3.1. Reducibility Investigation
The heat flow and the mass loss curves of reduction in the five C/Fe
2O
3 ratios investigated (
Figure S1) had behaviors comparable to those observed in the thermograms by Mombelli et al. [
32], showing two valleys between 200 and 400 °C due to the complete dehydroxylation of residual gibbsite and boehmite from the prior red mud drying step. The second peak in this range is also related to portlandite dihydroxylation [
59]. The valley at 650 °C is associated with the liming of magnesite and the dissociation of calcium carbonate [
60], while the weaker valley around 900 °C and the stronger one at 1000 °C correspond to the reduction of Fe
2O
3 into Fe
3O
4, and the subsequent full reduction and melting of the iron. Increasing the carbon content of the mixture decreases the reduction temperature peak due to an increase in reduction kinetics [
40,
61], as shown in
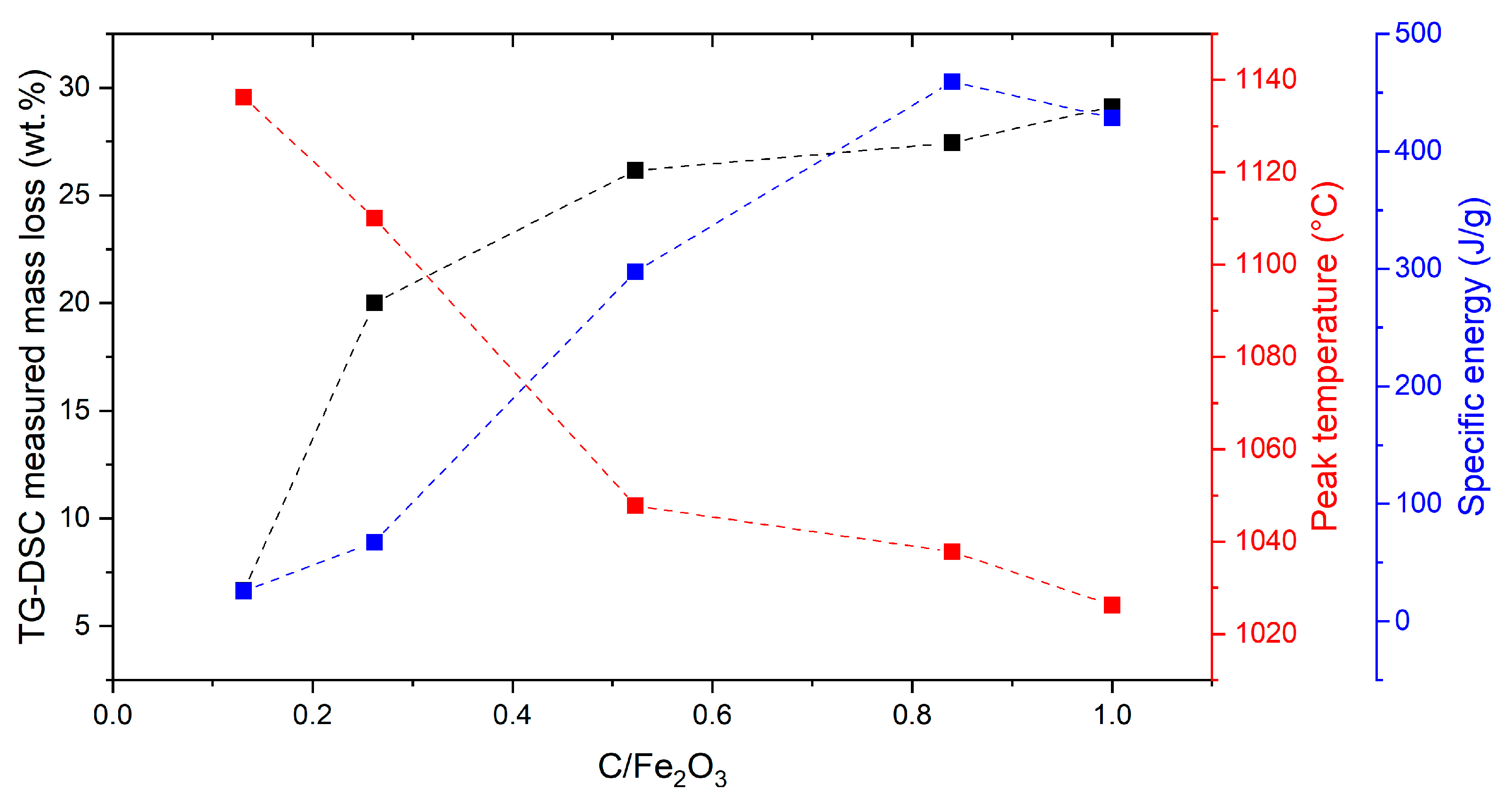
Figure 2, which also reports the mass loss measured through TG-DSC and the specific energy.
