Abstract
In this study, the solidification behavior of 310S stainless steel was systematically investigated by combining high-temperature confocal laser scanning microscopy (HT-CLSM), microstructural characterization, and thermodynamic calculations. The focus was on the formation and transformation of ferrite, secondary-phase precipitation, and elemental segregation behavior, with comparisons made with 304 stainless steel. The effects of an Al addition and cooling rate were also explored. The results show that the solidification sequence of 310S stainless steel is L → L + γ → L + γ + δ → δ + γ, in which austenite nucleates early and grows rapidly, followed by the precipitation of a small amount of δ-ferrite in the later stages of solidification. In contrast, 304 stainless steel solidifies according to L → L + δ → L + δ + γ → δ + γ, with a rapid δ → γ transformation occurring after solidification. Compared with 304, 310S stainless steel exhibits a reduced ferrite fraction and a significantly increased σ phase content. The σ phase primarily precipitates directly from δ-ferrite (δ → σ), while M23C6 preferentially forms at grain boundaries and δ/γ interfaces, where δ-ferrite not only provides fast diffusion pathways for Cr but also nucleation sites. The solidification segregation sequence in 310S stainless steel is Cr > Ni > Fe, with Cr and Ni showing positive segregation and Fe showing negative segregation. The addition of Al does not alter the solidification mode of 310S stainless steel but refines austenite grains, reduces interdendritic solute enrichment, decreases segregation, lowers both the size and fraction of ferrite, and suppresses the precipitation of σ and M23C6 phases. This effect is mainly attributed to the reduction of δ/γ interfaces, which weakens the preferred nucleation sites for M23C6. Increasing the cooling rate enhances non-equilibrium solute segregation, promotes ferrite formation, inhibits the δ → γ transformation, and ultimately retains more ferrite; the intensified segregation further accelerates the δ → σ transformation.
1. Introduction
Compared with conventional austenitic stainless steels, 310S stainless steel exhibits superior corrosion resistance, particularly in oxidizing environments. Its typical applications include high-temperature furnace components, pharmaceutical systems, nuclear power pipelines, and high-temperature corrosion-resistant parts in petrochemical equipment [1]. The Cr and Ni contents of 310S stainless steel are significantly higher than those of commonly used austenitic steels such as 304 and 316, making it more prone to dendritic segregation during solidification or welding [2]. Such segregation can not only reduce corrosion resistance but also increase susceptibility to hot cracking and thus must be controlled through process optimization or alloy composition design [3,4].
During the solidification of 310S stainless steel, local compositional enrichment can easily lead to the formation of δ-ferrite. Under normal circumstances, δ-ferrite can adversely affect high-temperature processing performance and corrosion resistance; therefore, it is often eliminated through subsequent heat treatment in the production of high-quality austenitic stainless steels [5,6]. The distribution of ferrite plays a critical role in controlling both surface and internal quality, as well as the hot working performance of the slabs [7]. Moreover, δ-ferrite can promote the precipitation of M23C6 carbides and the σ phase during subsequent cooling or service, thereby reducing the microstructural stability of the material [8]. For austenitic stainless steels that solidify through multiple mechanisms, the solidification sequence and subsequent phase transformation characteristics directly determine the degree of segregation and the distribution of residual ferrite, ultimately affecting the surface and internal quality of the final product [9].
The solidification microstructure of stainless steel is closely related to solidification conditions, among which the cooling rate is one of the key factors influencing the solidification mode, microstructure, segregation, and secondary-phase precipitation [10,11]. Fu, J.W et al. [12] investigated the effect of cooling rate on the microstructural evolution of AISI 304 austenitic stainless steel, and the results showed that at relatively low cooling rates, dendritic δ-ferrite is predominantly formed. As the cooling rate increases, the solidification mode gradually shifts from primary ferrite to primary austenite. Hao, Y et al. [13] studied the effects of different cooling rates on the solidification and segregation behavior of super-austenitic stainless steels. The results indicate that with increasing cooling rate, dendrite arm spacing decreases and segregation is mitigated, thereby suppressing eutectic formation in the interdendritic regions.
Alloying elements can influence the type, amount, size, and distribution of precipitates in stainless steels. By optimizing the alloy composition, the performance of stainless steel can be effectively improved. Among these elements, Al has received particular attention [14]. The addition of Al to 310 stainless steel can promote the formation of a stable passivation film, thereby enhancing high-temperature oxidation resistance and creep performance [15]. Studies have shown that in FeCrAl alloys, adding just 1 wt.% Al is sufficient to form an Al2O3 oxide layer at 650 °C [16]. Compared with commercial 310S stainless steel, Al-containing 310S exhibits superior oxidation resistance at 800 °C and can still form a dense passivation film after 120 h of corrosion, demonstrating enhanced corrosion resistance [1].
Previous studies have primarily focused on the high-temperature oxidation resistance and mechanical properties of 310S stainless steel, whereas its solidification and segregation behavior remain less explored [17,18,19]. Most prior investigations lacked direct observation methods to reveal the microstructural evolution during high-temperature processes. To address this, the present study combines high-temperature confocal laser scanning microscopy (HT-CLSM) with scanning electron microscopy (SEM), electron backscatter diffraction (EBSD), and energy-dispersive spectroscopy (EDS) to systematically investigate the microstructural evolution during the solidification of 310S austenitic stainless steel. This study focuses on the formation and transformation of ferrite, secondary-phase precipitation, and segregation behavior. Additionally, the effects of Al addition and cooling rate on the solidification microstructure of 310S stainless steel are examined, with comparisons made with 304 stainless steel. Understanding these transformation processes at the microscopic level provides insights for optimizing alloy composition and developing solidification strategies to improve material performance.
2. Experiment Procedure
The raw materials used in this study were hot-rolled plates, and their chemical compositions are listed in Table 1. The elemental contents were determined using inductively coupled plasma optical emission spectrometry (ICP-OES 730, Agilent Technologies Inc., Santa Clara, CA, United States). Specimens were cut from the hot-rolled plates and subjected to grinding with sandpaper, followed by mechanical polishing. The solidification and solid-state phase transformation processes of the experimental steels were observed in situ using a high-temperature confocal laser scanning microscope (VL2000DX HT-CLSM, Yonekura Manufacturing Co., Ltd., Yokohama, Kanagawa, Japan). Samples were first heated to 1520 °C and held for 5 min. For the three experimental steels, the cooling process was carried out in two stages: first, cooling at a rate of 15 °C/min to 1100 °C, followed by rapid cooling to room temperature. The resulting samples were designated as 304, 310S, and 310Al, respectively. The cooling rate was selected based on the results of preliminary continuous casting thermal simulation studies. This rate is close to that of the central region of the sample during simulated continuous casting. Since elemental segregation is more severe in the central region, this cooling rate was adopted as the simulation target to more accurately reflect the actual solidification microstructure and composition distribution. In addition, to investigate the effect of cooling rate on the solidification microstructure and secondary phases of 310Al, another sample was cooled at 30 °C/min to 1100 °C and labeled 310Al-H.

Table 1.
Chemical composition wt.%.
After the HT-CLSM experiments, the microstructure of the samples was analyzed using a ZEISS Gemini 500 (Carl Zeiss AG, Oberkochen, Germany) field emission scanning electron microscope (FE-SEM), and the elemental distribution of the microstructure was characterized with an attached ULTIM MAX 40 (Oxford Instruments NanoAnalysis, High Wycombe, UK) energy-dispersive spectrometer. The bottom surfaces of the HT-CLSM samples were subjected to electrolytic etching and observed under a LEICA DM2700M (Leica Microsystems GmbH, Wetzlar, Germany) optical microscope to examine the microstructure. The electrolytic etching was performed in a 10% oxalic acid solution at 2 V for 30 s. Ferrite content was quantified via image analysis using ImageJ (v1.8.0) software. Microstructural morphology was further characterized using FE-SEM, and electron backscatter diffraction analysis was conducted with an Oxford Symmetry S2 system (Oxford Instruments NanoAnalysis, High Wycombe, UK). After mechanical polishing, the samples were electrolytically polished in a solution of 10 vol.% perchloric acid and 90 vol.% anhydrous ethanol. Additionally, thermodynamic calculations of the experimental steels were performed using Thermo-Calc 2022b software (TCFE10 database, Thermo-Calc Software AB, Solna, Sweden) to aid in understanding their phase transformation behavior.
3. Results and Discussions
3.1. Solidification Mode
The microstructure formed during the solidification of austenitic stainless steel is primarily determined by the compositional balance between austenite-stabilizing and ferrite-stabilizing elements, with Ni and Cr contents being particularly critical [20]. These elements not only dictate the primary phase during solidification but also influence the final austenite-to-ferrite ratio in the microstructure. For quantitative description, researchers often use the concepts of equivalent nickel (Nieq) and equivalent chromium (Creq) to represent the combined effects of alloying elements and to predict different solidification modes, such as A, AF, FA, or F [21,22]. Using the classical Creq/Nieq formulas, the Nieq and Creq values of the experimental steels were calculated, as shown in Table 2. The 304 stainless steel used in this study has a Creq/Nieq value of 1.78, corresponding to the FA solidification mode (occurring when 1.48 < Creq/Nieq < 1.95). At the onset of solidification, the δ-ferrite forms first, while the remaining liquid, enriched in γ-stabilizing elements, transforms into γ. The solidification sequence can thus be described as liquid → (liquid + δ) → (liquid + δ + γ) → (δ + γ).

Table 2.
Equivalent nickel (Nieq) and equivalent chromium (Creq) of the experimental steels.
For 310S stainless steel, the Creq/Nieq value is 1.22, corresponding to the A-type solidification mode, in which austenite precipitates first from the liquid. The phase transformation sequence is liquid → (liquid + γ) → γ. In contrast, 310Al stainless steel has a Creq/Nieq value of 1.45, falling within the 1.25–1.48 range and exhibiting the AF-type solidification mode. In this mode, solidification begins with the formation of γ phase; as γ precipitates, the remaining liquid becomes Ni-depleted and Cr-enriched, promoting the precipitation of δ-ferrite in the interdendritic regions during the final stages of solidification. The typical phase transformation sequence is liquid → (liquid + γ) → (liquid + γ + δ) → (γ + δ). Under as-cast conditions, the microstructure consists of γ phase with a small amount of δ-ferrite, although the stability of δ-ferrite is influenced by cooling rate and subsequent heat treatments.
Nieq = Ni% + 30C% + 0.5Mn% + 0.25Cu%
Creq = Cr% + 1.5Mo% + 1.5Si% + 5.5Al%
3.2. Calculation of Non-Equilibrium Solidification
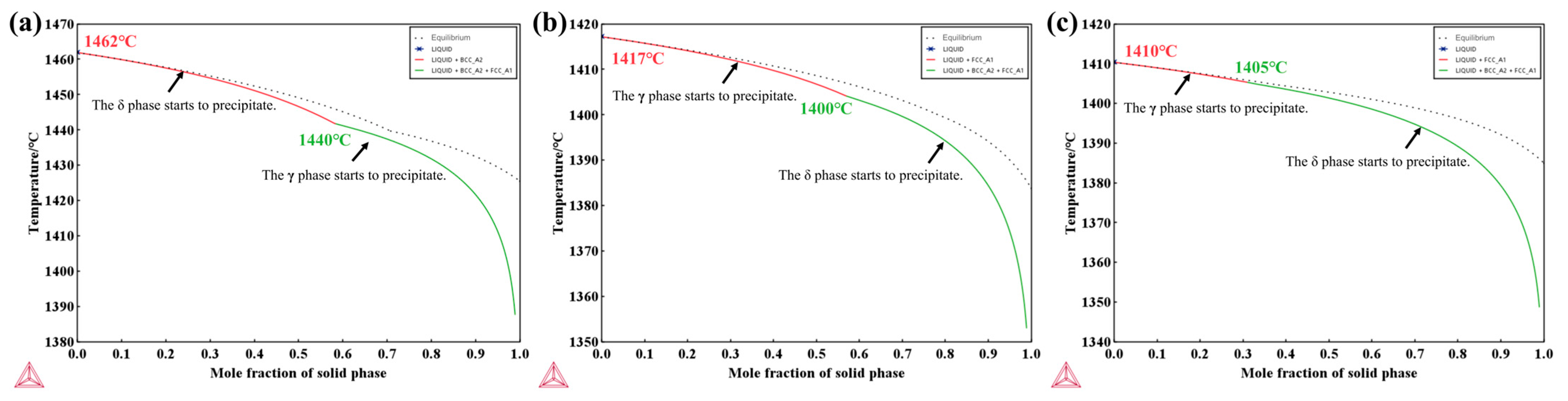
The solidification paths of the experimental steels were calculated using the Scheil–Gulliver module, as shown in Figure 1, with the initial precipitation temperatures of each phase indicated. The results show that the solidification sequence of 304 stainless steel is L → L + δ → L + γ + δ, with δ-ferrite forming preferentially at approximately 1462 °C, followed by the formation of austenite through the peritectic reaction (L + δ → γ). In contrast, the solidification sequence of 310S and 310Al stainless steels is L → L + γ → L + γ + δ, corresponding to the AF solidification mode, in which γ is the primary phase and δ precipitates during the final stage of solidification. The calculations also indicate that with increasing Al content, the precipitation temperature of δ-ferrite rises, suggesting that Al enhances the stability of δ-ferrite. Meanwhile, the liquidus temperature in this system decreases, as Al reduces the high-temperature stability of the γ phase, thereby lowering the equilibrium temperature between the liquid and γ phase.

Figure 1.
Scheil–Gulliver solidification paths: (a) 304; (b) 310S; (c) 310Al (calculated using Thermo-Calc software).
