Study on Constructing Indoor Accelerated Simulation Methods for Steel with Galvalume Coating Exposed to Marine Atmosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
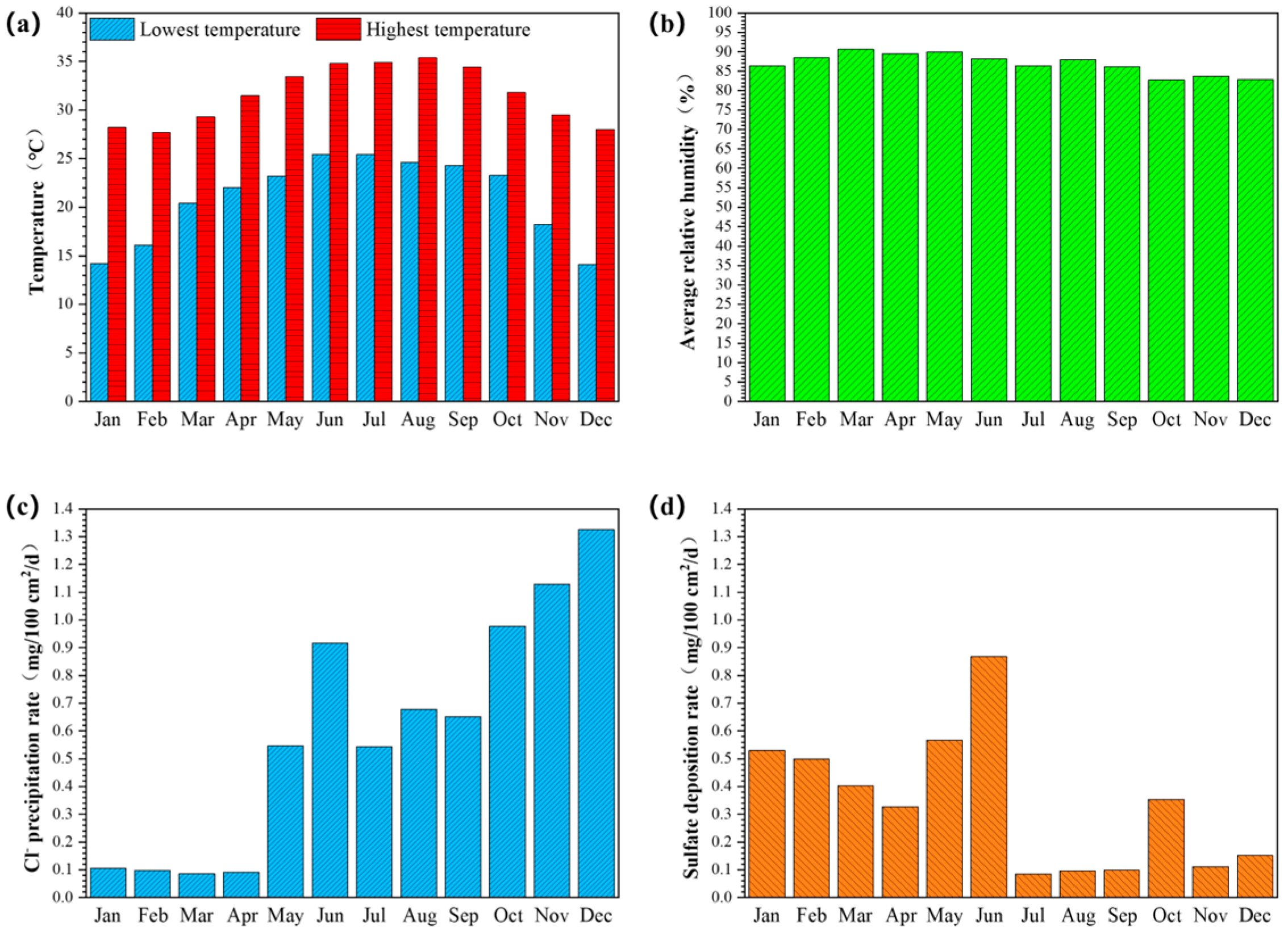
2.2. Outdoor Exposure Corrosion Test
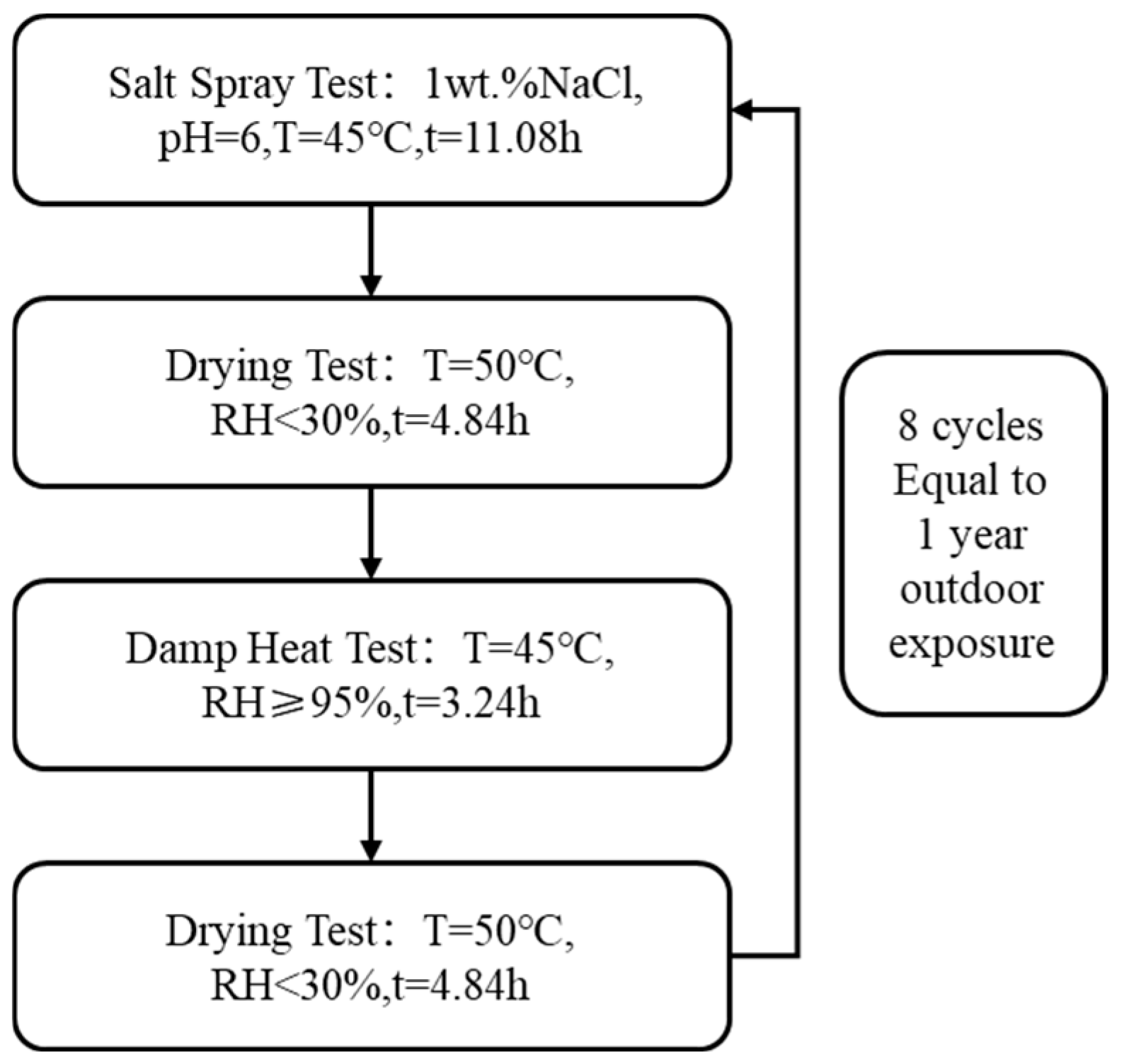
