Phase Equilibrium Relationship of CaO-Al2O3-Ce2O3-CaF2 Slag System at 1300~1500 °C
Abstract
1. Introduction
2. Experimental Procedure
2.1. Slag Sample Preparation
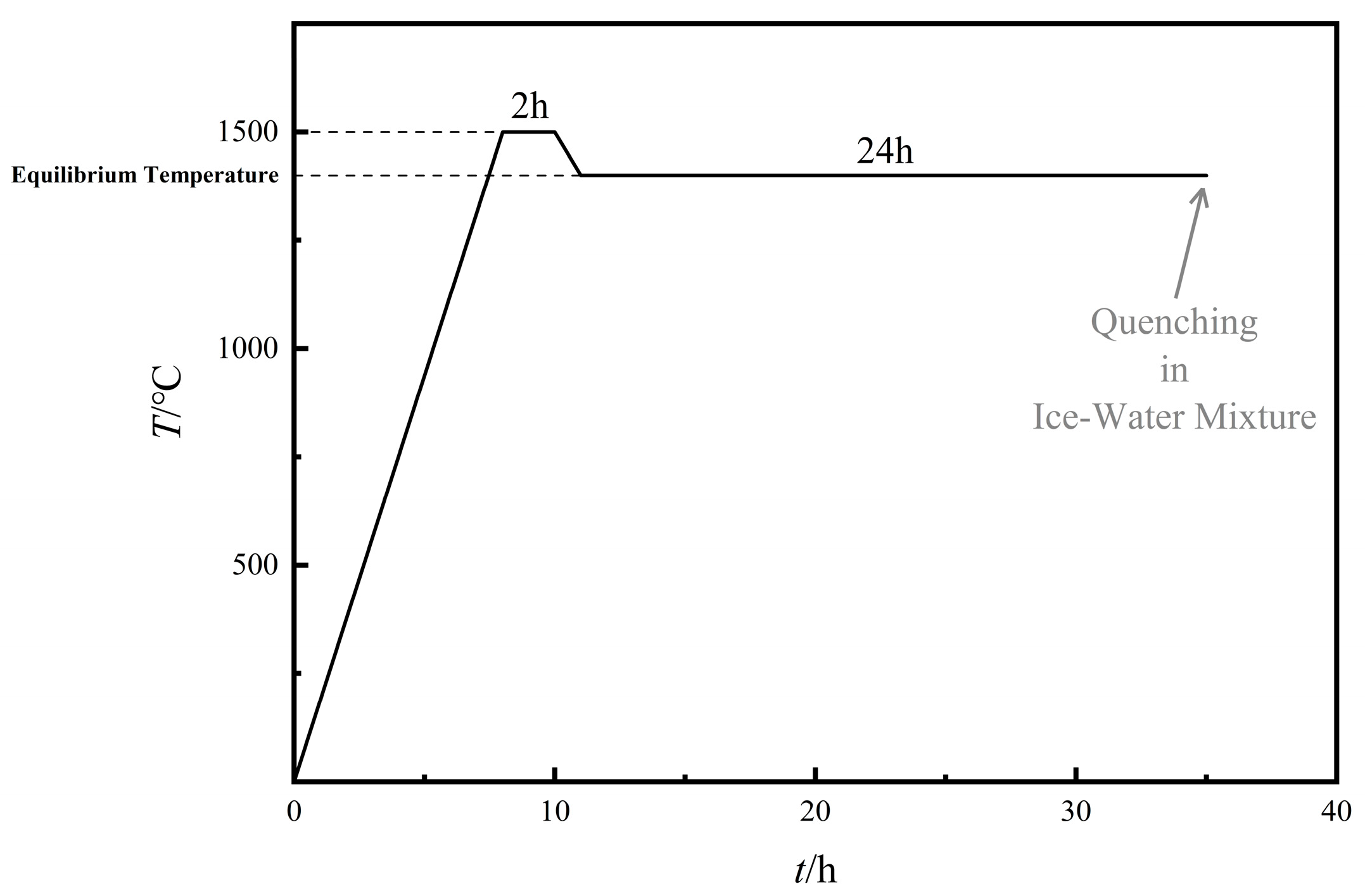
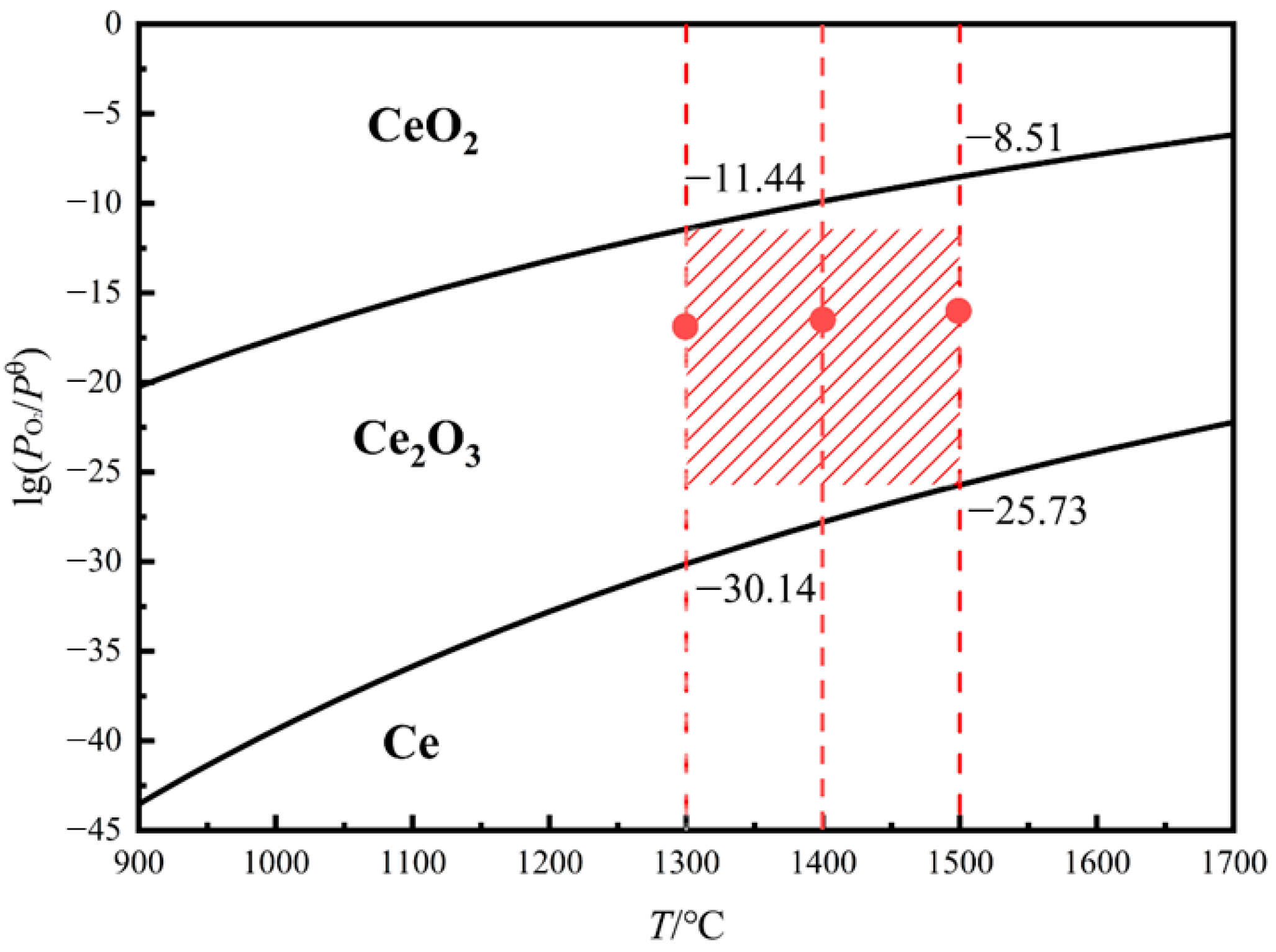
2.2. High Temperature Equilibrium Experiment
3. Phase Equilibrium Results
4. Drawing of Isothermal Phase Diagram
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Yu, Z.; Zhang, F. Handbook of Rare Earth Treated Steel; Metallurgical Industry Press: Beijing, China, 1993. [Google Scholar]
- China Association for Science and Technology; Chinese Society of Rare Earths. Development Report on Rare Earth Science and Technology; China Science and Technology Press: Beijing, China, 2016. [Google Scholar]
- Tang, X.; Song, W.; Yang, H.; Cheng, T.; Zeng, J. Technical Difficulties and Countermeasures in Casting of Rare Earth Treated Steel. Iron Steel Vanadium Titan. 2001, 22, 68–72. [Google Scholar]
- Wang, D. Study on the Influence of Rare Earth and Its Oxides on the Physicochemical Properties of Mold Fluxes for Continuous Casting. Ph.D. Thesis, Northeastern University, Shenyang, China, 2004. [Google Scholar]
- Liu, Z. Simulation Study on Mold Fluxes for Thin Slab Continuous Casting of Rare Earth Steel and Wire Feeding Process. Master’s Thesis, Chongqing University, Chongqing, China, 2006. [Google Scholar]
- Zeng, J.; Chen, T.; Zhao, Q.; Zhang, J.; Xiao, M. Development and Application of Mold Flux for Continuous Casting of Microalloyed Steel. Iron Steel Vanadium Titan. 2000, 4, 9–14. [Google Scholar]
- Xiang, S.; Wang, Y.; Xie, B. Effect of Rare Earth Oxides on Physicochemical Properties of Continuous Casting Mold Flux. Iron Steel Vanadium Titan. 2003, 2, 11–13. [Google Scholar]
- Blazek, K.; Yin, H.; Skoczylas, G.; McClymonds, M.; Frazee, M. Development and evaluation of lime alumina-based mold powders for casting high-aluminum TRIP steel grades. AIST Trans. 2011, 8, 232–240. [Google Scholar]
- Jeong, S.J.; Kim, T.S.; Park, J.H. Relationship between sulfide capacity and structure of MnO-SiO2-Al2O3-Ce2O3 system. Metall. Mater. Trans. B 2016, 48, 545–553. [Google Scholar] [CrossRef]
