Separation of REs from Ca and Mg Ions by Ammonium Bicarbonate Precipitation and the Influence of Fe and Al Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
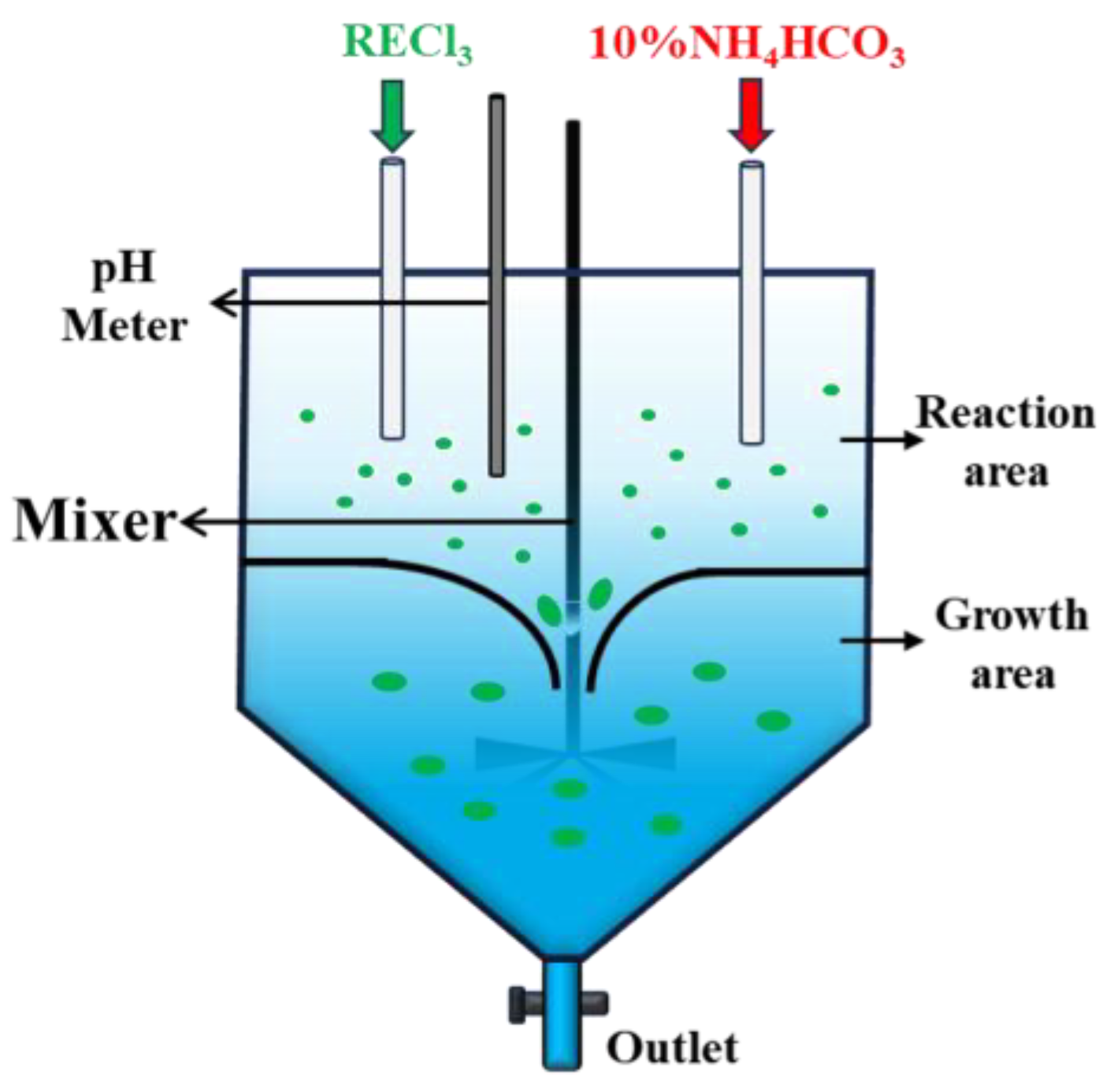
2.2. Experimental Process
2.3. Analytic Methods
2.4. Chemical Reactions and Formulas
3. Results and Discussion
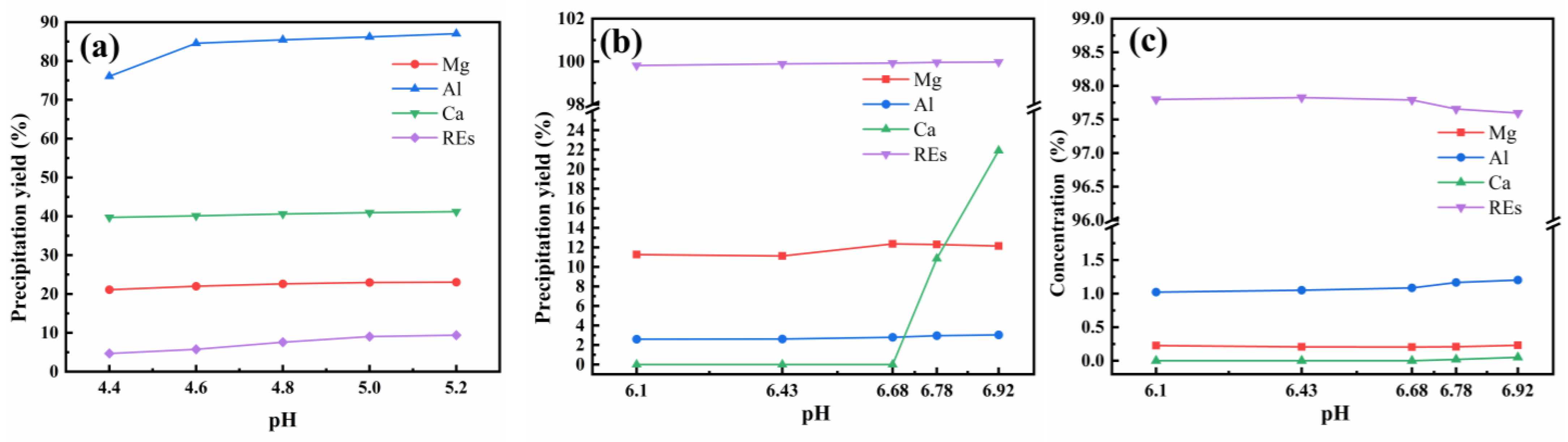
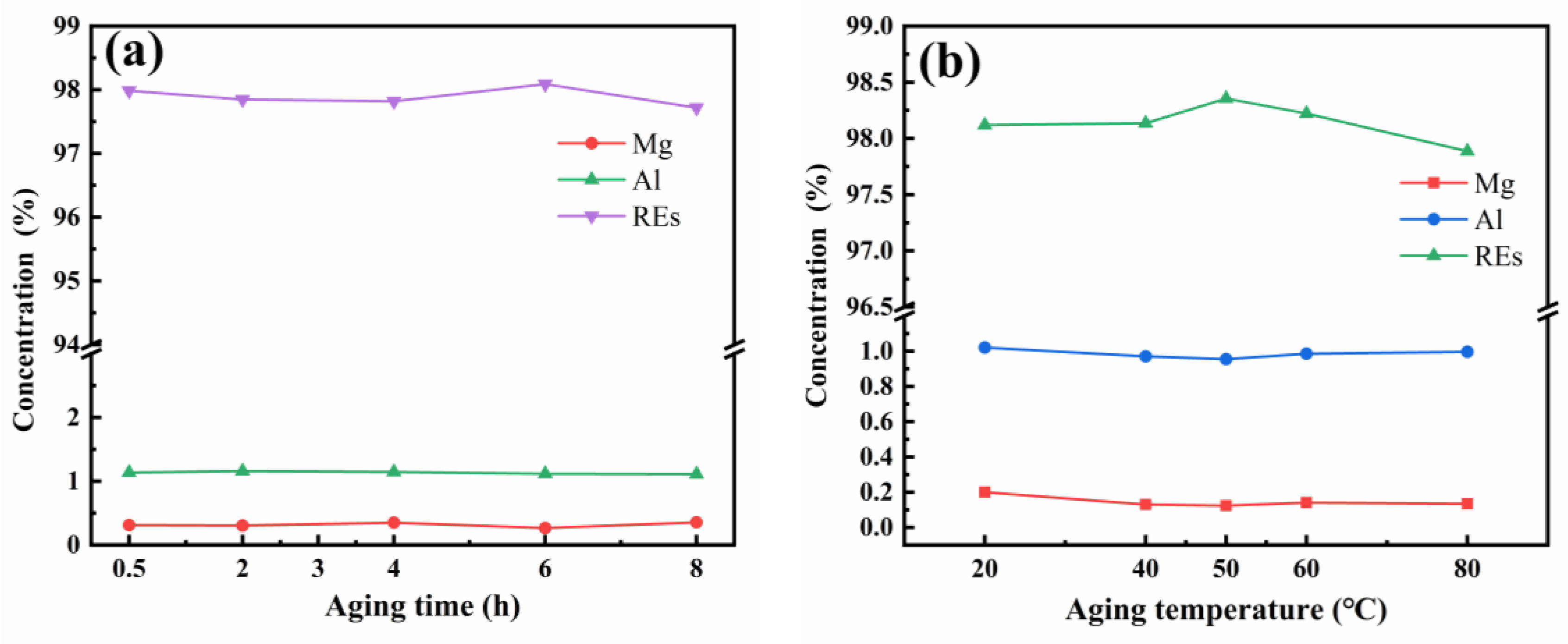
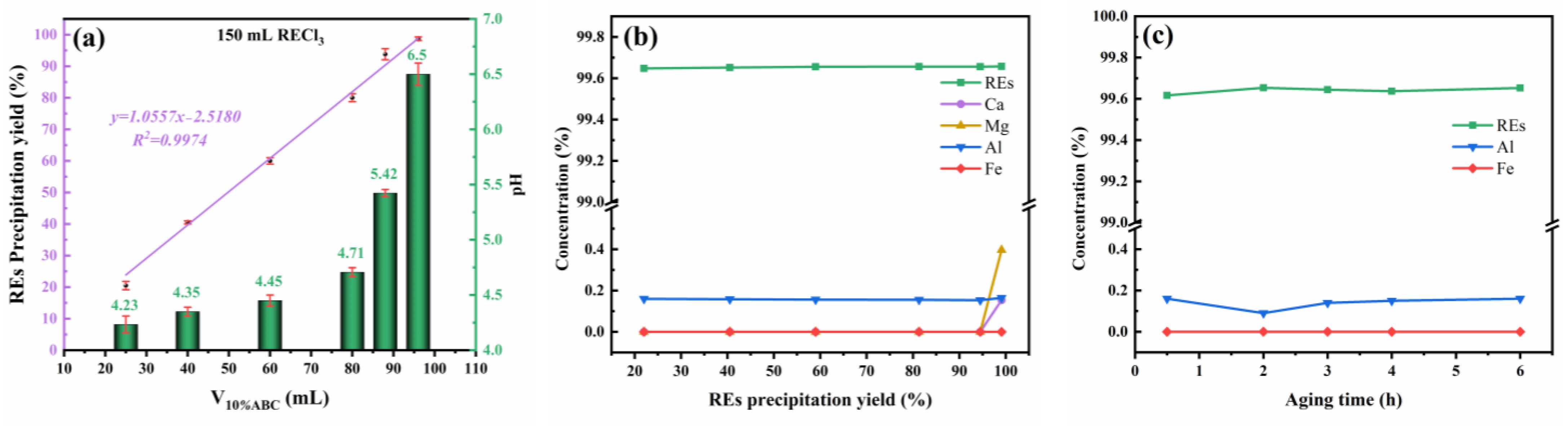
3.1. Method and Conditions for Separating REs from Ca and Mg by Ammonium Bicarbonate Precipitation
3.2. The Effect of Al and Fe on the Precipitation Separation of REs from Ca and Mg by Ammonium Bicarbonate
3.3. Purity of RE2O3 Obtained Using Ammonium Bicarbonate Stepwise Precipitation from a Solution of RECl3 Containing Ca, Mg, Al, and Fe
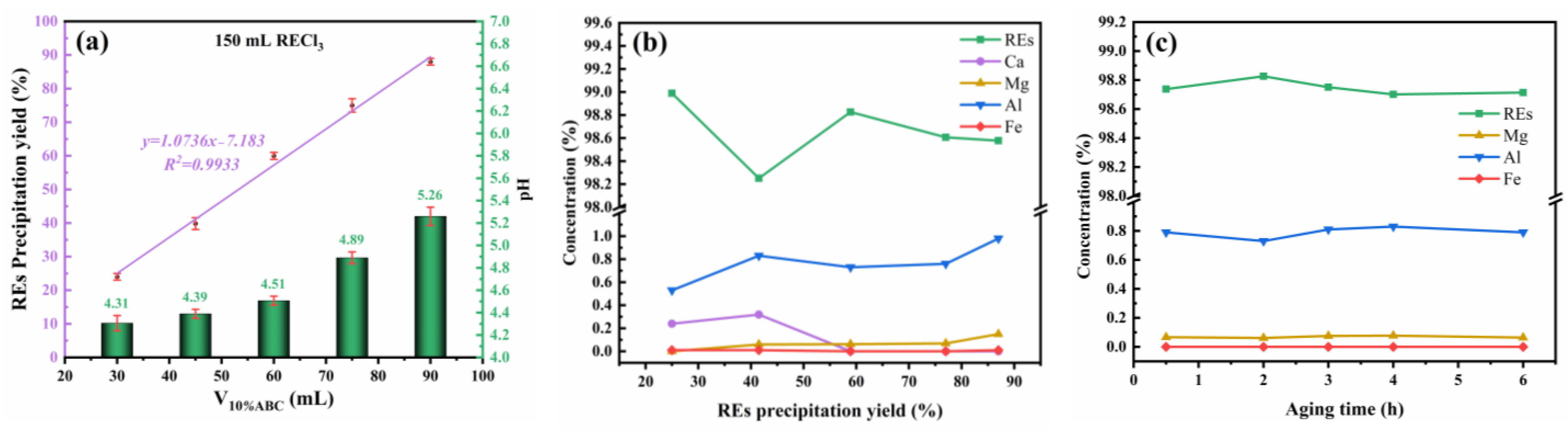
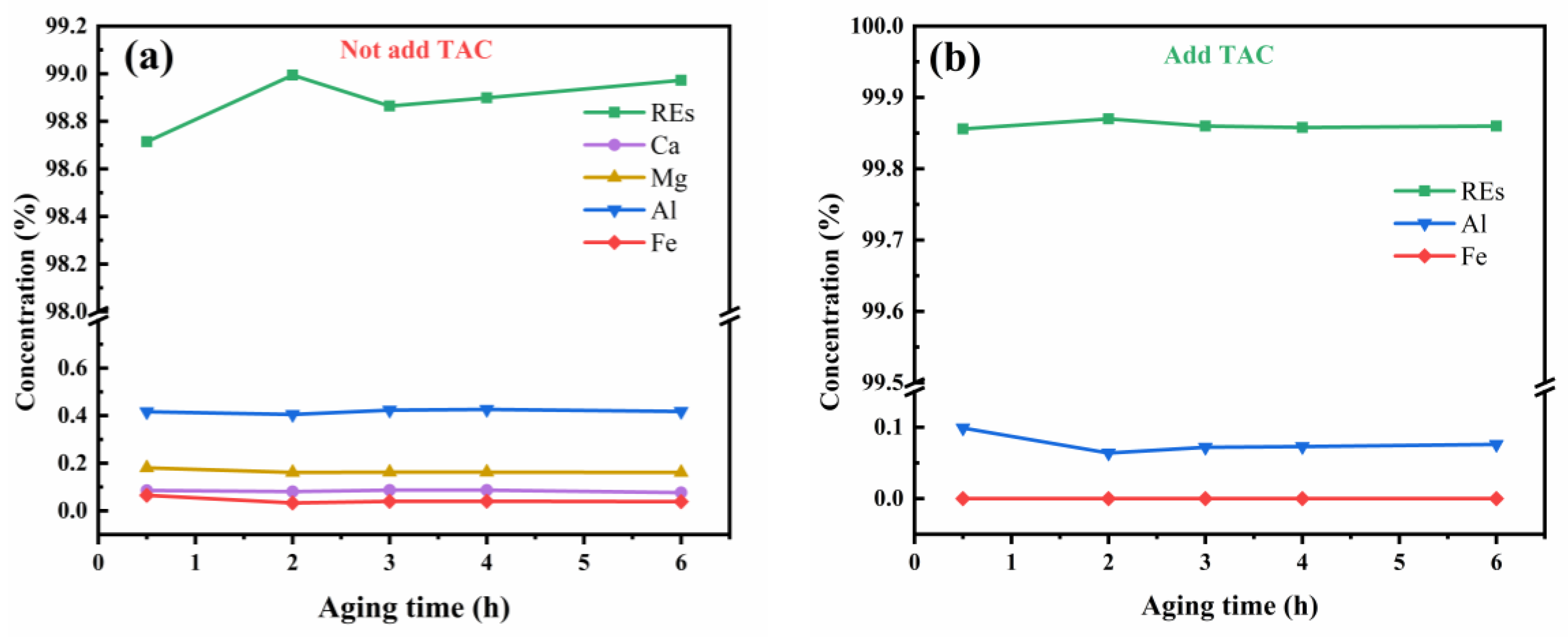
3.4. Triammonium Citrate-Assisted Ammonium Bicarbonate Precipitation Separation of REs from Ca, Mg, Al, and Fe
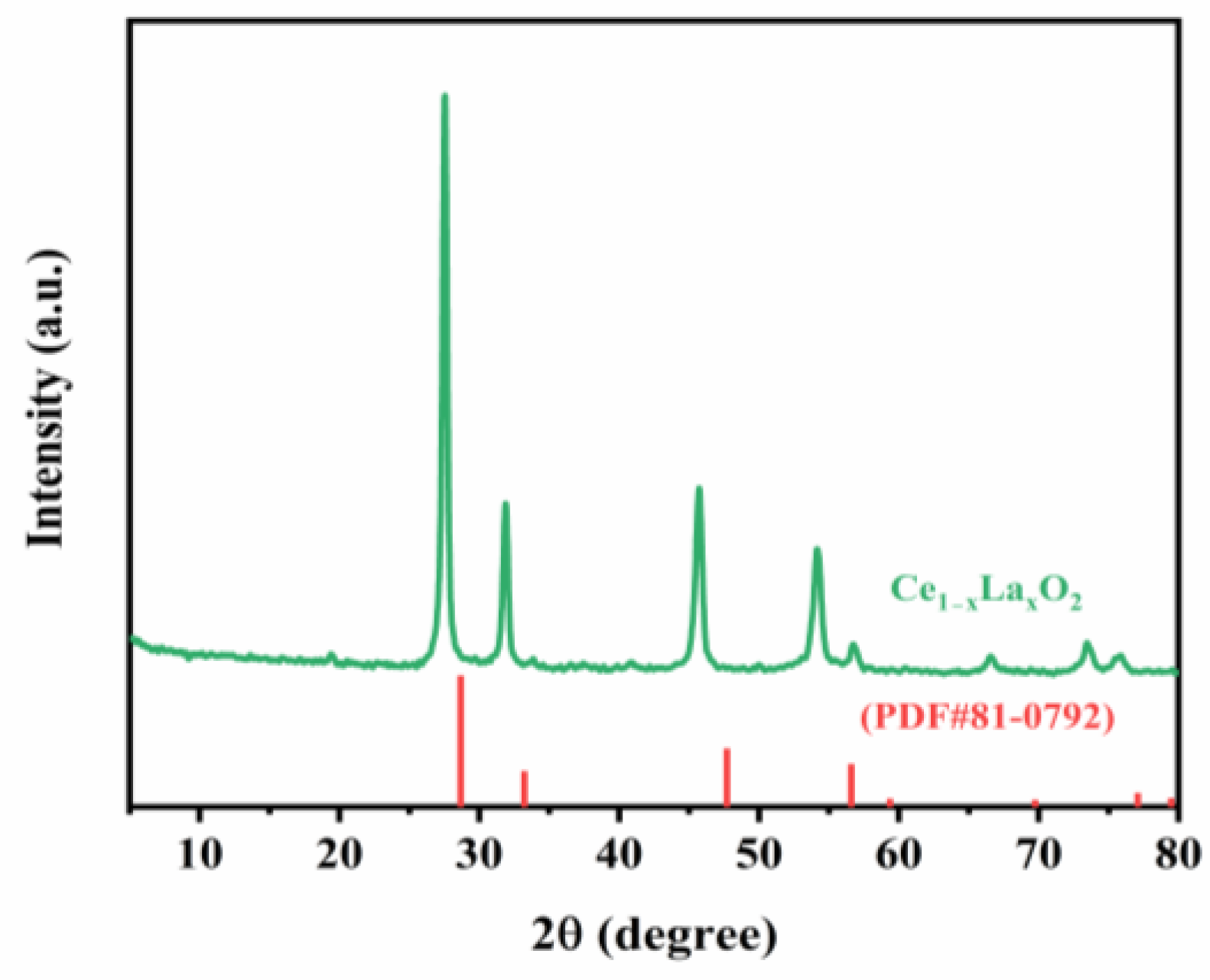
3.5. Triammonium Citrate Coordination Assistance Continuous Precipitation for Preparing High Purity of RE2O3
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RECl3 | rare earth chloride |
