Effect of ω-Phase Precipitation on Magnetic Susceptibility and Corrosion Resistance of Meta-Stable β-Phase Zr-Nb-Ti-Cr Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloy Design
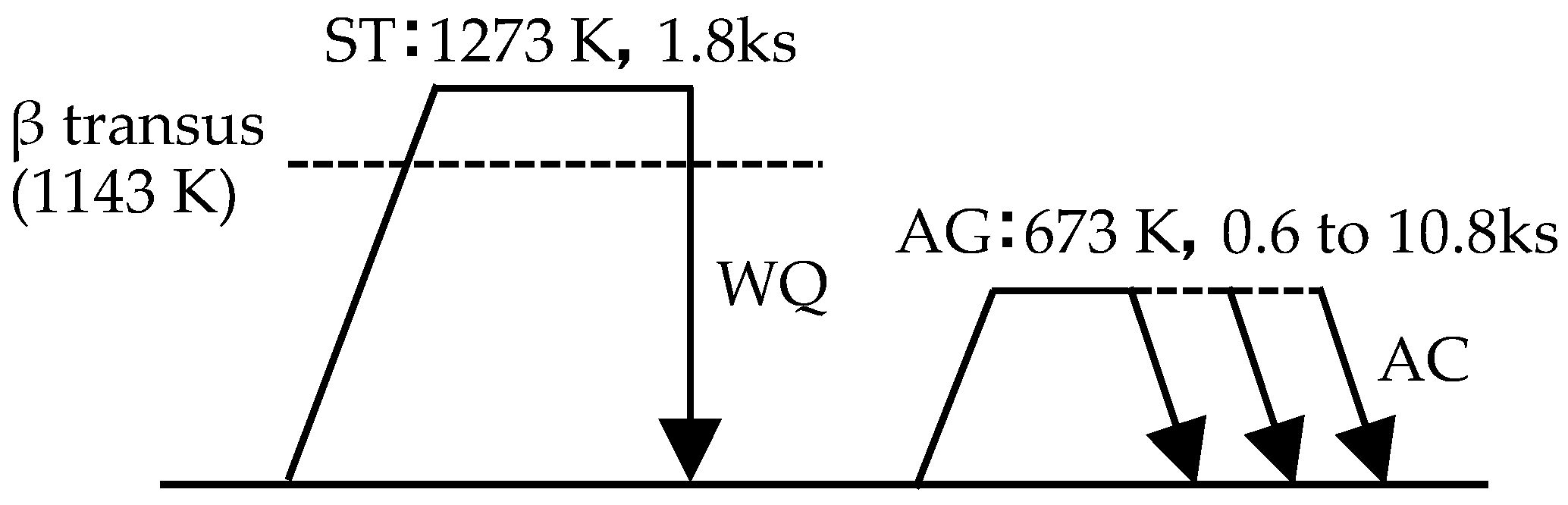
2.2. Alloy Preparation and Heat Treatment Conditions
2.3. Characterization of the Designed and Aged Alloys
3. Results
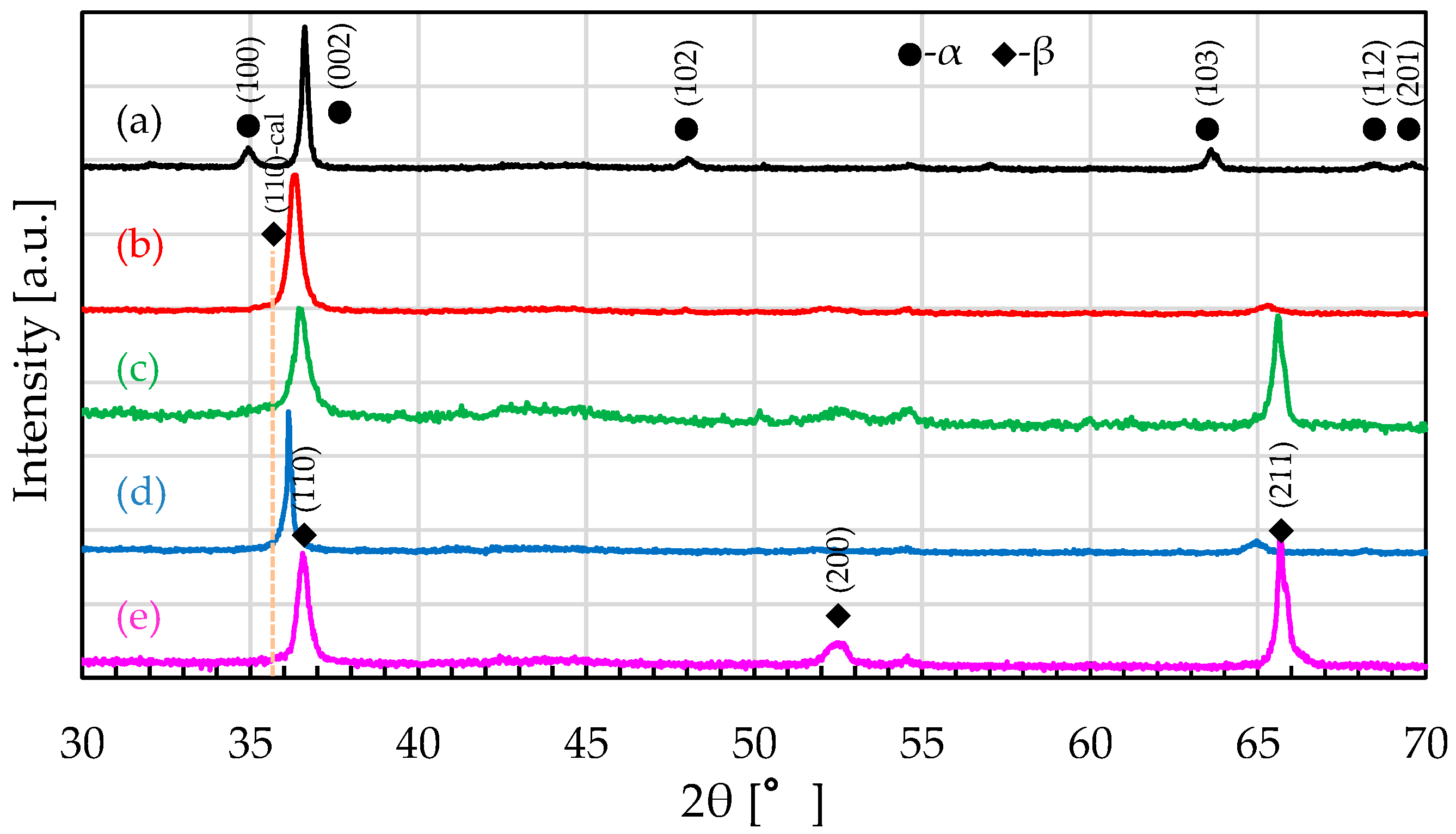
3.1. Phase Structure of Designed Alloys
3.2. Measurements of Magnetization
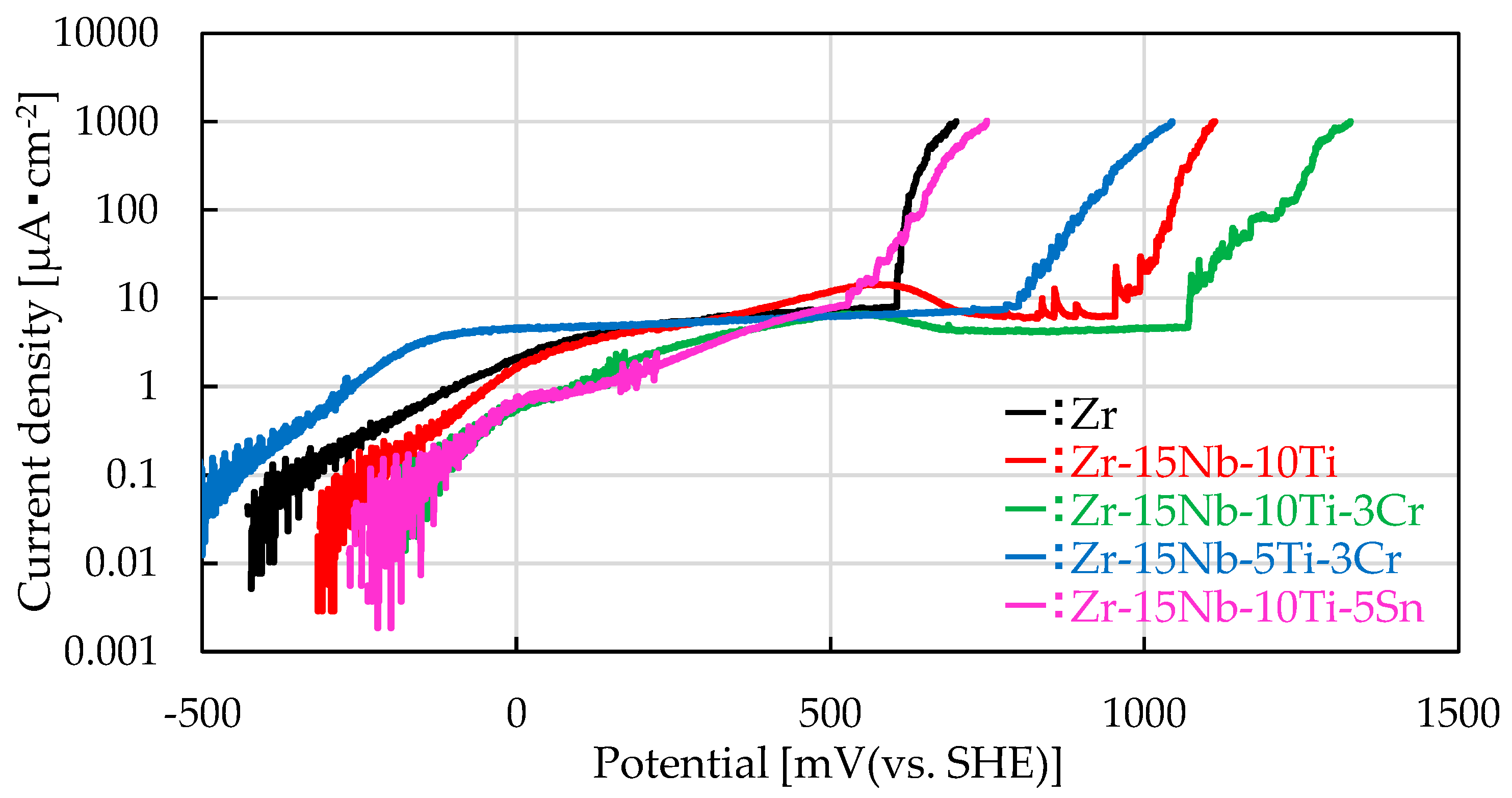
3.3. Measurement of Pitting Potential by the Anodic Polarization Test
4. Discussion
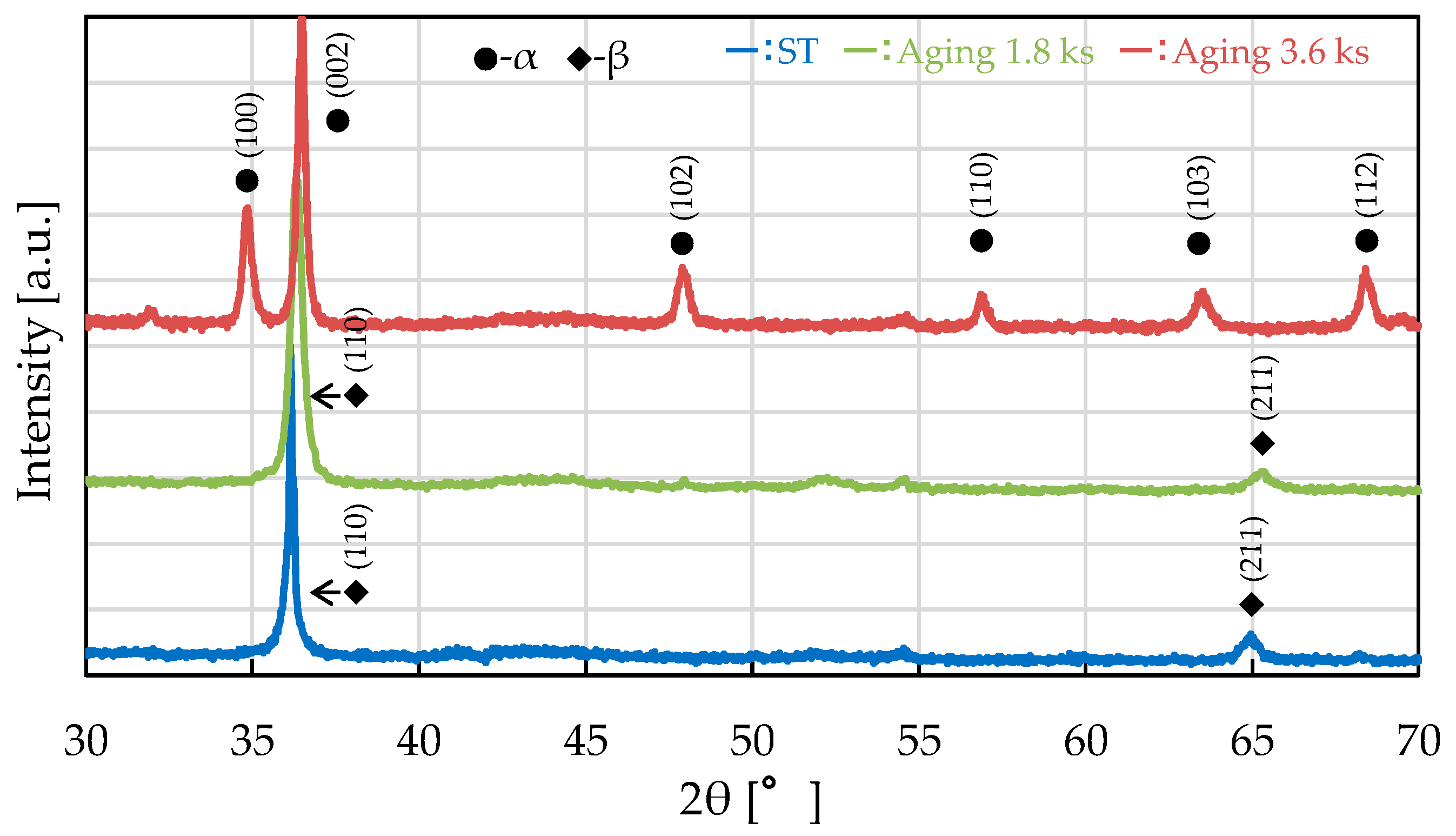
4.1. Change in Magnetic Susceptibility Due to Aging Heat Treatment
4.2. Effect of Changes in Phase Composition on Magnetic Susceptibility