- Shibata, E.; Nakamura, T.; Nishida, T.; Endo, M.; Itou, H.; Takasu, T. Evaluation of Desulphurization Flux in CaO-Al2O3-BaO-CeO2-MgO System. Steel Res. Int. 2004, 75, 308–313. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. Structural investigation of CaO-Al2O3 and CaO-Al2O3-CaF2 slags via Fourier transform infrared spectra. ISIJ Int. 2002, 42, 38–43. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, W.; Zhou, L.; Lu, B.; Kang, Y.-B. Effects of MnO on crystallization, melting, and heat transfer of CaO-Al2O3-based mold flux used for high Al-TRIP steel casting. Metall. Mater. Trans. B 2014, 45, 1510–1519. [Google Scholar] [CrossRef]
- Li, M.; Li, R.; Zhang, T. Phase equilibria of SiO2-Ce2O3-CaO-25 wt% Al2O3 system at 1673K-1773K. Ceram. Int. 2022, 48, 31614–31626. [Google Scholar] [CrossRef]
- Cao, J.; Li, Y.; Lin, W.; Che, J.; Zhou, F.; Tan, Y.; Li, D.; Dang, J.; Chen, C. Assessment of inclusion removal ability in refining slags containing Ce2O3. Crystals 2023, 13, 202. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Wang, Z.; Tan, Z.; Qu, B.; Cui, Y. Analysis on Volatilization Behavior of Typical Fluoride-Containing Slag Systems in Steelmaking. Steelmaking 2020, 36, 75–78. [Google Scholar]
- Jerebtsov, D.A.; Mikhailov, G.G. Phase diagram of CaO-Al2O3 system. Ceram. Int. 2001, 27, 25–28. [Google Scholar] [CrossRef]
- Wartenberg, H.V.; Eckhardt, K. Schmelzdiagramme chstfeuerfester Oxyde VIII. ZeitschdRfd Ranorganis Cheundall Gemeine Chem. 1937, 232, 179–187. [Google Scholar]
- Leonov, A.I.; Andreeva, A.B. High-temperature chemistry of cerium in the systems cerium oxides-Al2O3, Cr2O3, and Ga2O3. Izv. Akad. Nauk SSSR Neorg. Mater. 1996, 2, 517. [Google Scholar]
- Kim, D.G.; Van Hoek, C.; Liebske, C.; Van Der Laan, S.; Hudon, P.; Jung, I.H. Phase Diagram Study of the CaO-CaF2 System. ISIJ Int. 2012, 52, 1945–1950. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Korolyov, N.V.; Mogutnov, B.M. Phase equilibria in the CaF2-Al2O3-CaO system. J. Mater. Sci. 1991, 26, 1588–1600. [Google Scholar] [CrossRef]
- Ueda, S.; Morita, K.; Sano, N. Activity of AlO1.5 for the CaO-AlO1.5-CeO1.5 system at 1773 K. ISIJ Int. 1998, 38, 1292–1296. [Google Scholar] [CrossRef]
- Kitano, R.; Ishii, M.; Uo, M.; Morita, K. Thermodynamic properties of the CaO-AlO1.5-CeO1.5 system. ISIJ Int. 2016, 56, 1893–1901. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, H.; Huo, G.; Liu, C. Phase diagram of CaO-Al2O3-CeOx slag system at 1600 °C in reducing atmosphere and air atmosphere. Ceram. Int. 2023, 49, 20447–20455. [Google Scholar] [CrossRef]