2.3. Accelerated Corrosion Testing Method
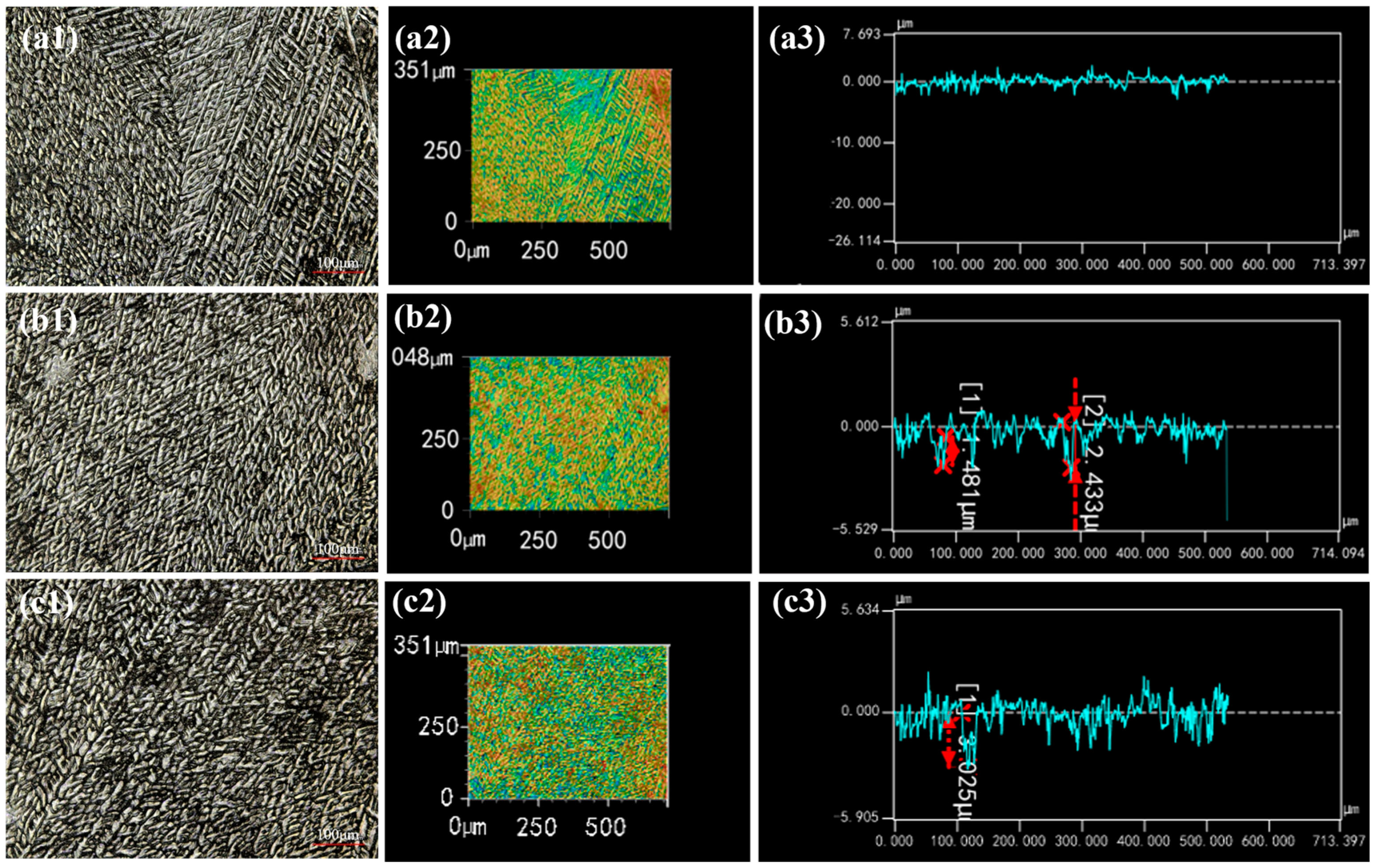
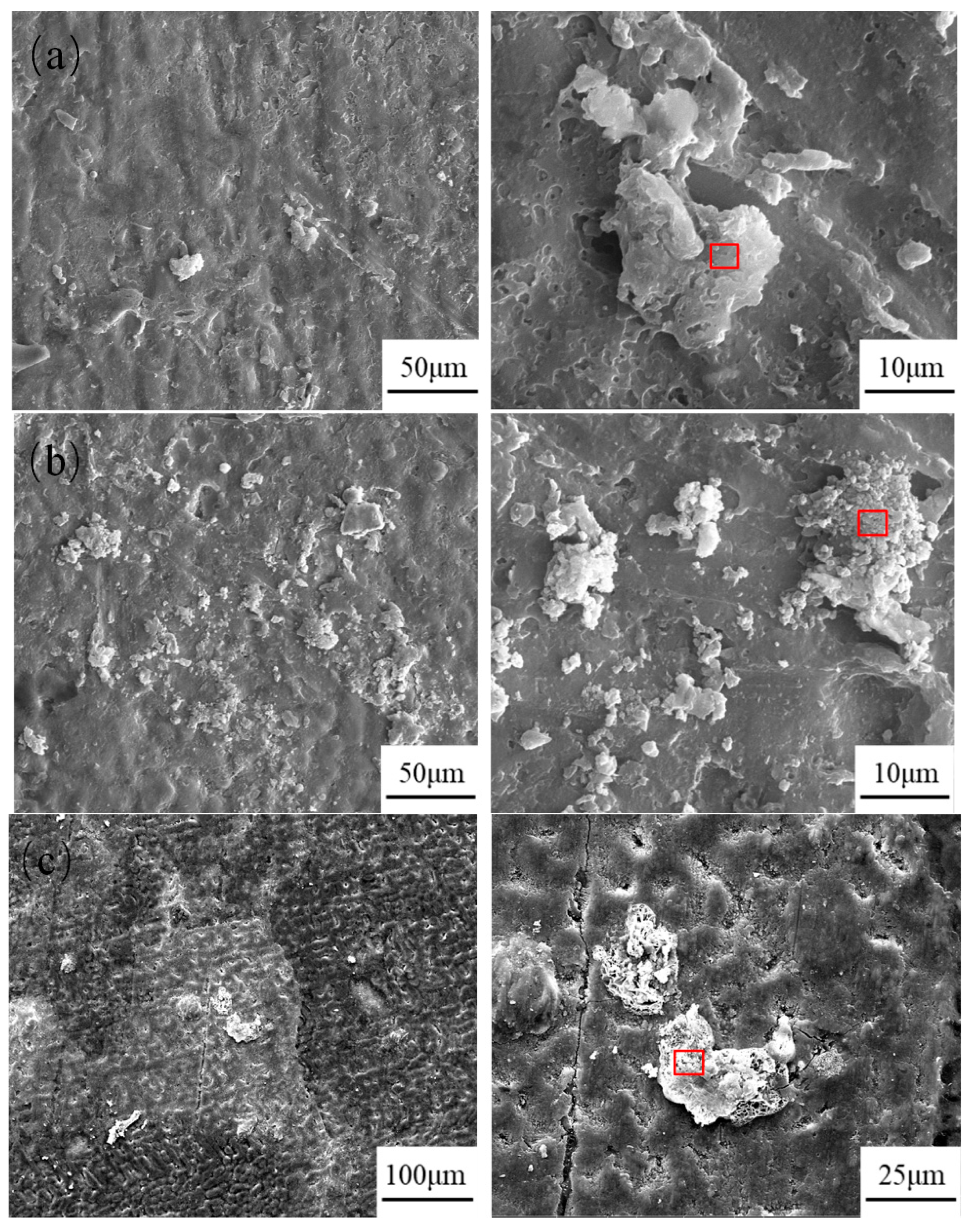
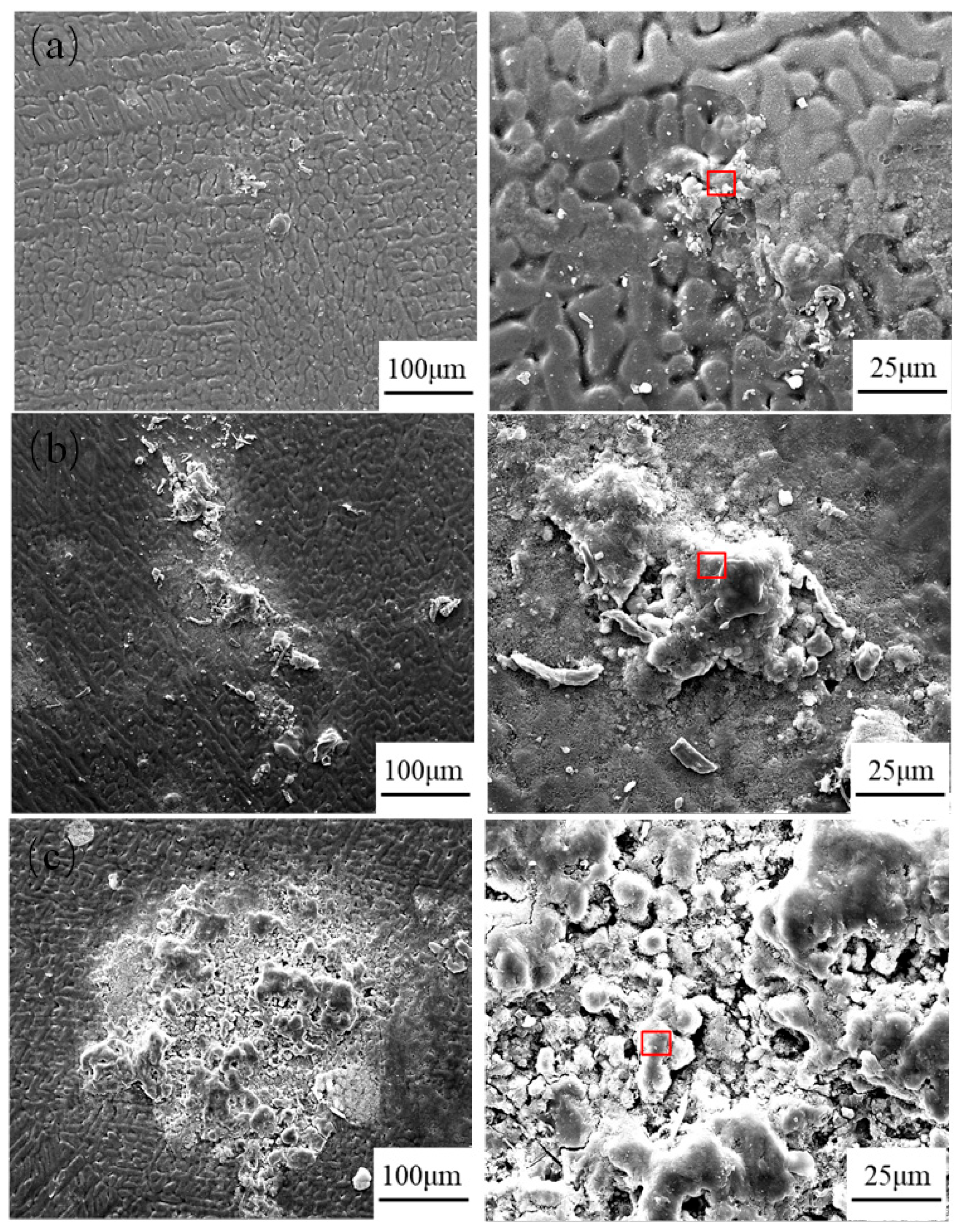
2.4. Morphology of Corrosion Products
2.5. Corrosion Kinetics
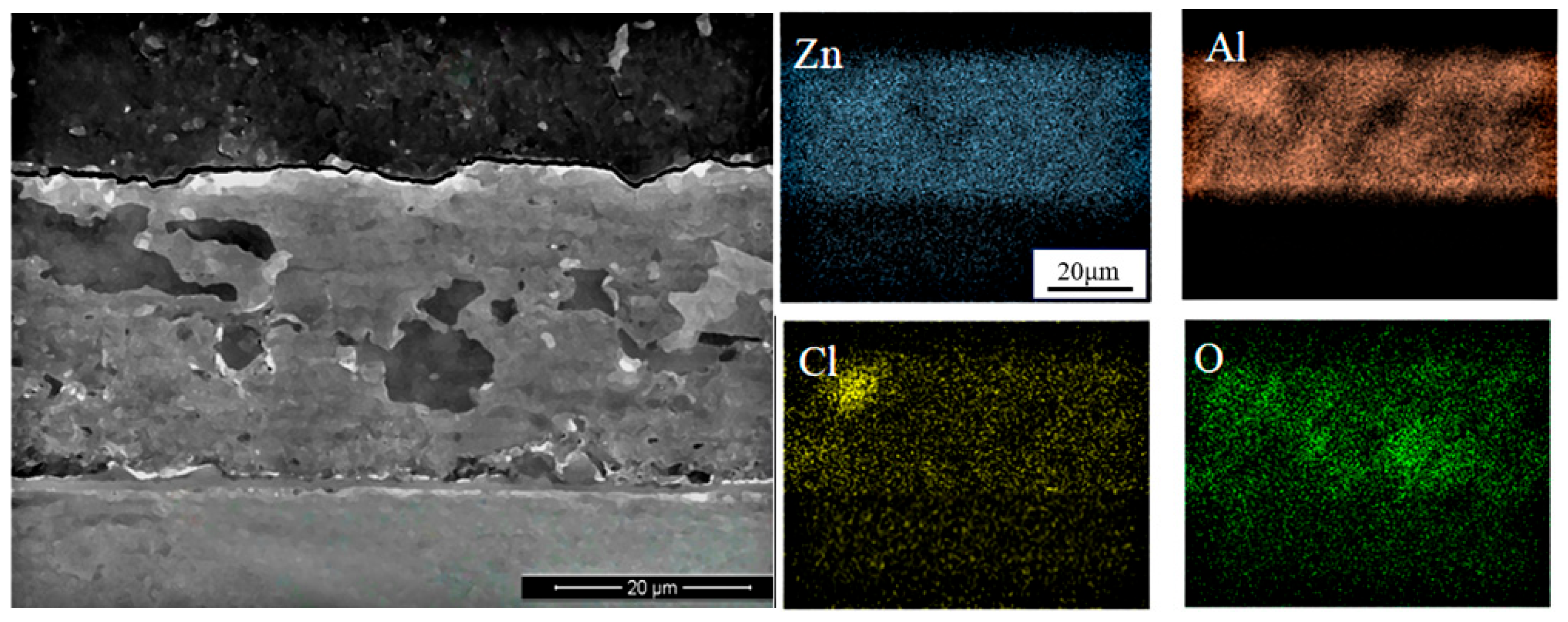
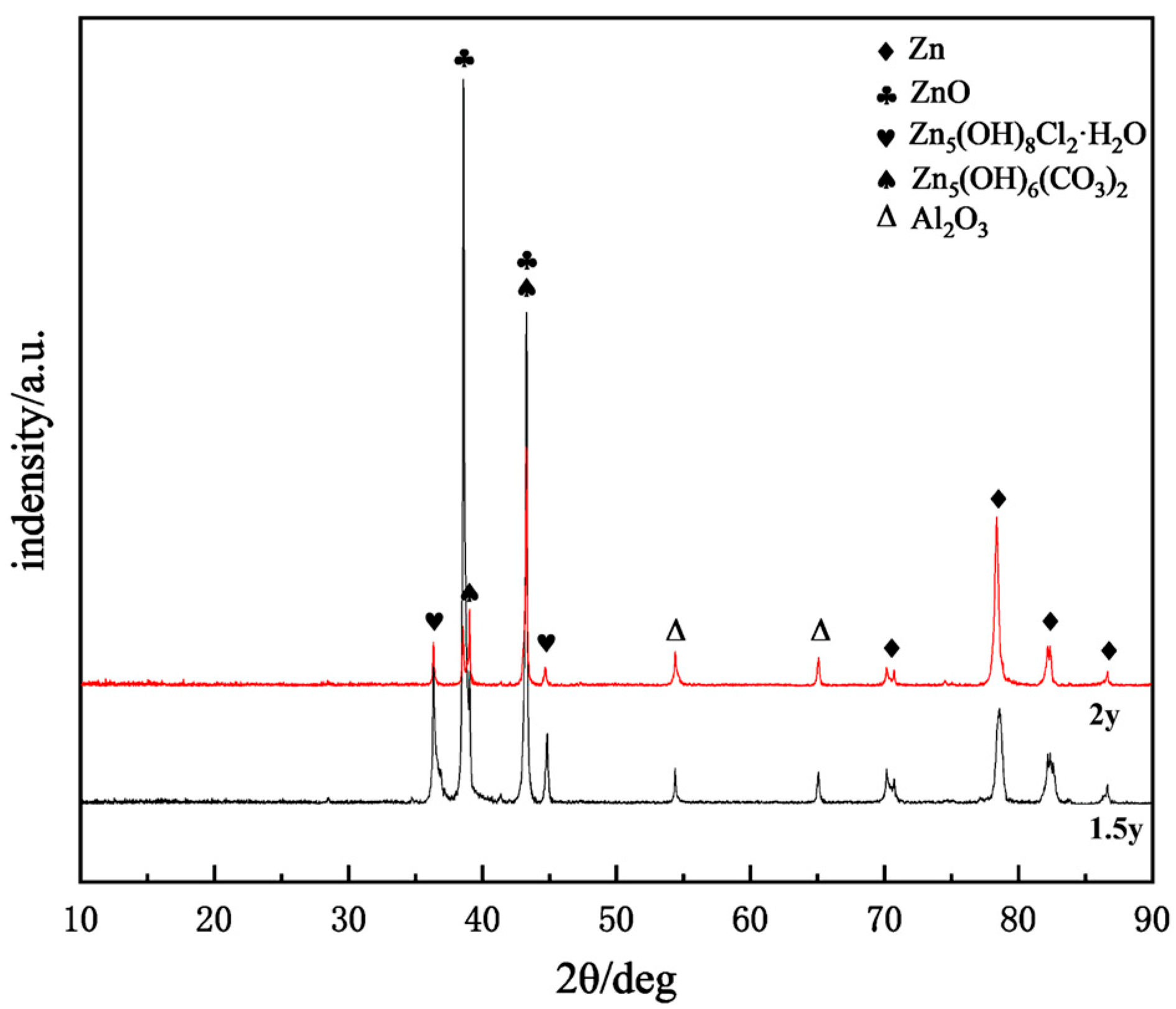