| RE2O3 | rare earth oxide |
| ABC | ammonium bicarbonate |
| TAC | triammonium citrate |
| REs | rare earths |
References
- Li, Y.X.; Zhu, W.C.; Zhang, L.Z.; Liu, Y.Z. Rare Earth Metallurgy and Environmental Protection, 1st ed.; Chemical Industrial Press: Beijing, China, 2024; Volume 1, ISBN 978-7-122-46001-1. [Google Scholar]
- Traore, M.; Gong, A.J.; Wang, Y.W.; Qiu, L.N.; Bai, Y.Z.; Zhao, W.Y.; Liu, Y.; Chen, Y.; Liu, Y.; Wu, H.L.; et al. Research progress of rare earth separation methods and technologies. J. Rare Earths 2023, 41, 182–189. [Google Scholar] [CrossRef]
- Pramanik, S.; Kaur, S.; Popovs, I.; Ivanov, A.S.; Jansone-Popova, S. Emerging rare earth element separation technologies. Eur. J. Inorg. Chem. 2024, 27, e202400064. [Google Scholar] [CrossRef]
- Khawassek, Y.M.; Eliwa, A.A.; Gawad, E.A.; Abdo, S.M. Recovery of rare earth elements from El-Sela effluent solutions. J. Radiat. Res. Appl. Sci. 2015, 8, 583–589. [Google Scholar] [CrossRef]
- Wu, S.X.; Wang, L.S.; Zhao, L.S.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.W.; Zhang, L.F. Recovery of rare earth elements from phosphate rock by hydrometallurgical processes–A critical review. Chem. Eng. J. 2018, 335, 774–800. [Google Scholar]
