Heat Capacity and Thermodynamic Properties of Photocatalitic Bismuth Tungstate, Bi2WO6
Abstract
1. Introduction
2. Literature Data
2.1. Structure Data
2.2. Thermodynamic Data
3. Experiment
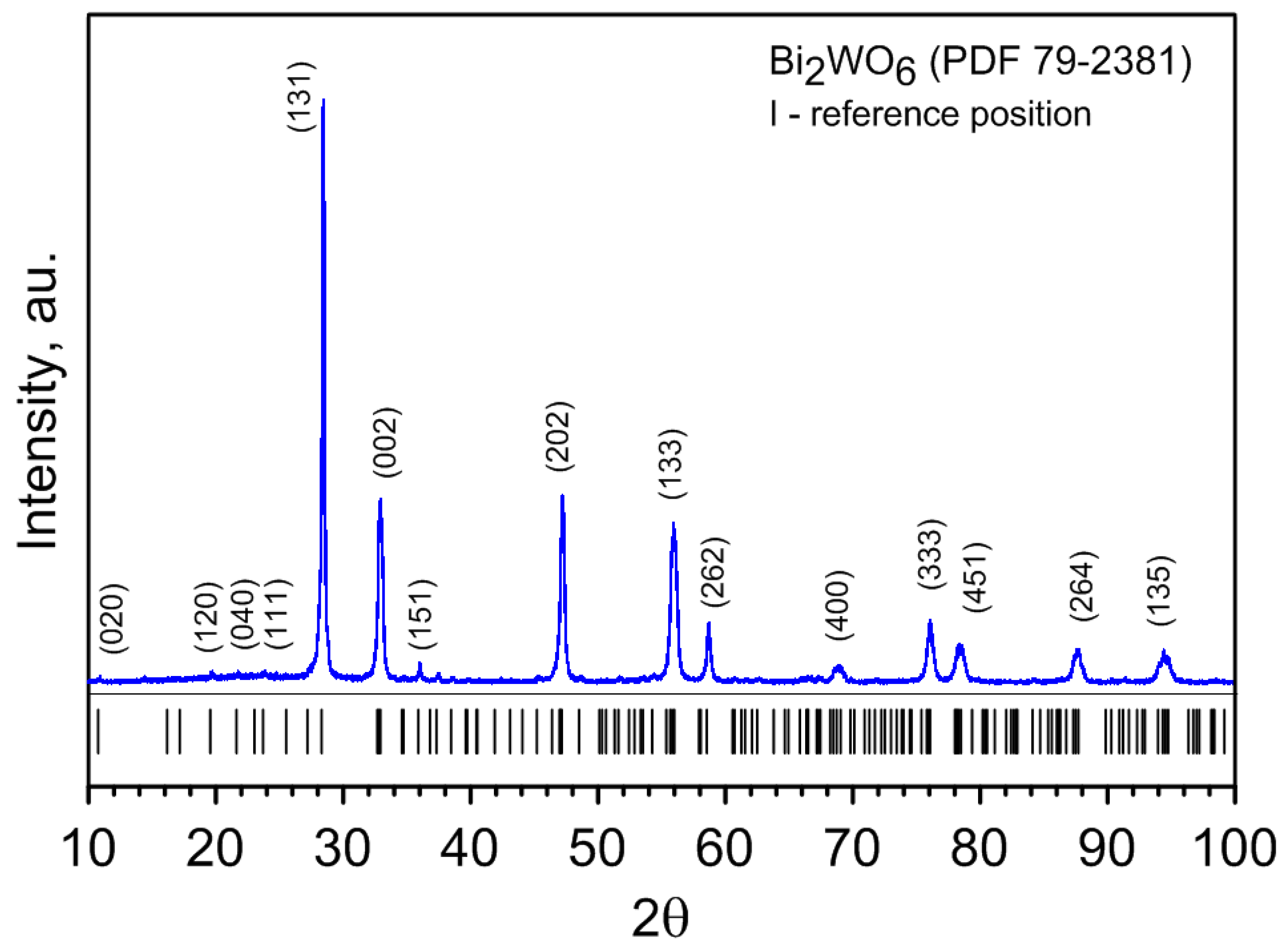
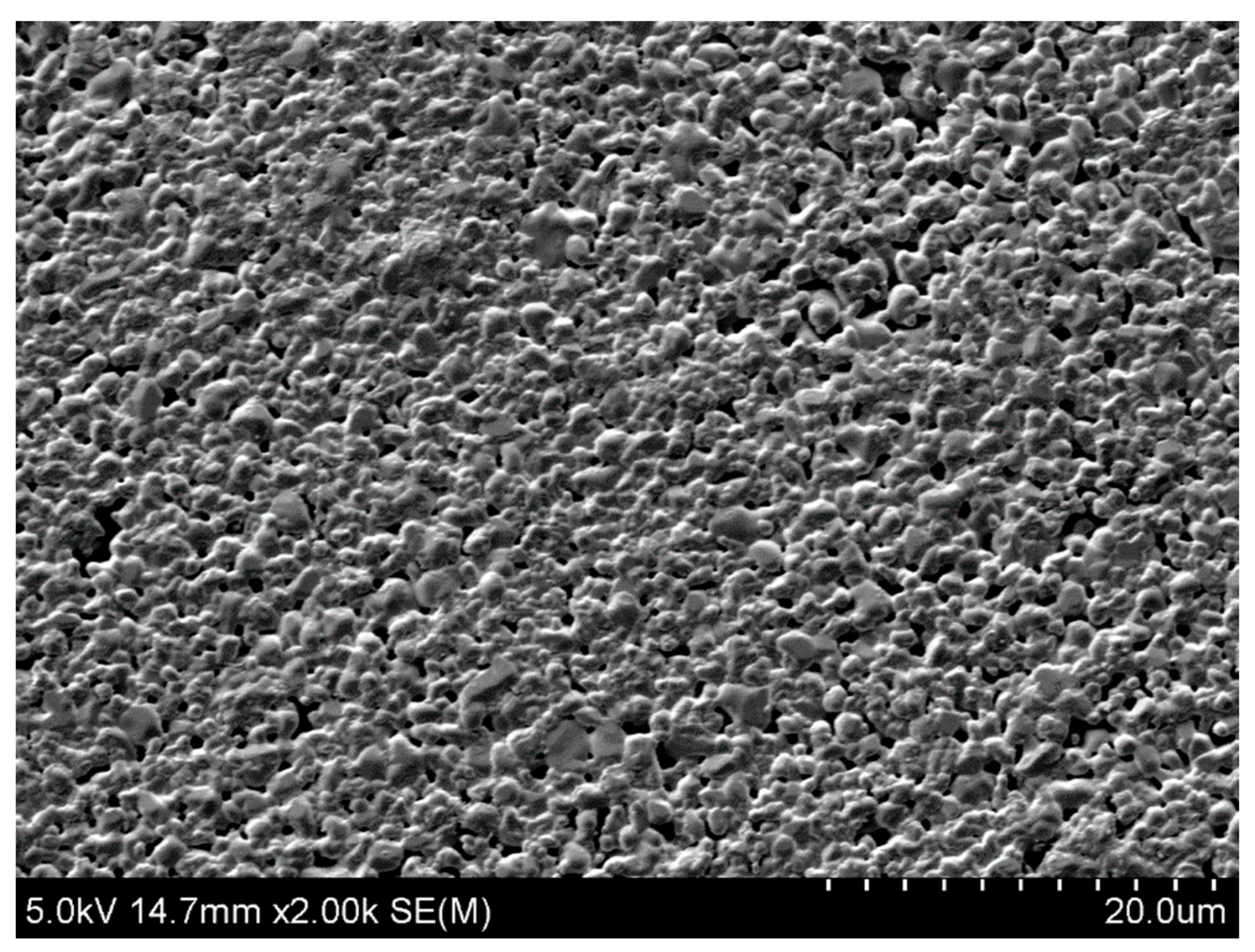
3.1. Material and Synthesis
3.2. Experimental Procedure
4. Results and Discussion
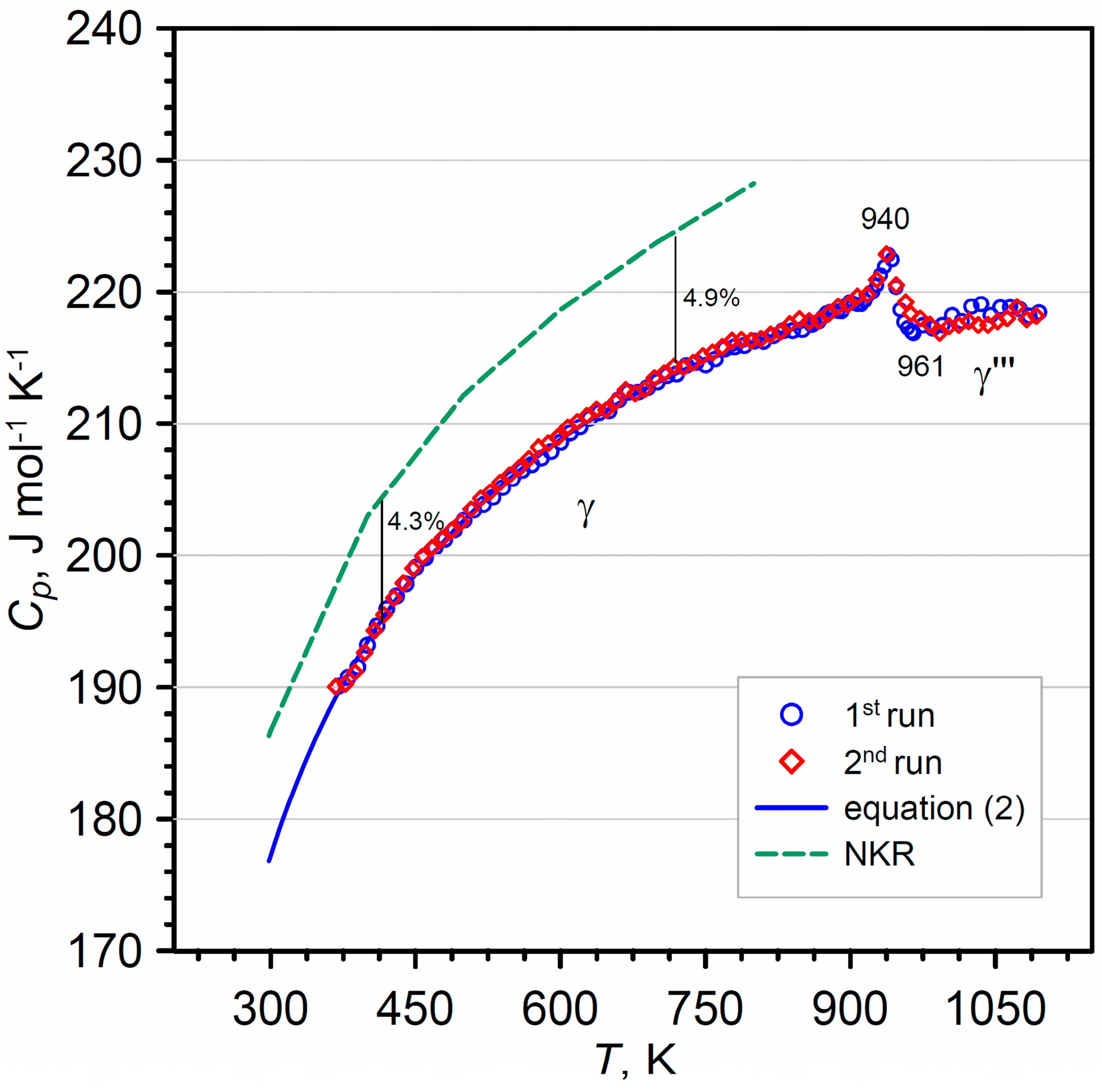
4.1. High-Temperature Heat Capacity Function
4.2. Extrapolation of Heat Capacity Function
4.3. Thermodynamic Functions of Bi2WO6
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| T, K | Cp(T) 2, J·mol−1·K−1 | T, K | Cp(T) 2, J·mol−1·K−1 | T, K | Cp(T) 2, J·mol−1·K−1 |
|---|---|---|---|---|---|
| 324 | 84.3 | 604 | 112.9 | 884 | 122.6 |
| 334 | 86.1 | 614 | 113.3 | 894 | 122.3 |
| 344 | 87.8 | 624 | 113.9 | 904 | 122.7 |
| 354 | 89.4 | 634 | 114.2 | 914 | 123.5 |
| 364 | 90.9 | 644 | 114.7 | 924 | 123.1 |
| 374 | 92.5 | 654 | 115.2 | 934 | 123.3 |
| 384 | 94.0 | 664 | 115.3 | 944 | 123.4 |
| 394 | 95.2 | 674 | 116.0 | 954 | 124.0 |
| 404 | 96.5 | 684 | 116.4 | 964 | 124.3 |
| 414 | 97.7 | 694 | 116.9 | 974 | 124.2 |
| 424 | 98.9 | 704 | 117.0 | 984 | 124.4 |
| 434 | 100.1 | 714 | 117.3 | 994 | 124.7 |
| 444 | 101.1 | 724 | 117.8 | 1004 | 124.4 |
| 454 | 102.0 | 734 | 118.2 | 1014 | 125.0 |
| 464 | 103.0 | 744 | 118.6 | 1024 | 125.0 |
| 474 | 104.0 | 754 | 118.9 | 1034 | 125.4 |
| 484 | 104.8 | 764 | 119.0 | 1044 | 125.5 |
| 494 | 105.6 | 774 | 119.4 | 1054 | 125.3 |
| 504 | 106.4 | 784 | 120.1 | 1064 | 126.0 |
| 514 | 107.1 | 794 | 120.2 | 1074 | 125.8 |
| 524 | 107.9 | 804 | 120.2 | 1084 | 126.0 |
| 534 | 108.6 | 814 | 120.6 | 1094 | 126.4 |
| 544 | 109.3 | 824 | 120.8 | 1104 | 126.7 |
| 554 | 109.8 | 834 | 121.3 | 1114 | 126.8 |
| 564 | 110.4 | 844 | 121.3 | 1124 | 126.9 |
| 574 | 111.1 | 854 | 121.6 | 1134 | 126.8 |
| 584 | 111.7 | 864 | 121.8 | 1144 | 126.7 |
| 594 | 112.4 | 874 | 122.1 | 1154 | 127.0 |
| First Run 2 | Second Run 2 | ||||||
|---|---|---|---|---|---|---|---|
| T, K | Cp(T), J·K−1·mol−1 | T, K | Cp(T), J·K−1·mol−1 | T, K | Cp(T), J·K−1·mol−1 | T, K | Cp(T), J·K−1·mol−1 |
| 379 | 190.5 | 749 | 215.4 | 375 | 190 | 745 | 214.7 |
| 389 | 191.3 | 759 | 215.8 | 385 | 191 | 755 | 214.6 |
| 399 | 192.7 | 769 | 215.8 | 395 | 192 | 765 | 215.4 |
| 409 | 194.1 | 779 | 216.8 | 405 | 194 | 775 | 215.7 |
| 419 | 195.6 | 789 | 216.4 | 415 | 195 | 785 | 215.9 |
| 429 | 196.8 | 799 | 216.3 | 425 | 197 | 795 | 215.6 |
| 439 | 197.7 | 809 | 216.5 | 435 | 197 | 805 | 216.5 |
| 449 | 198.6 | 819 | 216.6 | 445 | 198 | 815 | 216.4 |
| 459 | 199.5 | 829 | 216.9 | 455 | 200 | 825 | 217.1 |