- Zhu, D. Study and Drawing of Isothermal Phase Diagram of CaO-Al2O3-CeO2 Slag System at 1500 °C in Air Atmosphere. Master’s Thesis, Northeastern University, Shenyang, China, 2021; pp. 41–46. [Google Scholar]
- Nakada, H.; Susa, M.; Seko, Y.; Hayashi, M.; Nagata, K. Mechanism of heat transfer reduction by crystallization of mold flux for continuous casting. ISIJ Int. 2008, 48, 446–453. [Google Scholar] [CrossRef]
- Cho, J.W.; Emi, T.; Shibata, H.; Suzuki, M. Heat transfer across mold flux film in mold during initial solidification in continuous casting of steel. ISIJ Int. 1998, 38, 834–842. [Google Scholar] [CrossRef]
- Sridhar, S.; Mills, K.C.; Afrange, O.D.C.; Carli, L.R. Break temperatures of mould fluxes and their relevance to continuous casting. Ironmak. Steelmak. 2000, 27, 238–242. [Google Scholar] [CrossRef]
- Wang, H. Phase Equilibria and CALPHAD Study of CaO-Al2O3-Ce2O3 Slag System at 1600 °C. Master’s Thesis, Northeastern University, Shenyang, China, 2023; pp. 44–45. [Google Scholar]
- Brandaleze, E.; Di Gresia, G.; Santini, L.; Martín, A.; Benavidez, E. Mould fluxes in the steel continuous casting process. In Science and Technology of Casting Processes; IntechOpen: London, UK, 2012. [Google Scholar][Green Version]
- He, S.; Li, Z.; Chen, Z.; Wu, T.; Wang, Q. Review of mold fluxes for continuous casting of high-alloy (Al, Mn, Ti) steels. Steel Res. Int. 2019, 90, 1800424. [Google Scholar] [CrossRef]
- Van Ende, M.A.; Jung, I.H. Development of a thermodynamic database for mold flux and application to the continuous casting process. ISIJ Int. 2014, 54, 489–495. [Google Scholar] [CrossRef]
- Ni, P.; Tanaka, T.; Suzuki, M.; Nakamoto, M.; Ersson, M.; Jönsson, P.G. Mathematical Modelling Study of Dynamic Composition Change of Steel and Mold Flux in Continuous Casting of Steel. ISIJ Int. 2019, 59, 2024–2035. [Google Scholar] [CrossRef]
- Thu, H.L.; Annelies, M.; Bart, B.; Guo, M. Phase relations of the CaO-SiO2-Nd2O3 system and the Implication for rare earth recycling. Metall. Mater. Trans. B 2016, 47, 1736–1744. [Google Scholar]
- Chen, M.; Zhao, B. Phase equilibrium Studies of “Cu2O”-SiO2-Al2O3 system in equilibrium with metallic copper. J. Am. Ceram. Soc. 2013, 96, 3631–3636. [Google Scholar] [CrossRef]