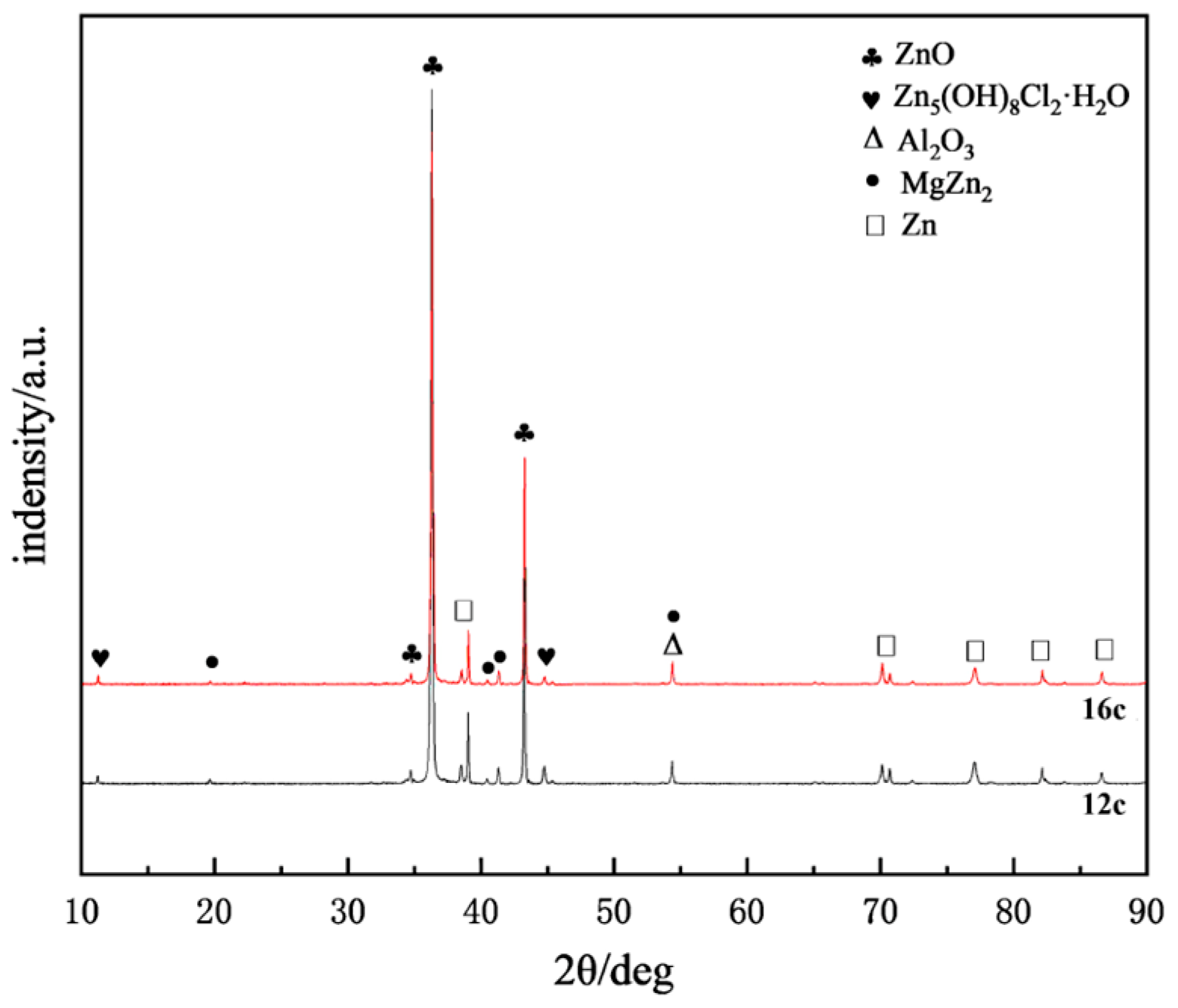
2.6. Corrosion Product Analysis
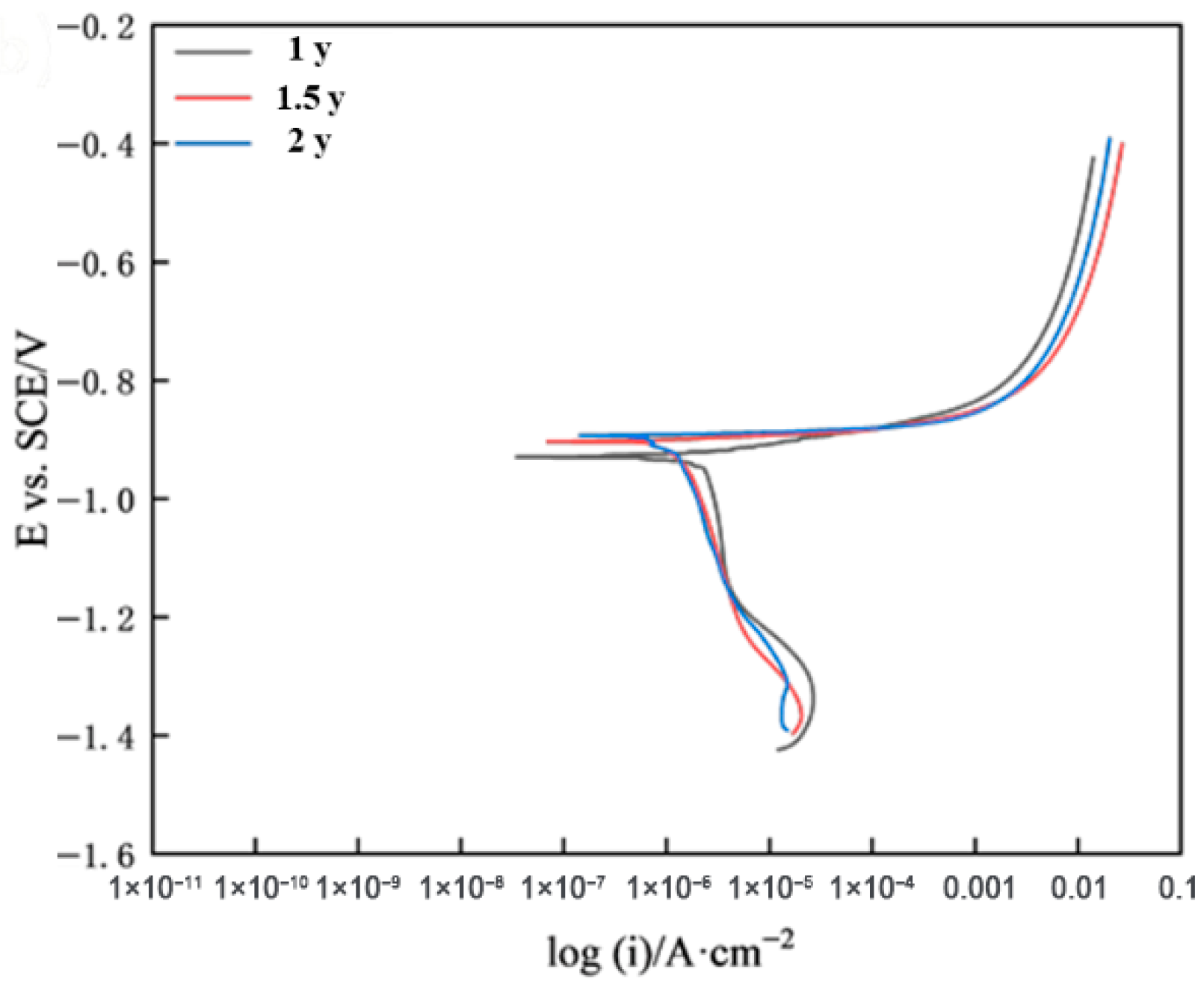
2.7. Electrochemical Test
3. Results
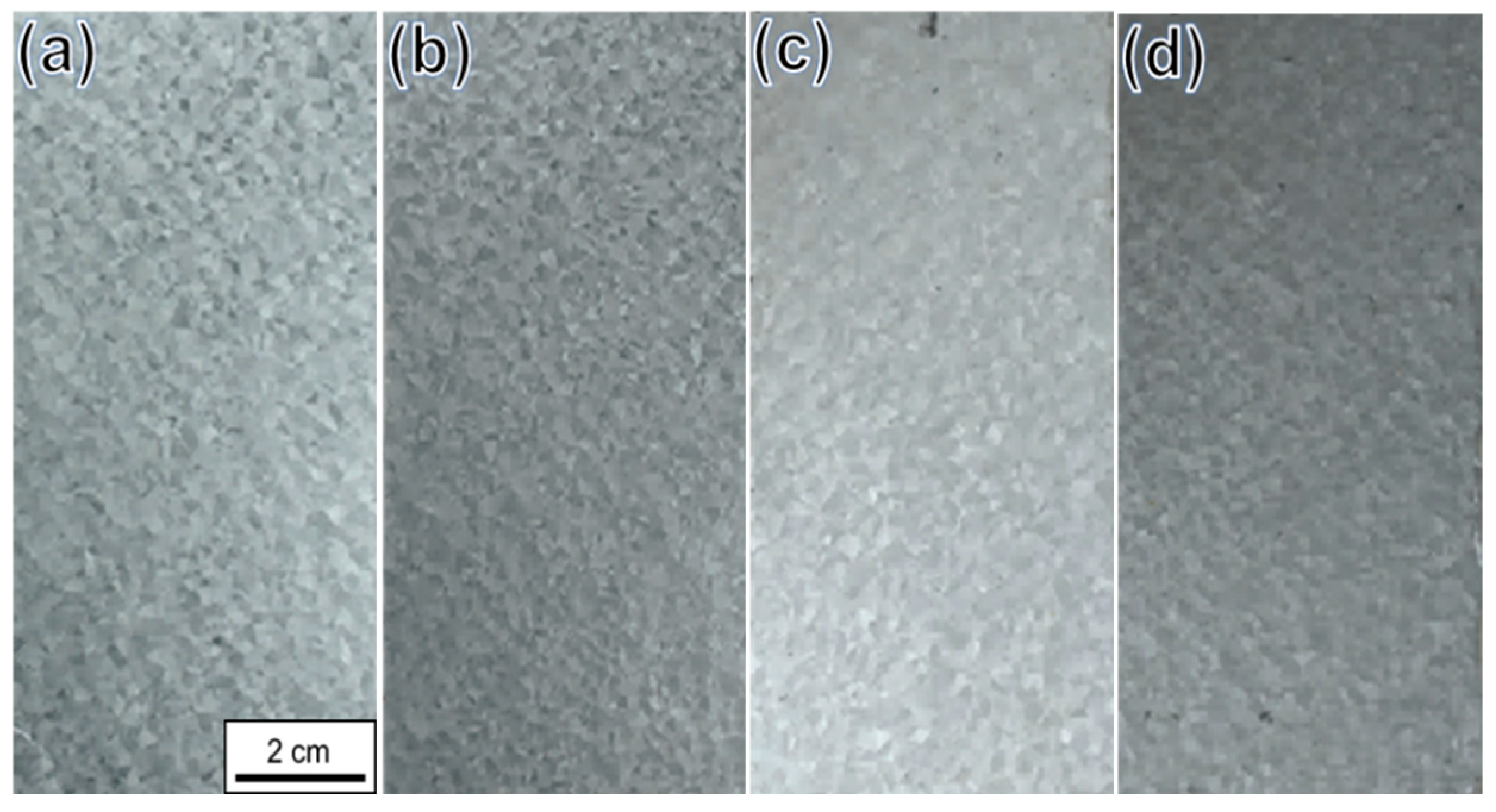
3.1. Marine Atmospheric Exposure Test
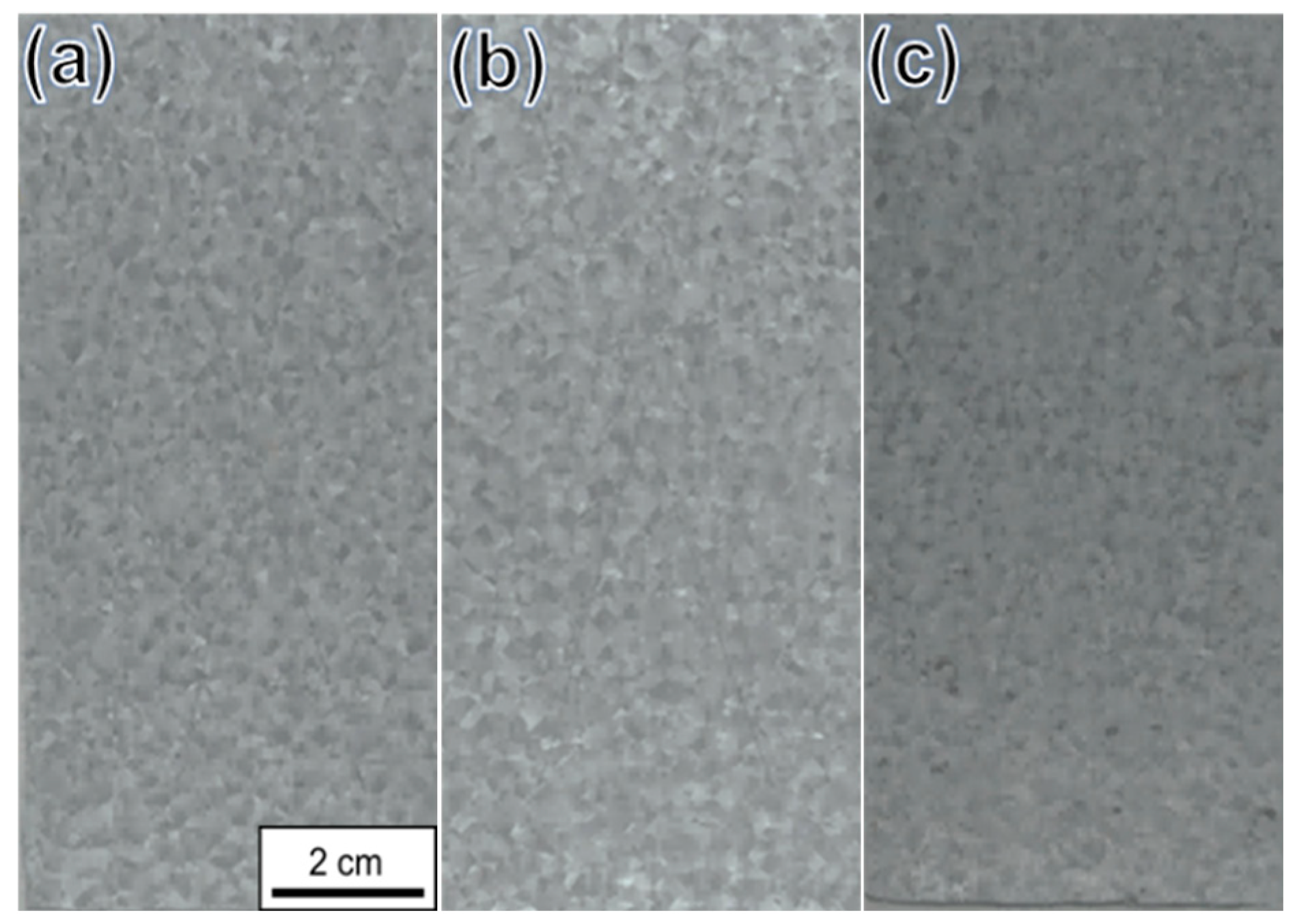
