Abstract
To promote the high-value recycling of machining return materials from powder metallurgy (P/M) FGH95 superalloy production, a vacuum induction melting refining process was developed to achieve gas impurity purification and compositional control. Cylindrical solid returns obtained from wire-cut electrical discharge machining were used as feedstock, and the effects of refining temperature (1550–1650 °C) and holding time (10–30 min) on impurity removal and element stability were systematically investigated. For each condition, three repeated melts were performed, and the average gas contents (mean ± SD) were evaluated by inert-gas fusion analysis. Results show that at 1650 °C, O decreased from 8 ppm to 6 ppm, N decreased from 6 ppm to 3 ppm, while H remained below the detection limit (<1 ppm). Prolonged refining caused slight compositional deviations, with Cr exhibiting measurable volatilization, whereas Al and Ti showed minor increases (<0.06 wt.%). A kinetic model describing O removal was established, yielding an apparent activation energy of 128 kJ·mol−1, confirming diffusion-controlled deoxidation behavior. The optimal refining condition—1650 °C for 10 min—achieved efficient removal of O and H while maintaining alloy compositional stability. This study provides both a practical refining route and a kinetic basis for the purification and reuse of machining returns in nickel-based P/M superalloys, contributing to cost reduction and sustainable manufacturing.
1. Introduction
Powder metallurgy (P/M) superalloys—particularly nickel-based grades such as FGH95—are essential materials in advanced aerospace engineering due to their outstanding high-temperature strength, fatigue resistance, oxidation resistance, and structural stability [1,2,3,4]. The fine and uniform microstructure achieved through powder processing enables the fabrication of high-performance turbine disks [5,6,7] and other critical components operating under extreme thermal and mechanical loads [8,9,10]. Consequently, P/M superalloys have become indispensable for aeroengine hot-section and gas turbine applications [11,12].
However, the growing demand for these alloys has led to an increasing amount of machining waste—including offcuts and swarf—generated during component manufacturing. These return materials contain high concentrations of valuable alloying elements such as Ni, Co, Cr, Al, Ti, and Nb [13,14]. Without efficient recycling strategies, a significant quantity of these strategic resources is lost, resulting in unnecessary material waste and increased production costs [15,16].
Unlike conventional cast or wrought superalloys, recycling of materials produced via powder metallurgy (P/M) processing remains rare due to the stringent cleanliness requirements and fine microstructural control inherent to this class of alloys. During machining, P/M components are prone to surface oxidation, adsorption of cutting fluids, and moisture-induced contamination, all of which introduce additional O and N into the return material. Such contamination can lead to the formation of oxide inclusions or microstructural heterogeneity during remelting, severely compromising fatigue and creep performance. Moreover, the compositional uniformity of P/M alloys makes them especially sensitive to minor elemental losses or contamination, posing a significant challenge for reuse.
Nevertheless, developing reliable and controllable recycling routes for P/M machining returns holds both technical and strategic importance. From an environmental and economic perspective, effective recycling enables the recovery of expensive alloying elements and reduces dependence on newly produced raw powders, thus lowering the life-cycle cost and environmental footprint of high-performance turbine materials. From a technological standpoint, achieving purification without degrading microstructural integrity can help establish a closed-loop manufacturing model for advanced superalloys. Therefore, the efficient recycling of machining return materials is critical not only for resource conservation and cost reduction but also for advancing sustainability and circular production within the superalloy industry [17,18].
Despite the evident benefits, the direct reuse of return materials in high-performance alloys presents several technical challenges. The most critical issue is the elevated O content, which arises from atmospheric exposure during handling and the introduction of O-bearing substances, such as cutting fluids, during machining. Excessive O leads to the formation of oxide inclusions and O-enriched phases during remelting, thereby degrading fatigue and creep properties [19]. Miller et al. reported that even trace levels of O markedly increased the crack growth rate of Inconel 718 under high-temperature conditions, reaching up to 4.5 orders of magnitude higher than in an inert environment, highlighting the critical influence of residual O on the high-temperature fatigue behavior of Ni-based superalloys [19]. In contrast, purification by high-temperature refining risks volatilization and loss of reactive elements such as Al, Ti, and B—elements that are vital for γ′ phase formation and long-term microstructural stability [20,21]. Striking a balance between efficient impurity removal and the retention of key alloying elements remains a central obstacle to the large-scale recycling of machining returns.
In Ni-based P/M superalloys such as FGH95, the principal strengthening phase is the ordered L12 γ′ precipitate with the nominal composition Ni3(Al,Ti) [22,23,24]. Al and Ti determine the γ′ volume fraction, size, and coarsening resistance, which govern high-temperature strength, creep life, and fatigue resistance [25,26,27,28]. A reduction in Al/Ti lowers the γ′ solvus temperature and volume fraction, diminishes anti-phase boundary energy, and weakens the precipitate network. Cr, on the other hand, serves as a γ-matrix stabilizer and enhances oxidation and corrosion resistance while affecting carbide formation and phase stability [29]. Loss of Cr below specification compromises environmental resistance and disrupts the γ/γ′ balance. Hence, controlling the volatilization or enrichment behavior of reactive elements (Al, Ti, Cr) during vacuum refining is crucial—Al/Ti loss deteriorates precipitation strengthening, while Cr depletion threatens compositional conformity and long-term durability [30,31]. Therefore, this study focuses on selective element retention while removing gas impurities during refining.
Previous studies on vacuum induction melting (VIM) of Ni-based superalloys have mainly focused on refining behavior and compositional control in conventional cast or powder metallurgy systems. For example, investigations on alloys such as Inconel 718, René 125, MAR-M247, and NiTiCu shape memory alloy have shown that higher refining temperatures and vacuum levels facilitate O and N removal, but may also lead to the volatilization of active elements such as Al and Ti [32,33,34,35]. Other works have examined crucible–melt interactions and inclusion evolution during VIM processing [25]. However, these studies have largely targeted virgin alloy feedstocks, and systematic investigations into the recycling and purification of machining return materials remain limited. The present work aims to address this gap by quantifying gas impurity removal and alloy element retention during VIM of FGH95 return materials, while also developing a deoxidation kinetics model to clarify the underlying mechanism.
The crucible material is another critical factor governing contamination control during high-temperature refining. Previous work by our group demonstrated that high-purity yttria (Y2O3) crucibles significantly improve purification efficiency during VIM by suppressing interfacial reactions between the melt and crucible walls [36,37]. Similarly, Ma et al. reported that the addition of 0.15 at.% yttrium reduced O pickup by approximately 42%, owing to the buoyant segregation of Y-rich inclusions [38]. The exceptional chemical inertness and thermal stability of yttria effectively prevent secondary contamination and promote gas impurity removal. Building upon these findings, the present study adopts this approach to further enhance refining performance and ensure the compositional integrity of remelted FGH95 alloys.