- Wang, N.; Huang, W.; Chen, S.; Chen, M. Effect of oxygen partial pressure on phase equilibria and liquidus in CaO-Al2O3-FeOx system. J. Iron Steel Res. Int. 2012, 19, 8–12. [Google Scholar] [CrossRef]
- Jang, K.; Ma, X.; Zhu, J.; Xu, H.; Wand, G.; Zhao, B. Phase equilibria in the system “FeO”-CaO-SiO2-Al2O3-MgO with CaO/SiO2 = 1.3. ISIJ Int. 2016, 56, 967–976. [Google Scholar] [CrossRef]
- Yu, Q.; Sugita, S.; Feng, X.; Mi, J. On the preparation of single crystals of 11CaO·7Al2O3·CaF2 and the confirmation of its crystal structure. Cem. Concr. Res. 1997, 27, 1439–1449. [Google Scholar]
- Kaptay, G. The Generalized Phase Rule, the Extended Definition of the Degree of Freedom, the Component Rule and the Seven Independent Non-Compositional State Variables: To the 150th Anniversary of the Phase Rule of Gibbs. Materials 2024, 17, 6048. [Google Scholar] [CrossRef] [PubMed]
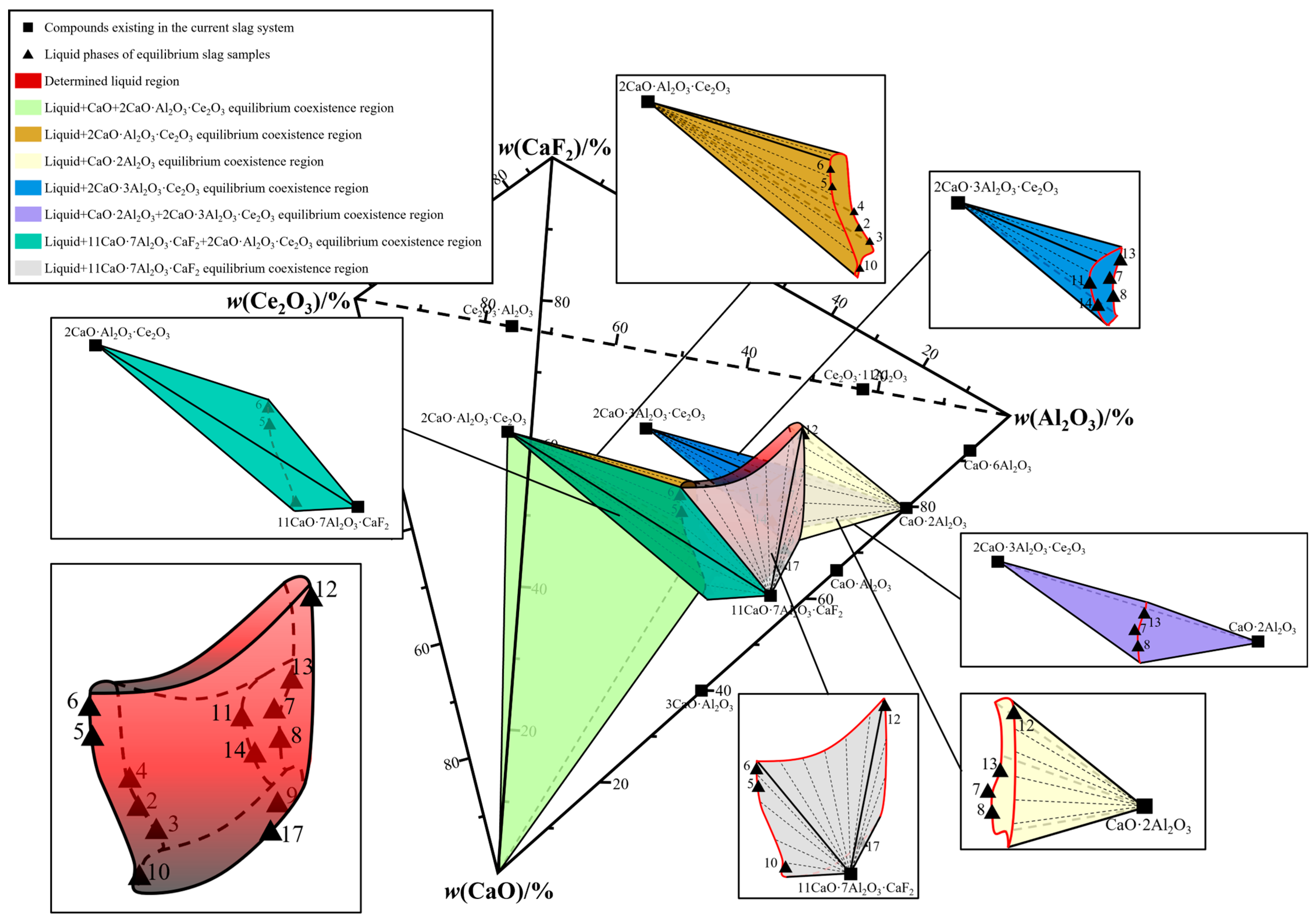
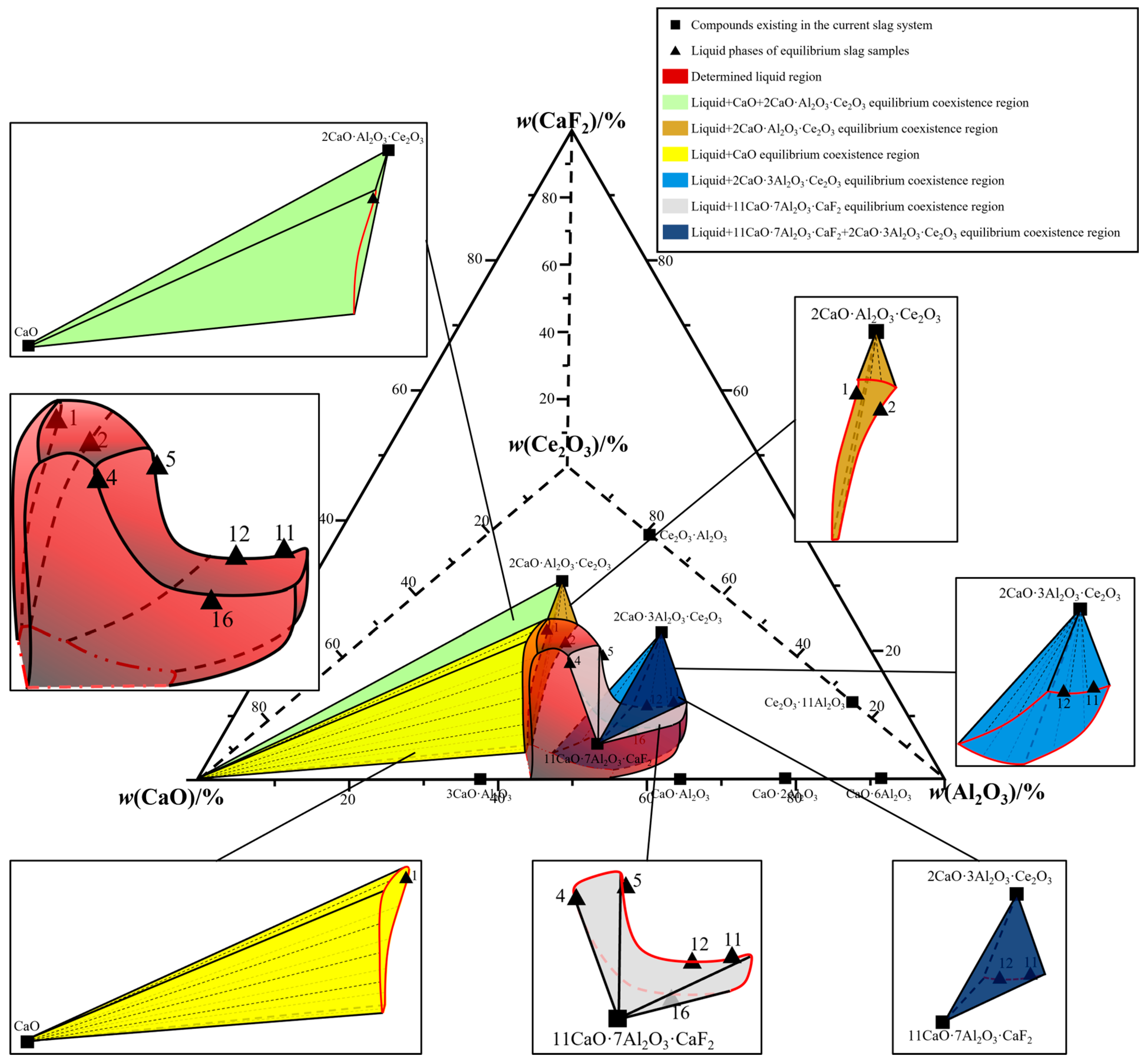
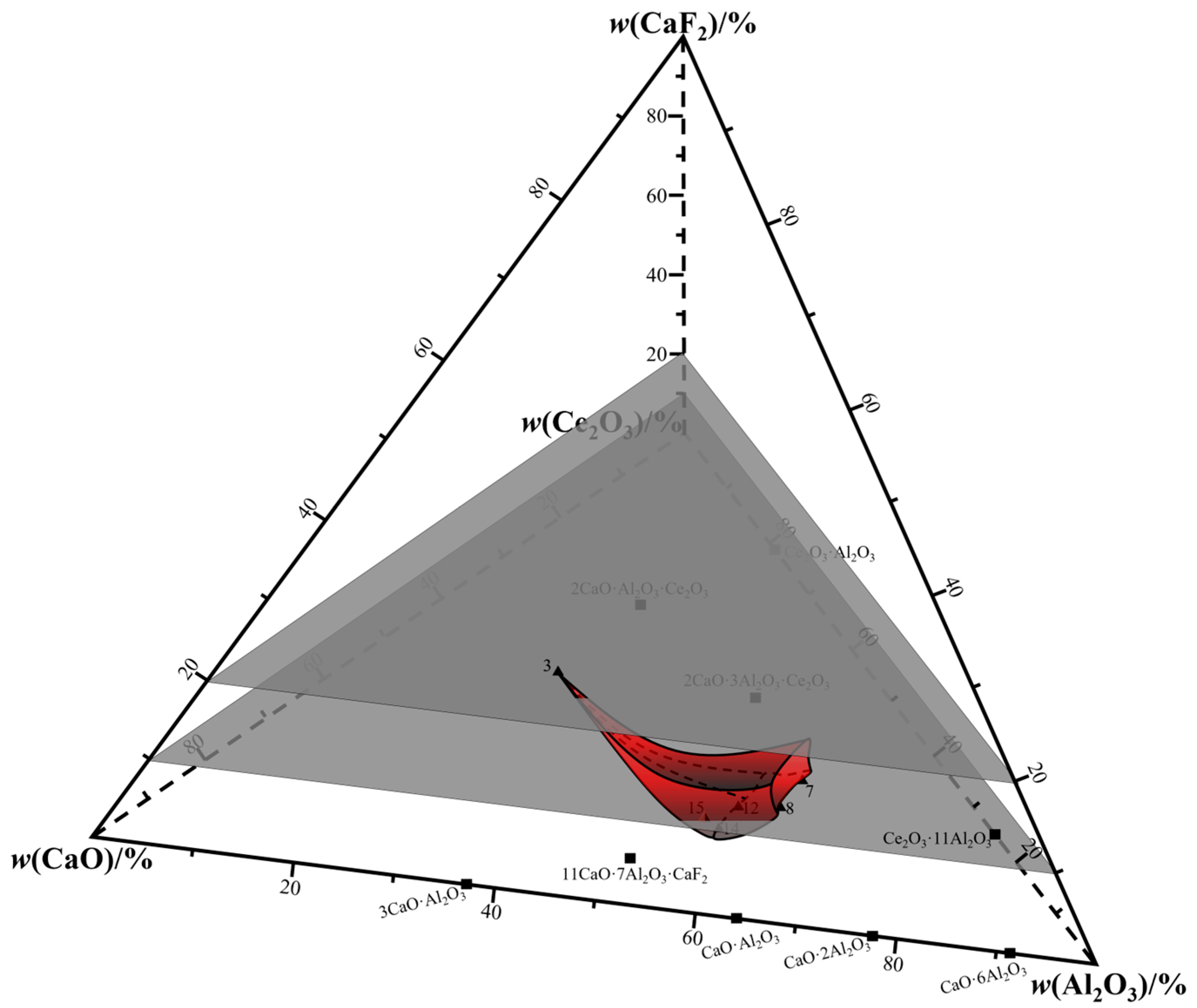

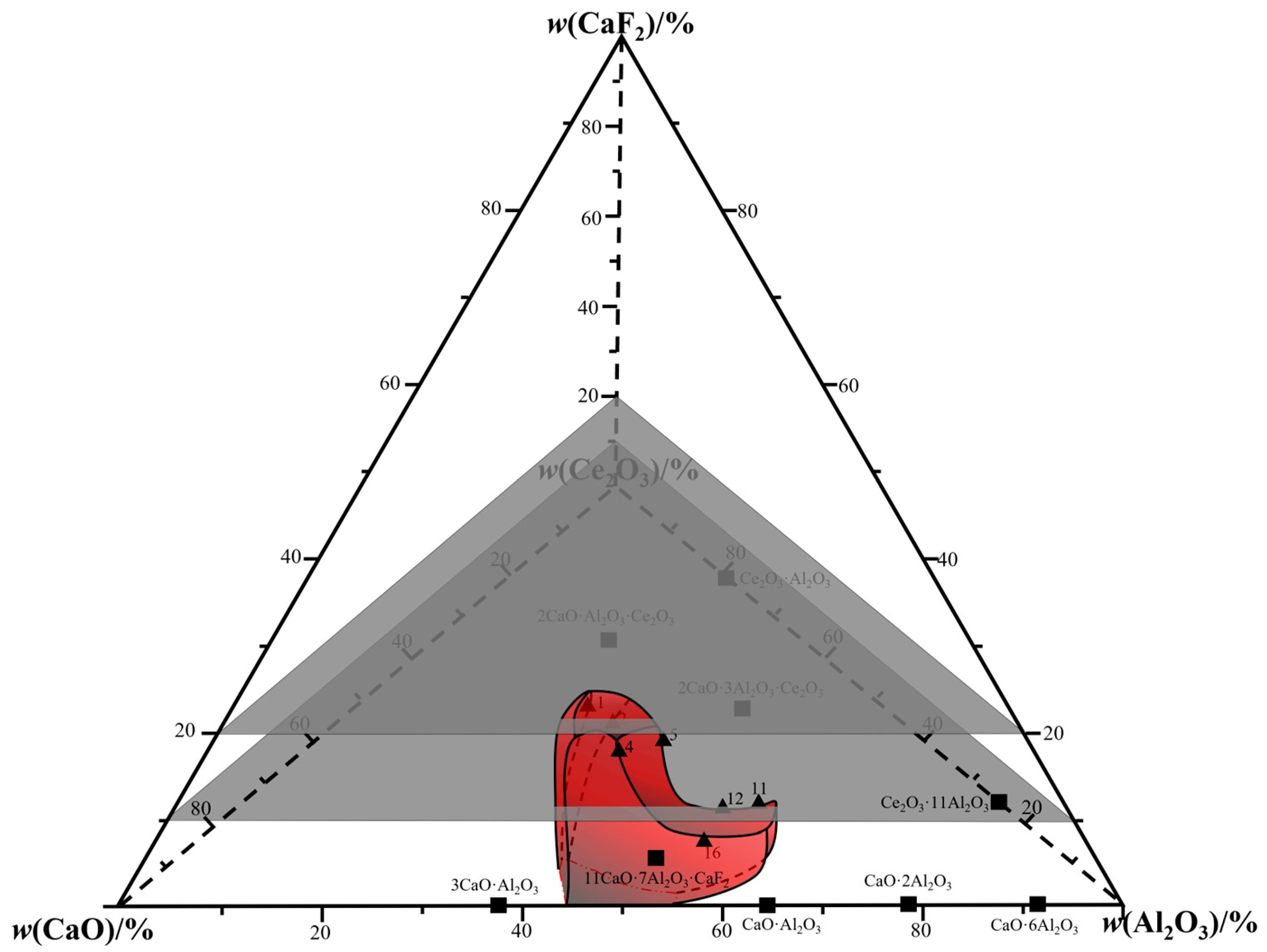
| No. | Detection Method | w(CaO)/% | w(Al2O3)/% | w(CeO2)/% | w(CaF2)/% | Error |
|---|---|---|---|---|---|---|
| 12 | EDX | 28.33 | 56.15 | 4.46 | 11.05 | 1.36% |
| EPMA | 28.86 | 55.78 | 4.51 | 10.85 | ||
| 13 | EDX | 20.11 | 61.07 | 11.44 | 7.38 | 1.41% |
| EPMA | 20.26 | 60.47 | 11.84 | 7.42 | ||
| 15 | EDX | 28.58 | 57.26 | 2.39 | 11.76 | 2.01% |
| EPMA | 29.13 | 56.90 | 2.47 | 11.50 |
| No. | CaO | Al2O3 | CeO2 | CaF2 | CaO/Al2O3 |
|---|---|---|---|---|---|
| 1 | 36.62 | 32.16 | 15.10 | 16.11 | 1.14 |
| 2 | 34.39 | 35.42 | 15.03 | 15.16 | 0.97 |
| 3 | 35.18 | 37.78 | 12.17 | 14.88 | 0.93 |
| 4 | 37.09 | 37.78 | 9.68 | 15.45 | 0.98 |
| 5 | 31.62 | 44.13 | 8.65 | 15.60 | 0.72 |
| 6 | 29.24 | 41.52 | 13.96 | 15.27 | 0.70 |
| 7 | 19.05 | 60.68 | 4.32 | 15.96 | 0.31 |
| 8 | 14.40 | 58.40 | 12.18 | 15.02 | 0.25 |
| 9 | 41.85 | 50.23 | 2.36 | 5.55 | 0.83 |
| 10 | 33.91 | 45.90 | 13.00 | 7.19 | 0.74 |
| 11 | 24.53 | 53.01 | 8.86 | 13.60 | 0.46 |
| 12 | 28.33 | 56.15 | 4.46 | 11.05 | 0.50 |
| 13 | 20.11 | 61.07 | 11.44 | 7.38 | 0.33 |
| 14 | 32.87 | 59.19 | 2.59 | 5.35 | 0.56 |
| 15 | 28.58 | 57.26 | 2.39 | 11.76 | 0.50 |
| 16 | 30.96 | 53.54 | 1.68 | 13.81 | 0.58 |
| 17 | 50.28 | 44.83 | 2.32 | 2.57 | 1.12 |
| Temperature | No. | Equilibrium Phase |
|---|---|---|