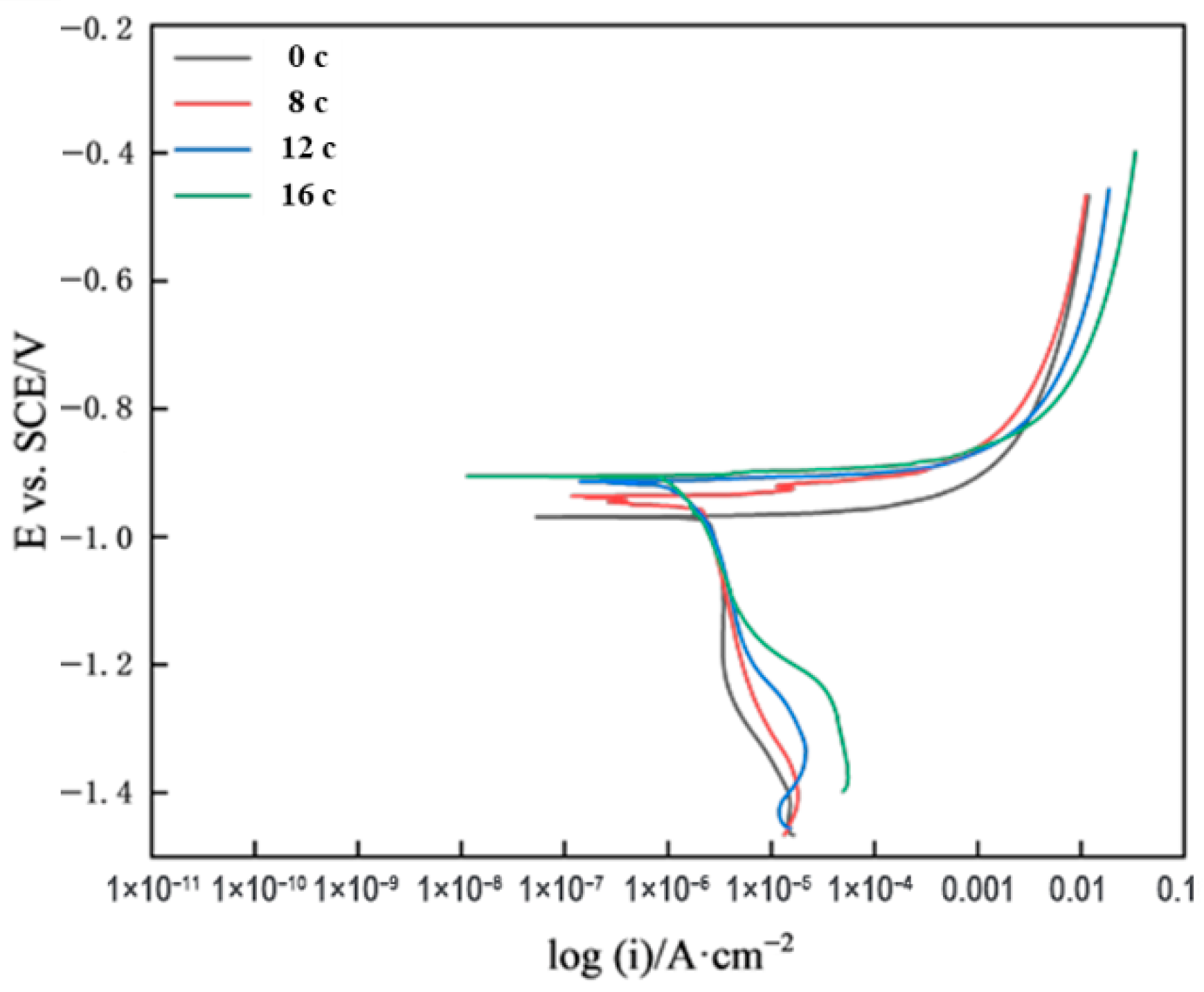
3.2. Accelerated Corrosion Test
3.3. Correlation Analysis Between Indoor Accelerated Tests and Outdoor Exposure Tests
- (1)
- Reference sequence and comparison sequence
- (2)
- Initialization of corrosion weight-loss data
- (3)
- Absolute difference sequence
- (4)
- Grey relational degree calculation between indoor and outdoor marine atmospheric tests
4. Conclusions
- (1)
- Throughout the entire test period, no significant corrosion products were observed on the surface, while needle-like products appeared in localized areas during the later stages. The main corrosion products were identified as ZnO, Zn5(OH)6(CO3)2, Zn5(OH)8Cl2·H2O, and Al2O3. Among them, ZnO was the most abundant phase and provided partial protection, whereas Zn5(OH)8Cl2·H2O and Al2O3 exhibited higher density, covering the corroded regions and hindering the ingress of aggressive ions.