3.3. HT-CLSM In Situ Observation
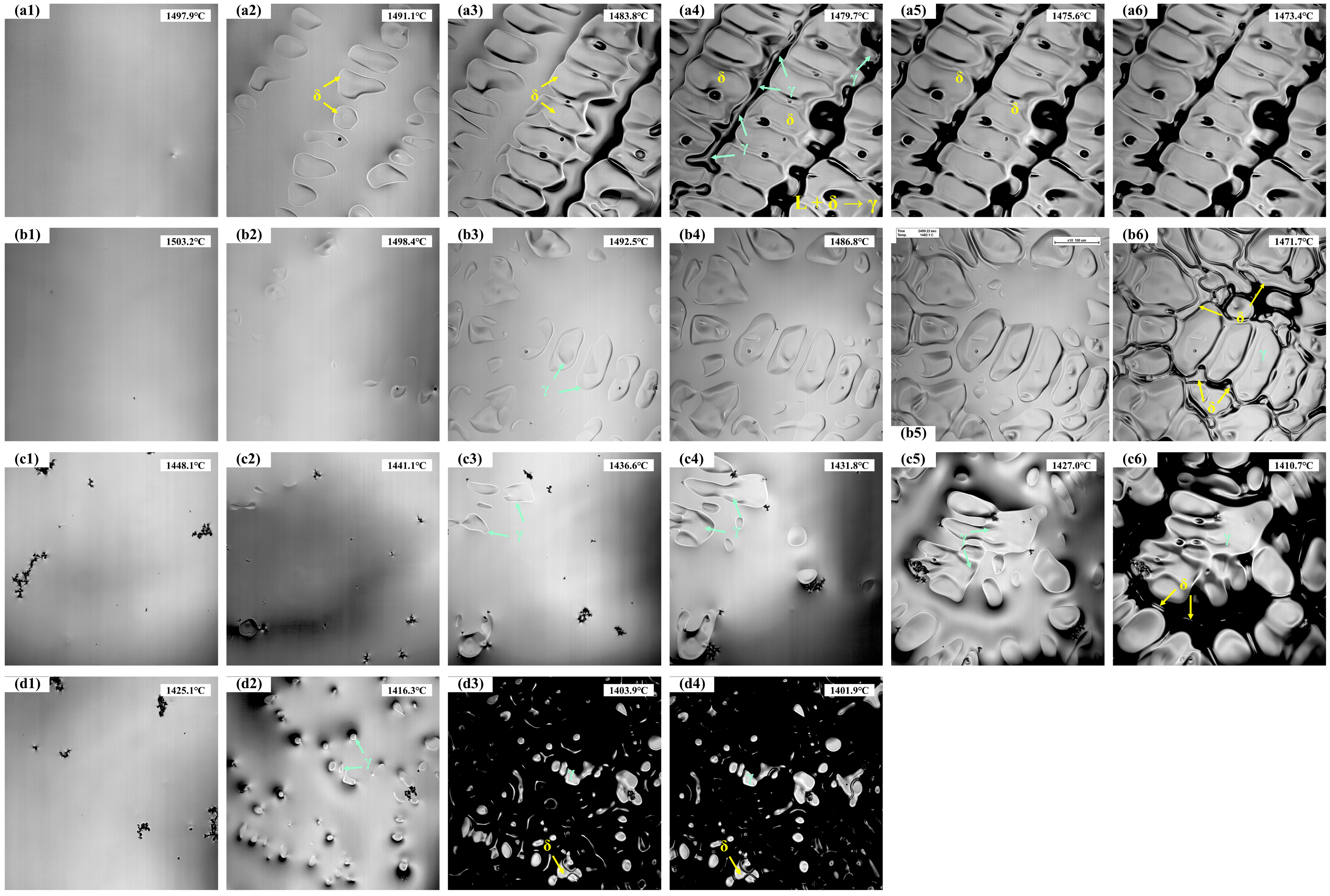
The in situ solidification of the experimental steels is shown in Figure 2, with the corresponding temperatures recorded in real time. The analysis results are summarized in Table 3, where Tn represents the initial nucleation temperature, Ts is the temperature of complete solidification, ΔT is the solidification temperature range, and t is the total solidification time. For 304 stainless steel, nucleation of solidification begins at 1497.9 °C (Figure 2(a1)). During the initial stage, δ-ferrite nucleates first from the liquid and grows rapidly, with the number of nuclei gradually increasing (Figure 2(a2)). As the temperature decreases further (Figure 2(a3)), the solid fraction increases, and the δ-ferrite develops into dendrites that grow into the liquid. Subsequently, as shown in Figure 2(a4–a6), a peritectic reaction occurs between the liquid and δ-ferrite (L + δ → γ), forming austenite at the δ/liquid interface, with the number of austenite grains gradually increasing and the remaining liquid being progressively consumed. Finally, 304 stainless steel is fully solidified at 1473.4 °C, with a solidification temperature range of approximately 24.5 °C and a total solidification time of 89.9 s.
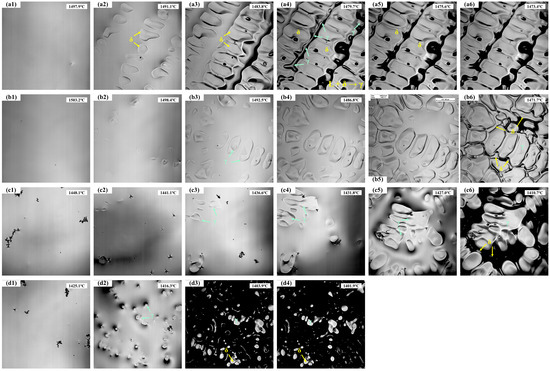
Figure 2.
The solidification process of the experimental steels: (a1–a6) 304; (b1–b6) 310S; (c1–c6) 310Al; (d1–d4) 310Al-H.

Table 3.
Analysis results of the solidification process.
As shown in Figure 2(b1–b6), 310S stainless steel exhibits distinct solidification behaviors at different stages. When the temperature decreases to 1503.2 °C, austenite nuclei first appear, followed by the rapid growth of austenite dendrites. As the solid fraction increases, the austenite continues to grow into the remaining liquid. In the later stages of solidification, Cr enrichment in the residual liquid (a δ-ferrite stabilizing element) leads to the precipitation of δ-ferrite in the interdendritic regions. δ-ferrite nucleates in the residual liquid and grows along the austenite grain boundaries, while the remaining liquid is gradually consumed until complete solidification. The 310S sample fully solidifies at 1471.7 °C, with a solidification temperature range of 31.5 °C and a total solidification time of 115.5 s.
As shown in Figure 2(c1–c6), the 310Al sample begins initial solidification at 1448.1 °C (Figure 2(c1)), which is significantly lower than that of the 310S sample, indicating that the increased Al content delays the nucleation of austenite. Similar to the 310S sample, austenite dendrites form first during solidification, and in the final stages, δ-ferrite precipitates in the interdendritic regions due to the enrichment of δ-stabilizing elements in the residual liquid. The 310Al sample fully solidifies at 1410.7 °C (Figure 2(c6)), with a solidification temperature range of 37.4 °C and a total solidification time of 133.1 s. This demonstrates that the addition of Al broadens the solidification temperature range and prolongs the solidification process. Al, as a typical ferrite-forming element, significantly enhances the stability of δ-ferrite. With increasing Al content, the γ-phase region shrinks and the initial nucleation temperature of γ decreases, leading to a lower solidification onset temperature.
As shown in Figure 2(d1–d4), when the cooling rate is increased from 15 °C/min to 30 °C/min, the initial nucleation temperature of 310Al-H decreases to 1425.1 °C. Austenite dendrites still form, and δ-ferrite precipitates in the interdendritic regions during the final stage; however, the solidification temperature range is reduced, and the total solidification time is shortened.
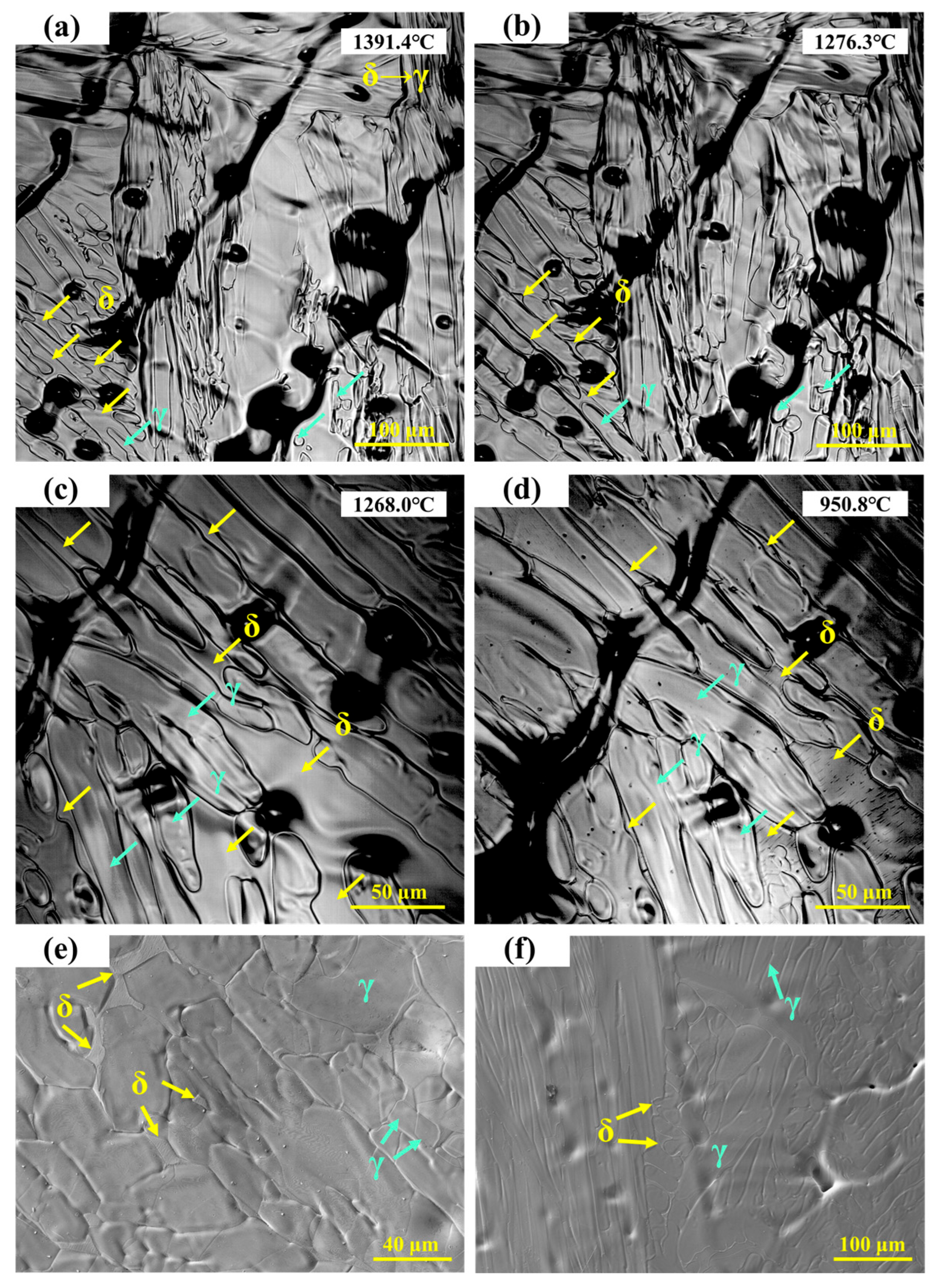
The in situ observation of the solid-state phase transformation in 304 stainless steel is shown in Figure 3a–d, where green arrows indicate γ-austenite and yellow arrows indicate δ-ferrite. Grain boundary migration can be clearly observed, with fine lines representing traces left during the migration process. The emergence of new grain boundaries can be regarded as a precursor to the δ → γ solid-state transformation [23]. During this process, grain boundary migration occurs simultaneously with the δ → γ phase transformation, which proceeds at a very high rate and forms the characteristic feather-like morphology. Because γ-austenite has a relatively high shrinkage coefficient, the phase transformation is accompanied by volumetric contraction.
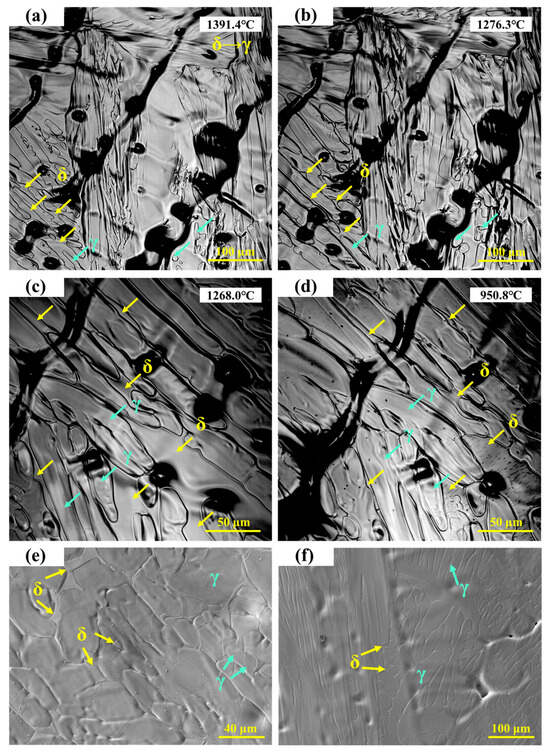
Figure 3.
(a–d) In situ observation of solid-state transformation in 304 stainless steel; (e,f) SEM microstructure of 304 stainless steel.
