Formation and Characterization of Ti-Al Intermetallic and Oxide Layers on Ti6Al4V as Interlayers for Hydroxyapatite Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
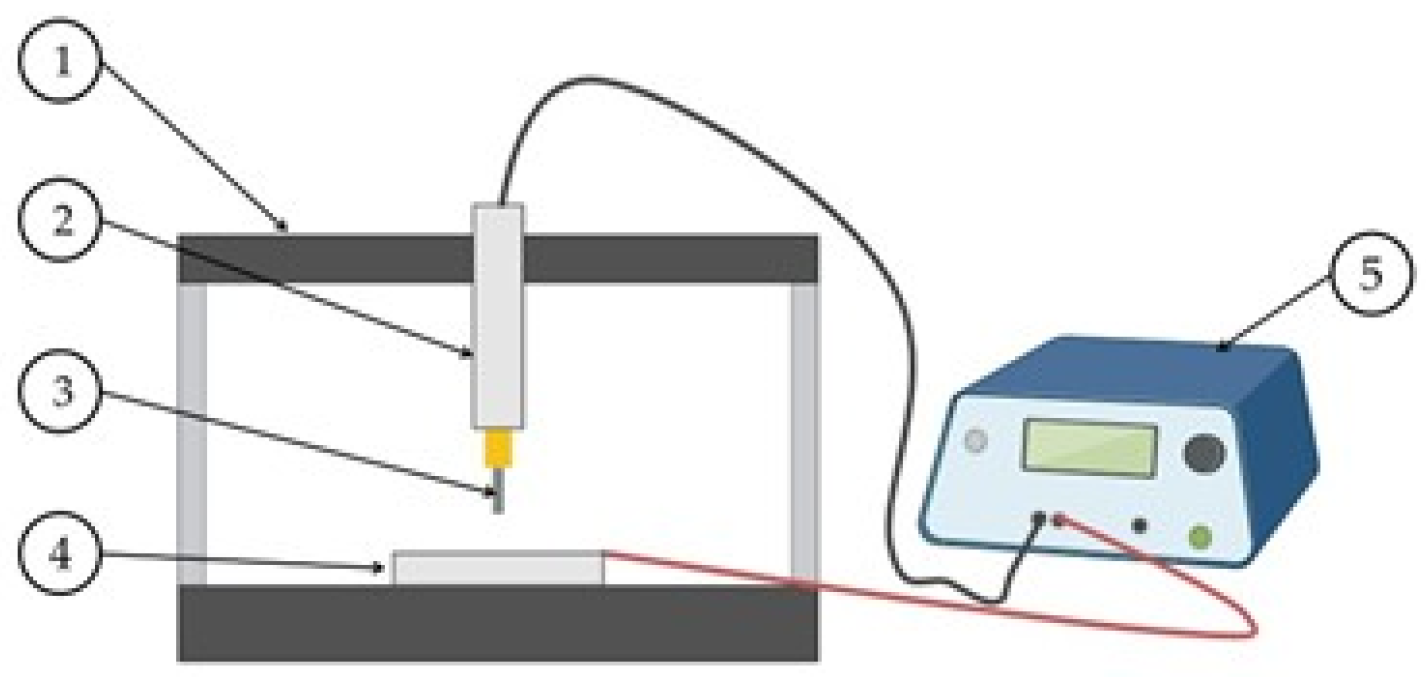
2.2. Aluminum Deposition via EDM
2.3. Post-Deposition Treatments
2.4. Characterization Techniques
3. Results and Interpretations
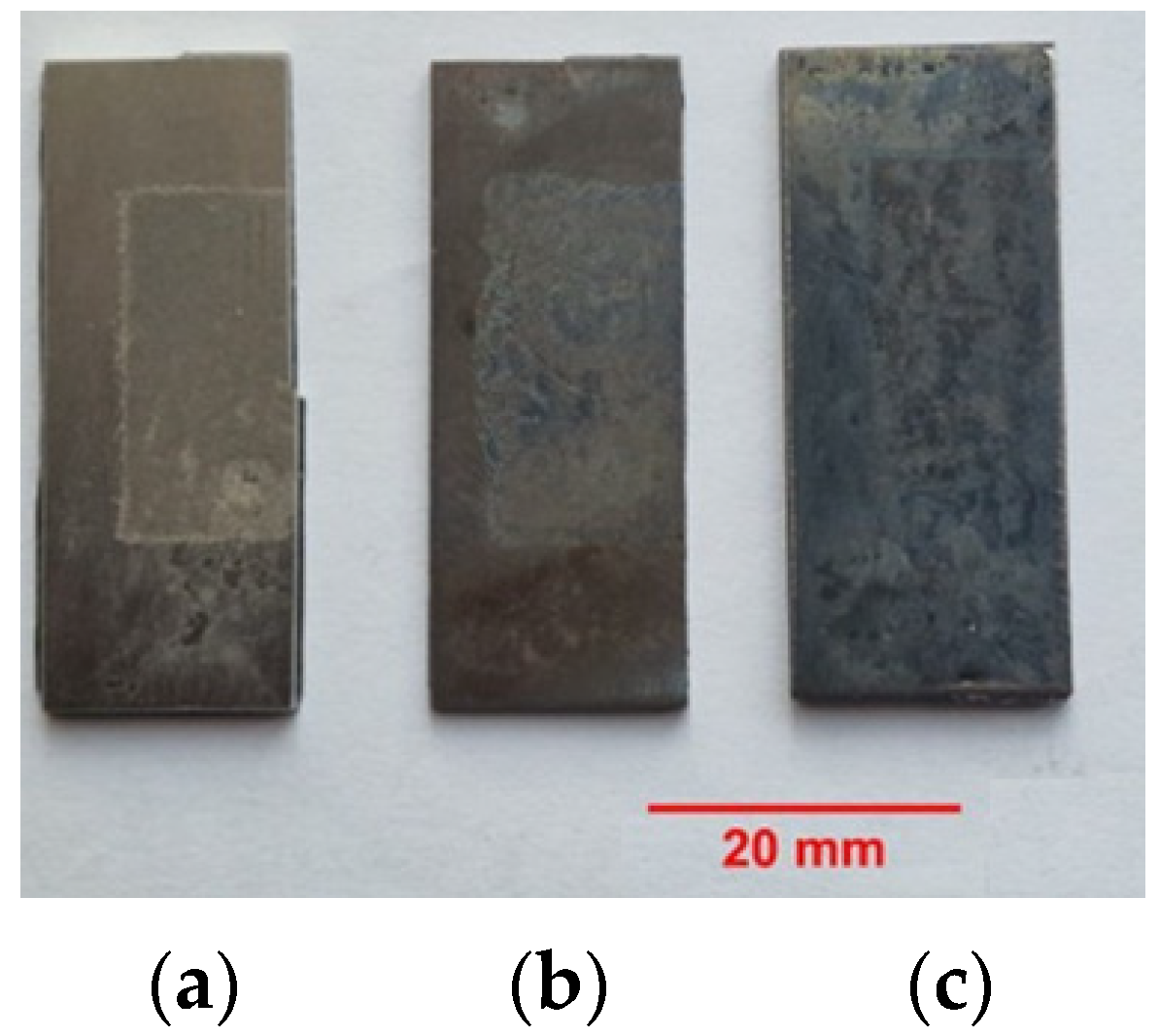
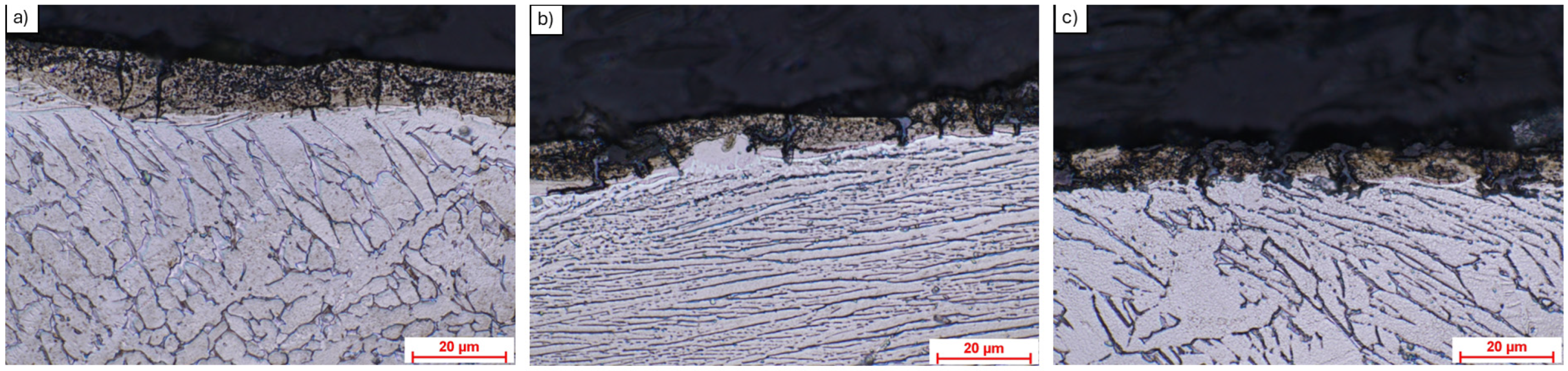
3.1. Optical Microscopy Analysis
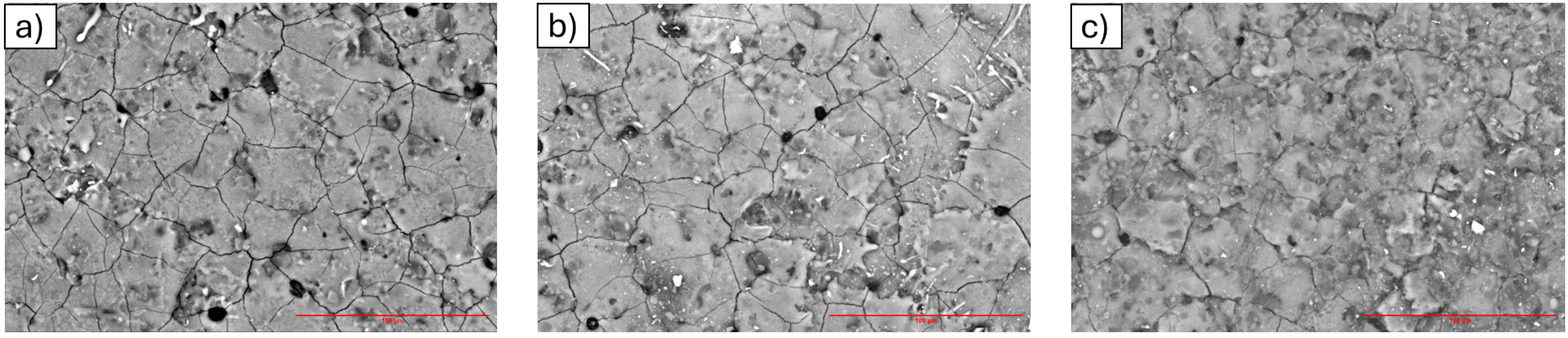
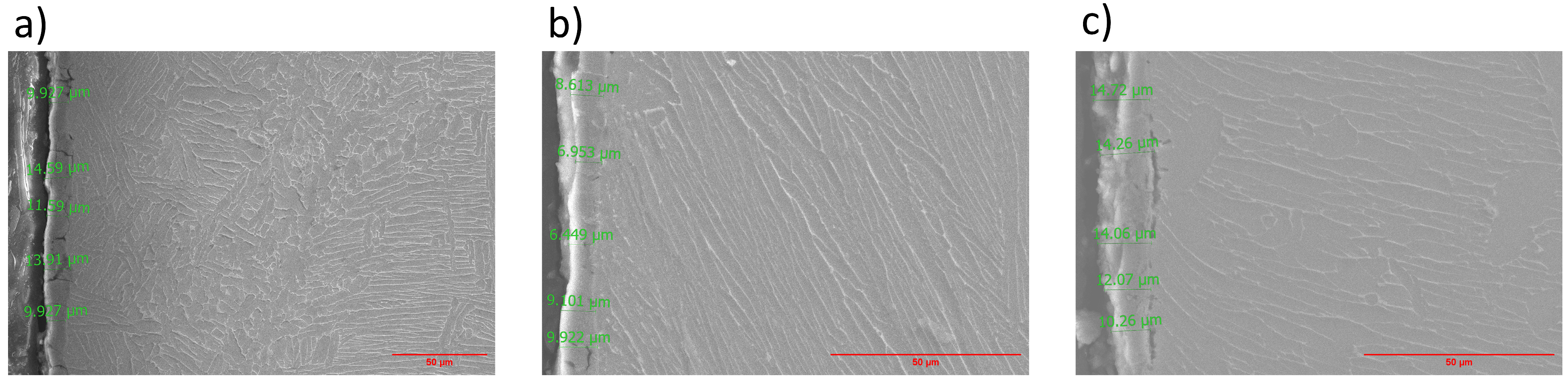
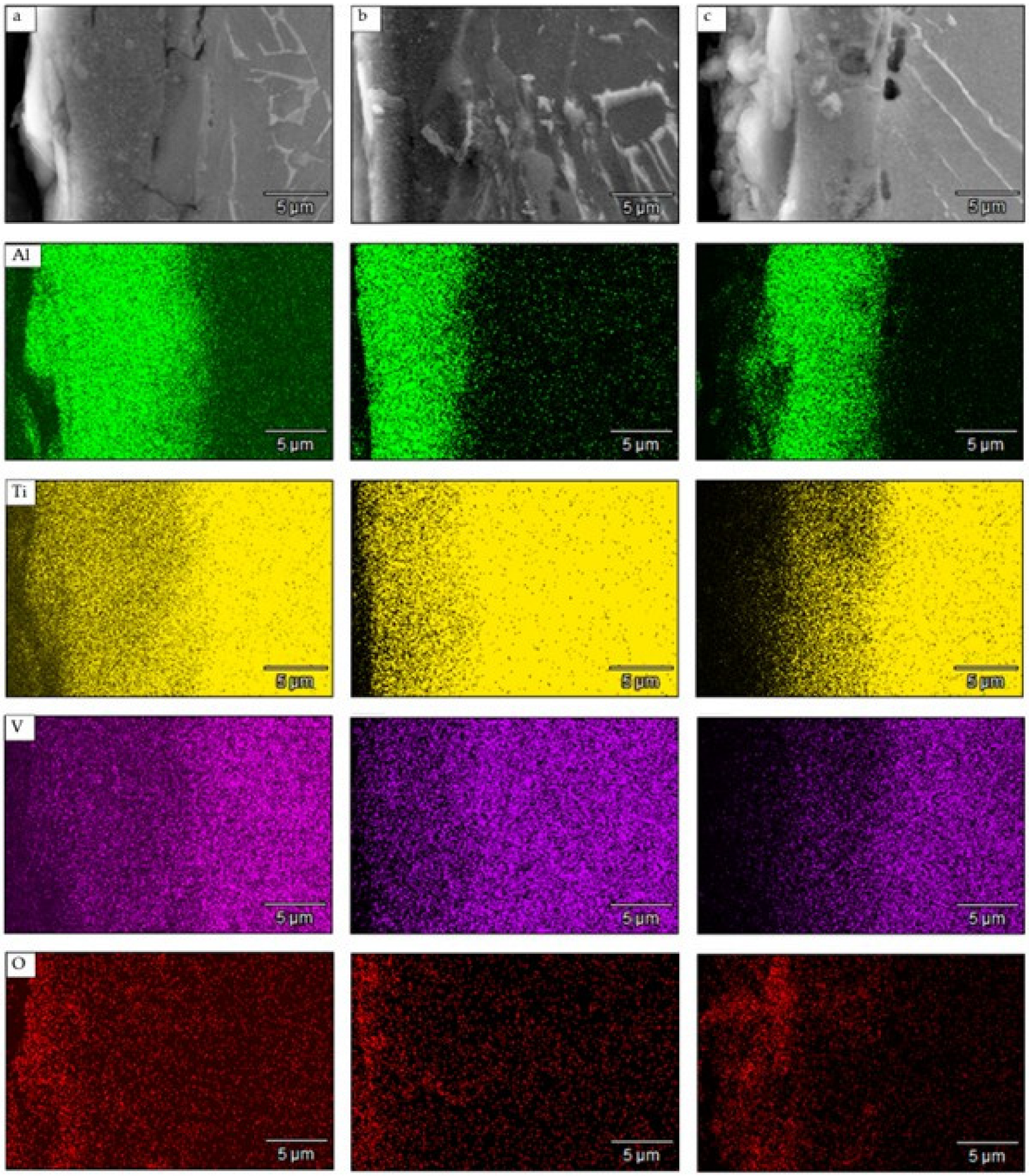
3.2. Scanning Electron Microscopy (SEM) and EDS Analysis
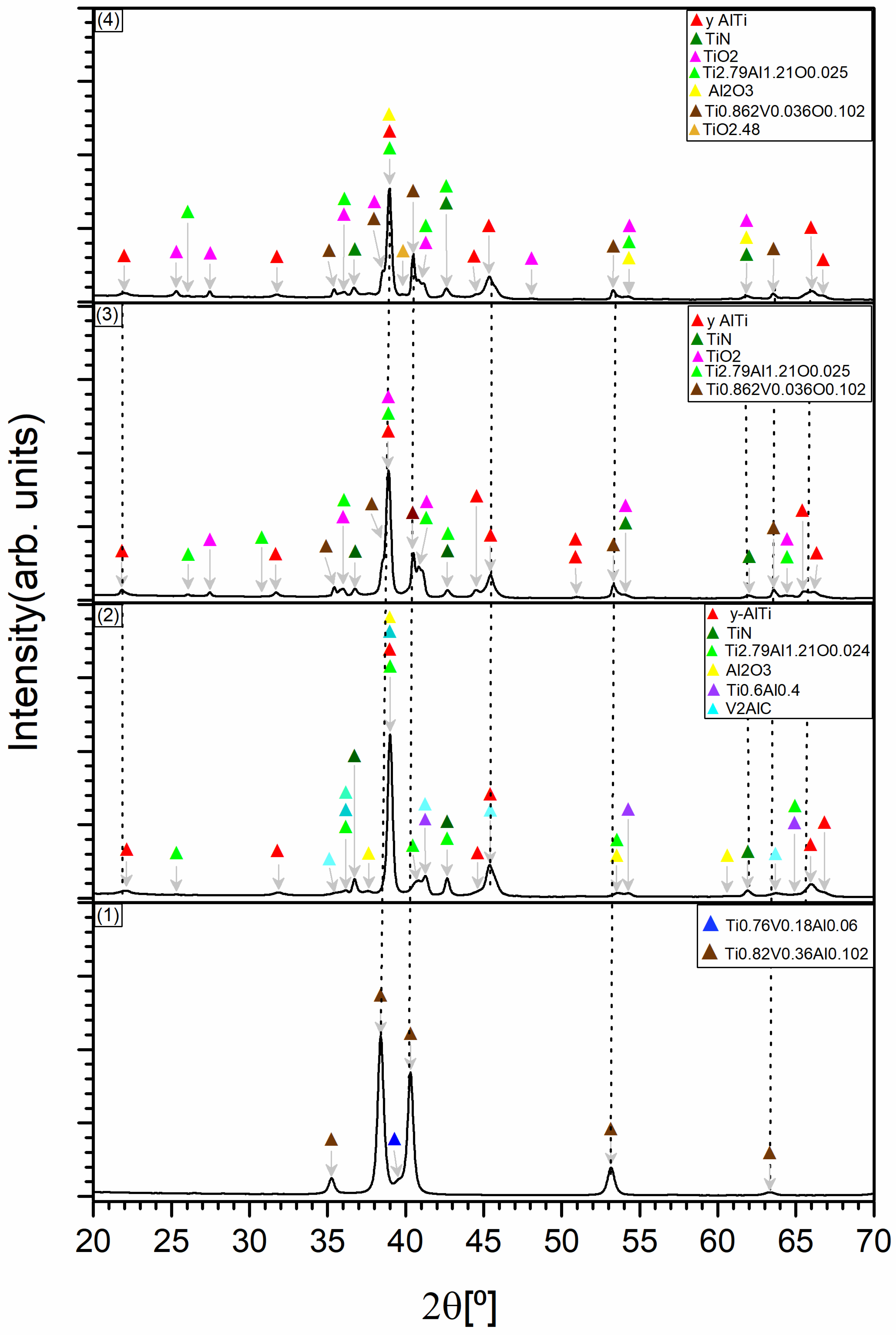
3.3. X-Ray Diffraction (XRD) Analysis
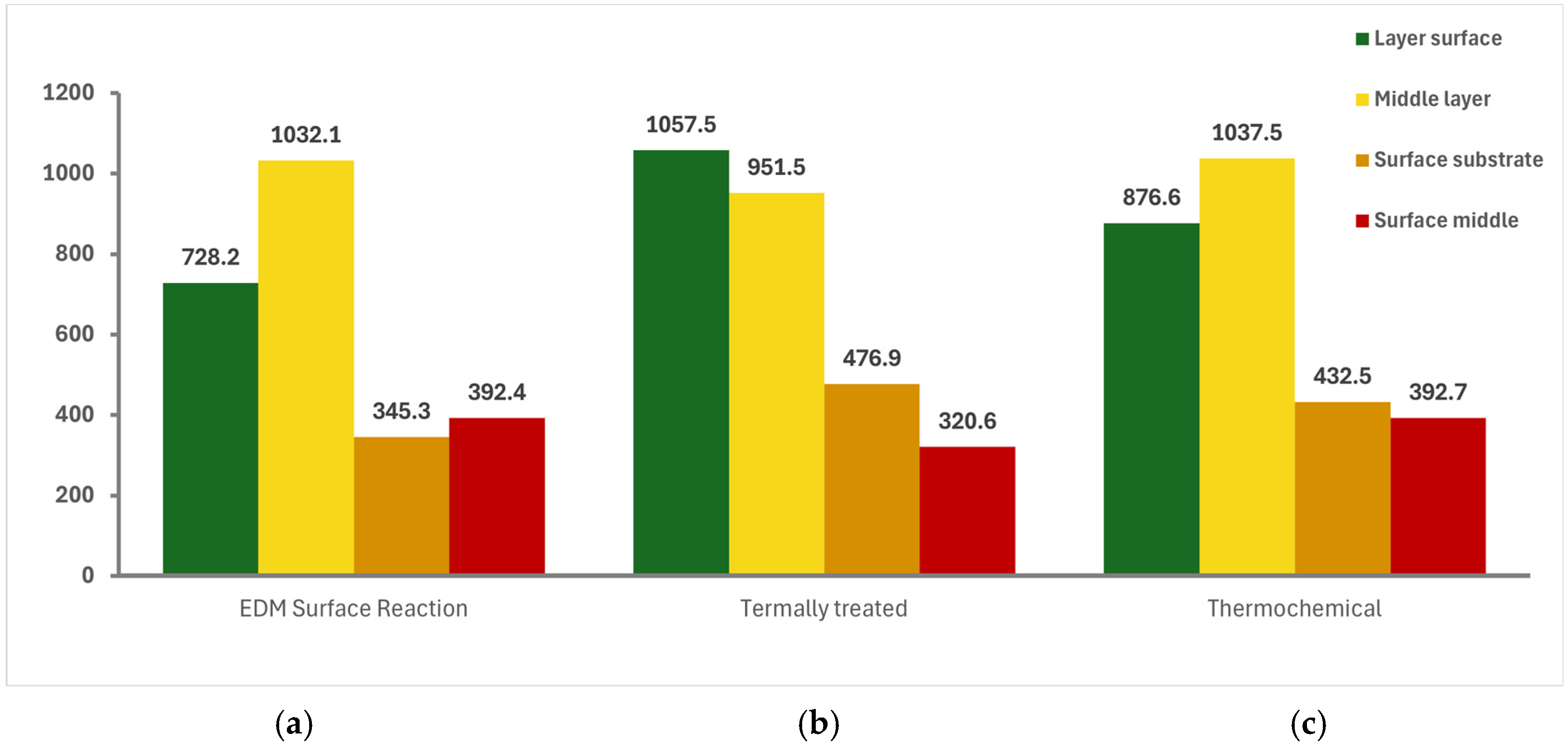
3.4. Microhardness Evaluation
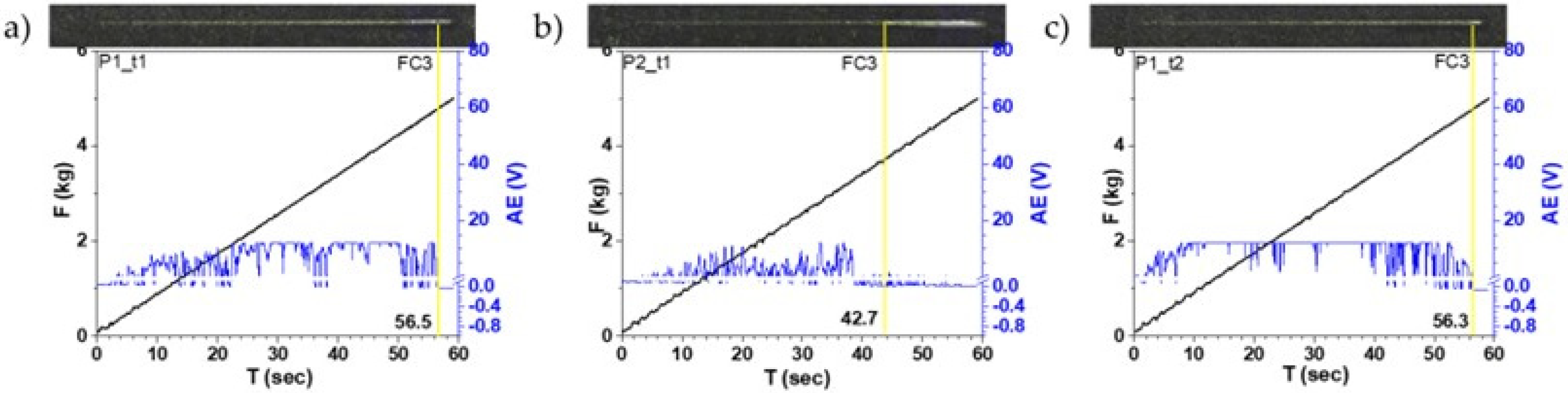
3.5. Adhesion Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Balla, V.K.; Bodhak, S.; Bose, S.; Bandyopadhyay, A. Porous tantalum structures for bone implants: Fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010, 6, 3349–3359. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Crăciunescu, E.; Sinescu, C.; Negruțiu, M.L.; Pop, D.M.; Lauer, H.C.; Rominu, M. Shear Bond Strength Tests of Zirconia Veneering Ceramics after Chipping Repair. J. Adhes. Sci. Technol. 2016, 30, 666–676. [Google Scholar] [CrossRef]
- Xia, W.; Lindahl, C.; Lausmaa, J.; Engqvist, H. Biomimetic Hydroxyapatite Deposition on Titanium Oxide Surfaces for Biomedical Application. In Advances in Biomimetics; Cavrak, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 427–443. [Google Scholar] [CrossRef]
- Tsui, Y.C.; Doyle, C.; Clyne, T.W. Plasma Sprayed Hydroxyapatite Coatings on Titanium Substrates. Part 2: Optimisation of Coating Properties. Biomaterials 1998, 19, 2031–2043. [Google Scholar] [CrossRef]
- Kitsugi, T.; Nakamura, T.; Oka, M.; Yan, W.Q.; Goto, T.; Shibuya, T.; Kokubo, T.; Miyaji, S. Bone-Bonding Behavior of Titanium and Its Alloys When Coated with Titanium Oxide (TiO2) and Titanium Silicate (Ti5Si3). J. Biomed. Mater. Res. 1996, 32, 149–156. [Google Scholar] [CrossRef]