Although the recycling of Ni-based superalloys has been studied in general, investigations specifically addressing the purification behavior and compositional control of FGH95 return materials remain limited. In particular, the deoxidation mechanisms and kinetics during vacuum remelting have not been clearly elucidated. This knowledge gap constrains the optimization of process parameters and limits industrial application of FGH95 recycling.
To bridge this gap, the present study investigates the effects of refining temperature and holding time on gas impurity removal and alloy element retention during VIM of FGH95 machining returns. Quantitative gas analysis, SEM/EDS, and XRD characterizations were combined to examine the evolution of microstructure and elemental distribution. Furthermore, a deoxidation kinetics model was established to determine the apparent activation energy and reveal the governing mechanism of O removal during high-temperature refining.
2. Materials and Methods
2.1. Experimental Materials
FGH95 P/M superalloy machining return material, originating from cylindrical cores cut out of turbine disk billets during wire-cut electrical discharge machining (EDM), was used as the feedstock. The return material was in the form of solid cylinders with typical dimensions of approximately 60 mm in diameter × 140 mm in height. Before purification, the surface of the return material was lathe-turned to remove the oxidized layer, leaving a smooth metallic surface with a bright metallic luster.
The chemical composition of the return material was determined by inductively coupled plasma optical emission spectrometry (ICP-OES) for metallic elements and by inert-gas fusion ONH analysis for O, N, and H contents. The measured composition is listed in Table 1.

Table 1.
Element composition of FGH95 P/M superalloy return material (wt.%).
2.2. Experimental Procedure
Pretreatment. The as-received machining return materials were first subjected to surface pretreatment to remove oxide layers and machining contaminants. The returns were ultrasonically cleaned in a 5 wt.% DOWFAX 2A1 aqueous solution at 50 °C (40 kHz, 20 min), rinsed thoroughly with anhydrous ethanol, and vacuum-dried at 80 °C under 10−2 Pa for 15 min. After pretreatment, the returns exhibited a metallic luster and a clean surface free of oxidation films.
The refining experiments were conducted in a vacuum induction melting (VIM) furnace equipped with a high-purity Y2O3 crucible (outer diameter 165 mm, height 153 mm, wall thickness 10 mm). During refining, the chamber pressure was dynamically maintained within (5 × 10−3–1 × 10−2 Pa) after initial evacuation to below 0.01 Pa. The pressure history was monitored continuously throughout each melt and remained stable within ±10% of the set value.
Heating was performed using a medium-frequency induction power supply operating at 3–4 kHz and 80–100 kW output power, corresponding to a heating rate of approximately 15–20 °C·min−1 until reaching the target refining temperature. Induction stirring was controlled by adjusting the coil current to 60–70% of the rated level, which provided moderate melt convection sufficient for uniform temperature and mass transfer without excessive turbulence or oxidation risk. These parameters ensured consistent melt conditions and reproducible refining kinetics across all experiments.
Two sets of experiments were conducted without the addition of fluxes or refining agents: (i) the refining-time series, performed at 1650 °C for holding times of 10, 20, and 30 min, and (ii) the refining-temperature series, performed at 1550, 1600, and 1650 °C for a fixed holding time of 10 min.
After refining, the molten alloy was cooled to 1420 °C and poured into cylindrical H13 steel molds (Φ 50 mm), producing dense, cylindrical master alloy ingots. Samples for gas analysis, chemical composition determination, and microstructural characterization were extracted from positions approximately 30 mm above the ingot bottom to ensure uniformity among all specimens.
2.3. Analysis & Testing
Gas impurity analysis. The contents of O, N, and H were determined using an inert-gas fusion elemental analyzer (LECO ONH836, St. Joseph, MI, USA). Calibration was performed using certified reference materials (LECO 502-891). The detection limits (LOD) of the analyzer were 0.5 ppm for O and N, and 0.1 ppm for H, with measurement uncertainties below ±0.3 ppm. The overall repeatability of ONH measurements was better than ±1 ppm for O and N.
Chemical composition analysis. Major and trace alloying elements were quantified using a combination of analytical instruments, including a carbon/sulfur analyzer (LECO CS844es, St. Joseph, MI, USA), an atomic fluorescence spectrometer (Haiguang Instrument HGF-V2, Beijing, China), an inductively coupled plasma mass spectrometer (ICP-MS, Thermo Fisher iCAP RQ, Waltham, MA, USA), and an inductively coupled plasma optical emission spectrometer (ICP-OES, Thermo Fisher iCAP 7200 Radial, Waltham, MA, USA).
For ICP-based analyses, approximately 0.1 g of each sample was dissolved in a mixed acid solution of volume ratio under controlled heating until complete dissolution. The resulting solutions were diluted with ultrapure water and matrix-matched with standard solutions of similar nickel-base alloy composition to minimize spectral interference. Spike recovery tests were conducted using certified reference standards [39], yielding recoveries between 98.5% and 102.3%, which confirmed the accuracy of the analytical procedure. Calibration curves for the major alloying elements were established using multi-element standards (Merck, Darmstadt, Germany), with correlation coefficients (R2 > 0.999).
The LODs for metallic elements were in the range of 0.1–0.5 ppm, and the measurement repeatability and uncertainty were better than ±0.05 wt.% for all reported elements. These analytical conditions ensured high accuracy, reproducibility, and trace-level detection capability for all major alloying constituents.
Experimental repeatability. To ensure the reproducibility of the results, three independent melts were conducted under each refining condition (defined by refining temperature and holding time). Samples for gas and chemical analyses were taken from equivalent positions in each ingot to maintain consistency. The statistical evaluations of ONH and ICP results confirm the high reproducibility and accuracy of the reported data.
Microstructure & phases. Optical microscopy (Shanghai Optical SG-51, Shanghai, China), scanning electron microscopy (ZEISS EVO 10, Oberkochen, Germany) with EDS (Oxford Xplore 30, Abingdon, UK), and X-ray diffraction (Bruker D8 Advance, Ettlingen, Germany) were used to evaluate grain morphology, γ/γ′ phase characteristics, elemental distributions, and phase constitution before and after refining.