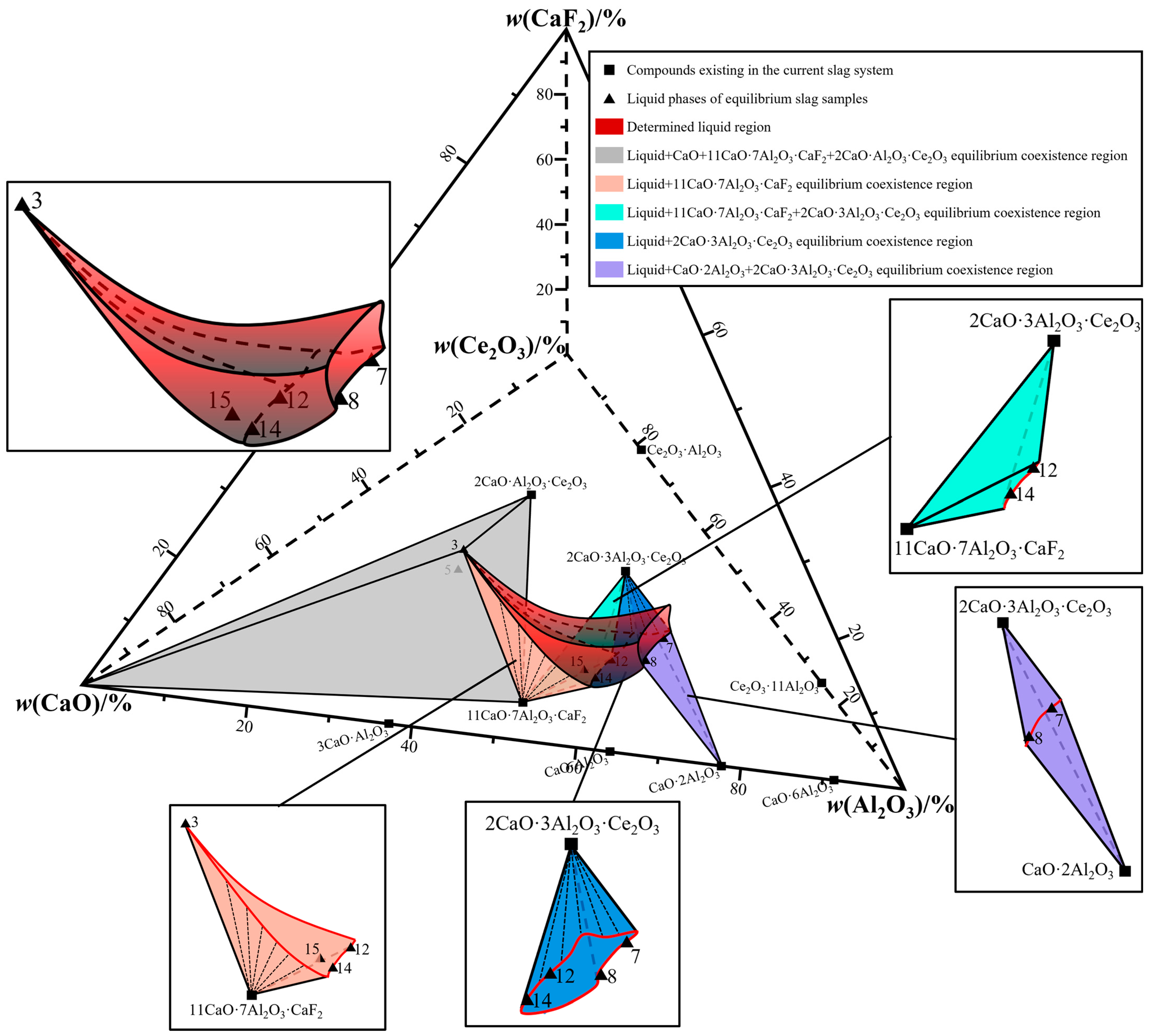
| 1300 °C | 3, 5 | Liquid + CaO + 11CaO·7Al2O3·CaF2 + 2CaO·Al2O3·Ce2O3 |
| 7, 8 | Liquid + CaO·2Al2O3 + 2CaO·3Al2O3·Ce2O3 | |
| 12, 14 | Liquid + 11CaO·7Al2O3·CaF2 + 2CaO·3Al2O3·Ce2O3 | |
| 15 | Liquid + 11CaO·7Al2O3·CaF2 | |
| 1400 °C | 9 | Liquid + 11CaO·7Al2O3·CaF2 |
| 11 | Liquid + 2CaO·3Al2O3·Ce2O3 | |
| 12 | Liquid + CaO·2Al2O3 + 11CaO·7Al2O3·CaF2 | |
| 14 | Liquid + 2CaO·3Al2O3·Ce2O3 + 3CaO·3Al2O3·CaF2 | |
| 17 | Liquid + 3CaO·Al2O3 + 11CaO·7Al2O3·CaF2 | |
| 2, 3, 4 | Liquid + CaO + 2CaO·Al2O3·Ce2O3 | |
| 5, 6, 10 | Liquid + 2CaO·Al2O3·Ce2O3 + 11CaO·7Al2O3·CaF2 | |
| 7, 8, 13 | Liquid + CaO·2Al2O3 + 2CaO·3Al2O3·Ce2O3 | |
| 1500 °C | 6 | Liquid |
| 2 | Liquid + 2CaO·Al2O3·Ce2O3 | |
| 1 | Liquid + CaO + 2CaO·Al2O3·Ce2O3 | |
| 11, 12 | Liquid + 2CaO·3Al2O3·Ce2O3 + 11CaO·7Al2O3·CaF2 | |
| 4, 5, 16 | Liquid + 11CaO·7Al2O3·CaF2 |
| Temperature | No. | Equilibrium Phase | Stoichiometric | CaO | Al2O3 | Ce2O3 | CaF2 | SEM | XRD |
|---|---|---|---|---|---|---|---|---|---|
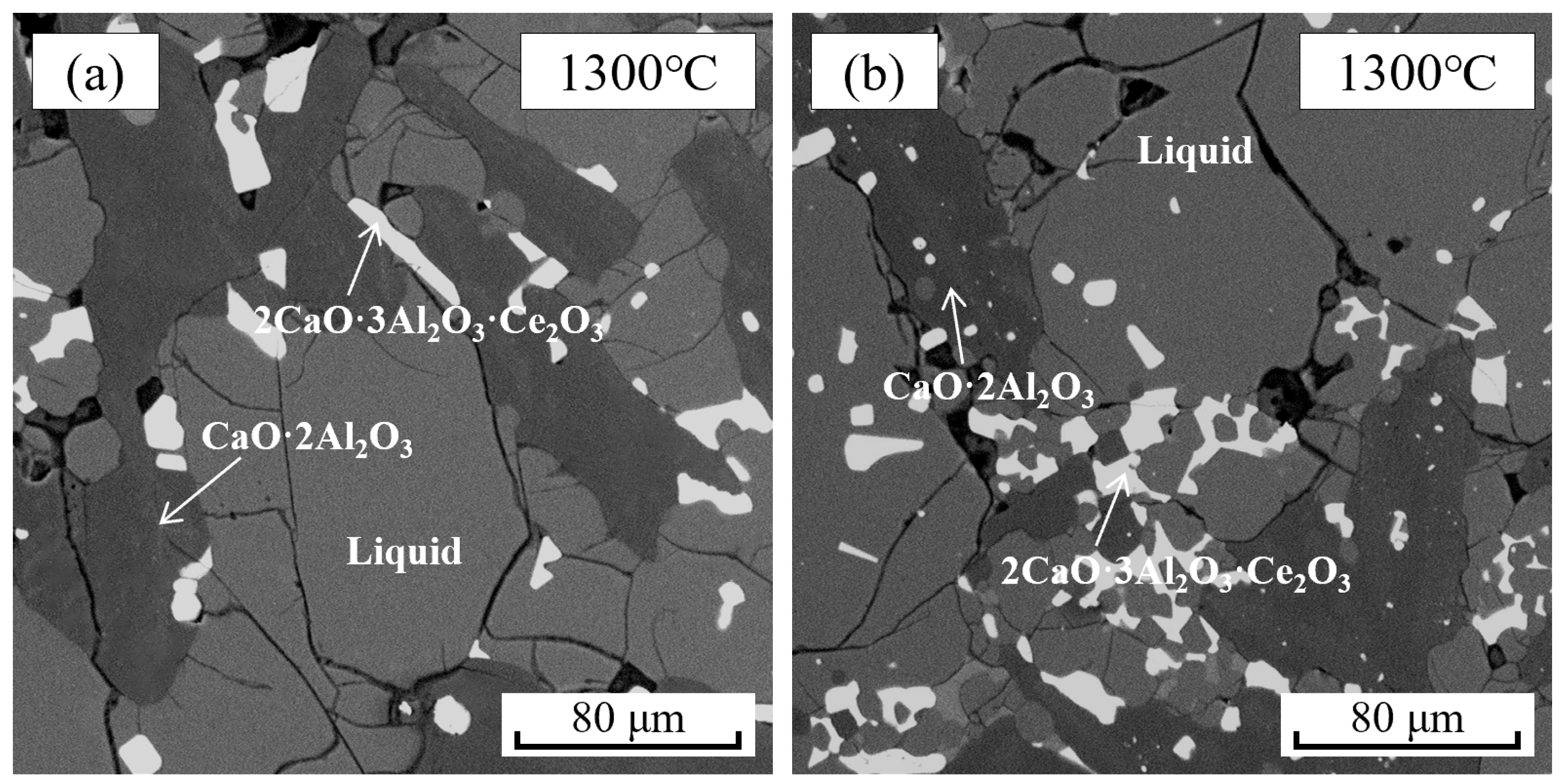
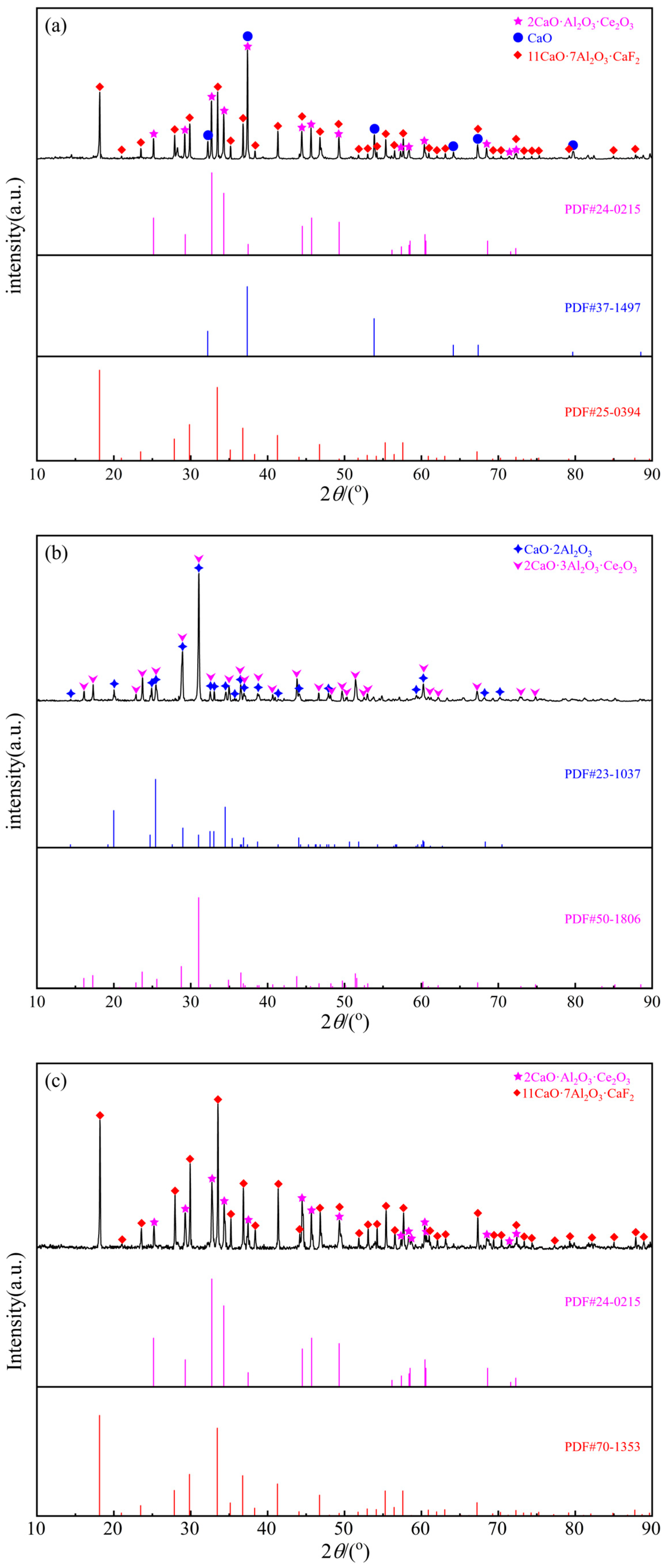
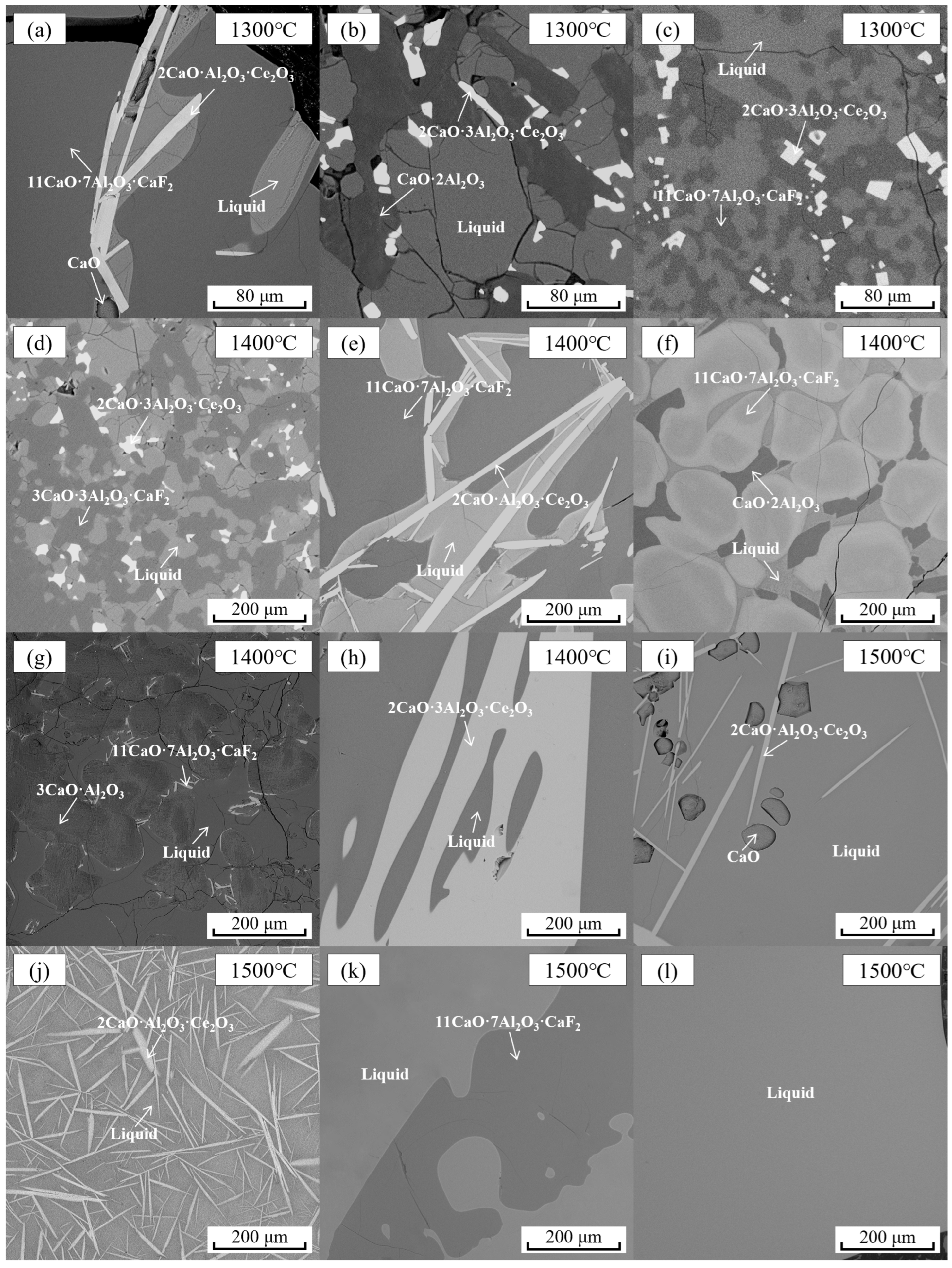
| 1300 °C | 3 | Liquid | 41.27 | 29.25 | 8.02 | 21.46 | Figure 6a | Figure 5a | |
| CaO | actual | 81.05 | 11.57 | 2.68 | 4.70 | ||||
| ideal | 100 | - | - | - | |||||
| 11CaO·7Al2O3·CaF2 | actual | 37.61 | 53.50 | 2.10 | 6.78 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 2CaO·Al2O3·Ce2O3 | actual | 21.63 | 28.78 | 38.10 | 11.48 | ||||
| ideal | 21.31 | 19.37 | 59.32 | - | |||||
| 5 | Liquid | 43.06 | 30.17 | 7.89 | 18.88 | - | - | ||
| CaO | actual | 88.70 | 6.27 | 2.16 | 2.87 | ||||
| ideal | 100 | - | - | - | |||||
| 11CaO·7Al2O3·CaF2 | actual | 36.38 | 55.60 | 1.31 | 6.71 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 2CaO·Al2O3·Ce2O3 | actual | 21.63 | 27.16 | 40.76 | 10.44 | ||||
| ideal | 21.31 | 19.37 | 59.32 | - | |||||
| 7 | Liquid | 21.65 | 60.57 | 1.63 | 16.15 | Figure 6b | Figure 5b | ||
| CaO·2Al2O3 | actual | 18.66 | 79.38 | 1.05 | 0.90 | ||||
| ideal | 35.48 | 64.52 | - | - | |||||
| 2CaO·3Al2O3·Ce2O3 | actual | 12.11 | 51.00 | 29.87 | 7.02 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
| 8 | Liquid | 24.75 | 59.86 | 4.60 | 10.79 | - | - | ||
| CaO·2Al2O3 | actual | 19.59 | 77.10 | 1.91 | 1.40 | ||||