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Niinomi, M.; Akahori, T. Improvement of the fatigue life of titanium alloys for biomedical devices through microstructural control. Expert Rev. Med. Devices 2010, 7, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Development of high biocompatible titanium alloys. Funct. Mater. 2000, 20, 36–44. [Google Scholar] [CrossRef]
- Niinomi, M. Recent Titanium R&D for Biomedical Applications in Japan. JOM 1999, 51, 32–34. [Google Scholar] [CrossRef]
- Niinomi, M. Development of Beta Titanium Alloys for Biomedical Applications. Mater. Jpn. 1998, 37, 843–846. [Google Scholar] [CrossRef]
- Sanderson, P.L.; Ryan, W.; Turner, P.G. Complications of metalwork removal. Injury 1992, 23, 29–30. [Google Scholar] [CrossRef]
- Seipel, R.C.; Pintar, F.A.; Yoganandan, N.; Boynton, M.D. Biomechanics of Calcaneal Fractures A Model for the Motor Vehicle. Clin. Orthop. Relat. Res. 2001, 388, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Inoue, T.; Konno, H.; Sasaki, M. Quantification of susceptibility artifacts produced on high-field magnetic resonance images by various biomaterials used for neurosurgical implants. J. Neurosurg. 2002, 97, 1472–1475. [Google Scholar] [CrossRef]
- Matsuura, H.; Inoue, T.; Ogasawara, K.; Sasaki, M.; Konno, H.; Kuzu, Y.; Nishimoto, H.; Ogawa, A. Quantitative Analysis of Magnetic Resonance Imaging Susceptibility Artifacts Caused by Neurosurgical Biomaterials: Comparison of 0.5, 1.5, and 3.0 Tesla Magnetic Fields. Neurol. Med.-Chir. 2005, 45, 395–399. [Google Scholar] [CrossRef]
- Ernstberger, T.; Heidrich, G.; Buchhorn, G. Postimplantation MRI with cylindric and cubic intervertebral test implants: Evaluation of implant shape, material, and volume in MRI artifacting—An in vitro study. Spine J. 2007, 7, 353–359. [Google Scholar] [CrossRef]