SEM observations were conducted in backscattered electron (BSE) mode at a magnification of 3000×, which provided enhanced atomic-number contrast for distinguishing γ and γ′ phases. Representative regions were analyzed under identical imaging conditions to ensure comparability across all samples.
3. Results
3.1. Effect of Refining Time on Purification
Figure 1 presents the variations in gas impurities of the remelted ingots refined for 10, 20, and 30 min at a fixed refining temperature of 1650 °C, and Table 2 lists the major chemical composition of the purified ingots. At 1650 °C, extending the refining time from 10 min to 30 min resulted in a gradual decrease in the O content, from 8 ppm at 10 min to 6 ppm at 30 min. The N content also decreased steadily, from 6 ppm at 10 min to 5 ppm at 20 min, and further to 3 ppm at 30 min. H remained consistently below the detection limit (<1 ppm) throughout all refining durations. These results indicate that a 10 min refining duration is sufficient to achieve effective removal of both O and H, while further extending the time contributes to only marginal improvement in O removal but provides a more noticeable benefit for N reduction.
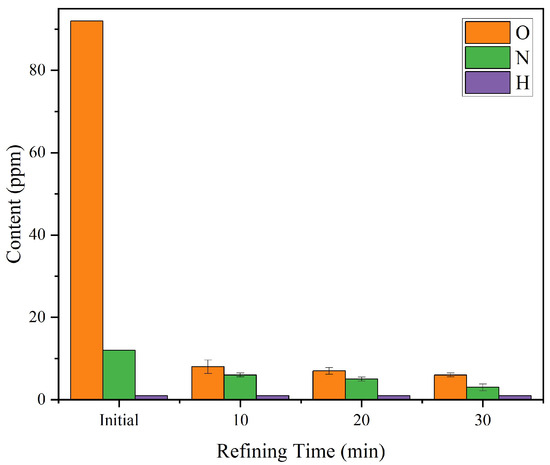
Figure 1.
Variation of gas impurity contents (O, N, H) in FGH95 remelted ingots with refining time (10–30 min) at 1650 °C. Each data point represents the mean ±standard deviation (n = 3).

Table 2.
Element composition after refining at 1650 °C for 10–20 min (wt.%).
In terms of alloy composition, all key elements—including Cr, Co, Al, Ti, Mo, Nb, W, Zr, B, and Ni—remained within the specified limits of the FGH95 P/M superalloy standard after 10 min of refining, as shown in Table 2. However, with extended refining times of 20 min and 30 min, the Cr content dropped below the lower specification limit, indicating notable volatilization loss of this element. In contrast, some other active elements, such as Al and Ti, showed slight increases with time (e.g., Al increased from 3.56 wt.% to 3.61 wt.%, Ti from 2.58 wt.% to 2.62 wt.%), with overall fluctuations remaining within ±0.06 wt.%. These results suggest that prolonged refining primarily promotes volatilization of certain elements like Cr, rather than all active elements, and may thus compromise compositional accuracy if refining is excessively extended.
The observed Cr depletion at longer refining times (20–30 min) is consistent with selective volatilization behavior of active elements under high vacuum. Based on the measured compositional change in Table 2, the Cr content decreased from 12.45 wt.% to 11.86 wt.%, corresponding to a relative loss of approximately 4.7%. This magnitude is in line with vapor pressure estimates: at 1650 °C, the equilibrium vapor pressure of Cr is on the order of 10−2 Pa, which becomes comparable to the operating chamber pressure (5 × 10−3–1 × 10−2 Pa), enabling partial evaporation of Cr from the melt surface.
Furthermore, the use of a Y2O3 crucible, known for its high chemical inertness, minimizes melt–crucible interfacial reactions; thus, the observed Cr loss is primarily attributed to vacuum evaporation rather than crucible consumption. No evidence of Y or O enrichment was detected in the ingot surface layer or slag residues, further confirming negligible crucible interaction. Therefore, the prolonged refining time beyond 10 min, while beneficial for marginal O reduction, leads to increased volatilization of Cr and a risk of deviation from compositional specifications. These results highlight the need to balance refining duration to maintain both gas purity and alloy compositional integrity.
Balancing gas purification performance with compositional stability, a 10 min refining time is sufficient to meet the target requirements of O ≤ 20 ppm, N ≤ 20 ppm, and H ≤ 10 ppm, while maintaining all alloying elements within the FGH95 P/M superalloy specification range. Therefore, a refining time of 10 min is recommended as the optimal process condition for this alloy system.
3.2. Effect of Refining Temperature on Purification
Figure 2 shows the results of gas impurities when refining at 1550 °C, 1600 °C, and 1650 °C with a fixed refining time of 10 min, and Table 3 shows the major chemical composition of purified ingot. As the refining temperature increased from 1550 °C to 1650 °C, a significant downward trend was observed in the O and N contents of the remelted ingots, while the H content consistently remained below the detection limit (<1 ppm). Specifically, when the temperature was raised from 1550 °C to 1600 °C, the O content decreased from 12 ppm to 9 ppm and the N content from 7 ppm to 4 ppm. Further increasing the temperature to 1650 °C resulted in a slight reduction to 8 ppm and 3 ppm for O and N, respectively. These results indicate that refining temperatures above 1600 °C substantially enhance the removal efficiency of dissolved gases. This improvement is attributed to the elevated temperature reducing melted viscosity and interfacial tension, thereby promoting the transfer of dissolved gas molecules into bubbles and their subsequent floatation and removal. However, the marginal benefit of gas removal from 1600 °C to 1650 °C becomes limited, suggesting diminishing returns at higher temperatures. Therefore, 1600 °C can be considered an optimal refining temperature that balances effective gas purification with energy efficiency.
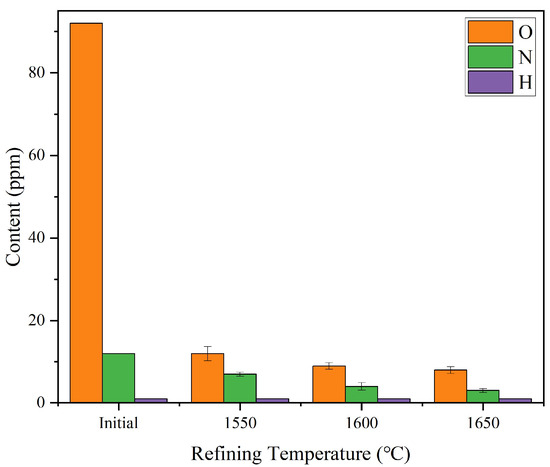
Figure 2.