| 469 | 200.4 | 839 | 217.5 | 465 | 200 | 835 | 217.0 |
| 479 | 201.1 | 849 | 217.6 | 475 | 201 | 845 | 217.2 |
| 489 | 201.7 | 859 | 218.0 | 485 | 202 | 855 | 217.4 |
| 499 | 202.4 | 869 | 218.2 | 495 | 202 | 865 | 217.7 |
| 509 | 203.1 | 879 | 218.3 | 505 | 203 | 875 | 218.4 |
| 519 | 203.6 | 889 | 219.0 | 515 | 204 | 885 | 218.6 |
| 529 | 204.3 | 899 | 218.8 | 525 | 204 | 895 | 219.0 |
| 539 | 205.0 | 909 | 219.8 | 535 | 205 | 905 | 219.0 |
| 549 | 205.7 | 919 | 220.5 | 545 | 205 | 915 | 219.4 |
| 559 | 206.1 | 929 | 221.4 | 555 | 206 | 925 | 220.2 |
| 569 | 206.8 | 939 | 223.0 | 565 | 207 | 935 | 221.9 |
| 579 | 207.3 | 949 | 219.7 | 575 | 207 | 945 | 221.5 |
| 589 | 208.1 | 959 | 219.2 | 585 | 208 | 955 | 217.7 |
| 599 | 208.8 | 969 | 218.2 | 595 | 208 | 965 | 216.9 |
| 609 | 209.4 | 979 | 217.6 | 605 | 209 | 975 | 217.5 |
| 619 | 209.7 | 989 | 217.1 | 615 | 210 | 985 | 217.2 |
| 629 | 210.4 | 999 | 217.0 | 625 | 210 | 995 | 217.5 |
| 639 | 210.8 | 1009 | 217.2 | 635 | 211 | 1005 | 218.2 |
| 649 | 211.1 | 1019 | 217.3 | 645 | 211 | 1015 | 217.8 |
| 659 | 211.7 | 1029 | 217.0 | 655 | 211 | 1025 | 218.9 |
| 669 | 212.1 | 1039 | 217.1 | 665 | 212 | 1035 | 219.1 |
| 679 | 212.5 | 1049 | 217.2 | 675 | 212 | 1045 | 218.2 |
| 689 | 213.0 | 1059 | 217.3 | 685 | 213 | 1055 | 218.8 |
| 699 | 213.4 | 1069 | 217.4 | 695 | 213 | 1065 | 218.9 |
| 709 | 213.5 | 1079 | 217.9 | 705 | 213 | 1075 | 218.7 |
| 719 | 213.6 | 1089 | 217.5 | 715 | 214 | 1085 | 218.2 |
| 729 | 214.3 | 1099 | 218.0 | 725 | 214 | 1095 | 218.5 |
| 739 | 214.7 | 735 | 214 | ||||
References
- Bhat, S.S.M.; Jang, H.W. Recent Advances in Bismuth-Based Nanomaterials for Photoelectrochemical Water Splitting. ChemSusChem 2017, 10, 3001–3018. [Google Scholar] [CrossRef]
- Lee, D.K.; Lee, D.; Lumley, M.A.; Choi, K.-S. Progress on Ternary Oxide-Based Photoanodes for Use in Photoelectrochemical Cells for Solar Water Splitting. Chem. Soc. Rev. 2019, 48, 2126–2157. [Google Scholar] [CrossRef]
- Zhou, L.; Shinde, A.; Guevarra, D.; Haber, J.A.; Persson, K.A.; Neaton, J.B.; Gregoire, J.M. Successes and Opportunities for Discovery of Metal Oxide Photoanodes for Solar Fuels Generators. ACS Energy Lett. 2020, 5, 1413–1421. [Google Scholar] [CrossRef]
- Aurivillius, B. Mixed bismuth oxides with layer lattices. 2. Structure of Bi4Ti3O12. Arkiv. Kemi 1950, 1, 499–512. [Google Scholar]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-Light-Induced Degradation of Rhodamine B by Nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef]
- Ren, J.; Wang, W.; Zhang, L.; Chang, J.; Hu, S. Photocatalytic inactivation of bacteria by photocatalyst Bi2WO6 under visible light. Catal. Commun. 2009, 10, 1940–1943. [Google Scholar] [CrossRef]