S.H. Kim et al. [24] reported that during the initial stage of δ-ferrite dissolution, over 50% of δ-ferrite transforms rapidly, particularly at high temperatures where interface migration is fast. This rapid dissolution is mainly attributed to the excessive consumption of Cr within the δ-ferrite. The dissolution kinetics of δ-ferrite are controlled by the diffusion of Cr and Ni in the austenite phase [25]. As the temperature decreases, the δ-ferrite gradually transforms into γ, with the phase boundary continuing to migrate toward the δ-ferrite. As shown in Figure 3c,d, the ferrite fraction further decreases. When the temperature drops to 950.8 °C, parallel lath-like structures are observed to precipitate within the ferrite.
The microstructure of 304 stainless steel observed under high-temperature in situ confocal microscopy is shown in Figure 3e. Irregular δ-ferrite is distributed in the interdendritic regions, slightly recessed from the surface, corresponding to the ferrite structures observed in Figure 3d. In the final room-temperature microstructure, both the austenite matrix and a small amount of residual δ-ferrite are retained. As shown in Figure 3f, some δ-ferrite remains as lath-like structures in the interdendritic regions, while other δ-ferrite completely transforms into austenite during cooling, leaving fine depressions in the interdendritic areas after transformation.
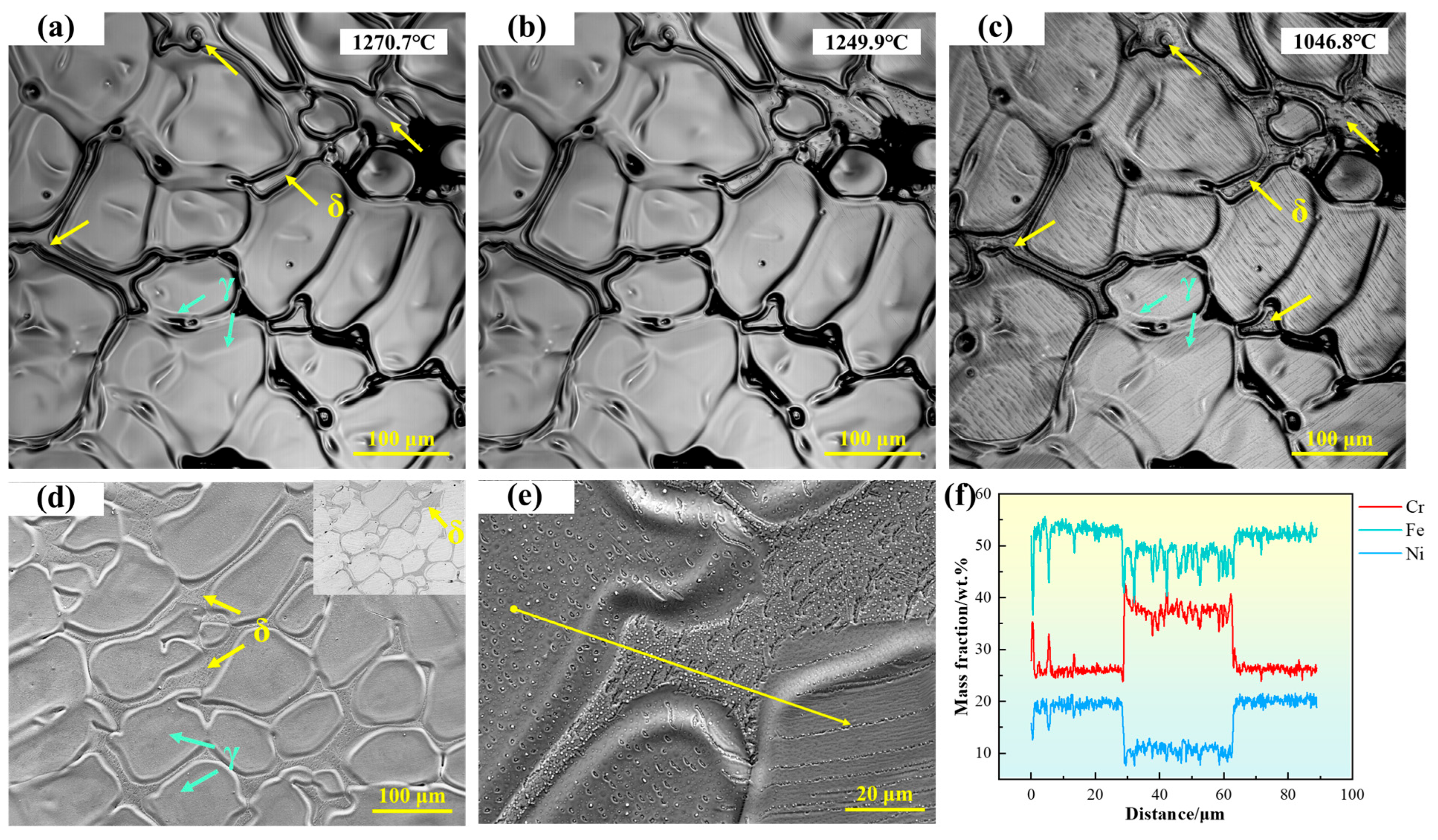
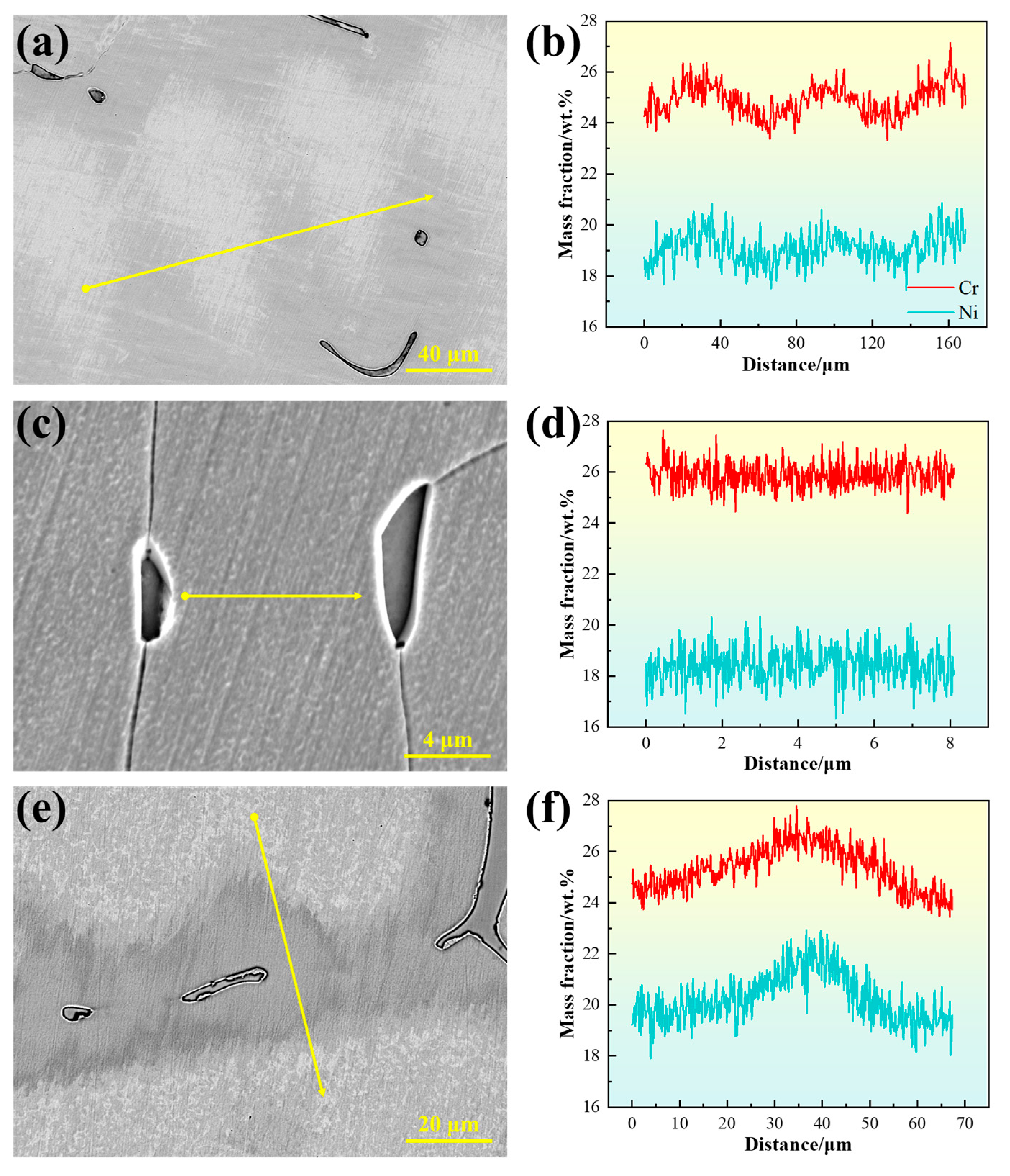
As shown in Figure 4a, the microstructure of 310S stainless steel is primarily composed of austenite, with irregular δ-ferrite distributed in the interdendritic regions. As the temperature decreases, the size of δ-ferrite does not change significantly, but black particles gradually appear on its surface. The high-temperature in situ confocal microscopy micrograph is shown in Figure 4d, with the upper right corner displaying the corresponding backscattered electron (BSE) image. The morphology of the austenite dendrites can be clearly observed, with δ-ferrite located in the recesses between dendrites. In the BSE image, δ-ferrite exhibits a darker contrast. To further verify the composition of δ-ferrite, EDS line scans were performed, as shown in Figure 4e,f. The results indicate that δ-ferrite is rich in Cr, whereas the austenite is relatively enriched in Ni and Fe.
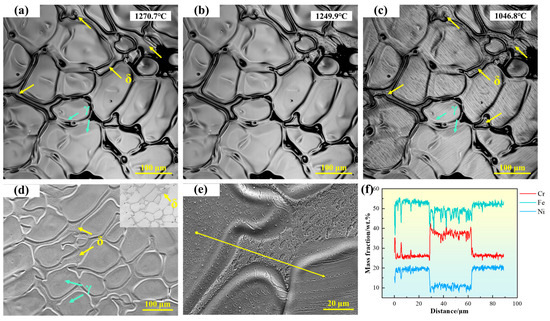
Figure 4.
(a–c) In situ observation of solid-state transformation in 310S stainless steel; (d) SEM microstructure of 310S stainless steel; (e,f) EDS line scan analysis of 310S stainless steel. (The yellow arrow in the figure indicates the position of the EDS line scan analysis).
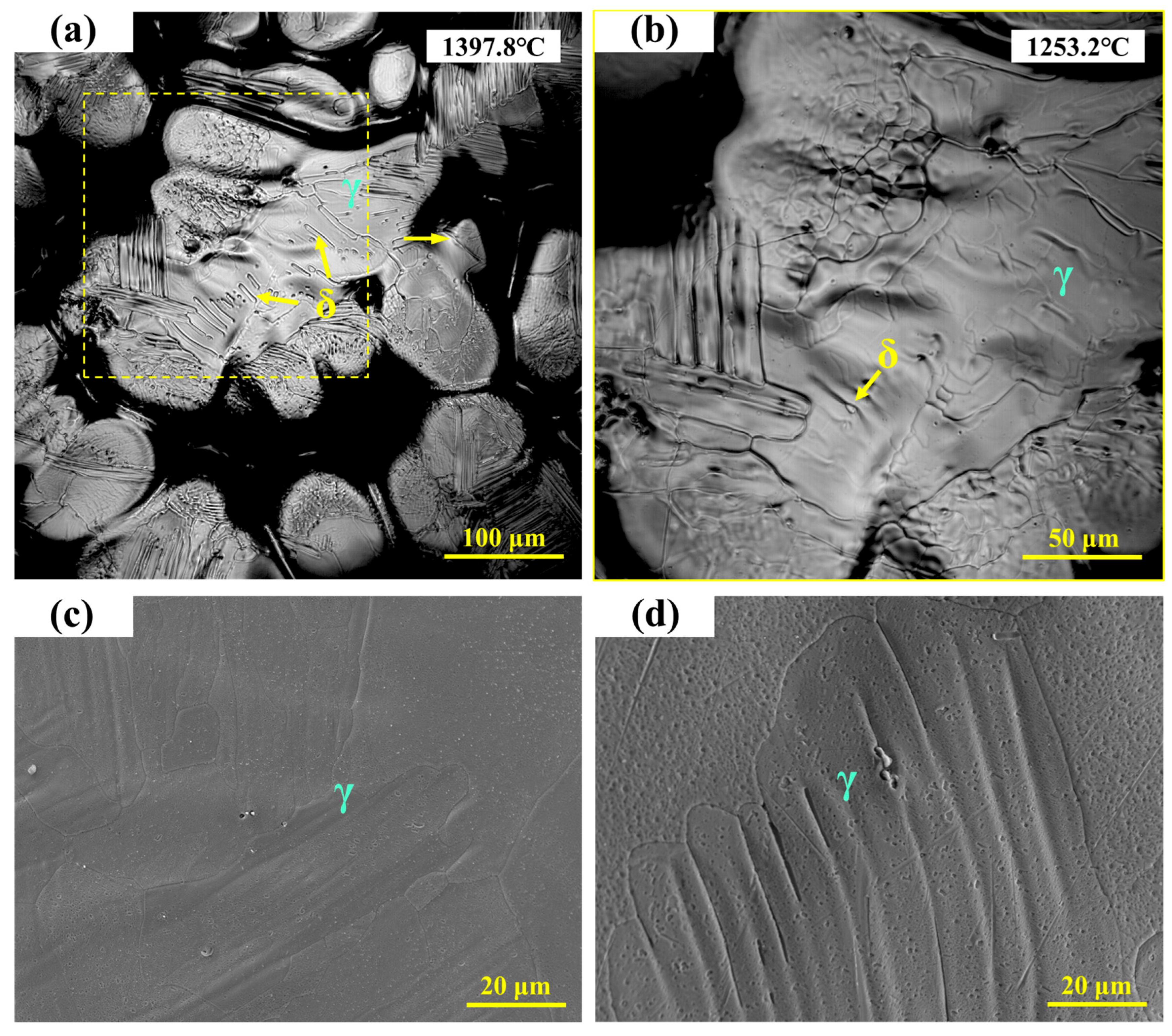
As shown in Figure 5a, the microstructure of 310Al stainless steel consists of austenite and a small amount of granular ferrite precipitated on the matrix, with ferrite particles significantly smaller than those in 310S stainless steel. Figure 5b presents a locally enlarged view, showing that as the temperature decreases from 1397.8 °C to 1253.2 °C, the fine ferrite particles gradually transform into austenite, leaving only a very small amount of residual ferrite. The high-temperature in situ confocal microscopy micrographs of 310Al stainless steel are shown in Figure 5c,d, revealing a typical austenite dendritic microstructure. In the imaged area, δ-ferrite almost completely transforms into austenite during cooling, leaving fine depressions in the interdendritic regions after transformation.