Variation of gas impurity contents (O, N, H) in FGH95 remelted ingots with refining temperature (1550–1650 °C) for 10 min. Each data point represents the mean ±standard deviation (n = 3).

Table 3.
Element composition after refining at 1550–1650 °C for 10 min (wt.%).
Under all three tested temperature conditions, the measured concentrations of key alloying elements—including Cr, Co, Al, Ti, Mo, Nb, W, Zr, B, and Ni—were within the specified compositional range for FGH95 P/M superalloy, with no deviations beyond standard limits; the results are shown in Table 3. For instance, the contents of Al and Ti at 1550 °C were 3.56 wt.% and 2.58 wt.%, respectively, which slightly decreased to 3.52 wt.% and 2.54 wt.% at 1600 °C and then remained stable at 1650 °C. Across all elements, compositional fluctuations were within ±0.06 wt.%, indicating that the vaporization loss of active elements under high-temperature vacuum conditions is well-controlled and does not compromise the compositional conformity of the alloy. Notably, although higher temperatures may theoretically intensify the evaporation of active elements like Al and Ti, within the 1600–1650 °C range, no significant impact on compositional accuracy was observed.
In summary, considering both gas removal efficiency and alloy composition stability, the refining process at 1600 °C effectively achieves the target thresholds of O ≤ 20 ppm, N ≤ 20 ppm, and H ≤ 10 ppm, while ensuring that all alloying elements remain strictly within the FGH95 P/M superalloy specification limits. This temperature condition not only offers efficient gas purification but also minimizes alloying element loss, making 1600 °C the recommended standard refining temperature for this process.
3.3. Microstructural Characterization
The microstructure of FGH95 P/M superalloy return materials was examined before and after refining using optical microscopy, SEM/EDS, and XRD. Across all observations, the results consistently indicate that the purification process preserves the alloy’s intrinsic phase morphology and compositional stability.
Optical microscopy (Figure 3) shows that both the as-received and refined samples exhibit a typical γ/γ′ dual-phase microstructure. Dendritic features are present in both conditions, with no obvious differences in grain size or secondary dendrite arm spacing. The γ′ precipitates are uniformly distributed, and no harmful phases or abnormal grain growth were detected.
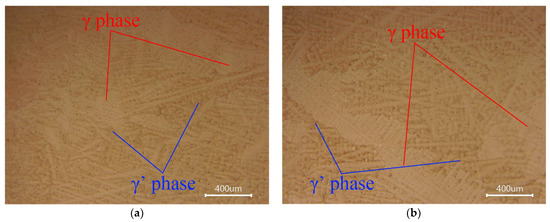
Figure 3.
Optical micrographs of FGH95 P/M superalloy before and after refining. (a) As-received P/M return material; (b) Remelted ingot after vacuum purification (1600 °C, 10 min).
SEM analysis (Figure 4) further confirmed these observations: both the machining return material and the refined alloy exhibited dense, well-bonded microstructures with clearly distinguishable γ and γ′ regions. The γ′ precipitates showed similar size, morphology, and spatial distribution before and after refining, demonstrating that high-temperature vacuum treatment did not alter the fundamental phase framework.
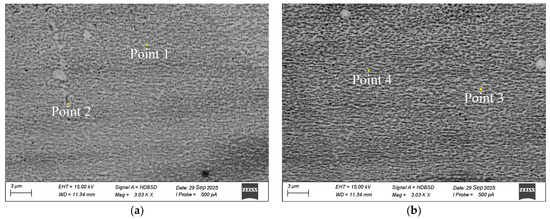
Figure 4.
SEM images of FGH95 P/M superalloy. (a) As-received P/M return material; (b) Remelted ingot after vacuum purification (1600 °C, 10 min).
EDS mapping (Table 4) of regions exhibiting contrast differences in the matrix identified them as γ and γ′ phases. Specifically, points 1 and 3 correspond to the γ matrix, while points 2 and 4 correspond to the γ′ precipitates. The compositional variations revealed by EDS—higher concentrations of Cr and Co in the γ phase, and enrichment of Al and Ti in the γ′ phase—are consistent with the expected phase partitioning behavior in Ni-based superalloys. These differences confirm correct phase identification and indicate that the morphology and elemental distribution of both phases remained essentially unchanged after purification.

Table 4.
EDS point analysis results of the FGH95 P/M superalloy for selected regions.
Quantitative image analysis of the SEM micrographs further supported these findings. The γ′ phase fraction in the machining return material was approximately 40.1%, whereas that in the purified alloy was about 38.2%, showing only a slight decrease after refining. This minor variation demonstrates that the γ′ precipitates were effectively retained during the purification process and that gas impurity removal did not compromise the alloy’s γ/γ′ structural stability. These results are consistent with the XRD patterns, which confirmed the preservation of the γ/γ′ two-phase constitution without the formation of new phases.
XRD patterns (Figure 5) corroborated the microscopic analyses. The diffraction results of both conditions showed sharp peaks corresponding to the γ matrix (FCC Ni-based solid solution) and the γ′ strengthening phase (Ni3(Al,Ti)), with no additional peaks from oxides or new intermetallics. The near-identical peak positions and intensities confirm that the purification process neither introduced residual stress nor altered the phase constitution.
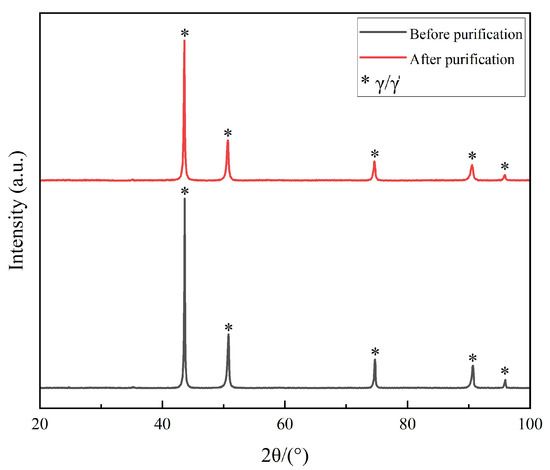
Figure 5.
XRD patterns of FGH95 P/M superalloy before and after purification.
4. Discussion
The deoxidation behavior of FGH95 P/M superalloy return materials during VIM is critically influenced by both refining temperature and refining time. These two parameters jointly determine the thermodynamic driving force and kinetic pathway for impurity gas removal, especially for O, which is the most critical contaminant affecting alloy performance.