- Kudo, A.; Hijii, S. H2 or O2 Evolution from Aqueous Solutions on Layered Oxide Photocatalysts Consisting of Bi3+ with 6s2 Configuration and D0 Transition Metal Ions. Chem. Lett. 1999, 28, 1103–1104. [Google Scholar] [CrossRef]
- Hill, J.C.; Choi, K.-S. Synthesis and Characterization of High Surface Area CuWO4 and Bi2WO6 Electrodes for Use as Photoanodes for Solar Water Oxidation. J. Mater. Chem. A 2013, 1, 5006–5014. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Chang, X.; Li, A.; Gong, J. Fabrication of Porous Nanoflake BiMOx (M = W, V, and Mo) Photoanodes via Hydrothermal Anion Exchange. Chem. Sci. 2016, 7, 6381–6386. [Google Scholar] [CrossRef]
- Chae, S.Y.; Lee, E.S.; Jung, H.; Hwang, Y.J.; Joo, O.-S. Synthesis of Bi2WO6 Photoanode on Transparent Conducting Oxide Substrate with Low Onset Potential for Solar Water Splitting. RSC Adv. 2014, 4, 24032–24037. [Google Scholar] [CrossRef]
- Buttrey, D.J.; Vogt, T.; Wildgruber, U.; Robinson, W.R. Structural Refinement of the High Temperature Form of Bi2MoO6. J. Solid State Chem. 1994, 111, 118–127. [Google Scholar] [CrossRef]
- Bégué, P.; Enjalbert, R.; Galy, J.; Castro, A. Single-crystal X-ray investigations of the structures of γ(H)Bi2MoO6 and its partially substituted As3+ and Sb3+ homologues. Solid State Sci. 2000, 2, 637–653. [Google Scholar] [CrossRef]
- Zhu, Z.; Wan, S.; Zhao, Y.; Qin, Y.; Ge, X.; Zhong, Q.; Bu, Y. Recent progress in Bi2WO6-based photocatalysts for clean energy and environmental remediation: Competitiveness, challenges, and future perspectives. Nano Sel. 2021, 2, 187–215. [Google Scholar] [CrossRef]
- Frit, B.; Mercurio, J.P. The Crystal Chemistry and Dielectric Properties of the Aurivillius Family of Complex Bismuth Oxides with Perovskite-Like Layered Structures. J. Alloys Compd. 1992, 188, 27–35. [Google Scholar] [CrossRef]
- Wolfe, R.W.; Newham, R.E.; Kay, M.I. Crystal structure of Bi2WO6. Solid State Commun. 1969, 7, 1797–1801. [Google Scholar] [CrossRef]
- Islam, M.S.; Lazure, S.; Vannier, R.-N.; Nowogrocki, G.; Mairesse, G. Structural and Computational Studies of Bi2WO6 Based Oxygen Ion Conductors. J. Mater. Chem. 1998, 8, 655–660. [Google Scholar] [CrossRef]
- Knight, K.S. The crystal structure of russellite; a re-determination using neutron powder diffraction of synthetic Bi2WO6. Min. Mag. 1992, 56, 399–409. [Google Scholar] [CrossRef]
- Yoneda, Y.; Kohara, S.; Takeda, H.; Tsurumi, T. Local Structure Analysis of Bi2WO6. Jap. J. Appl. Phys. 2012, 51, 09LE06. [Google Scholar] [CrossRef]