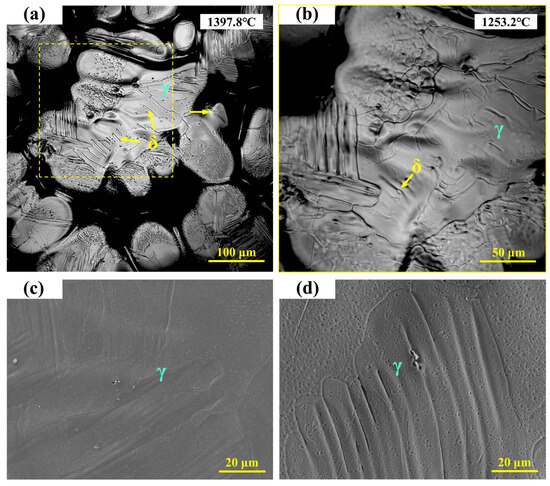
Figure 5.
(a,b) In situ observation of solid-state transformation in 310Al stainless steel; (c,d) SEM microstructure of 310Al stainless steel.
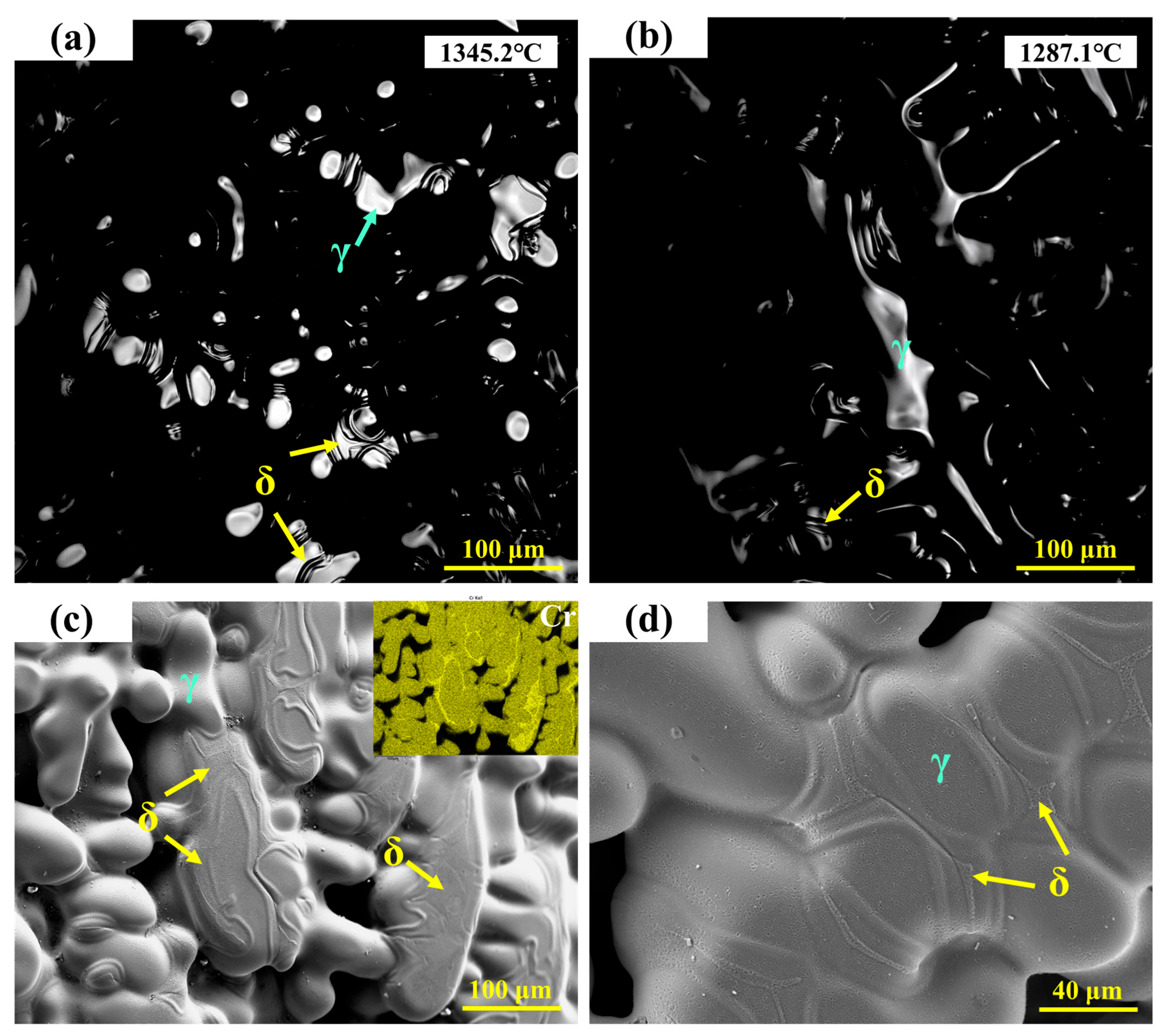
Figure 6a,b show the in situ observations of the solid-state phase transformation in 310Al-H stainless steel. It can be seen that the ferrite formed at the final stage of solidification gradually transforms into austenite during cooling. The high-temperature in situ confocal microscopy micrographs of the 310Al-H sample are shown in Figure 6c,d, revealing a microstructure primarily composed of austenite dendrites, with a small amount of residual ferrite still visible in the interdendritic regions. The EDS area scan shown in the upper right corner of Figure 6c further confirms that these ferrite regions are enriched in Cr.
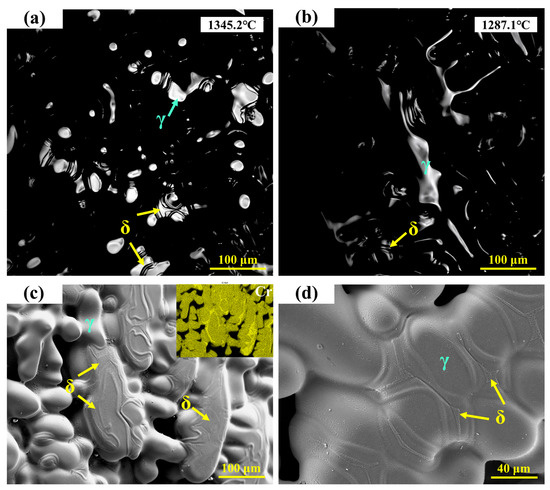
Figure 6.
(a,b) In situ observation of solid-state transformation in 310Al-H stainless steel; (c,d) SEM microstructure of 310Al-H stainless steel.
3.4. Microstructure
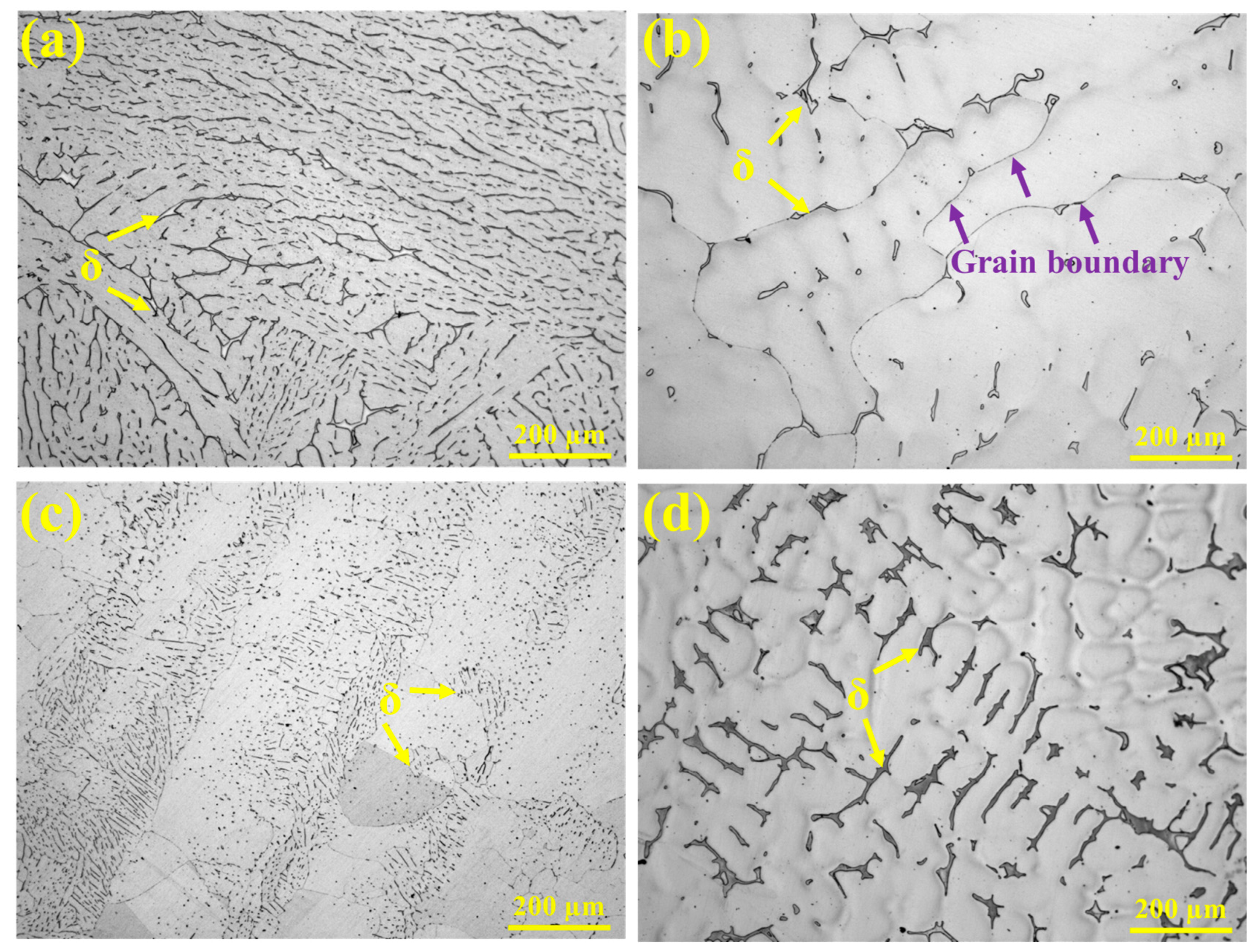
The HT-CLSM experiments revealed differences in the microstructure and alloy element distribution among the experimental steels. The following analysis combines OM, SEM, and EBSD results to highlight the microstructural characteristics. The metallographic microstructures of the experimental steels observed under high-temperature in situ confocal microscopy are shown in Figure 7. All samples exhibit an austenitic matrix, but the morphology of ferrite differs significantly. In 304 stainless steel, ferrite is relatively abundant, forming a discontinuous network structure with some degree of parallel alignment. In 310S stainless steel, ferrite is mainly concentrated in the interdendritic regions and at grain boundaries, where black, discontinuous secondary phases are visible along the boundaries (indicated by purple arrows). In 310Al stainless steel, ferrite appears as fine, granular particles dispersed throughout the austenite matrix, indicating that the increased Al content reduces ferrite size. The 310Al-H sample exhibits a pronounced dendritic structure, with ferrite irregularly distributed in the interdendritic regions of the austenite.
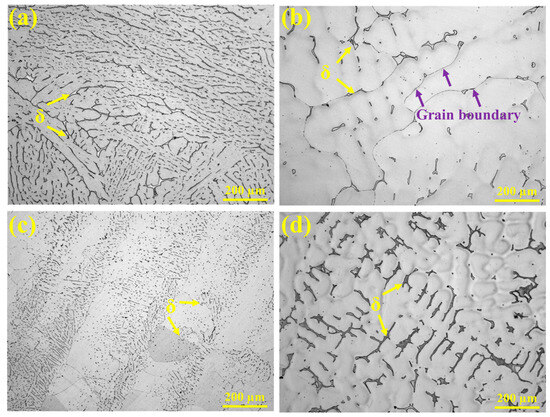
Figure 7.
OM morphologies: (a) 304, (b) 310S, (c) 310Al, and (d) 310Al-H.
The SEM microstructures of the samples observed after high-temperature in situ confocal microscopy are shown in Figure 8. In 304 stainless steel (Figure 8a), ferrite is distributed in a lath-like morphology, and no carbide precipitation is observed. In 310S stainless steel (Figure 8b), ferrite is irregularly distributed along the grain boundaries, with discontinuous M23C6 precipitates present at the boundaries. The higher Cr content promotes the formation of M23C6 in 310S stainless steel. In the 310Al sample (Figure 8c), the ferrite size is significantly reduced, appearing as either spherical or fine elongated particles. M23C6 is also present along grain boundaries, but both its size and quantity are lower than in 310S stainless steel. This is because the γ/δ interface is a favorable site for M23C6 precipitation, and as the δ-ferrite content decreases, the γ/δ interface is reduced, thereby limiting M23C6 formation. In the 310Al-H sample (Figure 8d), ferrite exhibits a parallel arrangement with significantly increased size, and M23C6 is mainly distributed along the γ/δ interfaces.
