rd Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 30 May–2 June 2023. [Google Scholar]
- Liu, G.; Tao, J.; Li, F.; Bao, R.; Liu, Y.; Li, C.; Yi, J. Optimizing the interface bonding in Cu matrix composites by using functionalized carbon nanotubes and cold rolling. J. Mater. Res. 2019, 34, 2600–2608. [Google Scholar] [CrossRef]
- Xiu, Z.; Ju, B.; Zhan, J.; Zhang, N.; Wang, Z.; Mei, Y.; Liu, J.; Feng, Y.; Guo, Y.; Kang, P.; et al. Microstructure evolution of graphene and the corresponding effect on the mechanical/electrical properties of graphene/Cu composite during rolling treatment. Materials 2022, 15, 1218. [Google Scholar] [CrossRef]
- Guiderdoni, C.; Estournès, C.; Peigney, A.; Weibel, A.; Turq, V.; Laurent, C. The preparation of double-walled carbon nanotube/Cu composites by spark plasma sintering, and their hardness and friction properties. Carbon 2011, 49, 4535–4543. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, H.-B.; Du, M.; Lu, Y.-H. Surface modification of carbon nanotubes and its application in copper matrix composites. J. Funct. Mater. 2019, 50, 4201–4206. [Google Scholar]
- Yan, Q.; Chen, B.; Zhou, X.; Kondoh, K.; Li, J. Effect of Metal Powder Characteristics on Structural Defects of Graphene Nanosheets in Metal Composite Powders Dispersed by Ball Milling. Crystals 2021, 11, 260. [Google Scholar] [CrossRef]
- Zhao, W.M.; Bao, R.; Yi, J.H.; Hou, X.H.; Fang, D.; Liu, C.X. Fabrication of RGO/Cu composites based on electrostatic adsorption. Trans. Nonferrous Met. Soc. China 2020, 30, 982–991. [Google Scholar] [CrossRef]
- Nie, H.; Fu, L.; Zhu, J.; Yang, W.; Li, D.; Zhou, L. Excellent tribological properties of lower reduced graphene oxide content copper composite by using a one-step reduction molecular-level mixing process. Materials 2018, 11, 600. [Google Scholar] [CrossRef]
- Yang, T.; Chen, W.; Zhang, H.; Ma, L.; Fu, Y.Q. In-situ generated graphene from wheat flour for enhancing mechanical and electrical properties of copper matrix composites. Mater. Sci. Eng. A 2022, 835, 142662. [Google Scholar] [CrossRef]
- Chung, T.F.; Shen, T.; Cao, H.; Jauregui, L.A.; Wu, W.; Yu, Q.; Newell, D.; Chen, Y.P. Synthetic graphene grown by chemical vapor deposition on copper foils. Int. J. Mod. Phys. B 2013, 27, 1341002. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef]
- Liu, L.; Ryu, S.; Tomasik, M.R.; Stolyarova, E.; Jung, N.; Hybertsen, M.S.; Steigerwald, M.L.; Brus, L.E.; Flynn, G.W. Graphene oxidation: Thickness-dependent etching and strong chemical doping. Nano Lett. 2008, 8, 1965–1970. [Google Scholar] [CrossRef]
- Chu, K.; Wang, J.; Liu, Y.; Geng, Z. Graphene defect engineering for optimizing the interface and mechanical properties of graphene/copper composites. Carbon 2018, 140, 112–123. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Agrawal, A.; Charleston, J.; Mirzaeifar, R. Experimental investigation of mechanical behavior at the microstructure of copper-graphene thin films. Mater. Sci. Eng. A 2023, 862, 144492. [Google Scholar] [CrossRef]
- Dong, L.L.; Fu, Y.Q.; Liu, Y.; Lu, J.W.; Zhang, W.; Huo, W.T.; Jin, L.H.; Zhang, Y.S. Interface engineering of graphene/copper matrix composites decorated with tungsten carbide for enhanced physico-mechanical properties. Carbon 2021, 173, 41–53. [Google Scholar] [CrossRef]