- He, Q.; Qiu, J.; Chen, J.F.; Zan, M.M.; Xiao, Y.F. Progress in green and efficient enrichment of rare earth from leaching liquor of ion adsorption type rare earth ores. J. Rare Earths 2022, 40, 353–364. [Google Scholar] [CrossRef]
- He, Y.F.; Gong, J.A.; Sun, J.E. Study on the extraction separation methods of rare earth elements. Huaxue Tongbao 2023, 86, 386–396. [Google Scholar]
- Hu, Y.W.; Wang, L.M.; Cao, Z.; Zhang, W.B. Research progress on rare earth ore metallurgy and separation technology in China. Conserv. Util. Miner. Resour. 2020, 40, 151–161. [Google Scholar]
- Preston, J.S.; Du Preez, A.C. The separation of europium from a middle rare earth concentpercentage by combined chemical reduction, precipitation and solvent-extraction methods. J. Chem. Technol. Biotechnol. 1996, 65, 93–101. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Ahmed, M.; Al-Heniti, S.H.; Sayyed, M.I.; Rammah, Y.S. Rare earth co-doped tellurite glass ceramics: Potential use in optical and radiation shielding applications. Ceram. Int. 2020, 46, 19198–19208. [Google Scholar] [CrossRef]
- Li, L.; Li, G.l.; Tian, F.; Wang, Z.Q.; Yan, S.H.; Li, X.G. Preparation of high purity Gd for novel functional materials. Mater. Prot. 2013, 46 (Suppl. S2), 28–29. [Google Scholar]