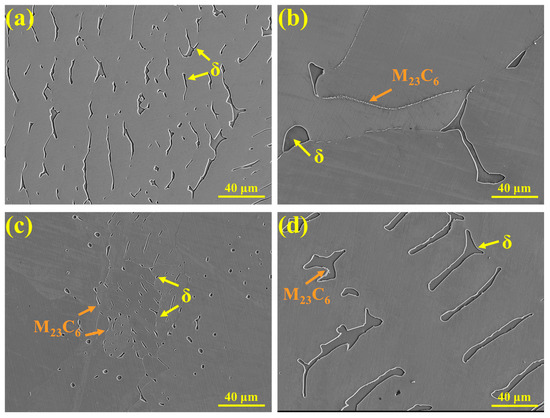
Figure 8.
SEM morphologies: (a) 304, (b) 310S, (c) 310Al, and (d) 310Al-H.
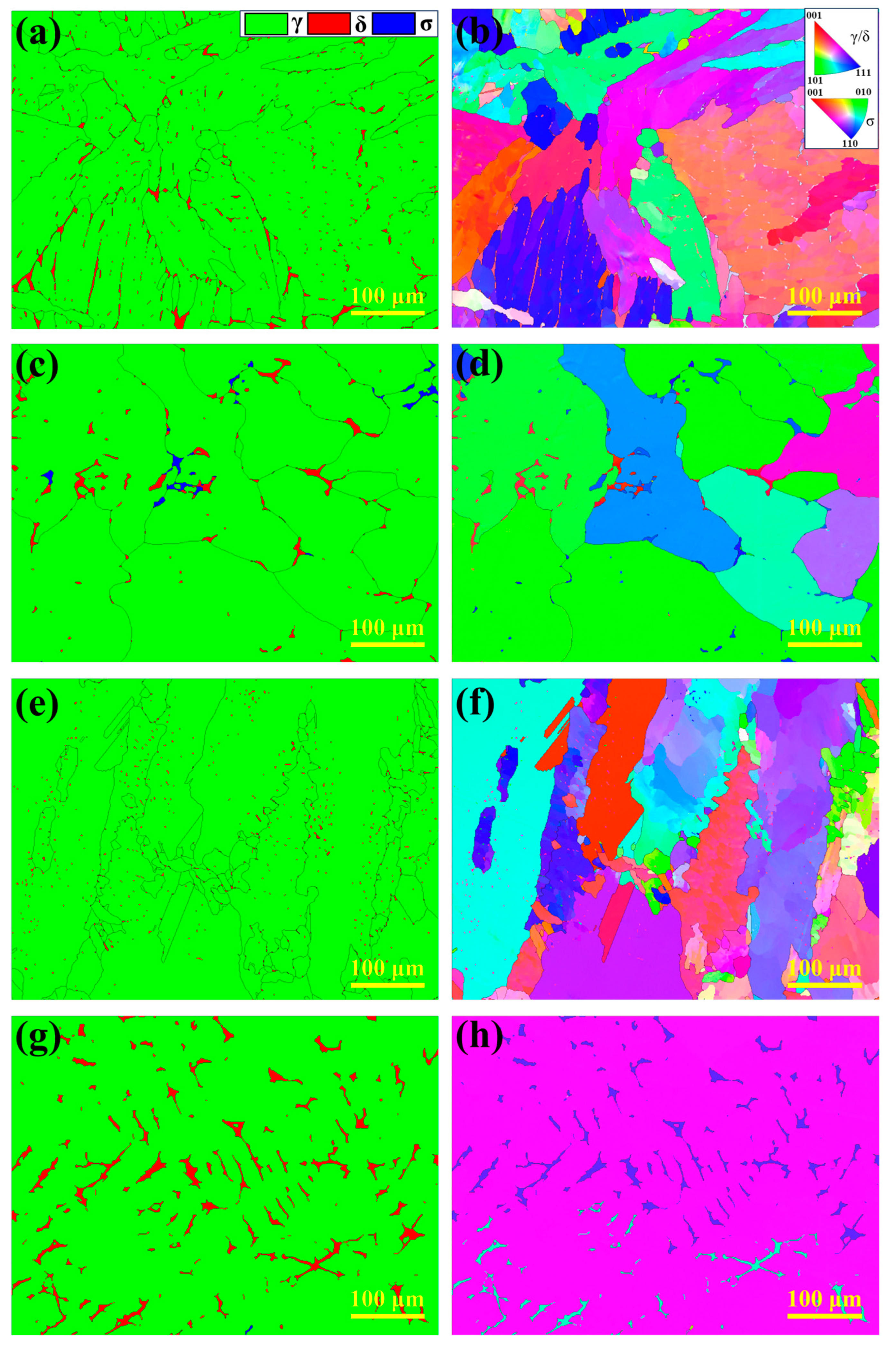
To further investigate the microstructural characteristics of the HT-CLSM samples, EBSD analysis was performed, as shown in Figure 9. Figure 9a,c,e,g display the phase distribution of the experimental steels (austenite in green, ferrite in red, and σ phase in blue), while Figure 9b,d,f,h show the corresponding inverse pole figure (IPF) maps. The results indicate significant differences in phase distribution among the experimental steels. In 304 stainless steel, ferrite content is the highest, primarily appearing as small blocky and short lath-like structures, consistent with its FA solidification mode. In 310S stainless steel, ferrite is mainly distributed along austenite grain boundaries and grows continuously along them, with the σ phase precipitating nearby. In contrast, ferrite in 310Al stainless steel is mainly short laths, with significantly reduced content, and the austenite grains are refined. No σ phase precipitation is observed, indicating that Al suppresses σ phase formation. In the 310Al-H sample, ferrite exhibits irregular blocky morphology and grows along the thermal gradient direction, forming a dendritic structure as shown in Figure 9g, with ferrite content significantly higher than in 310Al. Moreover, the high cooling rate promotes the preferential growth of columnar grains, reducing the total number of grains.
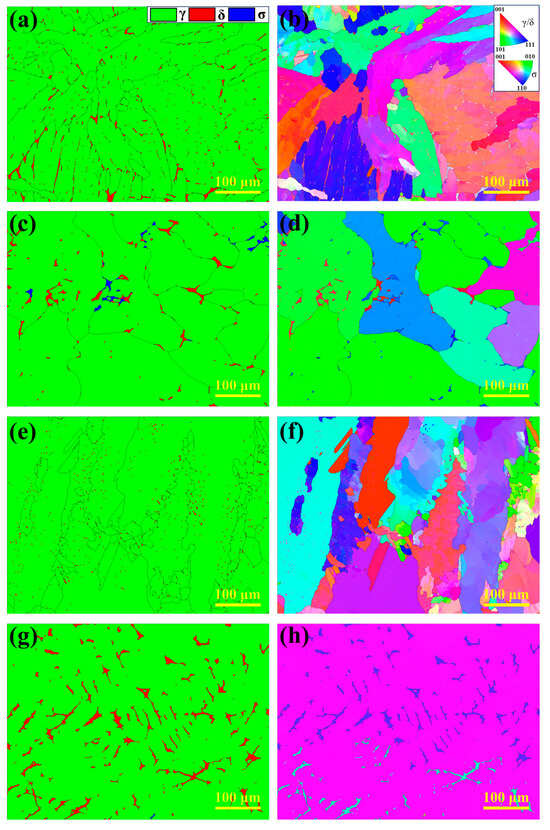
Figure 9.
EBSD analysis results: (a,b) 304; (c,d) 310S; (e,f) 310Al; (g,h) 310Al-H.
3.5. Microsegregation
The compositional inhomogeneity caused by dendritic segregation leads to microstructural differences, which can significantly affect material properties [26]. Figure 10 presents the line scan results of the experimental steels. In the 310S sample, Cr and Ni exhibit pronounced fluctuations, with relative enrichment in the interdendritic regions, indicating positive segregation. This occurs because the dendrite cores form first from the liquid during solidification, with compositions close to the initial alloy average, while elements with partition coefficients less than 1 gradually enrich in the residual liquid, eventually leading to segregation in the interdendritic regions. For steels solidifying in the austenite mode, segregation has a more pronounced effect on material properties because alloying elements that segregate to grain boundaries cannot be readily redistributed during subsequent solid-state transformations, thus being retained in the solidified microstructure.
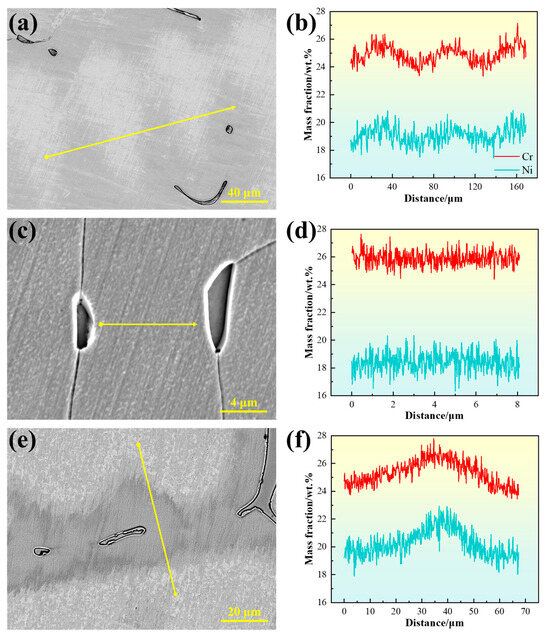
Figure 10.
Line scan results between dendrites: (a,b) for 310S sample; (c,d) for 310Al sample; (e,f) for 310Al-H sample. (The yellow arrow in the figure indicates the position of the EDS line scan analysis).
In contrast, for steels solidified in the F or FA mode, the primary ferrite can act as a channel for element redistribution during the subsequent δ → γ solid-state transformation, thereby alleviating dendritic segregation to some extent [9]. However, in the case of 310S stainless steel solidified in the AF mode, due to the enrichment of ferrite-stabilizing elements such as Cr and Mo in the residual liquid phase, a small amount of ferrite precipitates at the final stage of solidification, with its fraction being significantly lower than that of austenite. During the subsequent δ → γ transformation, the δ phase needs to absorb austenite-stabilizing elements such as Ni and C while rejecting part of the ferrite-stabilizing elements such as Cr and Mo. This back-diffusion can locally mitigate solidification segregation. Nevertheless, because of the relatively low diffusion rates of Cr and Mo in austenite and the limited fraction of δ phase, the complete transformation is kinetically constrained, and thus the overall effect of this process on relieving microsegregation is relatively limited.
With increasing Al content, the distribution of Cr and Ni in the 310Al sample becomes more uniform. Conversely, in the 310Al-H sample, Cr and Ni exhibit the most pronounced compositional fluctuations, indicating that at higher cooling rates, insufficient solute diffusion exacerbates segregation.
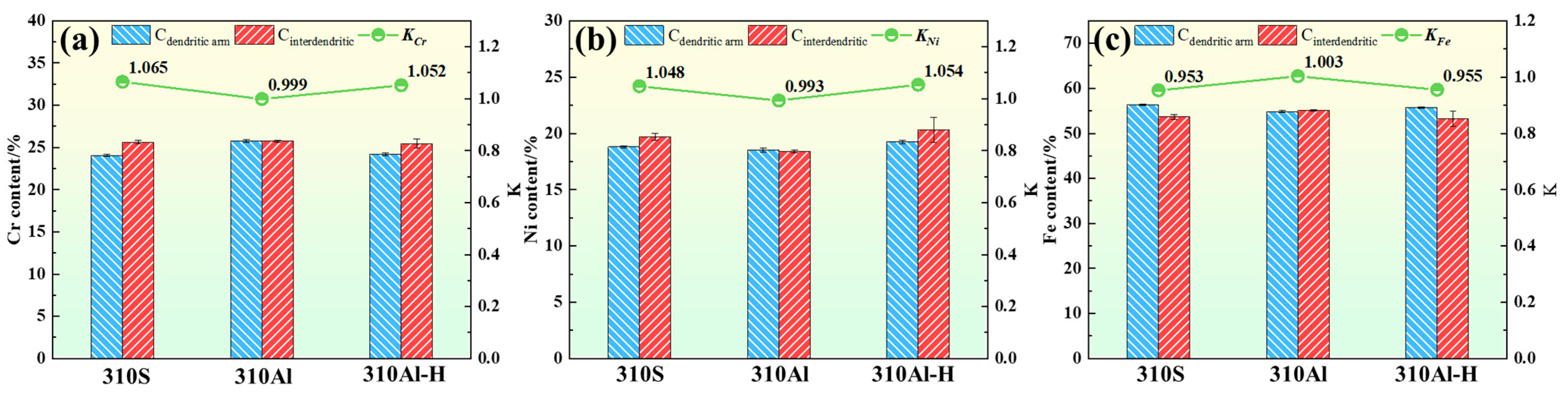
To characterize the degree of microsegregation in the solidified microstructure, EDS point scans were used to determine the segregation ratio of the experimental steels:
Here, represents the average elemental concentration in the interdendritic regions, and represents the average concentration in the dendrite arm regions. The ratio of these two values is used to quantify the degree of microsegregation in the solidified microstructure. The results are shown in Figure 11. In 310S stainless steel, the degree of elemental segregation follows the order Cr > Ni > Fe. Both Cr and Ni exhibit positive segregation, with segregation ratios of 1.065 and 1.048, respectively, while Fe exhibits negative segregation.