| ideal | 35.48 | 64.52 | - | - | |||||
| 2CaO·3Al2O3·Ce2O3 | actual | 12.89 | 50.77 | 30.63 | 5.71 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
| 12 | Liquid | 33.57 | 54.38 | 4.53 | 7.52 | Figure 6c | - | ||
| 11CaO·7Al2O3·CaF2 | actual | 26.87 | 59.83 | 0.00 | 13.30 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 2CaO·3Al2O3·Ce2O3 | actual | 18.13 | 49.91 | 31.96 | 0.00 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
| 14 | Liquid | 34.87 | 55.16 | 3.66 | 6.31 | - | - | ||
| 11CaO·7Al2O3·CaF2 | actual | 25.16 | 61.92 | 0.00 | 12.92 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 2CaO·3Al2O3·Ce2O3 | actual | 18.49 | 52.20 | 29.31 | 0.00 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
| 15 | Liquid | 32.93 | 53.26 | 6.95 | 6.86 | - | - | ||
| 11CaO·7Al2O3·CaF2 | actual | 25.96 | 60.74 | 0.89 | 12.40 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 1400 °C | 2 | Liquid | 41.66 | 36.31 | 11.29 | 10.73 | - | - | |
| CaO | actual | 89.93 | 4.09 | 1.79 | 4.19 | ||||
| ideal | 100 | - | - | - | |||||
| 2CaO·Al2O3·Ce2O3 | actual | 19.99 | 27.70 | 42.82 | 9.48 | ||||
| ideal | 21.31 | 19.37 | 59.32 | - | |||||
| 3 | Liquid | 38.69 | 37.28 | 9.81 | 14.22 | - | - | ||
| CaO | actual | 87.69 | 7.28 | 2.81 | 2.22 | ||||
| ideal | 100 | - | - | - | |||||
| 2CaO·Al2O3·Ce2O3 | actual | 22.52 | 25.52 | 42.02 | 9.94 | ||||
| ideal | 21.31 | 19.37 | 59.32 | - | |||||
| 4 | Liquid | 40.38 | 34.28 | 9.88 | 15.46 | - | - | ||
| CaO | actual | 90.49 | 4.41 | 4.10 | 1.00 | ||||
| ideal | 100 | - | - | - | |||||
| 2CaO·Al2O3·Ce2O3 | actual | 18.99 | 24.23 | 45.73 | 11.05 | ||||
| ideal | 21.31 | 19.37 | 59.32 | - | |||||
| 5 | Liquid | 34.78 | 35.84 | 10.68 | 18.70 | Figure 6e | Figure 5c | ||
| 11CaO·7Al2O3·CaF2 | actual | 36.32 | 55.32 | 0.80 | 7.55 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 2CaO·Al2O3·Ce2O3 | actual | 21.35 | 27.01 | 40.77 | 10.87 | ||||
| ideal | 21.31 | 19.37 | 59.32 | - | |||||
| 6 | Liquid | 32.56 | 35.13 | 10.38 | 21.93 | - | - | ||
| 11CaO·7Al2O3·CaF2 | actual | 34.70 | 55.60 | 1.90 | 7.80 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 2CaO·Al2O3·Ce2O3 | actual | 17.39 | 26.52 | 45.10 | 10.99 | ||||
| ideal | 21.31 | 19.37 | 59.32 | - | |||||
| 7 | Liquid | 25.79 | 59.07 | 3.70 | 11.45 | - | Figure 5e | ||
| CaO·2Al2O3 | actual | 18.69 | 79.97 | 0.30 | 1.04 | ||||