Looking at the specific energy associated with the main valley at 1000 °C, which is considered as a performance index of the iron recovery, it increased linearly up to 0.840 C/Fe
2O
3 ratio, which can be considered the most performing sample, and then the energy decreased, adding more carbon in the mixture. Indeed, the increased amount of blast furnace sludge implied an inevitable decrease in red mud. The excess of carbon totally reduced the iron oxides, but the reduced iron was small in amount, resulting in a weak endothermic valley [
32]. The specific energy of 450 J g
−1 for the 0.840 C/Fe
2O
3 confirmed the reducibility investigation performed in the previous study of Mombelli et al. [
32], which highlighted how the use of the steelmaking waste is less energy demanding than a conventional reducing agent (800 J g
−1 for the graphite [
32]).
Contrary to the work of Mombelli et al. [
32], which observed the highest mass loss in correspondence with a C/Fe
2O
3 ratio of 0.85 with graphite, in this work, the mass loss increased sharply between the 0.131 and 0.523 C/Fe
2O
3 ratios, after which the value remained almost constant. The 0.523 C/Fe
2O
3 ratio can be considered, as well as 0.840, a best reduction condition, because a lower amount of carbon would not allow the complete reduction of the available iron oxide, and further carbon would be unnecessary inside the mixture from a metallurgical point of view, acting as inert material [
62]. The slightly higher mass loss of the mixture with the highest C/Fe
2O
3 ratio (1.000) was associated with the excess of carbon, which burned [
62], and the greater amount of blast furnace sludge and its volatile matter. The increase in mass loss due to the volatilization of volatile matter was also supported by the higher mass loss of BFS than graphite, keeping the same C/Fe
2O
3 ratio (30 vs. 19 wt.%, considering 0.523 as the C/Fe
2O
3 ratio [
32], respectively).
However, both of the selected C/Fe2O3 ratios (0.523 and 0.840) seem worthy of investigation. From one perspective, 0.523 should optimize the process from a practical point of view, i.e., by minimizing the drawbacks of carbon excess. From the other perspective, 0.840 should optimize the yield of the process, i.e., by increasing iron production, which is the revenue-generating product.
The differences in mass loss comparing this work with Mombelli et al. [
32] highlighted how, despite the similar heat flow, thermogravimetry and first-order derivative of thermogravimetry (dTG) (
Figure S1 and Figure S2, respectively), it is not possible to generalize the ideal C/Fe
2O
3 ratio, but it is necessary to perform a reducibility investigation for each new reducing agent.