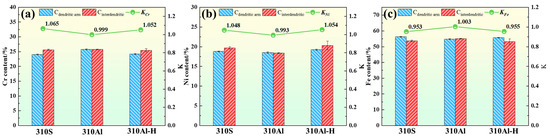
Figure 11.
Segregation ratios of the elements in the samples: (a) Cr, (b) Ni, and (c) Fe.
With the increase in Al content, the degree of elemental segregation in 310Al alloys is significantly reduced. The addition of Al can promote nucleation, refine austenite grains, and decrease dendrite arm spacing, thereby shortening the solute diffusion distance and facilitating solute redistribution, which reduces enrichment and segregation between dendrites. Meanwhile, Al also alters the thermodynamic and kinetic conditions of the alloy, suppresses grain boundary migration, and inhibits grain growth, further stabilizing the refined microstructure [27,28]. Liu, L et al. reported that the presence of Al can modify the stability of the solid–liquid interface, suppress excessive growth of columnar grains, and reduce solidification segregation, thereby significantly improving microstructural homogeneity [29].
The volume fractions of secondary phases in 310S and 310Al were statistically analyzed, as shown in Table 4. The results indicate that with increasing Al content, the precipitation of σ phase is suppressed, while the volume fraction of M23C6 decreases from 0.21% to 0.07%. This reduction in segregation decreases the tendency for δ-ferrite formation and inhibits carbide precipitation, especially that of the σ phase. Even a small amount of σ phase precipitation can significantly reduce the material’s toughness and cause embrittlement [30]. Chandra, K. et al. [31] found that in 347 heat-resistant steel aged at 650 °C, M23C6 and σ phases precipitated at grain boundaries, significantly increasing the material’s sensitization.

Table 4.
Statistical results of the volume fraction of the second phase/%.
At higher cooling rates, the solidification time is shortened, and Cr and Ni atoms do not have sufficient time to diffuse ahead of the solid–liquid interface, leading to their enrichment in the residual liquid. As a result, the Cr and Ni content in the interdendritic regions after solidification is significantly higher than in the dendrite cores, resulting in a marked increase in the segregation ratio.
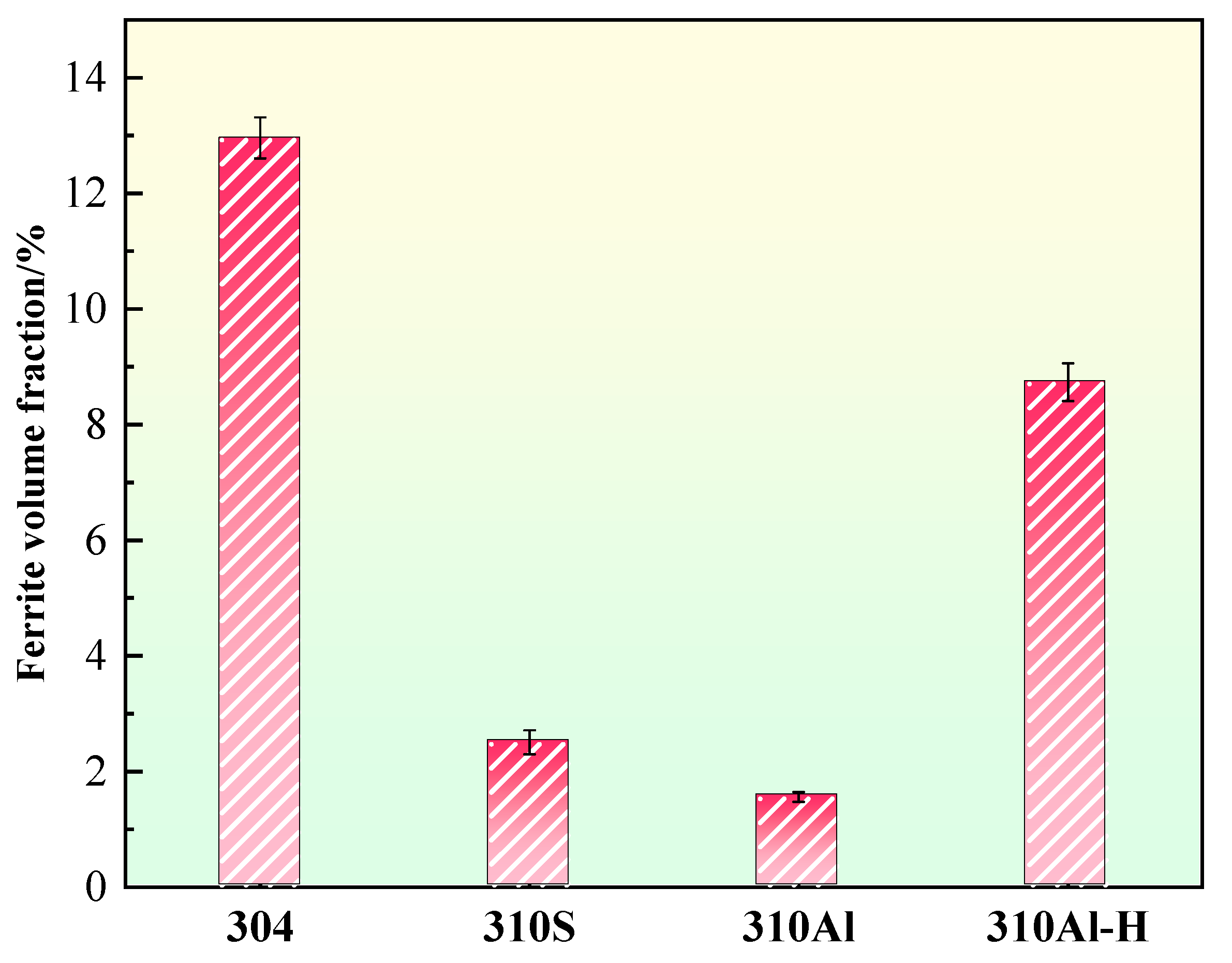
The ferrite content of the samples is summarized in Figure 12. 304 stainless steel exhibits the highest ferrite content, reaching 13.0%, which is consistent with its FA solidification mode, where ferrite forms preferentially during solidification and partially remains. With increasing Al content, the ferrite content in the 310Al sample decreases, as Al reduces solute segregation and thereby suppresses δ-ferrite formation. In contrast, the ferrite content in the 310Al-H sample significantly increases under higher cooling rates. Rapid cooling limits the diffusion of solute elements according to thermodynamic equilibrium, exacerbating segregation and promoting δ-ferrite formation. Moreover, the faster cooling rate suppresses the subsequent δ → γ solid-state transformation, resulting in more δ-ferrite being retained in the final microstructure, often with a coarser morphology. By comparison, samples cooled at lower rates complete the solid-state transformation at higher temperatures, facilitating δ-ferrite dissolution and yielding a lower final ferrite content. Overall, the final ferrite content is governed by the combined effects of solidification mode, alloy composition, and cooling rate [32]. It is noteworthy that previous studies have shown that higher δ-ferrite content can significantly increase the intergranular corrosion susceptibility of austenitic stainless steels, posing potential risks to service safety [8,19].
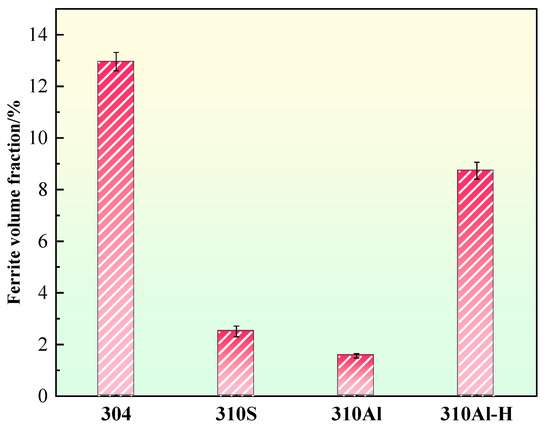
Figure 12.
Ferrite content.
3.6. Characterization of Precipitated Phase
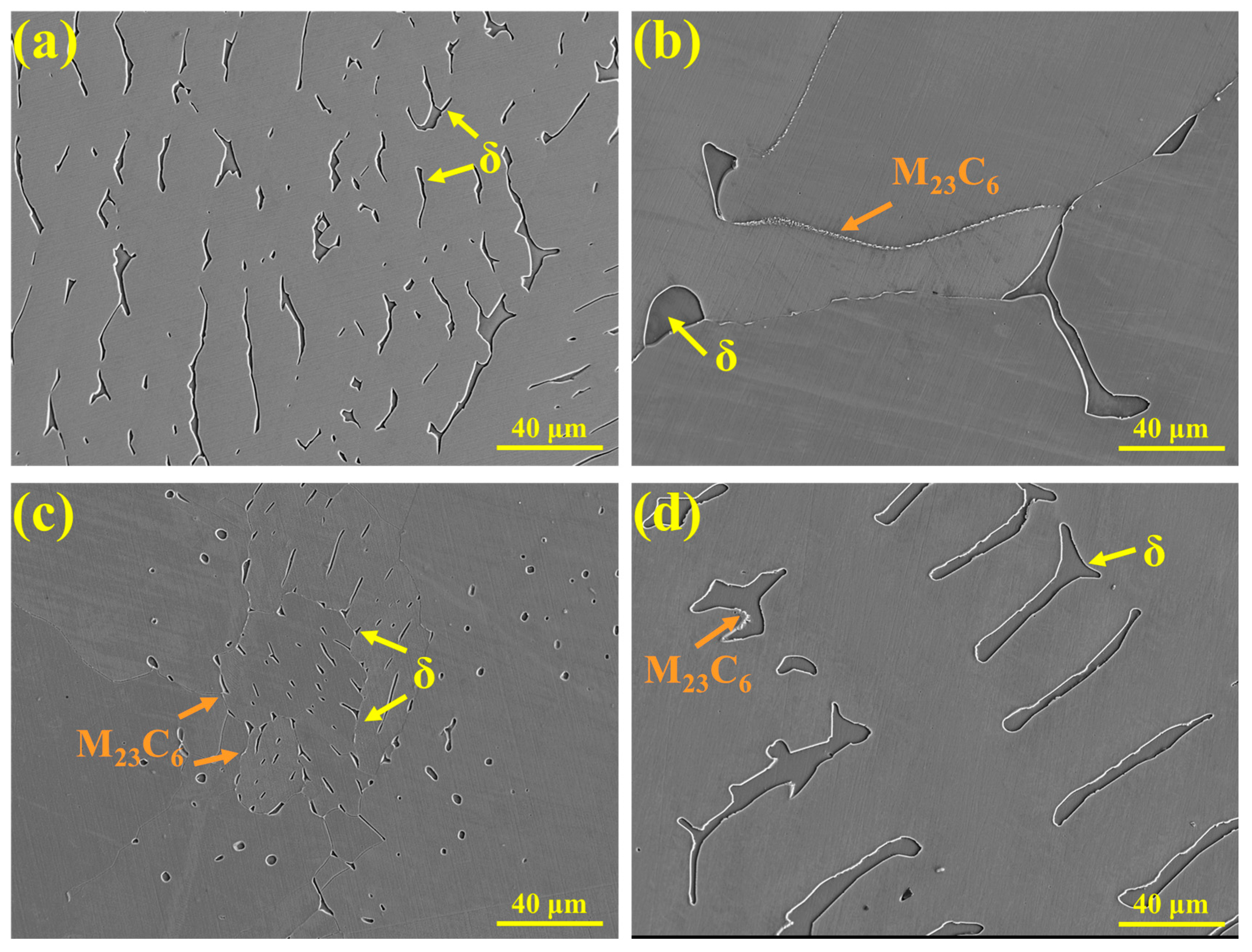
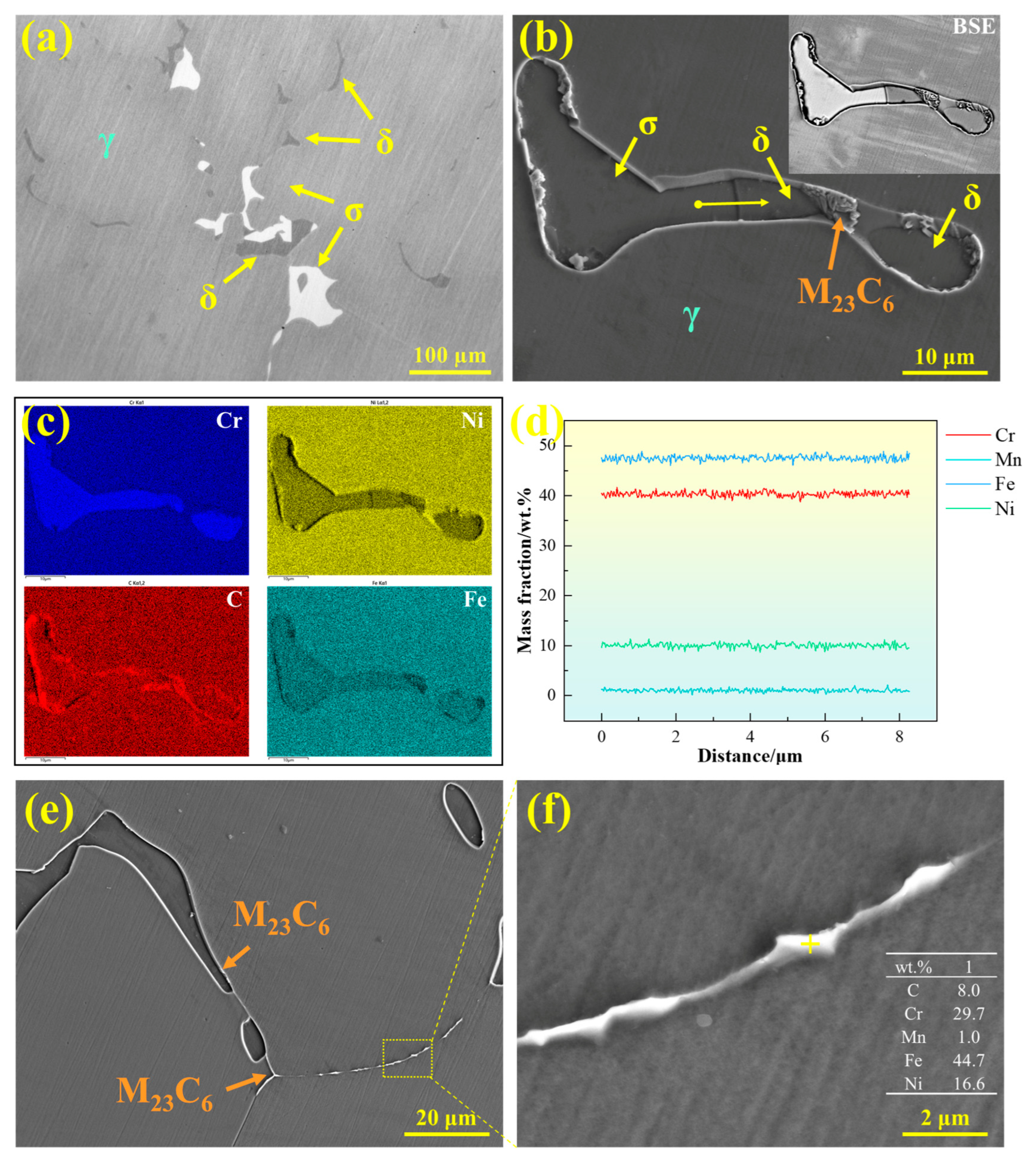
The precipitates in 310S stainless steel were characterized, as shown in Figure 13. Figure 13a presents a metallographic image, revealing two types of blocky precipitates distributed on the austenite matrix with similar morphology: one gray phase corresponding to ferrite and one white phase corresponding to σ phase. Figure 13b shows the SEM micrograph of the precipitates in 310S stainless steel, with the upper right corner displaying the corresponding BSE contrast image. Different contrast levels clearly distinguish the precipitates: the white-contrast blocky phase corresponds to σ phase, while the gray-contrast blocky phase corresponds to δ-ferrite. Additionally, clustered M23C6 precipitates are formed at the interfaces between ferrite and austenite. Figure 13c presents the EDS area scan analysis, indicating that both δ-ferrite and σ phase are enriched in Cr, with Fe and Ni depleted, whereas M23C6 is enriched in Cr and C. Figure 13d shows the EDS line scan across the precipitates, revealing that the major alloying elements in δ-ferrite and σ phase are similar and that there is no significant compositional fluctuation at the interfaces.