| ideal | 35.48 | 64.52 | - | - | |||||
| 2CaO·3Al2O3·Ce2O3 | actual | 10.98 | 51.71 | 29.84 | 7.46 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
| 8 | Liquid | 27.17 | 60.76 | 3.40 | 8.68 | - | - | ||
| CaO·2Al2O3 | actual | 19.61 | 78.82 | 1.11 | 0.46 | ||||
| ideal | 35.48 | 64.52 | - | - | |||||
| 2CaO·3Al2O3·Ce2O3 | actual | 15.95 | 51.56 | 32.49 | 0.00 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
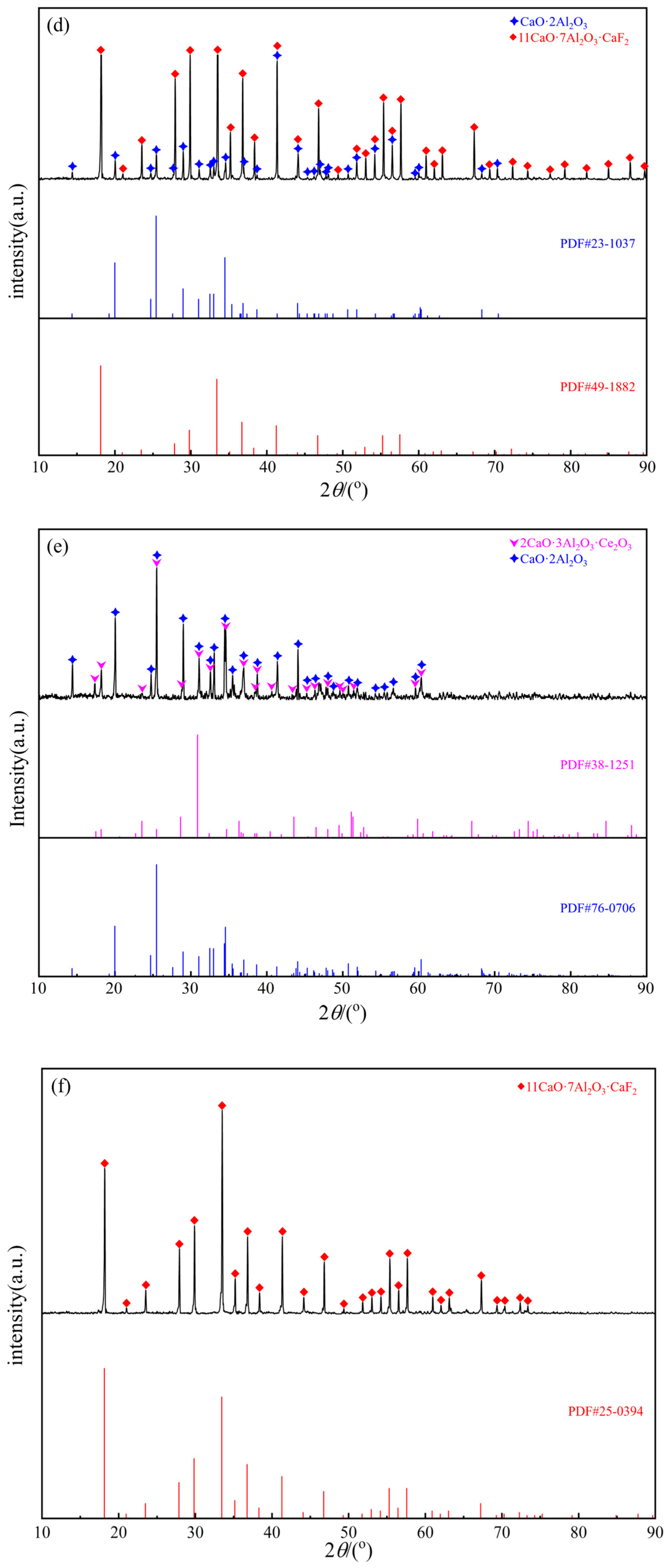
| 9 | Liquid | 43.45 | 30.29 | 9.26 | 17.00 | - | - | ||
| 11CaO·7Al2O3·CaF2 | actual | 38.89 | 55.12 | 0.89 | 5.09 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 10 | Liquid | 50.53 | 42.48 | 5.94 | 1.04 | - | -- | ||
| 2CaO·Al2O3·Ce2O3 | actual | 19.00 | 27.53 | 45.87 | 7.60 | ||||
| ideal | 21.31 | 19.37 | 59.32 | - | |||||
| 11CaO·7Al2O3·CaF2 | actual | 37.77 | 56.30 | 0.56 | 5.38 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 11 | Liquid | 28.21 | 54.72 | 3.77 | 13.30 | Figure 6h | - | ||
| 2CaO·3Al2O3·Ce2O3 | actual | 13.28 | 47.98 | 32.88 | 5.87 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
| 12 | Liquid | 15.55 | 55.39 | 2.51 | 26.55 | Figure 6f | Figure 5d | ||
| CaO·2Al2O3 | actual | 17.59 | 80.59 | 0.70 | 1.11 | ||||
| ideal | 35.48 | 64.52 | - | - | |||||
| 11CaO·7Al2O3·CaF2 | actual | 25.60 | 55.15 | 5.96 | 13.29 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 13 | Liquid | 24.25 | 61.53 | 1.17 | 13.05 | - | - | ||
| CaO·2Al2O3 | actual | 18.69 | 77.36 | 2.04 | 1.91 | ||||
| ideal | 35.48 | 64.52 | - | - | |||||
| 2CaO·3Al2O3·Ce2O3 | actual | 13.35 | 48.18 | 32.76 | 5.71 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
| 14 | Liquid | 30.75 | 56.81 | 2.59 | 9.85 | Figure 6d | - | ||
| 2CaO·3Al2O3·Ce2O3 | actual | 12.37 | 51.81 | 28.00 | 7.83 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
| 3CaO·3Al2O3·CaF2 | actual | 20.95 | 62.03 | 0.68 | 16.34 | ||||
| ideal | 30.47 | 55.39 | - | 14.14 | |||||
| 17 | Liquid | 36.82 | 55.83 | 0.90 | 6.45 | Figure 6g | - | ||
| 3CaO·Al2O3 | actual | 57.24 | 29.87 | 9.58 | 3.31 | ||||
| ideal | 62.26 | 37.74 | - | - | |||||
| 11CaO·7Al2O3·CaF2 | actual | 27.73 | 52.73 | 4.85 | 14.70 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 1500 °C | 1 | Liquid | 37.76 | 32.16 | 13.45 | 16.64 | Figure 6i | - | |