4.1. Effect of Refining Time on Deoxidation Kinetics
At a constant temperature of 1650 °C, varying the refining time from 10 to 30 min revealed that O removal is most rapid during the initial 10 min. O content dropped from 8 ppm to 6 ppm over 30 min, but the most substantial improvement occurred in the first 10 min. This behavior can be explained by:
- Initial Reaction-Controlled Stage: During the first few minutes, deoxidation proceeds rapidly due to high O concentration gradients, efficient bubble nucleation, and strong thermodynamic driving force.
- Transition to Diffusion-Controlled Stage: As O content decreases, further removal becomes kinetically limited by the diffusion of O atoms through the melt. At this stage, the rate of gas transport to the surface becomes the limiting factor, explaining the marginal benefit of prolonged refining time.
Notably, longer refining times also increased the risk of vaporization losses of active alloying elements such as Cr, Ti, and Al. The Cr content, in particular, fell below specification at 30 min, highlighting the trade-off between deoxidation and element retention.
4.2. Effect of Refining Temperature on Deoxidation Kinetics
To further interpret the influence of refining temperature on O removal behavior, both experimental observations and thermodynamic analysis were considered.
The experimental results demonstrate a clear temperature dependence in the deoxidation efficiency. As refining temperature increased from 1550 °C to 1650 °C, the O content of the remelted ingots significantly decreased—from 12 ppm to 8 ppm—showing that higher temperatures enhance O removal. This is primarily attributed to two thermodynamic and kinetic factors:
- Increased O Activity and Reduced Solubility: According to Sieverts’ law, the solubility of gases in molten metal decreases with increasing temperature under vacuum. Higher temperatures elevate the activity coefficient of O, making it more prone to escape from the melt.
- Enhanced Kinetic Conditions: Elevated temperature reduces melt viscosity and surface tension, facilitating the diffusion and migration of dissolved O to the melt surface where it can be evacuated by the vacuum environment. This promotes bubble nucleation and flotation, accelerating deoxidation kinetics. However, as the temperature approaches 1650 °C, the rate of improvement diminishes. This plateau effect suggests that the system approaches a diffusion-limited regime, where further temperature increases yield marginal benefits while risking the loss of volatile alloying elements (e.g., Al, Ti).
The observed decrease in O content with increasing refining temperature and time is governed by the equilibrium O potential in the molten Ni-based system rather than a simple Sieverts-type solubility relationship. Under high vacuum, the O partial pressure (≈10−5–10−6 Pa) is greatly reduced, shifting the deoxidation equilibrium toward the formation of stable oxides. The dissolved O in the melt is therefore controlled by the equilibrium between metal–oxide reactions such as:
The equilibrium O content can be expressed as:
where is the equilibrium constant, is the activity of the deoxidizing element (Al or Ti), and are stoichiometric coefficients. As refining temperature increases, both the activity coefficients of Al and Ti and the deoxidation equilibrium constants promote stronger O removal. Thus, the O potential in the melt decreases, enabling more complete oxide separation into the slag/crucible interface.
This behavior aligns with the thermodynamic trend predicted for Ni-Al-Ti systems, where Al and Ti exhibit the highest deoxidation affinity due to their large negative Gibbs free energies of oxide formation [40,41]. Consequently, the observed O reduction in this study primarily reflects equilibrium-controlled deoxidation by Al/Ti rather than simple gas-metal dissolution governed by Sieverts’ law.
Overall, the combined thermodynamic and kinetic effects account for the observed temperature dependence of deoxidation efficiency, confirming that effective O removal can be achieved without compromising alloy compositional stability.
4.3. Mathematical Model of Deoxidation Kinetics
The effectiveness of the refining process for FGH95 P/M superalloy return materials was evaluated by examining the relationship between refining parameters—particularly refining temperature and holding time—and O removal efficiency. Based on the O content data measured at different temperatures and times, a mathematical model was established to describe the deoxidation behavior under vacuum conditions.
Initial modeling efforts assumed a first-order kinetic process, expressed as:
where is the initial O content, is the O content after refining time , and is the deoxidation rate constant. Although this model offered a basic trend description, it lacked sufficient flexibility to capture nonlinear changes in O removal at different refining stages.
To improve accuracy, an alternative logarithmic model incorporating both refining time and temperature dependence was proposed:
where is a fitting coefficient representing the maximum theoretical O removal potential, is the pre-exponential factor, is the apparent activation energy, is the gas constant, and is the refining temperature in Kelvin.
Fitting this model to the experimental data yielded the following parameters:
The proposed model was validated using multiple experimental data points and demonstrated good consistency with the observed deoxidation behavior. As shown in Figure 6 and Figure 7, the simulated prediction curves closely match the measured O contents for two representative conditions: refining at 1650 °C for varying durations and refining at a fixed duration of 10 min across a temperature range of 1500–1700 °C, both at an initial O content of 92 ppm. The alignment between simulated prediction and experiment further confirms the model’s reliability.
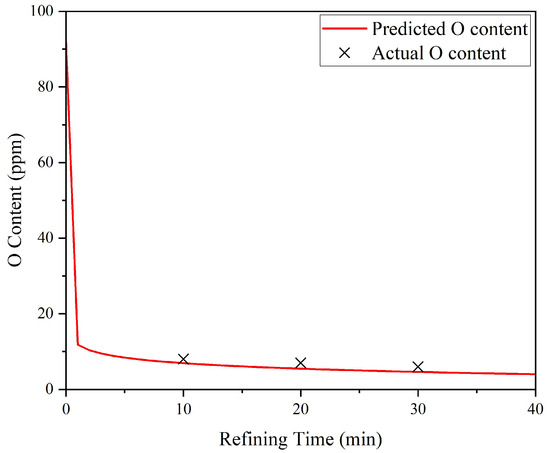
Figure 6.
Comparison between simulated prediction curves and actual O contents of FGH95 P/M superalloy return materials refined at 1650 °C for varying durations, with an initial O content of 92 ppm.
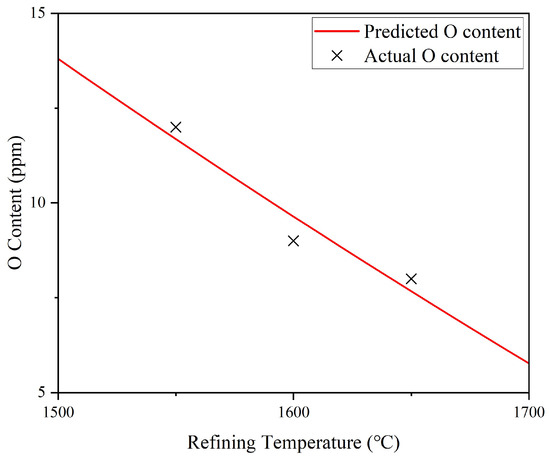
Figure 7.