- Rausch, M.A.; Shokoohi-Tabrizi, H.; Wehner, C.; Pippenger, B.E.; Wagner, R.S.; Ulm, C.; Moritz, A.; Chen, J.; Andrukhov, O. Impact of Implant Surface Material and Microscale Roughness on Initial Attachment and Proliferation of Primary Human Gingival Fibroblasts. Biology 2021, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Kawaguchi, H.; Mutoh, Y. Cyclic Delamination Behavior of Plasma-Sprayed Hydroxyapatite Coating on Ti–6Al–4V Substrates in Simulated Body Fluid. Mater. Sci. Eng. C 2016, 67, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.A.; Pencea, I.; Stanciu, S.G.; Hristu, R.; Antoniac, I.; Ciovica, E.; Sfat, C.E. Structural Characterization and Adhesion Appraisal of TiN and TiCN Coatings Deposited by CAE-PVD on a Carbide Composite Tool. J. Adhes. Sci. Technol. 2015, 29, 2576–2589. [Google Scholar] [CrossRef]
- Habibovic, P.; Barrere, F.; van Blitterswijk, C.A.; de Groot, K. Biomimetic Hydroxyapatite Coating on Metal Implants. J. Am. Ceram. Soc. 2002, 85, 517–522. [Google Scholar] [CrossRef]
- Appel, F.; Paul, J.D.H.; Oehring, M. Gamma Titanium Aluminide Alloys: Science and Technology; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar] [CrossRef]