- Djani, H.; Hermet, P.; Ghosez, P. First-Principles Characterization of the P21ab Ferroelectric Phase of Aurivillius Bi2WO6. J. Phys. Chem. C 2014, 118, 13514–13524. [Google Scholar] [CrossRef]
- Okudera, H.; Sakai, Y.; Yamagata, K.; Takeda, H. Structure of Russellite (Bi2WO6): Origin of Ferroelectricity and the Effect of the Stereoactive Lone Electron Pair on the Structure. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2018, 74, 295–303. [Google Scholar] [CrossRef]
- Rae, A.D.; Thompson, J.G.; Withers, R.L. Structure refinement of commensurately modulated bis muth tungstate, Bi2WO6. Acta Crystallogr. Sect. B. 1991, 47, 870–881. [Google Scholar] [CrossRef]
- Knight, K.S. The Crystal Structure of Ferroelectric Bi2WO6 at 961 K. Ferroelectrics 1993, 150, 319–330. [Google Scholar] [CrossRef]
- Mohn, C.E.; Stølen, S. Influence of the Stereochemically Active Bismuth Lone Pair Structure on Ferroelectricity and Photocalytic Activity of Aurivillius Phase Bi2WO6. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 83, 014103. [Google Scholar] [CrossRef]
- Elaouni, A.; El Ouardi, M.; BaQais, M.; Arab, M.; Saadi, M.; Ahsaine, H.A. Bismuth tungstate Bi2WO6: A review on structural, photophysical and photocatalytic properties. RSC Adv. 2023, 13, 17476. [Google Scholar] [CrossRef] [PubMed]
- Speranskaya, E.I. The Bi2O3–WO3 System. Inorg. Mater. 1970, 6, 127–129. [Google Scholar]
- Hoda, Y.N.; Chang, L.L.Y. Phase Relations in the System Bi2O3-WO3. J. Am. Ceram. Soc. 1974, 57, 323–326. [Google Scholar] [CrossRef]
- Ling, C.D.; Withers, R.L.; Schmid, S.; Thompson, J.G. A Review of Bismuth-Rich Binary Oxides in the Systems Bi2O3–Nb2O5, Bi2O3–Ta2O5, Bi2O3–MoO3, and Bi2O3–WO3. J. Solid State Chem. 1998, 137, 42–61. [Google Scholar] [CrossRef]
- Finlayson, A.P.; Ward, E.; Tsaneva, V.N.; Glowacki, B.A. Bi2O3–WO3 compounds for photocatalytic applications by solid state and viscous processing. J. Power Sources 2005, 145, 667–674. [Google Scholar] [CrossRef]
- Zhou, W. Defect Fluorite Superstructures in the Bi2O3-WO3 System. J. Solid State Chem. 1994, 108, 381–394. [Google Scholar] [CrossRef]
- Muktha, B.; Row, T.N.G. Crystal structure and ionic conductivity of a new bismuth tungstate, Bi3W2O10.5. J. Chem. Sci. 2006, 118, 43–46. [Google Scholar] [CrossRef]
- Phapale, S.; Das, D.; Mishra, R. Standard molar enthalpy of formation of Bi2WO6(s) and Bi2W2O9(s) compounds. J. Chem. Thermodyn. 2013, 63, 74–77, Erratum in J. Chem. Thermodyn. 2015, 89, 312–313. [Google Scholar] [CrossRef]
- Maier, C.G.; Kelley, K.K. An equation for the representation of high-temperature heat content data. J. Am. Chem. Soc. 1932, 54, 3243–3246. [Google Scholar] [CrossRef]