Comparison between simulated prediction curves and actual O contents of FGH95 P/M superalloy return materials refined at 1500–1700 °C for 10 min, with an initial O content of 92 ppm.
The deoxidation kinetics of O in the molten FGH95 alloy were analyzed using Equation (5), substituting the above-obtained data. The regression achieved an excellent correlation with R2 = 0.986 and a root-mean-square error (RMSE) of 0.13 ppm, demonstrating the strong agreement between the experimental and predicted O contents. The 95% confidence intervals (CIs) of the fitted parameters were within ±8% for and ±6% for , indicating high statistical reliability.
For comparison, a first-order kinetic model was also fitted, yielding a lower correlation (R2 = 0.946) and a higher error (RMSE = 0.18 ppm), which verifies that the proposed formulation provides a superior description of the deoxidation process.
It should be noted that the obtained kinetic parameters are valid under the current experimental conditions—initial O concentration of 90–100 ppm, charge mass of approximately 5 kg, and vacuum pressure of 10−3–10−1 Pa. Extrapolation beyond these ranges, such as to higher O levels, larger melt volumes, or weaker vacuum environments, may require recalibration of the kinetic constants to account for changes in mass transfer coefficients and melt surface area. Nonetheless, within the studied range, the proposed model accurately captures the thermally activated O removal mechanism and provides quantitative insight into the refining kinetics of FGH95 P/M return materials.
This improved kinetic model successfully captures the nonlinear time dependence of the deoxidation process while integrating the pronounced effect of refining temperature. It offers a robust predictive framework that can be used to optimize refining parameters. The coupled temperature–time model highlights the importance of considering both variables simultaneously when aiming for effective impurity removal in recycled FGH95 P/M superalloy materials.
The results indicate that a refining temperature of 1600 °C combined with a holding time of 10 min provides an optimal balance between O removal efficiency and alloy compositional stability for FGH95 P/M superalloy returns. This kinetic framework not only enhances the understanding of the underlying deoxidation mechanisms but also serves as a practical tool for guiding process design. Overall, the model supports the development of a more efficient and reliable recycling strategy for high-value superalloy return materials, contributing to improved resource utilization and industrial sustainability.
4.4. Elemental Stability During Refining
In accordance with the compositional data presented in the Results Section, the evolution of reactive elements during refining exhibits a selective volatilization behavior. With increasing refining time, Cr content decreased continuously and fell below the lower specification limit at 20–30 min, whereas Al and Ti showed slight increases (≤0.06 wt.%). This indicates that element volatilization under vacuum is not uniform across all active species. Owing to its higher vapor pressure and stronger affinity for O, Cr is more prone to evaporation and interfacial reaction losses during prolonged high-temperature exposure. In contrast, Al and Ti remain relatively stable under the same conditions, and their marginal increases are likely attributed to concentration effects associated with O removal and the chemical inertness of the Y2O3 crucible, which effectively suppresses parasitic reactions that would otherwise consume these elements.
These observations suggest that prolonged refining improves impurity removal efficiency but simultaneously promotes selective Cr depletion, thereby compromising compositional accuracy. Consequently, a refining duration of 10 min represents an optimal compromise, ensuring sufficient deoxidation and degassing while minimizing undesired volatilization of key alloying elements.
4.5. Implications for Industrial Recycling
The results indicate that, for FGH95 P/M superalloy machining returns, a refining temperature of 1600 °C and a holding time of 10 min provide the optimal balance between gas impurity removal efficiency and alloy composition stability. The developed kinetic model not only elucidates the underlying purification mechanism but also serves as a predictive tool for process optimization, offering theoretical and practical guidance for scaling up the recycling of return materials in industrial P/M superalloy production. This contributes to improved process reliability, cost reduction, and sustainability in closed-loop manufacturing.
To assess the robustness of this optimized refining window (1600 °C/10 min), a process-tolerancing analysis was conducted. Using the mean ±2 SD values derived from three independent melts (n = 3) and linear interpolation between experimental points, an operating map was established to define the acceptable region that satisfies the ONH specifications (O ≤ 6 ppm, N ≤ 3 ppm, H < 1 ppm). As shown in Figure 8, the acceptable ranges are approximately 1580–1620 °C (±20 °C) and 8–13 min (±3 min) when the vacuum level is maintained at p ≤ 1 × 10−2 Pa (typical range 5 × 10−3–1 × 10−2 Pa). Relaxation of the vacuum beyond 1 × 10−2 Pa narrows the permissible temperature-time domain, underscoring the importance of maintaining a stable high-vacuum environment for consistent impurity control. This process-tolerancing view provides practical guidance for industrial refining operations and confirms that the optimized parameters offer sufficient flexibility for stable, reproducible production.
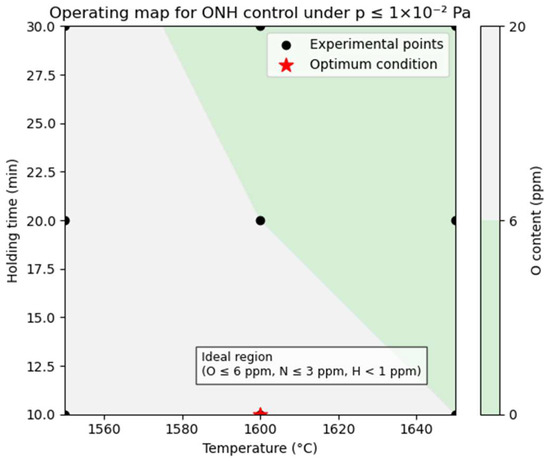
Figure 8.
Operating map showing the acceptable refining window (shaded region) satisfying ONH specifications (O ≤ 6 ppm, N ≤ 3 ppm, H < 1 ppm) as a function of temperature and holding time under p ≤ 1 × 10−2 Pa (5 × 10−3–1 × 10−2 Pa).
5. Conclusions
- A robust refining process window for recycling FGH95 P/M superalloy machining returns was established at 1600 ± 20 °C for 10 ±3 min under ≤ 1 × 10−2 Pa, achieving stable purification with O ≤ 20 ppm, N ≤ 20 ppm, and H < 10 ppm. A process tolerancing analysis confirmed that minor deviations in temperature and holding time do not compromise gas impurity control.