- Zhang, W.; Noble, A.; Ji, B.; Li, Q. Effects of contaminant metal ions on precipitation recovery of rare earth elements using oxalic acid. J. Rare Earths 2022, 40, 482–490. [Google Scholar] [CrossRef]
- Archambo, M.S.; Kawatra, S.K. Extraction of rare earths from red mud iron nugget slags with oxalic acid precipitation. Miner. Process. Extr. Metall. Rev. 2022, 43, 656–663. [Google Scholar] [CrossRef]
- Ahmad, N.; Yang, X.B.; Honaker, R. Parametric study and speciation analysis of rare earth precipitation using oxalic acid in a chloride solution system. Miner. Eng. 2022, 176, 107352. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhao, H.B.; Zhao, Y.; Shen, L.; Gu, G.H.; Qiu, G.Z. Effective recovery of rare earth from (bio) leaching solution through precipitation of rare earth-citpercentage complex. Water Res. 2023, 233, 119752. [Google Scholar] [CrossRef]
- Wang, Y.D.; Li, J.H.; Gao, Y.; Yang, Y.; Gao, Y.; Xu, Z.F. Removal of Al from rare-earth leaching solutions via a complexation-precipitation process. Hydrometallurgy 2020, 191, 105220. [Google Scholar] [CrossRef]