- Gamsjager, E.; Wiessner, M. Low temperature heat capacities and thermodynamic functions described by Debye-Einstein integrals. Monatsh. Chem. 2018, 149, 357–368. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.A.; Knight, K.S.; Lightfoot, P. Unusual High-Temperature Structural Behaviour in Ferroelectric Bi2WO6. Chem. Eur. J. 2006, 12, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Voronkova, V.I.; Kharitonova, E.P.; Rudnitskaya, O.G. Refinement of Bi2WO6 and Bi2MoO6 polymorphism. J. Alloys Compd. 2009, 487, 274–279. [Google Scholar] [CrossRef]
- Mączka, M.; Macalik, L.; Hermanowicz, K.; Kępiński, L.; Tomaszewski, P. Phonon properties of nanosized bismuth layered ferroelectric material—Bi2WO6. J. Raman Spectrosc. 2010, 41, 1059–1066. [Google Scholar] [CrossRef]
- Yanovskii, V.K.; Voronkova, V.I. Polymorphism and Properties of Bi2WO6 and Bi2MoO6. Phys. Stat. Sol. 1986, 93, 57–66. [Google Scholar] [CrossRef]
- Newkirk, H.W.; Quadflieg, P.; Liebertz, J.; Kockel, A. Growth, crystallography and dielectric properties of Bi2WO6. Ferroelectrics 1972, 4, 51–55. [Google Scholar] [CrossRef]
- Kubaschewski, O.; Alcock, C.B.; Spencer, P.J. Materials Thermochemistry, 6th ed.; Pergamon Press: Oxford, UK, 1993; pp. 267–323. [Google Scholar]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystal. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Day, N. Open-Access Collection of Crystal Structures of Organic, Inorganic, Metal-Organic Compounds and Minerals, Excluding Biopolymers. COD—Crystallography Open Database, University of Cambridge. Available online: http://www.crystallography.net/cod/result.php (accessed on 1 July 2025).
- ASTM Standard E1269-05; Determining Specific Heat Capacity by DSC. ASTM International: Philadelphia, PA, USA, 2007. [CrossRef]
- ASTM Standard E1269-11; Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry. ASTM International: Philadelphia, PA, USA, 2011. [CrossRef]
- Watanabe, A. Polymorphism in Bi2WO6. J. Solid State Chem. 1982, 41, 160–165. [Google Scholar] [CrossRef]
- Denisova, L.T.; Izotov, A.D.; Chumilina, L.G.; Kargin, Y.F.; Denisov, V.M. Heat capacity and thermodynamic properties of bismuth orthovanadate in the temperature range 356–980 K. Dokl. Phys. Chem. 2016, 467, 41–43. [Google Scholar] [CrossRef]
- Kopp, H. III. Investigations of the specific heat of solid bodies. Phil. Trans. R Soc. Lond. 1865, 155, 71–202. [Google Scholar] [CrossRef]
- Leitner, J.; Chuchvalec, P.; Semidubský, D.; Strejc, A.; Abrman, P. Estimation of heat capacities of solid mixed oxides. Thermochem. Acta 2003, 395, 27–46. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; VCH: Weinheim, Germany, 1995; pp. 202+1160. [Google Scholar]