| CaO | actual | 86.73 | 6.18 | 2.17 | 4.92 | ||||
| ideal | 100 | - | - | - | |||||
| 2CaO·Al2O3·Ce2O3 | actual | 28.57 | 27.76 | 29.10 | 14.57 | ||||
| ideal | 21.31 | 19.37 | 59.32 | - | |||||
| 2 | Liquid | 35.85 | 35.99 | 12.26 | 15.90 | Figure 6j | - | ||
| 2CaO·Al2O3·Ce2O3 | actual | 26.13 | 27.80 | 32.37 | 13.70 | ||||
| ideal | 21.31 | 19.37 | 59.32 | - | |||||
| 4 | Liquid | 39.47 | 38.50 | 9.03 | 13.01 | Figure 6k | Figure 5f | ||
| 11CaO·7Al2O3·CaF2 | actual | 37.26 | 51.73 | 3.86 | 7.15 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 5 | Liquid | 36.35 | 29.83 | 20.42 | 13.40 | - | - | ||
| 11CaO·7Al2O3·CaF2 | actual | 34.28 | 51.69 | 4.51 | 9.52 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 6 | Liquid | 29.15 | 43.71 | 11.58 | 15.57 | Figure 6l | - | ||
| 11 | Liquid | 19.83 | 57.28 | 4.65 | 18.24 | - | - | ||
| 11CaO·7Al2O3·CaF2 | actual | 31.12 | 52.81 | 6.78 | 9.30 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 2CaO·3Al2O3·Ce2O3 | actual | 12.83 | 48.20 | 31.60 | 7.37 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
| 12 | Liquid | 33.23 | 55.29 | 4.82 | 6.67 | - | - | ||
| 11CaO·7Al2O3·CaF2 | actual | 24.77 | 60.65 | 1.75 | 12.83 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 | |||||
| 2CaO·3Al2O3·Ce2O3 | actual | 12.77 | 49.77 | 30.12 | 7.34 | ||||
| ideal | 15.36 | 41.89 | 42.75 | - | |||||
| 16 | Liquid | 36.87 | 54.73 | 1.53 | 6.87 | - | - | ||
| 11CaO·7Al2O3·CaF2 | actual | 27.54 | 60.47 | - | 11.99 | ||||
| ideal | 43.79 | 50.67 | - | 5.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Ye, J.; Qiu, J.; Liu, C. Phase Equilibrium Relationship of CaO-Al2O3-Ce2O3-CaF2 Slag System at 1300~1500 °C. Metals 2025, 15, 1209. https://doi.org/10.3390/met15111209
Sun L, Ye J, Qiu J, Liu C. Phase Equilibrium Relationship of CaO-Al2O3-Ce2O3-CaF2 Slag System at 1300~1500 °C. Metals. 2025; 15(11):1209. https://doi.org/10.3390/met15111209
Chicago/Turabian StyleSun, Lifeng, Jiangsheng Ye, Jiyu Qiu, and Chengjun Liu. 2025. "Phase Equilibrium Relationship of CaO-Al2O3-Ce2O3-CaF2 Slag System at 1300~1500 °C" Metals 15, no. 11: 1209. https://doi.org/10.3390/met15111209
APA StyleSun, L., Ye, J., Qiu, J., & Liu, C. (2025). Phase Equilibrium Relationship of CaO-Al2O3-Ce2O3-CaF2 Slag System at 1300~1500 °C. Metals, 15(11), 1209. https://doi.org/10.3390/met15111209