- (2)
- The corrosion kinetics of galvalume coating steel in both marine atmospheric exposure and spectrum-based accelerated tests were consistent. In addition, the potentiodynamic polarization curves showed similar features under both conditions, with the corrosion process mainly controlled by cathodic reactions, and the trends of corrosion potential and corrosion current being consistent.
- (3)
- The corrosion kinetics and electrochemical mechanisms exhibited strong consistency between the indoor accelerated and outdoor exposure tests. The types of corrosion products were also the same, with aggressive ions enriched within the coating rather than penetrating into the substrate, indicating that the Zn–Al coating effectively acted as a barrier. Grey relational analysis further showed correlation coefficients greater than 0.6, confirming that the accelerated spectrum was well designed and that the indoor accelerated corrosion tests exhibited good correlation with outdoor exposure.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Liu, Q.; Wang, J.; Chen, N.; Chen, J.; Song, J.; Zhang, X.; Xiao, K. Study of the role of aluminium and corrosion mechanism in galvalume coating in the marine atmospheric environment. Anti-Corros. Methods Mater. 2024, 71, 286–294. [Google Scholar] [CrossRef]
- Qiu, P.; Leygraf, C.; Wallinder, I.O. Evolution of corrosion products and metal release from Galvalume coatings on steel during short and long-term atmospheric exposures. Mater. Chem. Phys. 2012, 133, 419–428. [Google Scholar] [CrossRef]
- Moreira, A.R.; Panossian, Z.; Camargo, P.; Moreira, M.F.; Da Silva, I.; De Carvalho, J.R. Zn/55Al coating microstructure and corrosion mechanism. Corros. Sci. 2006, 48, 564–576. [Google Scholar] [CrossRef]
- Yan, D.; Yang, Y.; Dong, Y.; Chen, X.; Wang, L.; Zhang, J.; He, J. Phase transitions of plasma sprayed Fe–Al intermetallic coating during corrosion in molten zinc at 640 °C. Intermetallics 2012, 22, 160–165. [Google Scholar] [CrossRef]
- Li, K.; Liu, Y.; Tu, H.; Su, X.; Wang, J. Effect of Si on the growth of Fe-Al intermetallic layer in Zn-11% Al-3% Mg coating. Surf. Coat. Technol. 2016, 306, 390–396. [Google Scholar] [CrossRef]
- AD, V.; Panda, B. Corrosion behaviour of galvanised steel in alkaline sodium chloride solution. Adv. Mater. Process. Technol. 2022, 8 (Suppl. 2), 826–840. [Google Scholar]
- Penney, D.J.; Sullivan, J.H.; Worsley, D.A. Investigation into the effects of metallic coating thickness on the corrosion properties of Zn–Al alloy galvanising coatings. Corros. Sci. 2007, 49, 1321–1339. [Google Scholar] [CrossRef]
- Edavan, R.P.; Kopinski, R. Corrosion resistance of painted zinc alloy coated steels. Corros. Sci. 2009, 51, 2429–2442. [Google Scholar] [CrossRef]
- Palma, E.; Puente, J.; Morcillo, M. The atmospheric corrosion mechanism of 55% Al-Zn coating on steel. Corros. Sci. 1998, 40, 61–68. [Google Scholar] [CrossRef]
- Kalendova, A. Mechanism of the action of zinc-powder in anticorrosive coatings. Anti-Corros. Methods Mater. 2002, 49, 173–180. [Google Scholar] [CrossRef]
- Zapico Álvarez, D.; Bertrand, F.; Mataigne, J.-M.; Giorgi, M.-L. Nature of the inhibition layer in GA baths. Metall. Res. Technol. 2014, 111, 9–15. [Google Scholar] [CrossRef]
- Gomes, C.; Mir, Z.; Sampaio, R.; Bastos, A.; Tedim, J.; Maia, F.; Rocha, C.; Ferreira, M. Use of ZnAl-layered double hydroxide (LDH) to extend the service life of reinforced concrete. Materials 2020, 13, 1769. [Google Scholar] [CrossRef]
- Panossian, Z.; Mariaca, L.; Morcillo, M.; Flores, S.; Rocha, J.; Peña, J.; Herrera, F.; Corvo, F.; Sanchez, M.; Rincon, O. Steel cathodic protection afforded by zinc, aluminium and zinc/aluminium alloy coatings in the atmosphere. Surf. Coat. Technol. 2005, 190, 244–248. [Google Scholar] [CrossRef]
- Yang, L.J.; Zeng, X.D.; Zhang, Y.M.; He, J.; Song, Z.L. Evolution of atmospheric corrosion of superplastic Zn-Al alloys exposed to an industrial environment. Mater. Corros. 2015, 66, 1169–1180. [Google Scholar] [CrossRef]
- Vu, T.N.; Volovitch, P.; Ogle, K. The effect of pH on the selective dissolution of Zn and Al from Zn–Al coatings on steel. Corros. Sci. 2013, 67, 42–49. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Q.; Wang, J.; Chen, N.; Chen, J.; Song, J.; Zhang, X.; Xiao, K. Study on corrosion mechanism of Al–Zn coatings in the simulated polluted marine atmosphere. J. Mater. Res. Technol. 2023, 25, 6446–6458. [Google Scholar] [CrossRef]
- LeBozec, N.; Thierry, D.; Persson, D.; Stoulil, J. Atmospheric corrosion of zinc-aluminum alloyed coated steel in depleted carbon dioxide environments. J. Electrochem. Soc. 2018, 165, C343. [Google Scholar] [CrossRef]
- ISO 9227 NSS; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests (NSS, AASS, CASS). International Organization for Standardization: Geneva, Switzerland, 2017.
- ASTM B117; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2019.
- Schürz, S.; Luckeneder, G.H.; Fleischanderl, M.; Mack, P.; Gsaller, H.; Kneissl, A.; Mori, G. Chemistry of corrosion products on Zn–Al–Mg alloy coated steel. Corros. Sci. 2010, 52, 3271–3279. [Google Scholar] [CrossRef]
- Schmidt, D.; Shaw, B.; Sikora, E.; Shaw, W.; Laliberte, L. Comparison of testing techniques used to analyze the corrosion resistance of sacrificial coating systems. Corrosion 2007, 63, 958–974. [Google Scholar] [CrossRef]
- DeForce, B.; Eden, T.J.; Pickering, H.W. Cold-sprayed aluminum coatings for magnesium aircraft components. Mater. Perform. 2009, 48, 40–44. [Google Scholar] [CrossRef]
- Morcillo, M.; Palma, E.; Fernández, B. Atmospheric galvanic protection, of 55% Al-Zn precoated steel. Mater. Corros. 1994, 45, 550–553. [Google Scholar] [CrossRef]
- Seré, P.; Zapponi, M.; Elsner, C.; Di Sarli, A. Comparative corrosion behaviour of 55Aluminium–zinc alloy and zinc hot-dip coatings deposited on low carbon steel substrates. Corros. Sci. 1998, 40, 1711–1723. [Google Scholar] [CrossRef]
- Wallinder, I.O.; He, W.; Augustsson, P.; Leygraf, C. Characterization of black rust staining of unpassivated 55% Al–Zn alloy coatings. Effect of temperature, pH and wet storage. Corros. Sci. 1999, 41, 2229–2249. [Google Scholar] [CrossRef]
- Hashemi, S.G.; Eghbali, B. Analysis of the formation conditions and characteristics of interphase and random vanadium precipitation in a low-carbon steel during isothermal heat treatment. Int. J. Miner. Metall. Mater. 2018, 25, 339–349. [Google Scholar] [CrossRef]
- Deng, C.-M.; Xia, D.-H.; Zhang, R.; Behnamian, Y.; Hu, W.; Birbilis, N. On the localized corrosion of AA5083 in a simulated dynamic seawater/air interface—Part 2: Effects of wetting time. Corros. Sci. 2023, 221, 111367. [Google Scholar] [CrossRef]
- Mansfeld, F. Tafel slopes and corrosion rates obtained in the pre-Tafel region of polarization curves. Corros. Sci. 2005, 47, 3178–3186. [Google Scholar] [CrossRef]
- Wang, M.; Ji, Y.; Xia, D.-H.; Zhou, D.; Zhu, Y.; Gao, Z.; Qin, Z.; Hu, W. Effect of substrate orientations on the electrochemical and localized corrosion behavior of a quad-layer Al alloy composite. J. Mater. Sci. Technol. 2024, 176, 57–68. [Google Scholar] [CrossRef]
- ASTM G85; Standard Practice for Modified Salt Spray (Fog) Testing. ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 20340; Paints and Varnishes—Performance Requirements for Protective Paint Systems for Offshore and Related Structures. International Organization for Standardization: Geneva, Switzerland, 2019.