- Li, Y.X.; He, X.B.; Gu, Z.Y.; Hu, P.G. Precipitation Reaction of RECl3 with NH4HCO3 and co-precipitation Behavior of accompanying impurity ions. Chin. Rare Earths 1999, 20, 21–24. [Google Scholar]
- Liu, Y.Z.; Xiao, H.F.; Liu, L.H.; Ye, X.F.; Hu, X.Q.; Ding, Y.R.; Li, Y.X. Selective leaching bastnaesite from Bayan Obo rare earth concentrate and the recovery process of rare earths, aluminum, fluoride and calcium. Metals 2025, 15, 431. [Google Scholar] [CrossRef]
- Luo, W.Q. Discussion on new processes for rare earth separation and purification in high-end application fields. Shanxi Metall. 2023, 46, 87–89. [Google Scholar]
- Anawati, J.; Azimi, G. Separation of rare earth elements from a South American ionic clay lixivium by sequential precipitation. Hydrometallurgy 2022, 213, 105946. [Google Scholar] [CrossRef]
- De Vasconcellos, M.E.; Queiroz, C.A.S.; Abrão, A. Sequential separation of the yttrium—Heavy rare earths by fractional hydroxide precipitation. J. Alloys Compd. 2004, 374, 405–407. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, M.; Wang, L.; Zhao, L.; Feng, Z.; Sun, X.; Huang, X. Preparation of crystalline mixed rare earth carbonates by Mg (HCO3)2 precipitation method. J. Rare Earths 2020, 38, 292–298. [Google Scholar] [CrossRef]