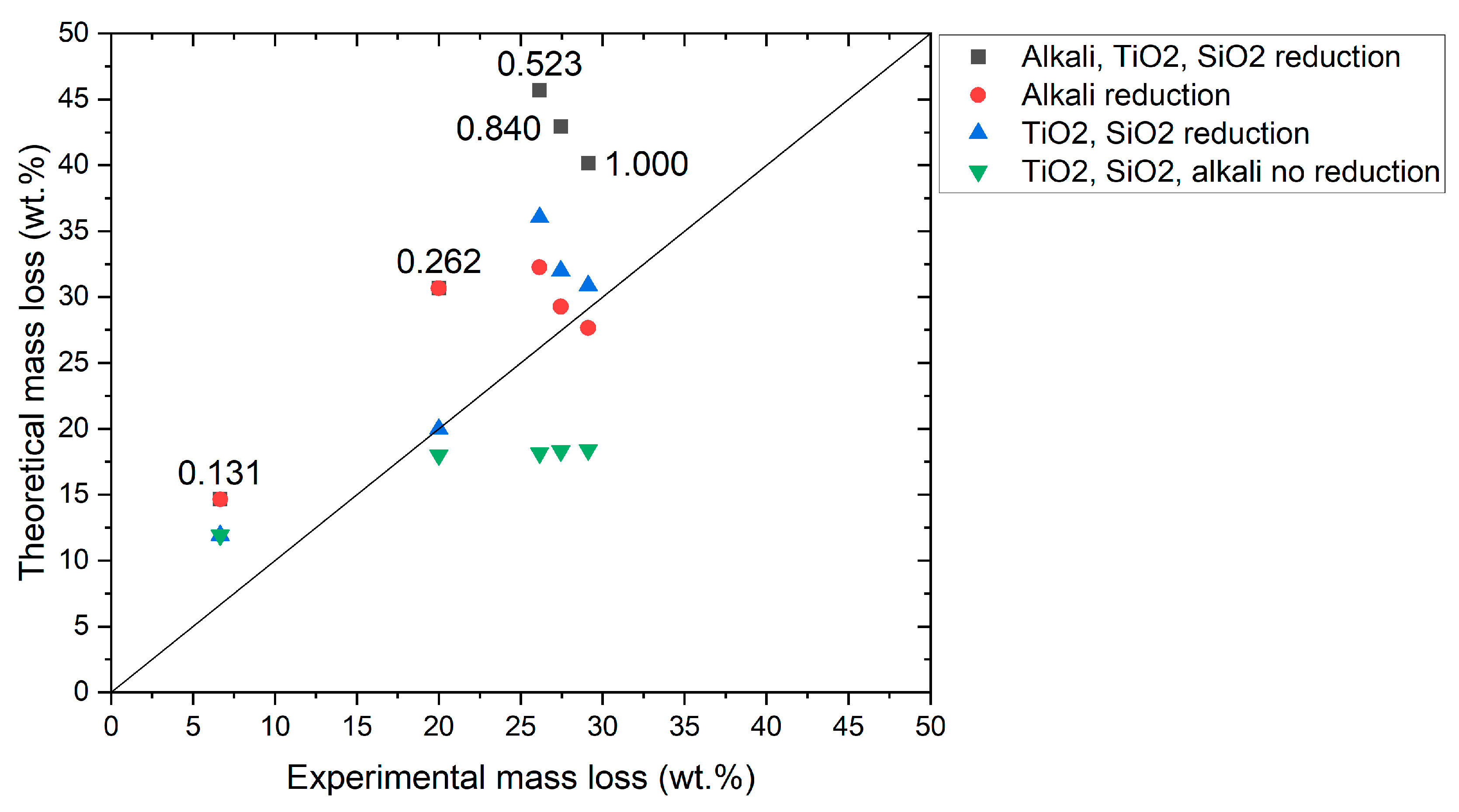
A theoretical model based on the chemical composition of the red mud and the blast furnace sludge (
Table 1 and
Table 2, respectively) has been developed to compare the data obtained through the thermal analysis with the predicted total mass loss during the reduction of each C/Fe
2O
3 ratio. The computation assumed the loss of oxygen contained in all the reducible oxides up to SiO
2, the direct reduction of hematite to iron and the loss of all carbon necessary for the reduction. For simplification, each oxide was considered not bonded with the other oxides. The comparison between the mass loss calculated through the theoretical model and the one obtained from the thermal analysis is shown in
Figure 3.
Considering the 0.131 C/Fe
2O
3 ratio, the “TiO
2 and SiO
2 reduction” and the “TiO
2, SiO
2, alkali no reduction” cases gave a more similar mass loss to the experimental one, with an overestimation of 78.98% with respect to the other two cases. Despite the mixture with 0.262 C/Fe
2O
3 presenting a perfect match considering the “TiO
2 and SiO
2 reduction” case, the low temperature and the insufficient carbon should not allow their complete reduction from a theoretical point of view. Therefore, it was also reasonable to consider a partial reduction of alkali. By increasing the C/Fe
2O
3 ratio, the “alkali reduction” case better explained the experimental mass loss with an overestimation of 37.82, 16.58 and 6.01% for 0.523, 0.840 and 1.000 C/Fe
2O
3 ratios, respectively. However, the incomplete overlap between the experimental and theoretical mass loss suggested that a more complex series of phenomena was occurring, such as the generation of a liquid phase which filled the porosities and avoided the contact between the iron oxide and the reducing CO [
63]. In addition, decreasing the carbon content, more slag was formed, affecting the final result [
64].