- GB/T 16545-2015; Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens. Standardization Administration of China: Beijing, China, 2015.
- Zhu, G.; Zhao, Y.; Liu, L.; Wang, L.; Wang, J.; Yu, S. Facile fabrication and evaluation of self-healing Zn-Al layered double hydroxide superhydrophobic coating on aluminum alloy. J. Mater. Sci. 2021, 56, 14803–14820. [Google Scholar] [CrossRef]
- Cao, J.; Li, Y.; Chen, X.; Cai, D.; Zhou, S.-F.; Zhan, G. Preparation and multiple applications of integrated Zn-Al layered double hydroxide@polydopamine nanocomposites. Mater. Today Commun. 2022, 31, 103650. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Bordbar-Khiabani, A.; Yarmand, B. Immobilization of rGO/ZnO hybrid composites on the Zn substrate for enhanced photocatalytic activity and corrosion stability. J. Alloys Compd. 2020, 845, 156219. [Google Scholar] [CrossRef]
- Deng, J.L. Introduction to grey system theory. J. Grey Syst. 1989, 1, 1–24. [Google Scholar]
- Cao, X.; Deng, H.; Lan, W. Use of the grey relational analysis method to determine the important environmental factors that affect the atmospheric corrosion of Q235 carbon steel. Anti-Corros. Methods Mater. 2015, 62, 7–12. [Google Scholar] [CrossRef]
- Fu, C.; Zheng, J.; Zhao, J.; Xu, W. Application of grey relational analysis for corrosion failure of oil tubes. Corros. Sci. 2001, 43, 881–889. [Google Scholar] [CrossRef]
- Kuruvila, R.; Kumaran, S.T.; Khan, M.A. Optimization of erosion–corrosion behavior of nichrome coated 2205 duplex stainless steel using grey relational analysis. Surf. Rev. Lett. 2022, 29, 2250087. [Google Scholar] [CrossRef]


| Sample | Coating Thickness (mm) | Coating Weight (g/m2) | Coated Steel Sheet Size (cm) |
|---|---|---|---|
| Galvalume coating steel | 0.5 | 120 | 150 × 75 × 1 |
| Time/Year | 1 | 1.5 | 2 |
|---|---|---|---|
| Corrosion mass loss per unit area (g/m2) | 15.27 | 23.86 | 27.12 |
| Average thickness loss (µm) | 2.09 | 3.27 | 3.72 |
| Average corrosion rate (µm/y) | 2.09 | 2.18 | 1.86 |
| Sample | O | Cl | C | Zn | Al |
|---|---|---|---|---|---|
| 1 y | 38.22 | 1.17 | 35.27 | 19.24 | 6.11 |
| 1.5 y | 9.33 | 6.43 | 48.05 | 23.15 | 13.04 |
| 2 y | 32.79 | 11.48 | 18.25 | 34.50 | 2.98 |
| Exposure Time | 1 y | 1.5 y | 2 y | |||
|---|---|---|---|---|---|---|
| Fitted data | Vcorr/mV | icorr/μA | Vcorr/mV | icorr/μA | Vcorr/mV | icorr/μA |
| −927 | 2.378 | −905 | 1.353 | −892 | 1.146 | |
| Cycles | 8 | 12 | 16 |
|---|---|---|---|
| Corrosion mass loss per unit area (g/m2) | 19.30 | 24.44 | 28.73 |
| Average thickness loss (µm) | 2.65 | 3.35 | 3.94 |
| Average corrosion rate (µm/y) | 2.65 | 2.23 | 1.97 |
| Experimental Cycles | O | Cl | C | Zn | Al |
|---|---|---|---|---|---|
| 8 | 40.71 | 0.48 | 18.06 | 25.92 | 14.83 |
| 12 | 43.68 | 0.32 | 19.91 | 20.92 | 15.18 |
| 16 | 15.30 | 0.42 | 13.07 | 14.46 | 56.75 |
| Experimental Cycles | 0 | 8 | 12 | 16 | ||||
|---|---|---|---|---|---|---|---|---|
| Fitted data | Vcorr/mV | icorr/μA | Vcorr/mV | icorr/μA | Vcorr/mV | icorr/μA | Vcorr/mV | icorr/μA |
| −969 | 2.827 | −936 | 2.132 | −911 | 1.696 | −904 | 1.332 | |
| Experiment Time | Galvalume Coating Steel | |
|---|---|---|
| Outdoor Exposure | Indoor Accelerated Test | |
| X0 | Xi | |
| 1 y/8 cycles | 4.02 | 4.33 |
| 1.5 y/12 cycles | 5.56 | 5.48 |
| 2 y/16 cycles | 6.98 | 6.44 |
| Experiment Time | Galvalume Coating Steel | |
|---|---|---|
| Outdoor Exposure | Indoor Accelerated Test | |
| X0 | Xi | |
| 1 y/8 cycles | 1.74026 | 1.72510 |
| 1.5 y/12 cycles | 2.406926 | 2.183267 |
| 2 y/16 cycles | 3.021645 | 2.565737 |
| Experiment Time | Galvalume Coating Steel |
|---|---|
| D2 | |
| 1 y/8 cycles | 0.015160 |
| 1.5 y/12 cycles | 0.223659 |
| 2 y/16 cycles | 0.455908 |
| Materials | Accelerated Testing |
|---|---|
| Galvalume coating Galvalume Steel | 0.6789 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, H.; Li, B.; Yu, H.; Zhang, H.; Chen, J.; Yin, C.; Xiao, K. Study on Constructing Indoor Accelerated Simulation Methods for Steel with Galvalume Coating Exposed to Marine Atmosphere. Metals 2025, 15, 1143. https://doi.org/10.3390/met15101143
Wang L, Wang H, Li B, Yu H, Zhang H, Chen J, Yin C, Xiao K. Study on Constructing Indoor Accelerated Simulation Methods for Steel with Galvalume Coating Exposed to Marine Atmosphere. Metals. 2025; 15(10):1143. https://doi.org/10.3390/met15101143
Chicago/Turabian StyleWang, Luntao, Hongkai Wang, Bo Li, Hao Yu, Hao Zhang, Junhang Chen, Chenghui Yin, and Kui Xiao. 2025. "Study on Constructing Indoor Accelerated Simulation Methods for Steel with Galvalume Coating Exposed to Marine Atmosphere" Metals 15, no. 10: 1143. https://doi.org/10.3390/met15101143
APA StyleWang, L., Wang, H., Li, B., Yu, H., Zhang, H., Chen, J., Yin, C., & Xiao, K. (2025). Study on Constructing Indoor Accelerated Simulation Methods for Steel with Galvalume Coating Exposed to Marine Atmosphere. Metals, 15(10), 1143. https://doi.org/10.3390/met15101143