- Dawod, N.; Miculescu, M.; Antoniac, I.V.; Miculescu, F.; Agop-Forna, D. Metal–Ceramic Compatibility in Dental Restorations According to the Metallic Component Manufacturing Procedure. Materials 2023, 16, 5556. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, Processing, Microstructure, Properties, and Applications of Advanced Intermetallic TiAl Alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Liu, R.; Dang, X.; Gao, Y.; Wu, T.; Zhu, Y. The Wear Behavior of the Laser-Cladded Ti-Al-Si Composite Coatings on Ti-6Al-4V Alloy with Additional TiC. Materials 2021, 14, 4567. [Google Scholar] [CrossRef]
- Hanawa, T. Research and Development of Metals for Medical Devices Based on Clinical Needs. Sci. Technol. Adv. Mater. 2012, 13, 064102. [Google Scholar] [CrossRef]
- Yao, C.; Slamovich, E.B.; Webster, T.J. Enhanced Osteoblast Functions on Anodized Titanium with Nanotube-like Structures. J. Biomed. Mater. Res. A 2008, 85A, 157–166. [Google Scholar] [CrossRef]
- Liu, D.M.; Troczynski, T.; Tseng, W.J. Water-Based Sol–Gel Synthesis of Hydroxyapatite: Process Development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef]