- Inui, S.; Uyama, E.; Hamada, K. Volume magnetic susceptibility design and hardness of Au–Ta alloys and Au–Nb alloys for MRI-compatible biomedical applications. Biomed. Phys. Eng. Express 2017, 3, 015025. [Google Scholar] [CrossRef]
- Nomura, N.; Tanaka, Y.; Suyalatu; Kondo, R.; Doi, H.; Tsutsumi, Y.; Hanawa, T. Effects of Phase Constitution of Zr-Nb Alloys on Their Magnetic Susceptibilities. Mater. Trans. 2009, 50, 2466–2472. [Google Scholar] [CrossRef]
- Kobayashi, E.; Ando, M.; Tsustumi, Y.; Doi, H.; Yoneyama, T.; Kobayashi, M.; Hanawa, T. Inhibition Effect of Zirconium Coating on Calcium Phosphate Precipitation of Titanium to Avoid Assimilation with Bone. Mater. Trans. 2007, 48, 301–306. [Google Scholar] [CrossRef]
- Tsutsumi, Y. Corrosion Resistance of Zr as Metallic Biomaterials. Zair. Kankyo 2014, 63, 360–364. [Google Scholar] [CrossRef]
- Collings, E.W. Magnetic studies of phase equilibria in Ti-Ai (30 to 57 At. Pct) alloys. Metall. Trans. A 1979, 10, 463–474. [Google Scholar] [CrossRef]
- Chui, P. Near β-type Zr-Nb-Ti biomedical alloys with high strength and low modulus. Vacuum 2017, 143, 54–58. [Google Scholar] [CrossRef]
- Morinaga, M. Local lattice distortion and martensitic transformation near alloying elements in titanium and iron. Toyota Res. Rep. 2017, 70, 35–44. [Google Scholar]
- Hong, S.-P.; Ko, Y.-M.; Kim, C.-S. Magnetic Susceptibility of Zr-Cu Binary Alloys. Mater. Trans. 2014, 55, 1634–1636. [Google Scholar] [CrossRef]
- Xue, R.-H.; Wang, D.; Yue-yan, T.; Zi-xuan, D.; Li-bin, L.; Li-gang, Z. Effect of Sn on elastic modulus and magnetic susceptibility of Zr-16Nb-xTi (x = 4 wt%, 6 wt%) alloys. J. Cent. South Univ. 2023, 30, 412–418. [Google Scholar] [CrossRef]
- JIS T 0302; Method for Evaluating Corrosion Resistance of Metallic Biomaterials by Anodic-Polarization Test. JSA GROUP Webdesk: Tokyo, Japan, 2000.