- Rahman, M.M.; Awual, M.R.; Asiri, A.M. Preparation and evaluation of composite hybrid nanomaterials for rare-earth elements separation and recovery. Sep. Purif. Technol. 2020, 253, 117515. [Google Scholar] [CrossRef]
- Uysal, E.; Emil-Kaya, E.; Dursun, H.N.; Papakci, M.; Gürmen, S.; Friedrich, B. Recovery of samarium from waste SmCo magnets via selective precipitation with ammonium bicarbonate: Optimization of process efficiency. Metals 2024, 14, 1363. [Google Scholar] [CrossRef]
- Behera, S.S.; Parhi, P.K. Leaching kinetics study of neodymium from the scrap magnet using acetic acid. Sep. Purif. Technol. 2016, 160, 59–66. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van-Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Li, T.; Cui, K.H.; Sui, N.; Huang, K. Interface salt effect based on competition adsorption ofco-existing cations in process of rare earth solvent extraction. Chin. J. Nonferrous Met. 2023, 33, 4201–4213. [Google Scholar]
- Cai, L.X. Study on the influence of citric acid dosage and pH value on the leaching percentage of rare earth and Al during ammonium sulfate leaching of ion-type rare earth. World Nonferrous Met. 2024, 19, 193–195. [Google Scholar]