- Chirca, O.; Biclesanu, C.; Florescu, A.; Stoia, D.I.; Pangica, A.M.; Burcea, A. Adhesive-Ceramic Interface Behavior in Dental Restorations: FEM Study and SEM Investigation. Materials 2021, 14, 5048. [Google Scholar] [CrossRef]
- Xie, B.; Gao, K. Research Progress of Surface Treatment Technologies on Titanium Alloys: A Mini Review. Coatings 2023, 13, 1486. [Google Scholar] [CrossRef]
- Singh, A.; Ghosh, A. A Thermo-Electric Model of Material Removal during Electric Discharge Machining. Int. J. Mach. Tools Manuf. 1999, 39, 669–682. [Google Scholar] [CrossRef]
- Kunieda, M.; Lauwers, B.; Rajurkar, K.P.; Schumacher, B.M. Advancing EDM through Fundamental Insight into the Process. CIRP Ann. Manuf. Technol. 2005, 54, 64–87. [Google Scholar] [CrossRef]
- Kumar, S.S.; Varol, T.; Canakci, A.; Kumaran, S.T.; Uthayakumar, M. A Review on the Performance of Materials by Surface Modification through EDM. Int. J. Lightweight Mater. Manuf. 2021, 4, 127–144. [Google Scholar] [CrossRef]