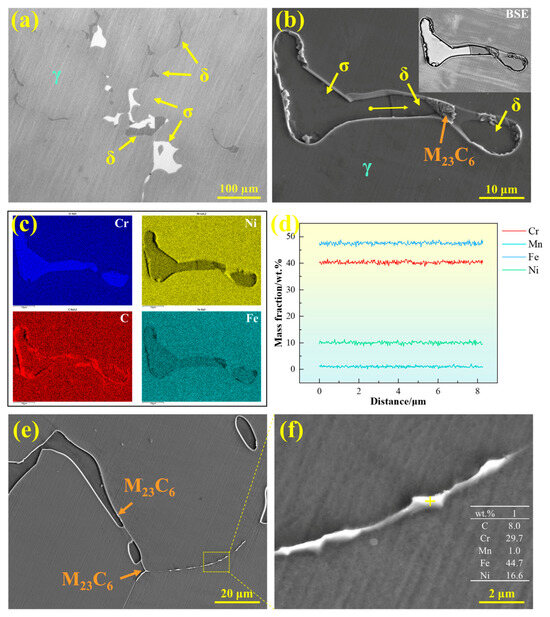
Figure 13.
Precipitates in 310S stainless steel: (a) OM microstructure, (b) SEM microstructure, (c) EDS elemental distribution map, (d) EDS line scan, and (e,f) morphology of M23C6 carbides.
The σ phase possesses very high hardness, and even a small amount of precipitation can significantly reduce material toughness and induce embrittlement [30]. In stainless steels, the formation mechanisms of σ phase can be classified into three main types [33,34]: (1) direct continuous or discontinuous precipitation from δ-ferrite (δ → σ); (2) eutectoid decomposition of δ-ferrite (δ → γ2 + σ); and (3) eutectoid decomposition of austenite (γ → γ2 + σ).
The σ phase formed via the common eutectoid decomposition pathway typically exhibits a honeycomb-like morphology. However, in the 310S stainless steel studied here, the σ phase was observed to be blocky, with morphology and compositional characteristics similar to those of δ-ferrite. Therefore, it can be inferred that the σ phase primarily precipitates directly through the solid-state transformation of δ-ferrite (δ → σ). During the δ → σ transformation, the σ phase precipitates in the Cr-rich regions of δ-ferrite and forms directly within the δ-ferrite grains [33]. Similarly, Li Yunong et al. reported in 254SMO superaustenitic stainless steel that the σ phase originates from the δ-ferrite transformation, and its fraction decreases with increasing cooling rate, exhibiting a trend opposite to that of the δ phase [35].
As a Cr-rich BCC phase, δ-ferrite provides favorable diffusion pathways for alloying elements and thus serves as the preferred nucleation site for σ phase. When δ-ferrite-containing microstructures are exposed to high temperatures for extended periods, this phase gradually decomposes into σ phase and other brittle intermetallic compounds, severely degrading mechanical properties. Similar behavior has been reported in previous studies. For example, AB Rhouma et al. [36] observed that during long-term thermal exposure of 316L welds at 550–700 °C, δ-ferrite decomposed into σ and χ phases, adversely affecting the mechanical properties of the weld joints. Perron A et al. [37] reported that the σ phase is rarely observed in austenitic stainless steels with Cr content below 20 wt.%, while it forms rapidly when Cr content is in the range of 25–30 wt.%. Although the σ phase can be eliminated through prolonged homogenization heat treatment, long-term high-temperature exposure may lead to abnormal grain growth and negatively impact subsequent corrosion resistance. Therefore, controlling σ phase formation during solidification through careful alloy design and process optimization is of critical importance.
In addition to the σ phase, M23C6 carbides also precipitate in 310S stainless steel. As shown in Figure 13e,f, M23C6 is primarily distributed discontinuously along grain boundaries or precipitates at the γ/δ phase interfaces. Figure 13f presents the chemical composition of this phase, showing that it is enriched in Cr and C compared with the matrix. The precipitation of such carbides not only reduces microstructural stability but may also compromise the corrosion resistance of the grain boundaries.
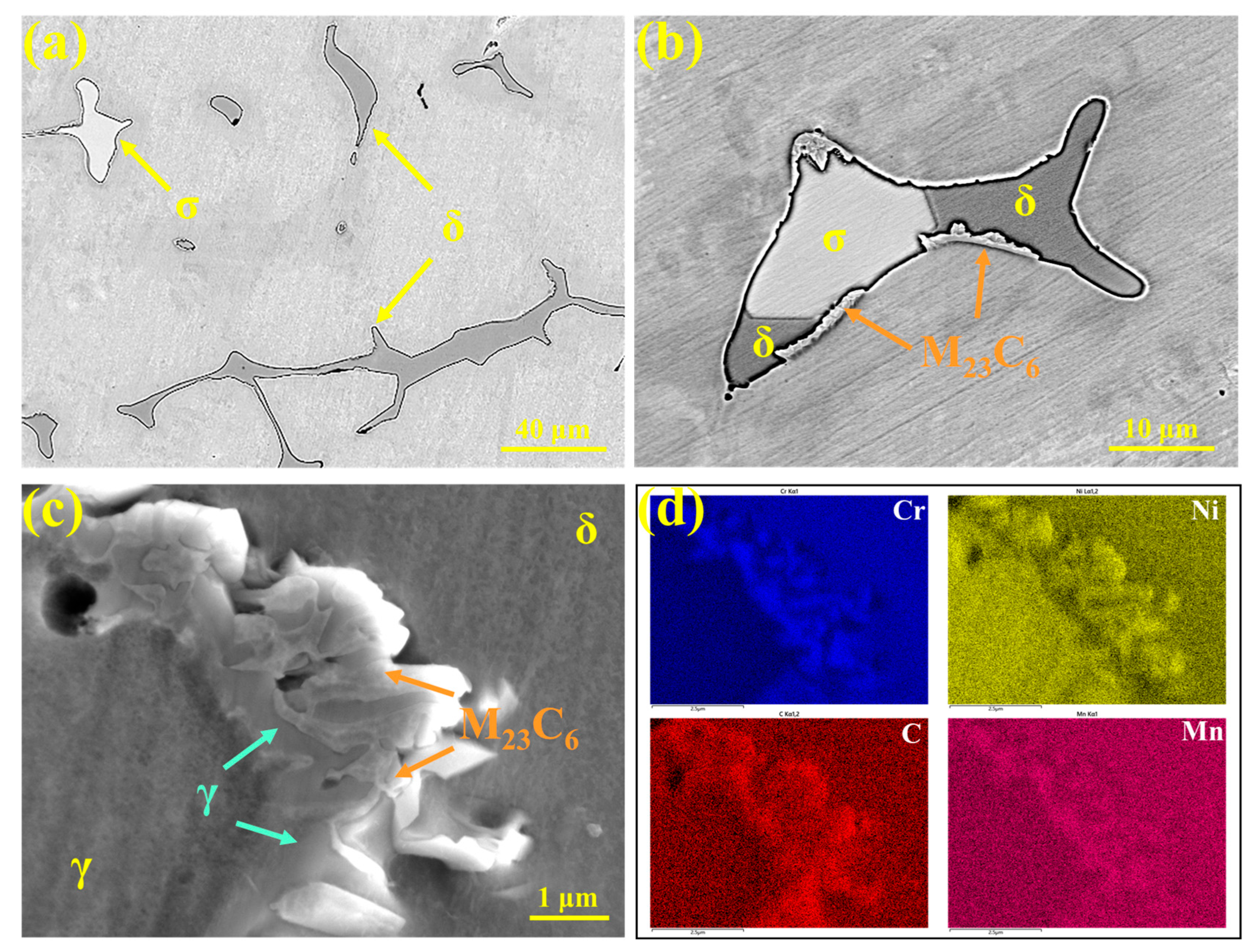
The precipitates in 310Al-H stainless steel were characterized, as shown in Figure 14. Figure 14a presents the SEM image, revealing δ-ferrite (gray) and σ phase (white) distributed on the austenite matrix. The phase composition is consistent with that of 310S stainless steel; however, the amount of σ phase increases significantly with the higher cooling rate. This is attributed to the accelerated elemental segregation and increased δ-ferrite content under rapid cooling, which promotes the δ → σ transformation.
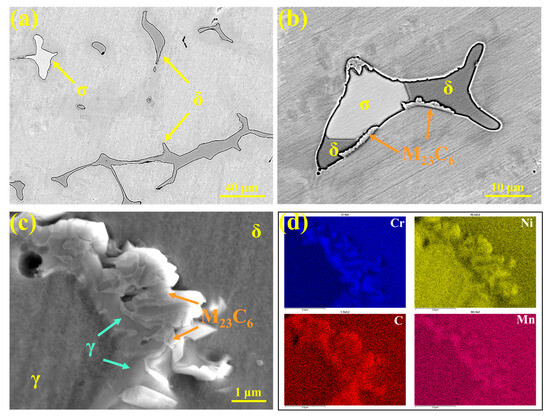
Figure 14.
(a–c) Microstructures of precipitates in 310Al-H stainless steel; (d) EDS area scan results.
As shown in Figure 14b, a large number of M23C6 carbides are distributed along the phase interfaces. Figure 14c presents the SEM morphology of M23C6 and the corresponding EDS area scan, showing that M23C6 exhibits a typical eutectic-like structure and is primarily enriched at the δ/γ phase interfaces, with Cr and C contents significantly higher than the matrix. This indicates that the precipitation of M23C6 is strongly influenced by the ferrite–austenite interface structure and local elemental segregation. After solidification, the microstructure remains in the high-temperature γ + δ two-phase region, where C and Cr segregate to grain boundaries or interdendritic regions, and dislocations and vacancies at the boundaries also facilitate M23C6 nucleation. As the temperature decreases and the solubility of C and Cr in austenite declines, M23C6 precipitates extensively along the grain boundaries at approximately 900–1100 °C, creating locally Cr-depleted regions and thereby increasing intergranular corrosion susceptibility [38].
A portion of δ-ferrite remains after solidification and undergoes the δ → γ transformation during subsequent cooling. During this process, local elemental redistribution occurs, and when the composition reaches the M23C6 stability range, preferential nucleation and growth of M23C6 at the δ/γ interface are promoted. δ-Ferrite not only provides a diffusion pathway for Cr but also serves as a nucleation site for M23C6. During the growth of M23C6, continuous Cr supply from δ-ferrite is required, with the diffusion rate of Cr acting as the rate-limiting step [39]. Because the diffusion coefficient of Cr in δ-ferrite is approximately one order of magnitude higher than in austenite, M23C6 primarily grows into δ-ferrite, leading to Cr depletion within δ-ferrite. This reduces the thermodynamic stability of δ-ferrite, making it more susceptible to transformation into austenite at higher temperatures [40]. Tseng et al. [41] reported that M23C6 nucleates at γ/δ interfaces at around 720 °C, and its precipitation can be described by the eutectoid reaction: δ → M23C6 + γ.