Taking into account all the previously discussed aspects, the optimal yield for the reduction process were found to be 0.523 and 0.840 C/Fe2O3, as these provided the highest mass loss and the highest specific energy while requiring the lowest temperature to metallize the iron, respectively. Furthermore, 0.840 was an optimal ratio since the mass loss was not further increased by the high volatile matter content in the BFS, as was the case with 1.000 C/Fe2O3, and the excess carbon did not have a negative impact on reducibility.
3.2. Briquettes Characterization
According to the reducibility investigation, the ratio of 0.840 C/Fe2O3 was selected for briquetting the powders. The second mixture chosen was 0.523, as this corresponded to the initial point on the mass loss plateau, meaning there would be no excess carbon. Additionally, the higher concentration of red mud enabled a better understanding of its mechanical properties in agglomerate form.
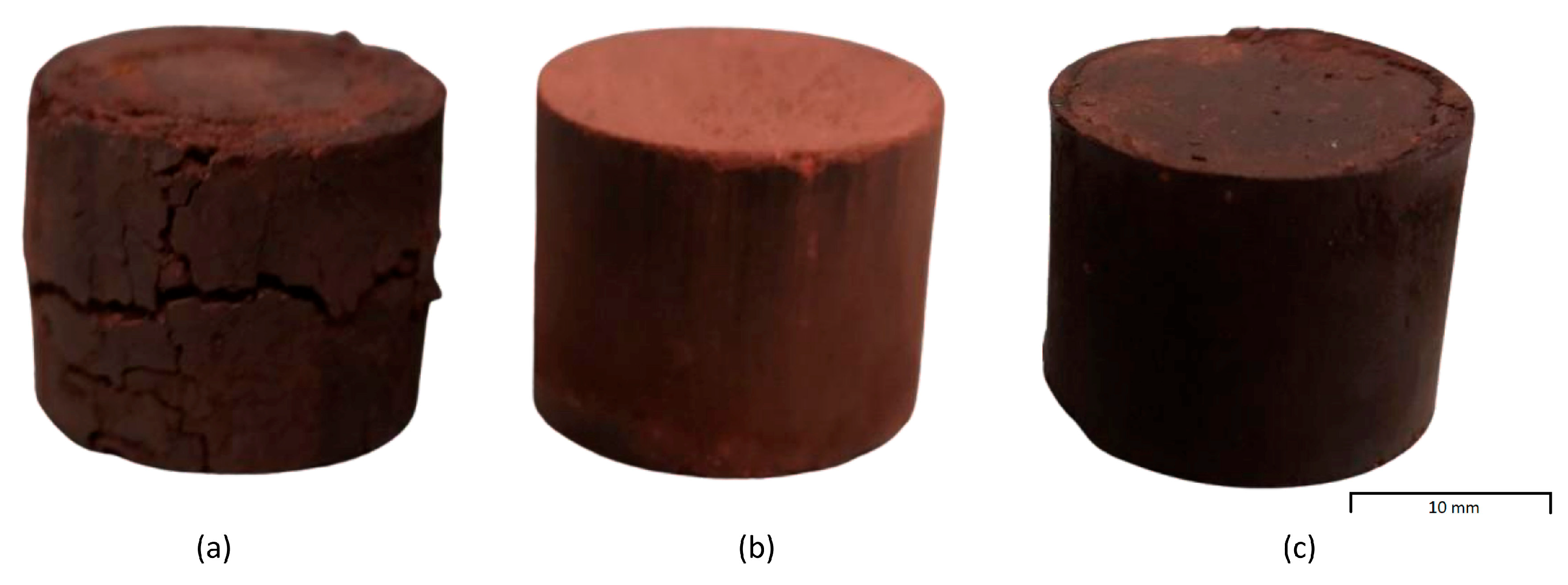
The mixtures showed different appearances according to the preparation procedures, resulting in different briquette surface textures, independent of the C/Fe
2O
3 ratio used. The visual appearance of the uncured 0.523 C/Fe
2O
3 ratio briquettes, taken as most representative, is shown in
Figure 4. The 1W mixture was very dry, and the resulting agglomerates showed large cracks along all their surface, appearing very fragile. A higher amount of moisture instead favored a better arrangement of particles during packing and increased the agglomeration efficiency [
65]. In addition, increasing the amount of water, the workability and fluidity of the mixture improved, as in the case of cement [
66,
67]. Indeed, the 2W mixture appeared well mixed and satisfyingly wet. The briquettes were more compact and had no significant damage to their surface. However, an excessive viscosity of the fluid, as in the case of 3W, caused the formation of burrs around the briquettes and problems during the briquetting process, such as the spilling of water from the funnel.