- Aiswarya, P.M.; Kumar, S.S.; Ganesan, R.; Gnanasekaran, T. Determination of thermodynamic properties of Bi2Mo3O12 (s), Bi2MoO6 (s) and Bi6Mo2O15 (s). J. Chem. Thermodyn. 2019, 139, 105886. [Google Scholar] [CrossRef]
- Aiswarya, P.M.; Narang, S.; Dawar, R.; Babu, P.D.; Mishra, R. Calorimetric investigation of ternary oxides in the CuO–V2O5 system. J. Therm. Anal. Calorim. 2025. [Google Scholar] [CrossRef]
- Ilhan, M.; Mergen, A.; Sarıoglu, C.; Yaman, C. Heat capacity measurements on BaTa2O6 and derivation of its thermodynamic functions. J. Therm. Anal. Calorim. 2017, 128, 707–711. [Google Scholar] [CrossRef]
- Denisova, L.T.; Belousova, N.V.; Denisov, V.M.; Galiakhmetova, N.A. High-temperature heat capacity of oxides of the CuO–V2O5 system. Phys. Solid State. 2017, 59, 1270–1274. [Google Scholar] [CrossRef]
- Denisova, L.T.; Kargin, Y.F.; Belousova, N.V.; Galiakhmetova, N.A.; Denisov, V.M. Synthesis and investigation of thermodynamic properties of Cu5V2O10. Russ. J. Inorg. Chem. 2019, 64, 725–728. [Google Scholar] [CrossRef]
- Leitner, J.; Vonka, P.; Sedmidubsky, D.; Svoboda, P. Application of Neumann-Kopp rule for the estimation of heat capacity of mixed oxides. Thermochim. Acta 2010, 497, 7–13. [Google Scholar] [CrossRef]
- Demidenko, A.F.; Koshchenko, V.I.; Medvedeva, Z.S.; Radchenko, A.F. Thermal capacities and thermodynamic functions of BAs and B6As. Izv. Akad. Nauk. SSSR Neorg. Mater. 1975, 11, 2117–2119. [Google Scholar]
- Danilchenko, B.A.; Paszkiewicz, T.; Wolski, S.; Jezowski, A.; Plackowski, T. Heat capacity and phonon mean free path of wurtzite GaN. Appl. Phys. Lett. 2006, 89, 061901. [Google Scholar] [CrossRef]
- Sedmidubsky, D.; Leitner, J. Calculation of the thermodynamic properties of AIII nitrides. J. Cryst. Growth 2006, 286, 66–70. [Google Scholar] [CrossRef]
- Sedmidubsky, D.; Leitner, J.; Svoboda, P.; Sofer, Z.; Macháček, J. Heat capacity and phonon spectra of AIIIN. J. Therm. Anal. Calorim. 2009, 95, 403–407. [Google Scholar] [CrossRef]
- Pässler, R. Non-Debye heat capacity formula refined and applied to GaP, GaAs, GaSb, InP, InAs, and InSb. AIP Adv. 2013, 3, 82108. [Google Scholar] [CrossRef]
- Szytuła, A.; Baran, S.; Przewoźnik, J.; Tyvanchuk, Y.; Kalychak, Y. Magnetic properties and specific heat data of R11Ni4In9 (R = Pr, Nd, Sm, Gd and Tb) compounds. J. Alloys Comp. 2014, 601, 238–244. [Google Scholar] [CrossRef]
- Jendrzejczyk-Handzlik, D.; Przewoźnik, J.; Onderka, B.; Kapusta, C.; Handzlik, P.; Fitzner, K. Thermodynamic properties of the novel photocatalyst BiSbO4 phase determined from 1.8 K to 1073 K. Ceram. Intern. 2017, 48, 29686–29694. [Google Scholar] [CrossRef]