- The nominal alloy composition and γ/γ′ two-phase microstructure were well preserved after refining. The concentrations of Al, Ti, and Cr remained within specification limits, and microstructural observations confirmed that the primary strengthening γ′ phase retained its morphology and distribution, ensuring mechanical integrity of the recycled alloy.
- A deoxidation kinetics model was developed to describe O removal under vacuum refining. The fitted parameters showed excellent correlation. The model accurately predicts O removal behavior within the studied temperature–time–vacuum range but requires recalibration for different initial O levels, melt sizes, or vacuum degrees.
Author Contributions
Conceptualization, H.Z. and X.W.; methodology, L.Z.; software, J.C.; validation, J.C., L.Z. and Y.C.; formal analysis, J.C.; investigation, P.F.; resources, L.Z.; data curation, J.C. and P.F.; writing—original draft preparation, J.C.; writing—review and editing, Y.C.; visualization, J.C.; supervision, H.Z. and X.W.; project administration, Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Authors Xuqing Wang and Lei Zhou were employed by AECC Beijing Institute of Aeronautical Materials. Author Peng Fu was employed by AECC South Industry Company Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hu, B.F.; Chen, H.M.; Song, D.; Li, H.Y. Research on the Carbides in FGH95 Superalloy Powders Prepared by PREP during Solidification. J. Mater. Eng. 2005, 41, 1042–1046. [Google Scholar]
- Gao, Y.; Zou, J.; Wang, X.; Wang, X.; Yang, J.; Wang, H. Microstructure and Mechanical Performance of Graphene Nanosheets Reinforced Nickel-Based Superalloy FGH95 Composite. Nanomaterials 2020, 10, 100. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, C.; Shu, Q. Microstructure, Tensile Properties and Heat Treatment Process of Spray Formed FGH95 Superalloy. J. Iron Steel Res. Int. 2013, 20, 59–63. [Google Scholar] [CrossRef]
- Hu, D.; Wang, R.; Fan, J.; Shen, X. Probabilistic Damage Tolerance Analysis on Turbine Disk through Experimental Data. Eng. Fract. Mech. 2012, 87, 73–82. [Google Scholar] [CrossRef]
- Yin, F.-Z.; Hu, B.-F.; Jin, K.-S.; Jia, C.-C. Effect of Hot Extrusion and Heat Treatment on the Gamma’ Phase Precipitation in FGH 95 Alloy. J. Mater. Eng. 2005, 52–55. [Google Scholar]
- Zhang, Y.; Yang, S.Z.; Li, L. Current Status of Research on PM Superalloy in China. Mater. Rev. 2002, 16, 14. [Google Scholar]
- Bianchi, L.M. Powder Production: Argon Atomized Superalloys for Jet Engine Discs. In Proceedings of the European Congress and Exhibition on Powder Metallurgy, European PM Conference Proceedings, Valencia, Spain, 20–22 October 2003; Volume 3, p. 1. [Google Scholar]
- Lu, Z.Z.; Liu, C.L.; Yue, Z.F.; Xu, Y.L. Probabilistic Safe Analysis of the Working Life of a Powder Metallurgical Turbine Disc. Mater. Sci. Eng. A 2005, 395, 153–159. [Google Scholar] [CrossRef]
- Bhaumik, S.K.; Bhaskaran, T.A.; Rangaraju, R.; Venkataswamy, M.A.; Parameswara, M.A.; Krishnan, R.V. Failure of Turbine Rotor Blisk of an Aircraft Engine. Eng. Fail. Anal. 2002, 9, 287–301. [Google Scholar] [CrossRef]
- Tan, L.; Huang, Z.; Liu, F.; He, G.; Wang, X.; Huang, L.; Zhang, Y.; Jiang, L. Effects of Strain Amount and Strain Rate on Grain Structure of a Novel High Co Nickel-Based Polycrystalline Superalloy. Mater. Des. 2017, 131, 60–68. [Google Scholar] [CrossRef]
- Grison, J.; Remy, L. Fatigue Failure Probability in a Powder Metallurgy Ni-Base Superalloy. Eng. Fract. Mech. 1997, 57, 41–55. [Google Scholar] [CrossRef]
- Semiatin, S.L.; McClary, K.E.; Rollett, A.D.; Roberts, C.G.; Payton, E.J.; Zhang, F.; Gabb, T.P. Microstructure Evolution during Supersolvus Heat Treatment of a Powder Metallurgy Nickel-Base Superalloy. Metall. Mater. Trans. A 2012, 43, 1649–1661. [Google Scholar] [CrossRef]
- Qiu, C.L.; Attallah, M.M.; Wu, X.H.; Andrews, P. Influence of Hot Isostatic Pressing Temperature on Microstructure and Tensile Properties of a Nickel-Based Superalloy Powder. Mater. Sci. Eng. A 2013, 564, 176–185. [Google Scholar] [CrossRef]
- Qiu, C.; Wu, X.; Mei, J.; Andrews, P.; Voice, W. Influence of Heat Treatment on Microstructure and Tensile Behavior of a Hot Isostatically Pressed Nickel-Based Superalloy. J. Alloys Compd. 2013, 578, 454–464. [Google Scholar] [CrossRef]
- Cui, F.; Wang, G.; Yu, D.; Gan, X.; Tian, Q.; Guo, X. Towards “Zero Waste” Extraction of Nickel from Scrap Nickel-Based Superalloy Using Magnesium. J. Clean. Prod. 2020, 262, 121275. [Google Scholar] [CrossRef]
- DeBarbadillo, J.J. Nickel-Base Superalloys; Physical Metallurgy of Recycling. Metall. Trans. A 1983, 14, 329–341. [Google Scholar] [CrossRef]
- Brooks, P.T.; Potter, G.M.; Martin, D.A. Chemical Reclaiming of Superalloy Scrap; US Department of Interior, Bureau of Mines: Washington, DC, USA, 1969; Volume 7316.