The 0.523-1W briquettes failed and broke during the 15-day curing period due to the dryness of the agglomerate. The water used in this procedure interacted with the starch to allow its proper gelatinization, but the moisture was not properly absorbed by the red mud, affecting its mechanical resistance. Despite their fragile appearance, the 0.840-1W briquettes probably survived the curing process because the mixture required less water absorption due to the reduced amount of RM.
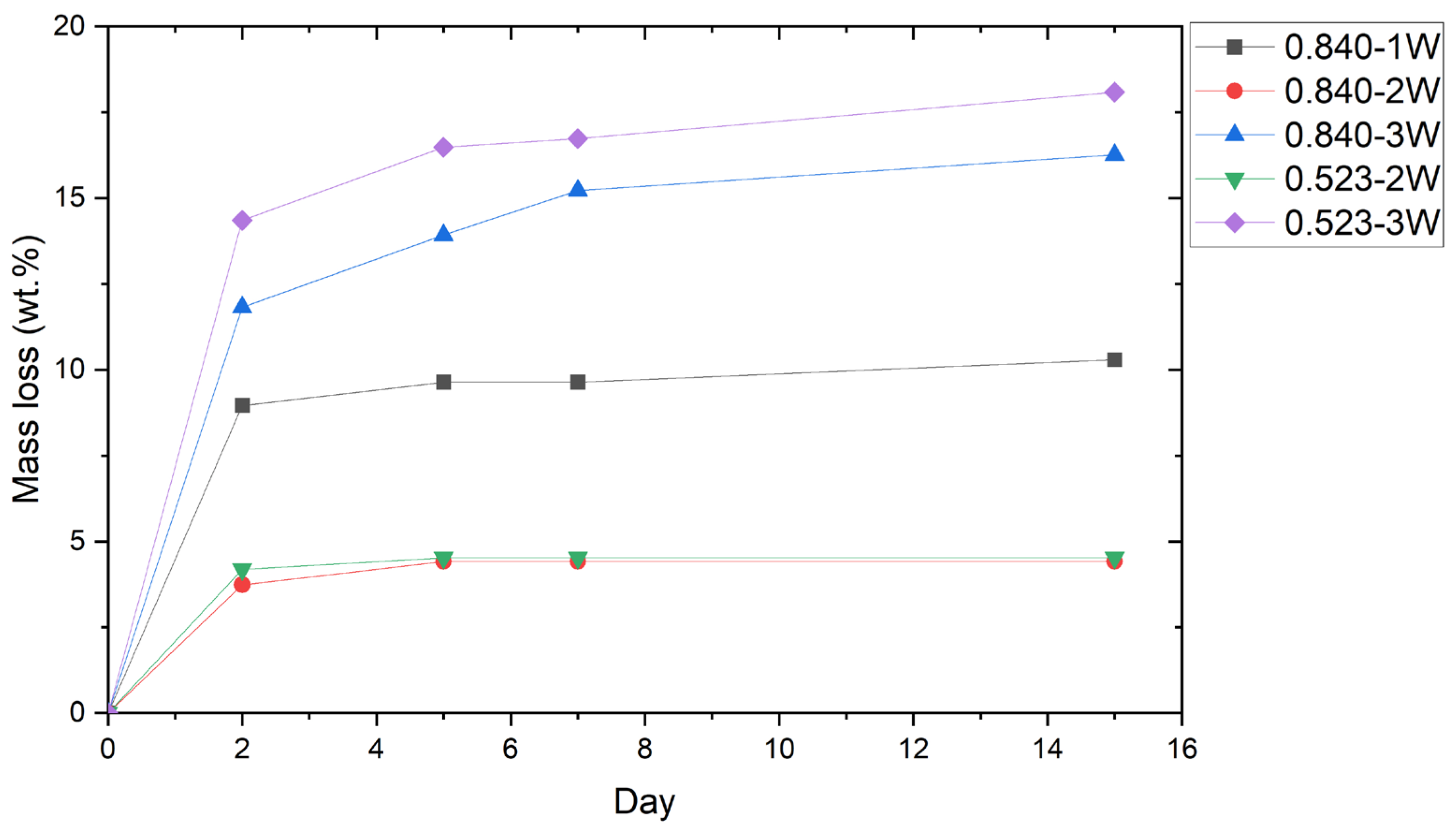
As shown in
Figure 5, the mass of all surviving briquettes stabilized in a few days since the hygroscopicity of the RM favored the progressive absorption of the water released during the starch retrogradation [
41]. Since the moisture in 1W was completely used for the gelatinization of starch, the mass loss of the briquettes was completely associated with the water released and not absorbed by the agglomerates during the binder retrogradation. The procedure of 2W instead caused a partial absorption of water by the red mud due to their direct interaction, and only the residual one was used for the starch gelatinization. The latter was probably incomplete, and it resulted in lower mass loss with respect to 1W due to partial binder retrogradation. The hypothesis that the mass loss of 1W and 2W was solely due to starch retrogradation was confirmed by the similarity of the curves, which showed greater loss during the first few days before stabilizing after five days. The 3W instead required nearly 7 days to stabilize, showing, however, a slight mass reduction in the following week. The higher mass loss of 3W (18.09% and 16.26% for 0.523 and 0.840, respectively) was due to the increased amount of water that had probably saturated the RM and was released during curing, in addition to that released during starch retrogradation.
3.2.1. Selection of Optimal Briquetting Process Based on Mechanical Characterization
The density of the cured briquettes, the results of the drop test and the UCS are reported in
Table 5.
In both C/Fe
2O
3 ratios, the highest density was shown by the 2W briquettes. The balance between the amount of water required for starch gelatinization and the amount absorbed by the red mud led to better agglomeration conditions, resulting in the highest density. Indeed, the density improvement of 3% between 0.840-1W and 2W was given by the higher content of moisture, which favored the particle arrangement and deformation, leading to a better packing [
68]. The excess of water in 3W was instead ejected, leading to a higher mass loss and consequently lower density (−8% than 0.840-1W).