- Voronin, G.F.; Kutsenok, I.B. Universal Method for Approximating the Standard Thermodynamic Functions of Solids. J. Chem. Eng. Data 2013, 58, 2083–2094. [Google Scholar] [CrossRef]
- Chen, Q.; Sundman, B. Modelling of thermodynamic properties for Bcc, Fcc, liquid, and amorphous iron. J. Phase Equil. 2001, 22, 631–644. [Google Scholar] [CrossRef]
- Chase, M.W.; Ansara, I.; Dinsdale, A.; Eriksson, G.; Grimvall, G.; Höglund, L.; Yokokawa, H. Workshop on thermodynamic models and data for pure elements and other endmembers of solutions, Schloss Ringberg. Calphad 1995, 19, 437–447. [Google Scholar] [CrossRef]
- Onderka, B. The heat capacity of bismuth silicates. Thermochim. Acta. 2015, 601, 68–74. [Google Scholar] [CrossRef]
- Mrovĕc, M.; Leitner, J.; Nevřiva, M.; Sedmidubský, D.; Stejskal, J. Thermochemical properties of MeCuO2 and Me2CuO3 (Me=Ca, Sr, Ba) mixed oxides. Thermochim. Acta 1998, 318, 63–70. [Google Scholar] [CrossRef]
- Aspiala, M.; Sukhomlinov, D.; Taskinen, P. Standard thermodynamic properties of Bi2O3 by a solid-oxide electrolyte EMF technique. J. Chem. Thermodyn. 2014, 75, 8–12. [Google Scholar] [CrossRef]
- Han, B.-Y.; Khoroshilov, A.V.; Tyurin, A.V.; Baranchikov, A.E.; Razumov, M.I.; Ivanova, O.S.; Gavrichev, K.S.; Ivanov, V.K. WO3 thermodynamic properties at 80–1256 K revisited. J. Therm. Anal. Calorim. 2020, 142, 1533–1543. [Google Scholar] [CrossRef]
- Harvey, J.-P.; Lebreux-Desilets, F.; Marchand, J.; Oishi, K.; Bouarab, A.-F.; Robelin, C.; Gheribi, A.E.; Pelton, A. On the Application of the FactSage Thermochemical Software and Databases in Materials Science and Pyrometallurgy. Processes 2020, 8, 1156. [Google Scholar] [CrossRef]
- Leitner, J.; Jakeš, V.; Sofer, Z.; Semidubský, D.; Ružicka, K.; Svoboda, P. Heat capacity, enthalpy and entropy of ternary bismuth tantalum oxides. J. Solid State Chem. 2011, 184, 241–245. [Google Scholar] [CrossRef]
- Kauwe, S.K.; Graser, J.; Vazquez, A.; Sparks, T.D. Machine Learning Prediction of Heat Capacity for Solid Inorganics. Integr. Mater. Manuf. Innov. 2018, 7, 43–51. [Google Scholar] [CrossRef]
- Chihaia, V.; Alexiev, V.; AlMatrouk, H.S. Assessment of the Heat Capacity by Thermodynamic Approach Based on Density Functional Theory Calculations. In Applications of Calorimetry; Rivera-Armenta, J.L., Flores-Hernández, C.G., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Mostafa, A.T.M.G.; Eakman, J.M.; Montoya, M.M.; Yarbro, S.L. Prediction of heat capacities of solid inorganic salts from group contributions. Ind. Eng. Chem. Res. 1996, 35, 343–348. [Google Scholar] [CrossRef]