- Habibpour, R.; Kashi, E. Ultrasound/Complexing agent-assisted solvent extraction behavior of Zn2+ with D2EHPA from sulfuric media containing transition and rare-earth (Fe, Ni, Cd, La, and Ce) metal ions. J. Sustain. Metall. 2021, 7, 1772–1789. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Nie, F.; Liu, L.L.; Ding, Y.R.; Ma, Y.; Ding, Z.X.; Zhao, Q.; Li, Y.X. High efficient separation of Al(Fe) from La(Ce) by ammonium bicarbonate precipitation with the cooperation of triammonium citrate. Hydrometallurgy 2025, 238, 106578. [Google Scholar] [CrossRef]
- Johnston, J. The Solubility-Product Constant of Calcium and Magnesium Carbonates. J. Am. Chem. Soc. 1915, 37, 2001–2020. [Google Scholar] [CrossRef]
- Hiemstra, T.; Mendez, J.C.; Li, J.Y. Evolution of the reactive surface area of ferrihydrite: Time, pH, and temperature dependency of growth by Ostwald ripening. Environ. Sci. Nano 2019, 6, 820–833. [Google Scholar] [CrossRef]
- Lee, H.C.; Kim, D.; Lee, J.A.; Heo, Y.W.; Kim, J.J.; Lee, J. Oxygen migration energy in La and Y Co-doped CeO2: Effect of lattice constant and grain boundary segregation. J. Sci. Adv. Mater. Devices 2022, 7, 100450. [Google Scholar] [CrossRef]

| Elements | Mg | Al | Ca | Fe | Co | Ni | Zn | Mn | Na |