All the briquettes survived to 10 drops except 0.840-1W, which did not exceed 2 drops, as imagined by the presence of cracks all along their surfaces. This result confirmed that the traditional method is not suitable for the agglomeration of RM since the briquettes 0.523-1W failed during the curing time, and 0.840-1W are not suitable to withstand their transport and management. Avoiding the interaction between red mud and free water, the workability is reduced and the mixture becomes stiffer, resulting in potential problems [
67]. The survived mixtures instead showed similar results in terms of the drop test, which was hence not the discriminant mechanical test to select the best preparation method and choose the best C/Fe
2O
3 ratio. All the industrial benchmarks were indeed largely satisfied, considering a minimum number of 4 drops [
69] and that the Italian standard for briquettes in industrial application is IRI of 97.7 [
55], and the highest compression strength is 9.8 MPa in case of a converter [
70]. However, a higher AIRI is shown in 3W for both C/Fe
2O
3 ratios (increase of about 10% and 12% for 0.523 and 0.840, respectively), highlighting a limited release of fine particles. On the contrary, increasing the amount of water, the UCS decreases because water weakens the bonding between mineral particles. When water infiltrates, pore pressure increases and friction between particles is reduced, making it easier for fractures to propagate under applied stress. Additionally, water can lead to the swelling of clay minerals and other constituents, further destabilizing the structure [
71]. This evidence could be correlated with the relationship between UCS and water-cement ratio of a concrete structure [
72]. The binding force of the agglomerate was given by the hydration of phases as calcium silicates since the H-bonds formed between the non-bridging oxygen and water are very strong [
73]. However, the excessive water decreased the mechanical properties because it makes the hydrated phases more brittle [
73]. Continuing a parallelism with cement, an elevated water/Ca ratio causes a shorter silicate chain, and the molecules of water significantly reduce the stiffness of the hydrated gel due to their partial replacement of Ca-O connections. In addition, an excess of water absorbed causes larger interlayer distances, resulting in lower Columbian attraction [
73]. The combined effect of these processes significantly compromises the mechanical stability of the agglomerate, resulting in a decline in uniaxial compressive strength as water content increases [
71].