- Gaultois, M.W.; Oliynyk, A.; Mar, A.; Sparks, T.D.; Mulholland, G.J.; Meredig, B. Perspective: Web-based machine learning models for real-time screening of thermoelectric materials properties. APL Mater. 2016, 4, 053213. [Google Scholar] [CrossRef]
- Seshadri, R.; Sparks, T.D. Perspective: Interactive material property databases through aggregation of literature data. APL Mater. 2016, 4, 053206. [Google Scholar] [CrossRef]
- Sparks, T.D.; Gaultois, M.W.; Oliynyk, A.; Brgoch, J.; Meredig, B. Data mining our way to the next generation of thermoelectrics. Scr. Mater. 2016, 111, 10–15. [Google Scholar] [CrossRef]
| i | K | fi | a, J·K−2·mol−1 | b, J·K−5·mol−1 |
|---|---|---|---|---|
| 1 | 757.4 ± 0.7 | 0.3725 ± 0.0423 | 0.0199 ± 0.0070 | −4.450·10−12 ± 3.295·10−12 |
| 2 | 172.0 ± 0.4 | 0.5570 ± 0.0208 |
| T, K | Cp(T) | So(T) − So(298.15) | So(T) | Ho(T) − Ho(298.15) | Ho(T) | Go(T) |
|---|---|---|---|---|---|---|
| J·K−1·mol−1 | J·K−1·mol−1 | J·K−1·mol−1 | kJ·mol−1 | kJ·mol−1 | kJ·mol−1 | |
| 298.15 | 176.8 | 0.0 | 226.3 | 0.0 | −1440.5 | −1508.0 |
| 300 | 177.3 | 1.1 | 227.4 | 0.3 | −1440.2 | −1508.4 |
| 350 | 186.7 | 29.2 | 255.5 | 9.4 | −1431.1 | −1520.5 |
| 400 | 193.4 | 54.6 | 280.9 | 19.0 | −1421.6 | −1533.9 |
| 450 | 198.6 | 77.7 | 304.0 | 28.8 | −1411.8 | −1548.6 |
| 500 | 202.7 | 98.8 | 325.1 | 38.8 | −1401.7 | −1564.3 |
| 550 | 206.0 | 118.3 | 344.6 | 49.0 | −1391.5 | −1581.0 |
| 600 | 208.8 | 136.3 | 362.6 | 59.4 | −1381.2 | −1598.7 |
| 650 | 211.1 | 153.1 | 379.4 | 69.9 | −1370.7 | −1617.3 |
| 700 | 213.1 | 168.9 | 395.2 | 80.5 | −1360.0 | −1636.7 |
| 750 | 214.8 | 183.6 | 409.9 | 91.2 | −1349.3 | −1656.8 |
| 800 | 216.2 | 197.5 | 423.8 | 102.0 | −1338.6 | −1677.6 |
| 850 | 217.4 | 210.7 | 437.0 | 112.8 | −1327.7 | −1699.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onderka, B.; Kula, A. Heat Capacity and Thermodynamic Properties of Photocatalitic Bismuth Tungstate, Bi2WO6. Metals 2025, 15, 1174. https://doi.org/10.3390/met15111174
Onderka B, Kula A. Heat Capacity and Thermodynamic Properties of Photocatalitic Bismuth Tungstate, Bi2WO6. Metals. 2025; 15(11):1174. https://doi.org/10.3390/met15111174
Chicago/Turabian StyleOnderka, Bogusław, and Anna Kula. 2025. "Heat Capacity and Thermodynamic Properties of Photocatalitic Bismuth Tungstate, Bi2WO6" Metals 15, no. 11: 1174. https://doi.org/10.3390/met15111174
APA StyleOnderka, B., & Kula, A. (2025). Heat Capacity and Thermodynamic Properties of Photocatalitic Bismuth Tungstate, Bi2WO6. Metals, 15(11), 1174. https://doi.org/10.3390/met15111174