- Yagi, R.; Okabe, T.H. Recovery of Nickel from Nickel-Based Superalloy Scraps by Utilizing Molten Zinc. Metall. Mater. Trans. B 2017, 48, 335–345. [Google Scholar] [CrossRef]
- Miller, C.F.; Simmons, G.W.; Wei, R.P. Mechanism for Oxygen Enhanced Crack Growth in Inconel 718. Scr. Mater. 2001, 44, 2405–2410. [Google Scholar] [CrossRef]
- Guo, W.; Wu, J.; Zhang, F.; Zhao, M. Microstructure, Properties and Heat Treatment Process of Powder Metallurgy Superalloy FGH95. J. Iron Steel Res. Int. 2006, 13, 65–68. [Google Scholar] [CrossRef]
- Utada, S.; Joh, Y.; Osawa, M.; Yokokawa, T.; Sugiyama, T.; Kobayashi, T.; Kawagishi, K.; Suzuki, S.; Harada, H. Creep Property and Phase Stability of Sulfur-Doped Ni-Base Single-Crystal Superalloys and Effectiveness of CaO Desulfurization. Metall. Mater. Trans. A 2018, 49, 4029–4041. [Google Scholar] [CrossRef]
- Vilar, R.; Almeida, A. Repair and Manufacturing of Single Crystal Ni-Based Superalloys Components by Laser Powder Deposition—A Review. J. Laser Appl. 2015, 27. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Song, F.; Zhang, M.; Luo, Y.; Zhao, H.; Tang, D. Effect of Trace Boron on Microstructural Evolution and High Temperature Creep Performance in Re-Contianing Single Crystal Superalloys. Prog. Nat. Sci. Mater. Int. 2020, 30, 371–381. [Google Scholar] [CrossRef]
- Long, H.; Mao, S.; Liu, Y.; Zhang, Z.; Han, X. Microstructural and Compositional Design of Ni-Based Single Crystalline Superalloys―A Review. J. Alloys Compd. 2018, 743, 203–220. [Google Scholar] [CrossRef]
- Luo, C.; Yang, X.; Weng, H.; Yuan, H. Degradation Mechanism of Nickel-Base Single Crystal Superalloy after Long-Term Aging: Roles of Nanomechanical Properties and Morphology of γ/Γ′ Phases. J. Mater. Sci. Technol. 2026, 248, 247–265. [Google Scholar] [CrossRef]
- Kangazian, J.; Shamanian, M.; Kermanpur, A.; Foroozmehr, E.; Badrossamay, M. Investigation of Microstructure-Tensile Behavior Relationship in Hastelloy X Ni-Based Superalloy Processed by Laser Powder-Bed Fusion: Insights into the Elevated Temperature Ductility Loss. Mater. Sci. Eng. A 2021, 823, 141742. [Google Scholar] [CrossRef]
- Weng, F.; Liu, Y.; Chew, Y.; Yao, X.; Sui, S.; Tan, C.; Ng, F.L.; Bi, G. IN100 Ni-Based Superalloy Fabricated by Micro-Laser Aided Additive Manufacturing: Correlation of the Microstructure and Fracture Mechanism. Mater. Sci. Eng. A 2020, 788, 139467. [Google Scholar] [CrossRef]
- Xie, X.; Li, J.; Jiang, W.; Dong, Z.; Tu, S.-T.; Zhai, X.; Zhao, X. Nonhomogeneous Microstructure Formation and Its Role on Tensile and Fatigue Performance of Duplex Stainless Steel 2205 Multi-Pass Weld Joints. Mater. Sci. Eng. A 2020, 786, 139426. [Google Scholar] [CrossRef]
- Zhuo, H.; Zheng, D.; Yang, C.; Guo, Y.; Han, J.; Wang, C.; Liu, X. Development of L12 Nanoparticles Strengthened Ni-Co-Al-Ta Based Superalloys with Excellent Comprehensive Properties. Intermetallics 2025, 187, 109007. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Zhuo, H.; Cai, W.; Chen, Y.; Yu, X.; Yang, S.; Li, Y.; Yang, Y.; Liu, X. Development of Co–Ni–Al–V–Ta–Cr-Based Superalloys with High-Temperature Strength and Excellent Oxidation Resistance. J. Mater. Res. Technol. 2023, 27, 8143–8150. [Google Scholar] [CrossRef]
- Wang, C.; Zhuo, H.; Zheng, D.; Yu, X.; Chen, X.; Chen, Y.; Chen, X.; Zhang, J.; Han, J.; Liu, X. Development of a Low-Density Co-Ni-Al-Ta-Cr Superalloy with High Mechanical Performance and Superior Oxidation Resistance. Mater. Des. 2024, 238, 112673. [Google Scholar] [CrossRef]
- Lapin, J.; Klimová, A. Vacuum Induction Melting and Casting of TiAl-Based Matrix in-Situ Composites Reinforced by Carbide Particles Using Graphite Crucibles and Moulds. Vacuum 2019, 169, 108930. [Google Scholar] [CrossRef]
- Wang, N.; Gao, J.; Yang, S.; Yang, S.; Liu, M.; Liu, W.; Qu, J. Numerical Simulation of the Denitrification Reaction of INCONEL 718 Superalloy During Vacuum Induction Melting. Met. Mater. Trans. B 2022, 53, 1474–1483. [Google Scholar] [CrossRef]
- Baldan, R.; da Rocha, R.L.P.; Tomasiello, R.B.; Nunes, C.A.; da Silva Costa, A.M.; Barboza, M.J.R.; Coelho, G.C.; Rosenthal, R. Solutioning and Aging of MAR-M247 Nickel-Based Superalloy. J. Mater. Eng. Perform. 2013, 22, 2574–2579. [Google Scholar] [CrossRef]
- Om, H.; Singh, S. Fabrication and Characterization of Vacuum Induction-Melted Cast NiTiCu Shape Memory Alloy. J. Mech. Sci. Technol. 2025, 39, 2681–2687. [Google Scholar] [CrossRef]
- Bai, P.; Zhang, H.; Li, Y.; Kong, B.; Zhang, H. Effect of Y2O3 Crucible on Purification of Ni3Al-Based Superalloy Scraps. RARE Met. Mater. Eng. 2019, 48, 406–410. [Google Scholar]
- Bian, W.; Zhang, H.; Gao, M.; Li, Q.; Li, J.; Tao, T.; Zhang, H. Influence of Yttrium and Vacuum Degree on the Purification of K417 Superalloy. Vacuum 2018, 152, 57–64. [Google Scholar] [CrossRef]
- Ma, L.; Tang, X.; Wang, B.; Jia, L.; Yuan, S.; Zhang, H. Purification in the Interaction between Yttria Mould and Nb-Silicide-Based Alloy during Directional Solidification: A Novel Effect of Yttrium. Scr. Mater. 2012, 67, 233–236. [Google Scholar] [CrossRef]
- HB 20241-2016; Methods for Spectrometric Analysis of Super Alloys. State Administration of Science, Technology and Industry for National Defence: Beijing, China, 2016.
- Tukdogan, E.T. Physical Chemistry of High Temperature Technology; Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Kubaschewski, O. Metallurgical Thermochemistry; Pergamon: Oxford, UK, 1976; ISBN 978-0-08-011887-1. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).