If the same amount of binder is used in all the mixtures, its effect on the mechanical properties of the RM and BFS agglomerates can be neglected. The stability of the RM agglomeration indeed was more sensitive to the mechanical reinforcement of the raw material, in particular to the amount of water absorbed by the aluminum by-product. In order to agglomerate red mud, the better condition is, hence, directly adding a sufficient amount of water to complete the phase hydration and guarantee a satisfying workability of the mixture. As already demonstrated in previous work [
48], the amount of water is fundamental to obtaining a good agglomeration, which results in the highest density and higher compression strength. The preparation conditions, the drop test results and UCSs calculated defined that the mixture with better mechanical properties was 0.840-2W.
3.2.2. Mechanical and Metallurgical Characterization of 0.840-2W Briquettes
The experimental campaign discussed before allowed the identification of the best mechanical properties for 0.840-2W briquettes. They were hence further characterized mechanically and metallurgically to provide more information about their suitability in industrial applications. In particular, metallurgical characterization determined the degree of reduction according to the temperature of the thermal treatment, while mechanical characterization provided information on the briquettes’ resistance. The optimization of both behaviors defines the best temperature to achieve a satisfactory iron oxide reduction and coalescence and obtain a relatively brittle agglomerate to favor the following magnetic separation.
The mechanical characterization of the pre-reduced briquettes is reported in
Table 6.
To check the eventual effect of the drop test on the compressed agglomerates discussed in the previous section, the compression test was directly performed on cured briquettes, resulting in 17.925 MPa. The UCS was even lower than the value collected in the previous section, suggesting that the drop test did not influence the cold compression strength, but, on the contrary, the result was strongly affected by the heterogeneity of the samples. However, the variation lower than 10% was considered as an acceptable experimental error.
Increasing the tumbler’s rotation rate caused the AI to increase and the TI to slightly decrease (147% and −1% from 300 to 900 rotations, respectively). However, the AI and TI at 100 and 300 rotations were similar. The strongest variation occurred at 900 rotations due to the disintegration of the briquettes. Nevertheless, the AI and TI, as well as the release of particles smaller than 500 µm, were lower than 4 wt.%, guaranteeing the briquettes’ ability to withstand a high number of rotations, indicating high abrasion resistance. If they were chemically compatible, the briquettes would be suitable for use in Midrex processes, showing tumbler resistance of more than 85%, releasing less than 6% by weight of fines, and having a UCS of more than 15 MPa [
74]. Considering the survival up to 7 drops and UCS higher than 0.2 MPa, the briquettes also largely exceed the requirements for the use of pellets in rotary heart furnaces [
75].