- Motooka, T.; Komatsu, A.; Tsukada, T.; Yamamoto, M. Effect of gamma radiolysis on pit initiation of zircaloy-2 in water containing sea salt. J. Nucl. Sci. Technol. 2014, 51, 987–995. [Google Scholar] [CrossRef][Green Version]
- Tsutsumi, Y.; Muto, I.; Nakano, S.; Tsukada, J.; Manaka, T.; Chen, P.; Ashida, M.; Sugawara, Y.; Shimojo, M.; Hara, N.; et al. Effect of Impurity Elements on Localized Corrosion of Zirconium in Chloride Containing Environment. J. Electrochem. Soc. 2020, 167, 141507. [Google Scholar] [CrossRef]
- Palit, G.C. Pit Simulation Study of Zirconium Under Simple Immersion Conditions in Oxidizing Chloride Solutions. Corrosion 2000, 56, 523–532. [Google Scholar] [CrossRef]
- Georg, C.; Feuerriegel, R. Sutter. Managing hardware-related metal artifacts in MRI: Current and evolving techniques. Skelet. Radiol. 2024, 53, 1737–1750. [Google Scholar] [CrossRef]
- Zhanal, P.; Harcuba, P.; Hajek, M.; Smola, B.; Strasky, J.; Smilauerova, J.; Vesely, J.; Janecek, M. Evolution of ω phase during heating of metastable β titanium alloy Ti–15Mo. J. Mater. Sci. 2018, 53, 837–845. [Google Scholar] [CrossRef]
- Prima, F.; Vermaut, P.; Ansel, D.; Debuigne, J. ω Precipitation in a Beta Metastable Titanium Alloy, Resistometric Study. Mater. Trans. 2000, 41, 1092–1097. [Google Scholar] [CrossRef]
- Settefrati, A.; Aeby-Gaytier, E.; Dehmas, M.; Geandier, G.; Appolaire, B.; Audion, S.; Delfosse, J. Precipitation in a near β titanium alloy on ageing: Influence of heating rate and chemical composition of the β-metastable phase. Solid State Phenom. 2011, 172–174, 760–765. [Google Scholar] [CrossRef]


| Alloys | Nb | Ti | Cr | Sn | Zr |
|---|---|---|---|---|---|
| Zr-15Nb-10Ti | 15 | 10 | 0 | 0 | 75 |
| 13.5 | 17.5 | 0 | 0 | 69.0 | |
| Zr-15Nb-10Ti-3Cr | 15 | 10 | 3 | 0 | 72 |
| 13.3 | 17.2 | 4.7 | 0 | 64.8 | |
| Zr-15Nb-5Ti-3Cr | 15 | 5 | 3 | 0 | 77 |
| 13.8 | 9.0 | 4.9 | 0 | 72.3 | |
| Zr-15Nb-10Ti-5Sn | 15 | 10 | 0 | 5 | 70 |
| 13.7 | 17.7 | 0 | 3.6 | 65.0 |
| Alloys | Zr | Zr-15Nb-10Ti | Zr-15Nb-10Ti-3Cr | Zr-15Nb-5Ti-3Cr | Zr-15Nb-10Ti-5Sn |
|---|---|---|---|---|---|
| Mass magnetic susceptibility [×10−6 cm3/g] | 1.29 | 2.13 | 2.17 | 1.79 | 1.91 |
| Alloys | Zr | Zr-15Nb-10Ti | Zr-15Nb-10Ti-3Cr | Zr-15Nb-5Ti-3Cr | Zr-15Nb-10Ti-5Sn |
|---|---|---|---|---|---|
| Pitting potential [mV (vs. SHE)] | 622 | 1045 | 1219 | 905 | 647 |
| Zr [at.%] | Cr [at.%] | Nb [at.%] | |
|---|---|---|---|
| β-phase | 76.0 | 2.80 | 11.24 |
| ω-phase | 85.5 | 0.86 | 6.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, S.; Kimura, T.; Aono, Y. Effect of ω-Phase Precipitation on Magnetic Susceptibility and Corrosion Resistance of Meta-Stable β-Phase Zr-Nb-Ti-Cr Alloy. Metals 2025, 15, 1208. https://doi.org/10.3390/met15111208
Tamura S, Kimura T, Aono Y. Effect of ω-Phase Precipitation on Magnetic Susceptibility and Corrosion Resistance of Meta-Stable β-Phase Zr-Nb-Ti-Cr Alloy. Metals. 2025; 15(11):1208. https://doi.org/10.3390/met15111208
Chicago/Turabian StyleTamura, Shinya, Tomonori Kimura, and Yasuhisa Aono. 2025. "Effect of ω-Phase Precipitation on Magnetic Susceptibility and Corrosion Resistance of Meta-Stable β-Phase Zr-Nb-Ti-Cr Alloy" Metals 15, no. 11: 1208. https://doi.org/10.3390/met15111208
APA StyleTamura, S., Kimura, T., & Aono, Y. (2025). Effect of ω-Phase Precipitation on Magnetic Susceptibility and Corrosion Resistance of Meta-Stable β-Phase Zr-Nb-Ti-Cr Alloy. Metals, 15(11), 1208. https://doi.org/10.3390/met15111208