- Rajurkar, K.P.; Levy, G.; Malshe, A.; Sundaram, M.M.; McGeough, J.; Hu, X.; Resnick, R.; DeSilva, A. Micro and Nano Machining by Electro Physical and Chemical Processes. CIRP Ann. Manuf. Technol. 2006, 55, 643–666. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, V.; Misra, J.P.; Singh, R.K.; Upadhyay, V. Surface Modification by Electrical Discharge Machining: A Systematic Literature Review and Bibliometric Analysis. Proc. Inst. Mech. Eng. E J. Process Mech. Eng. 2023, 239, 3045–3061. [Google Scholar] [CrossRef]
- Ahmed, T.; Rack, H.J. Phase Transformations during Cooling in α+β Titanium Alloys. Mater. Sci. Eng. A 1998, 243, 206–211. [Google Scholar] [CrossRef]
- Boyer, R.R.; Welsch, G.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Materials Park, OH, USA, 1994; ISBN 9780871703908. [Google Scholar]
- Uwanyuze, R.S.; Kanyo, J.; Myrick, S.F.; Schafföner, S. A Review on Alpha-Case Formation and Modeling of Mass Transfer during Investment Casting of Titanium Alloys. J. Alloys Compd. 2021, 865, 158558. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Iozzelli, F.; Pradelli, G. Improvement of Wear Resistance of Ti–6Al–4V Alloy by Means of Thermal Oxidation. Mater. Lett. 2005, 59, 2159–2162. [Google Scholar] [CrossRef]