| Content (g/L) | 1.30 | 2.31 | 2.02 | 0.23 | 0.01 | 0.01 | 0.02 | 0.01 | 1.23 |
| Elements | Y | La | Ce | Pr | Nd | Gd | Other REs | K | |
| Content (g/L) | 0.06 | 19.81 | 52.81 | 0.18 | 0.06 | 0.01 | 0.01 | 0.68 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, Z.; Nie, F.; Liu, L.; Shi, J.; Li, Y. Separation of REs from Ca and Mg Ions by Ammonium Bicarbonate Precipitation and the Influence of Fe and Al Ions. Metals 2025, 15, 1142. https://doi.org/10.3390/met15101142
Liu Y, Zhu Z, Nie F, Liu L, Shi J, Li Y. Separation of REs from Ca and Mg Ions by Ammonium Bicarbonate Precipitation and the Influence of Fe and Al Ions. Metals. 2025; 15(10):1142. https://doi.org/10.3390/met15101142
Chicago/Turabian StyleLiu, Yanzhu, Zhenghui Zhu, Fen Nie, Lihui Liu, Jinfei Shi, and Yongxiu Li. 2025. "Separation of REs from Ca and Mg Ions by Ammonium Bicarbonate Precipitation and the Influence of Fe and Al Ions" Metals 15, no. 10: 1142. https://doi.org/10.3390/met15101142
APA StyleLiu, Y., Zhu, Z., Nie, F., Liu, L., Shi, J., & Li, Y. (2025). Separation of REs from Ca and Mg Ions by Ammonium Bicarbonate Precipitation and the Influence of Fe and Al Ions. Metals, 15(10), 1142. https://doi.org/10.3390/met15101142