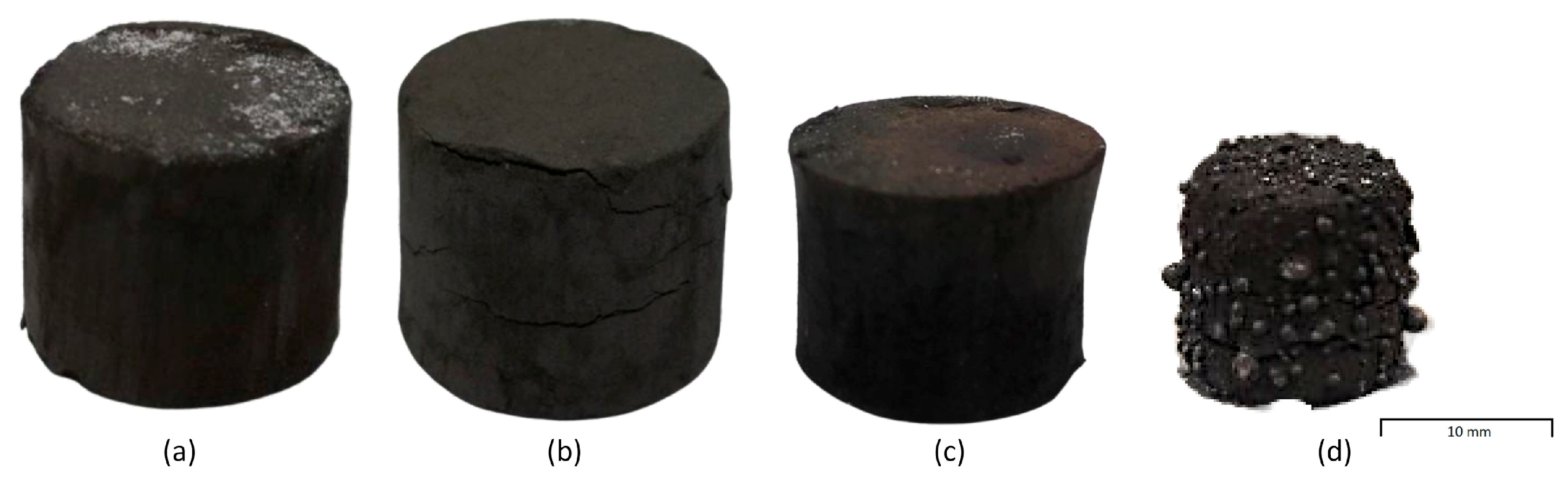
The briquettes were then thermally treated at 700, 950, 1200 and 1450 °C. Looking at the appearance of the briquettes during the thermal treatments reported in
Figure 6, no volume variation was shown until 950 °C. Further increasing the temperature, the negative swelling of −35.606 and −50.231% (1200 and 1450 °C, respectively) characterized the briquettes.
The degree of reduction of the briquettes at each temperature is reported in
Table 7.
As predicted by the reducibility investigation in the previous section, the reduction at 700 °C was limited (~20%) because of only the incipient stage of the iron oxide reduction, but when increasing the temperature, the carbothermic reaction proceeded, leading to RD of ~94%.
A better reduction behavior (96%) was only obtained by reducing red mud with 200% excess petroleum coke at 1050 °C [
76]. Not only could the different reducing agent affect the reduction capacity, with the coke being very reactive [
77], but it is important to notice that the latter was performed for a longer time (2 h) instead of the 15 min of this work, increasing, hence, the energy demand of the process. A total of 98% of iron recovery was instead obtained by mixing RM with graphite and heating the mixture at 1750 °C [
78]. This evidence suggested that RM probably requires a longer time and/or higher temperature to achieve the complete reduction of oxides. However, the chemical composition of the red mud also affects the reduction capability of the mixture, since a higher content of iron oxide allowed RM to have a metallization degree of 88% when reduced by semi-coke at 1100–1200 °C for 12–20 min [
79].
Following the thermal treatments, the briquettes were subjected to mechanical characterization to determine the force required to disintegrate them, with the aim of facilitating magnetic separation. RDI and UCS on the reduced briquettes at each temperature are summarized in
Table 8.
The compressive strength after thermal treatments depends only on the behavior of the briquettes during the thermal treatment, and it is not correlated with the green strength [
80]. Indeed, despite the fact that at 700 °C no volume variation or significant chemical reactions happened (as indicated by the low RD reported in
Table 7), the USC was reduced by about 75% compared to the cured briquette. This is due to the decomposition of the hydrated components at temperatures higher than 600 °C [
81]. Both the abrasion and compressive resistances decreased drastically at 950 °C, indicating that this was the most critical temperature for briquette resistance. This latter is indeed the typical temperature associated with significant swelling [
82] due to the popping of the particles during the transformation from the hexagonal structure of hematite to the isometric system of magnetite [
83], which causes crack development and detachment of grains [
80,
82]. Although no significant volume variation was identified in treated 0.840-2W briquettes at 950 °C, the beginning of the carbothermic reaction causes large pores and micro-fracture, which decrease the mechanical strength [
84]. The mechanical resistance then increased at 1200 °C due to the metallic iron formed, which strengthened the agglomerate [
80], but slightly decreased when the temperature of the thermal treatment further increased to 1400 °C.