- Hörling, A.; Hultman, L.; Odén, M.; Sjölén, J.; Karlsson, L. Mechanical Properties and Machining Performance of Ti1–xAlxN-Coated Cutting Tools. Surf. Coat. Technol. 2005, 191, 384–392. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, A.; Wang, Z.; Liu, H.; Wang, J.; Li, C. Advanced Surface Modification for 3D Printed Titanium Alloy Implant Interface Functionalization. Front. Bioeng. Biotechnol. 2022, 10, 850110. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of Micro Arc Oxidation of Titanium Alloys: Mechanism, Properties and Applications. J. Alloys Compd. 2023, 948, 169773. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Lv, Y.; Guo, S. Superhydrophilic surface on Ti6Al4V with good HA-inducing ability prepared via an eco-friendly two-step method. Vacuum 2022, 205, 111390. [Google Scholar] [CrossRef]
- EN 1071-3:2005; Advanced Technical Ceramics—Methods of Test for Ceramic Coatings Part 3: Determination of Adhesion and Other Mechanical Failure Modes by a Scratch Test. European Committee for Standardization: Brussels, Belgium, 2005.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laptoiu, S.A.; Miculescu, M.; Enescu, D.; Antoniac, I.; Miculescu, F. Formation and Characterization of Ti-Al Intermetallic and Oxide Layers on Ti6Al4V as Interlayers for Hydroxyapatite Coatings. Metals 2025, 15, 1159. https://doi.org/10.3390/met15101159
Laptoiu SA, Miculescu M, Enescu D, Antoniac I, Miculescu F. Formation and Characterization of Ti-Al Intermetallic and Oxide Layers on Ti6Al4V as Interlayers for Hydroxyapatite Coatings. Metals. 2025; 15(10):1159. https://doi.org/10.3390/met15101159
Chicago/Turabian StyleLaptoiu, Stefan Alexandru, Marian Miculescu, Diana Enescu, Iulian Antoniac, and Florin Miculescu. 2025. "Formation and Characterization of Ti-Al Intermetallic and Oxide Layers on Ti6Al4V as Interlayers for Hydroxyapatite Coatings" Metals 15, no. 10: 1159. https://doi.org/10.3390/met15101159
APA StyleLaptoiu, S. A., Miculescu, M., Enescu, D., Antoniac, I., & Miculescu, F. (2025). Formation and Characterization of Ti-Al Intermetallic and Oxide Layers on Ti6Al4V as Interlayers for Hydroxyapatite Coatings. Metals, 15(10), 1159. https://doi.org/10.3390/met15101159