- Jiang, L.; Wen, H.; Yang, H.; Hu, T.; Topping, T.; Zhang, D.; Lavernia, E.J.; Schoenung, J.M. Influence of length-scales on spatial distribution and interfacial characteristics of B4C in a nanostructured Al matrix. Acta Mater. 2015, 89, 327–343. [Google Scholar] [CrossRef]
- McQuade, G.A.; Plaut, A.S.; Usher, A.; Martin, J. The thermal expansion coefficient of monolayer, bilayer, and trilayer graphene derived from the strain induced by cooling to cryogenic temperatures. Appl. Phys. Lett. 2021, 118, 203101. [Google Scholar] [CrossRef]
- Abdel-Aal, S.K.; Beskrovnyi, A.I.; Ionov, A.M.; Mozhchil, R.N.; Abdel-Rahman, A.S. structure investigation by neutron diffraction and x-ray diffraction of graphene nanocomposite CuO–rGO prepared by low-cost method. Phys. Status Solidi (a) 2021, 218, 2100138. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, S.; Liu, Y.; Yan, Z.; Chen, G.; Yang, J.; Zhang, W. Effect of Temperature on the Mechanical Properties, Conductivity, and Microstructure of Multi-layer Graphene/Copper Composites Fabricated by Extrusion. J. Mater. Eng. Perform. 2025, 34, 7773–7785. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, L.; Wang, B.; Hu, Y.; Cai, Z.; Ren, J.; Liu, J.; Li, S. Microstructure and mechanical behavior of carbon fiber reinforced carbon, silicon carbide, and copper alloy hybrid composite fabricated by Cu-Si alloy melt infiltration. Adv. Compos. Hybrid Mater. 2023, 6, 25. [Google Scholar] [CrossRef]

| Reinforcement Type | Preparation Method | Carbon Source | Graphene Content (wt.%) | Yield Strength (MPa) | Conductivity (%IACS) | Reference |
|---|---|---|---|---|---|---|
| Gr | In situ growth | Highland barley powder | 0.70 | 175 | 70 | This work |
| Gr | In situ growth | Wheat flour | 0.75 | ~160 | ~75 | [19] |
| Gr | Ball milling | Commercial graphene | 0.50 | ~140 | ~65 | [16] |
| Gr | Molecular-level mixing | GO | 2.50 | ~210 | ~60 | [18] |
| Gr | CVD + PM | CH4 | 1.80 | ~190 | ~80 | [20] |
| CNT | Ball milling | Commercial CNT | 0.70 | 133 | (Not reported) | This work |
| Pure Cu | Powder metallurgy | - | 0 | 52 | 85 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Sun, C.; Tang, X.; Du, C.; Li, D.; Chen, C. The Effect of In-Situ-Grown Graphene from Highland Barley Powder on the Properties of Copper Matrix Materials. Metals 2025, 15, 1217. https://doi.org/10.3390/met15111217
Wang Z, Sun C, Tang X, Du C, Li D, Chen C. The Effect of In-Situ-Grown Graphene from Highland Barley Powder on the Properties of Copper Matrix Materials. Metals. 2025; 15(11):1217. https://doi.org/10.3390/met15111217
Chicago/Turabian StyleWang, Zhe, Changfei Sun, Xianglongtian Tang, Cheng Du, Denghui Li, and Cong Chen. 2025. "The Effect of In-Situ-Grown Graphene from Highland Barley Powder on the Properties of Copper Matrix Materials" Metals 15, no. 11: 1217. https://doi.org/10.3390/met15111217
APA StyleWang, Z., Sun, C., Tang, X., Du, C., Li, D., & Chen, C. (2025). The Effect of In-Situ-Grown Graphene from Highland Barley Powder on the Properties of Copper Matrix Materials. Metals, 15(11), 1217. https://doi.org/10.3390/met15111217