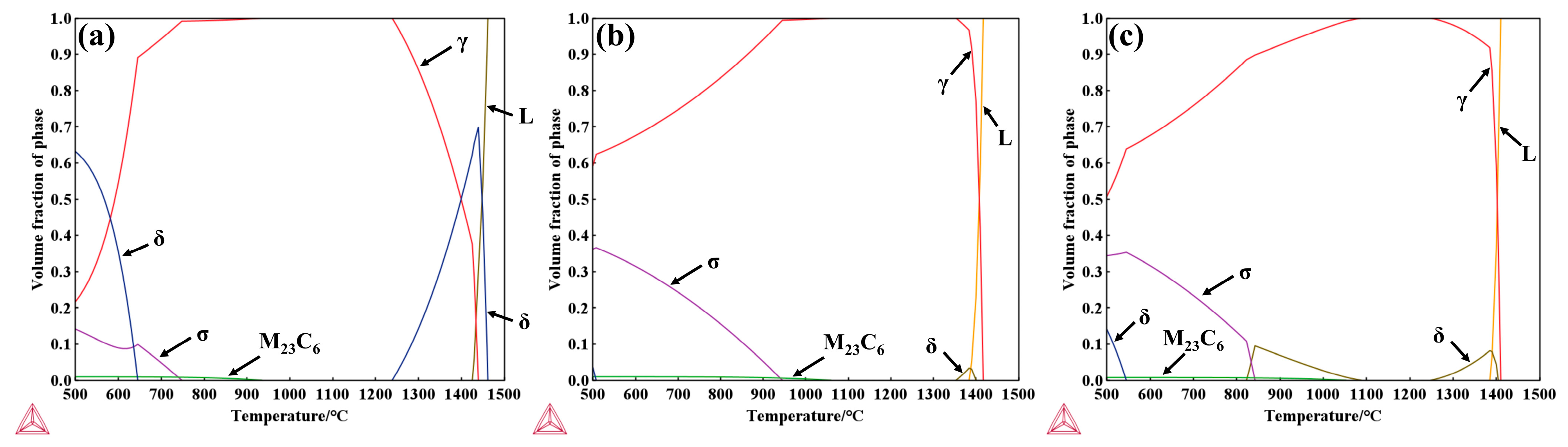
The study by A. Facco et al. [42] demonstrated that phase predictions for Al-containing steels using Thermo-Calc software are in good agreement with the experimentally observed microstructures. The temperature-dependent stable phase fractions of the experimental steels were calculated using Thermo-Calc, as shown in Figure 15, with the precipitation temperatures of each phase listed in Table 5. As shown in Figure 15a, above 1462 °C, only the liquid phase exists; when the temperature decreases below 1440 °C, the peritectic reaction L + δ → γ occurs, initiating γ-phase formation, which rapidly increases with further cooling. At 1426 °C, the liquid phase is completely solidified, after which δ-ferrite gradually transforms into the γ phase and completely disappears below 1239 °C. For 304 stainless steel, the matrix is primarily austenite, and the thermodynamically predicted precipitates include δ-ferrite, σ phase, and M23C6 carbides. Compared with 304, the δ-ferrite stability range and content in 310S alloys are reduced, whereas the σ-phase precipitation range and volume fraction are significantly increased. In addition, the onset temperature for M23C6 precipitation is higher, and it can fully dissolve above 1059 °C, consistent with the experimentally observed microstructural features.
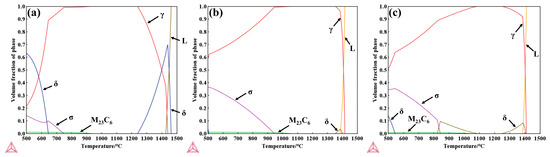
Figure 15.
The temperature-dependent stable phase fraction curves: (a) 304 stainless steel; (b) 310S stainless steel; (c) 310Al stainless steel (calculated using Thermo-Calc software).

Table 5.
The statistical results of the precipitation temperatures of each phase/°C.
4. Conclusions
- Based on thermodynamic calculations and the HT-CLSM observations, the solidification sequence of 310S stainless steel can be described as L → L + γ → L + γ + δ → δ + γ. In this sequence, austenite nucleates first and grows rapidly during the initial stage, followed by the precipitation of a small amount of δ-ferrite in the interdendritic regions during the later stage of solidification. In contrast, the solidification sequence of 304 stainless steel is L → L + δ → L + δ + γ → δ + γ. After solidification, a rapid δ → γ transformation occurs. The higher δ-ferrite content in 304 stainless steel is attributed to the preferential formation and partial retention of δ-ferrite during solidification;
- Both thermodynamic calculations and experimental results indicate that, compared with 304 stainless steel, 310S stainless steel exhibits a lower δ-ferrite content and a significantly higher σ-phase fraction. The σ phase primarily precipitates directly from δ-ferrite (δ → σ), with a chemical composition similar to that of δ. M23C6 carbides preferentially form at grain boundaries and δ/γ interfaces, where δ-ferrite not only provides rapid diffusion paths for Cr but also serves as nucleation sites for M23C6. At the δ/γ interface, M23C6 formation follows the eutectoid reaction: δ → M23C6 + γ. Furthermore, during the solidification of 310S stainless steel, the elemental segregation sequence is Cr > Ni > Fe, with Cr and Ni exhibiting positive segregation and Fe showing negative segregation;
- With increasing Al content, the solidification mode of 310S stainless steel remains unchanged, but its microstructural features are significantly altered. The addition of Al refines the austenite grains and reduces solute enrichment in the interdendritic regions, thereby markedly decreasing elemental segregation. As a result, both the size and fraction of δ-ferrite are reduced, while the precipitation of σ phase is suppressed. The amount of M23C6 carbides decreases because the addition of Al reduces the δ/γ interface area, thereby diminishing the number of preferred nucleation sites for M23C6;
- Cooling rate has a significant effect on microstructural evolution. As the cooling rate increases, insufficient solute diffusion causes the actual solute distribution to deviate from equilibrium, leading to enhanced segregation of elements such as Cr and Ni. This process promotes the formation of δ-ferrite. Simultaneously, rapid cooling suppresses the δ → γ phase transformation, resulting in a greater retention of δ-ferrite in the final microstructure. The increased δ-ferrite content, combined with its associated segregation, further facilitates the transformation of the δ phase into the σ phase.
Author Contributions
Conceptualization, J.X., D.W. and A.Z.; methodology, J.X. and K.C.; software, S.Y.; validation, S.Y., K.C. and J.W.; investigation, J.X. and S.Y.; resources, K.C. and S.Q.; visualization, S.Q. and K.C.; formal analysis, S.Q.; supervision, S.Y. and A.Z.; data curation, J.X., J.W. and A.Z.; writing—original draft preparation, J.X., S.Y. and A.Z.; writing—review and editing, J.W. and D.W.; project administration, A.Z.; funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities (Grant No. FRF-BD-23-02).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wei, Y.; La, P.; Jin, J.; Du, M.; Zheng, Y.; Zhan, F.; Sheng, J.; Yu, H.; Zhu, M. Corrosion Behavior of Aluminum-Forming Alloy 310S for Application in Molten Chloride Salt CSP Thermal Storage Tank. Front. Mater. 2022, 9, 886285. [Google Scholar] [CrossRef]
- Sah, S.P.; Tada, E.; Nishikata, A. Corrosion Behaviour of Austenitic Stainless Steels in Carbonate Melt at 923 K under Controlled CO2-O2 Environment. Corros. Sci. 2018, 133, 310–317. [Google Scholar] [CrossRef]
- Kim, D.C.; Ogura, T.; Tanabe, Y.; Yamashita, S.; Saida, K. Hot Cracking Susceptibility and Solidification Segregation Analysis by Computer Simulation in Duplex Stainless Steels. Weld. Int. 2019, 33, 231–240. [Google Scholar] [CrossRef]
- Sun, J.; Tang, H.; Wang, C.; Han, Z.; Li, S. Effects of Alloying Elements and Microstructure on Stainless Steel Corrosion: A Review. Steel Res. Int. 2022, 93, 2100450. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Yang, X.; Zhang, Z.; Wang, J.; Li, Z.; Chen, L.; Mu, W. Solidification Modes and Delta-Ferrite of Two Types of 316L Stainless Steels: A Combination of as-Cast Microstructure and HT-CLSM Research. J. Iron Steel Res. Int. 2025, 32, 426–436. [Google Scholar] [CrossRef]
- Almomani, A.; Mourad, A.-H.I.; Barsoum, I. Effect of Sulfur, Phosphorus, Silicon, and Delta Ferrite on Weld Solidification Cracking of AISI 310S Austenitic Stainless Steel. Eng. Fail. Anal. 2022, 139, 106488. [Google Scholar] [CrossRef]
- Zargar, T.; Sadeghi, F.; Kim, J.W.; Lee, J.S.; Heo, Y.-U.; Yim, C.H. Kinetic Model to Investigate the Effect of Cooling Rate on δ-Ferrite Behavior and Its Application in Continuous Casting of AISI 304 Stainless Steel. Met. Mater. Int. 2022, 28, 2263–2276. [Google Scholar] [CrossRef]
- Unnikrishnan, R.; Idury, K.S.N.S.; Ismail, T.P.; Bhadauria, A.; Shekhawat, S.K.; Khatirkar, R.K.; Sapate, S.G. Effect of Heat Input on the Microstructure, Residual Stresses and Corrosion Resistance of 304L Austenitic Stainless Steel Weldments. Mater. Charact. 2014, 93, 10–23. [Google Scholar] [CrossRef]
- Spaccarotella, A.; Ridolfi, M.R.; Picht, G.; Scheller, P.R. Control of δ-γ Transformation during Solidification of Stainless Steel Slabs in the Mould. Steel Res. Int. 2003, 74, 693–699. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Zhang, W.; Chou, K. Influence of Cooling Rate on Solidification Process Ce-High Mo Austenite Stainless Steel: Nucleation, Growth, and Microstructure Evolution. Metals 2023, 13, 246. [Google Scholar] [CrossRef]
- Li, Y.; Zou, D.; Li, M.; Tong, L.; Zhang, Y.; Zhang, W. Effect of Cooling Rate on Segregation Characteristics of 254SMO Super Austenitic Stainless Steel and Pitting Corrosion Resistance under Simulated Flue Gas Desulfurization Environment. J. Mater. Sci. 2023, 58, 4137–4149. [Google Scholar] [CrossRef]
- Fu, J.W.; Yang, Y.S.; Guo, J.J.; Tong, W.H. Effect of Cooling Rate on Solidification Microstructures in AISI 304 Stainless Steel. Mater. Sci. Technol. 2008, 24, 941–944. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Li, X.; Liu, W.; Cao, G.; Li, C.; Liu, Z. Influences of Cooling Rates on Solidification and Segregation Characteristics of Fe-Cr-Ni-Mo-N Super Austenitic Stainless Steel. J. Mater. Process. Technol. 2020, 275, 116326. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Li, Y.; Lu, S. The Influence of Niobium on the Plastic Deformation Behaviors of 310s Austenitic Stainless Steel Weld Metals at Different Temperatures. Mater. Sci. Eng. A 2019, 743, 648–655. [Google Scholar] [CrossRef]
- Wei, Y.; Cao, J.; Yu, H.; Sheng, J.; La, P. Effect of Mg Addition on Molten Chloride Salt Corrosion Resistance of 310S Stainless Steel with Aluminum. Metals 2024, 14, 1109. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Maziasz, P.J.; Liu, C.T.; Pint, B.A.; More, K.L.; Meyer, H.M.; Payzant, E.A. Creep-Resistant, Al2 O3 -Forming Austenitic Stainless Steels. Science 2007, 316, 433–436. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Lin, L.; Liu, K. High-Temperature Mechanical Properties Evaluation of 310S Stainless Steel. Mater. High Temp. 2023, 40, 479–491. [Google Scholar] [CrossRef]
- Jiao, Y.; Mahmood, J.; Zheng, W.; Singh, P.M.; Kish, J.R. Effect of Thermal Aging on the Intergranular Stress Corrosion Cracking Susceptibility of Type 310S Stainless Steel. Corrosion 2018, 74, 430–443. [Google Scholar] [CrossRef]
- Shalchi Amirkhiz, B.; Xu, S.; Scott, C. Microstructural Assessment of 310S Stainless Steel during Creep at 800 °C. Materialia 2019, 6, 100330. [Google Scholar] [CrossRef]
- Apolinario, L.H.R.; Wallerstein, D.; Montealegre, M.A.; Urtiga Filho, S.L.; Torres, E.A.; Hermenegildo, T.F.C.; Santos, T.F.A. Predominant Solidification Modes of 316 Austenitic Stainless Steel Coatings Deposited by Laser Cladding on 304 Stainless Steel Substrates. Met. Mater. Trans. A 2019, 50, 3617–3628. [Google Scholar] [CrossRef]
- Osuki, T.; Yonemura, M.; Ogawa, K.; Komizo, Y.; Terasaki, H. Analysis of the Solidification Process of Austenitic Stainless Steel Weld Metal Using Synchrotron Radiation. Weld World 2009, 53, R290–R303. [Google Scholar] [CrossRef]
- Ferrandini, P.L.; Rios, C.T.; Dutra, A.T.; Jaime, M.A.; Mei, P.R.; Caram, R. Solute Segregation and Microstructure of Directionally Solidified Austenitic Stainless Steel. Mater. Sci. Eng. A 2006, 435, 139–144. [Google Scholar] [CrossRef]
- Wang, T.; Phelan, D.; Wexler, D.; Yin, Y.; Guo, L.; Zhang, C.; Li, H. Detailed Analysis of Nucleation and Subsequent δ to γ Phase Transformation of a High N Super Austenitic Stainless Steel. Mater. Charact. 2023, 195, 112485. [Google Scholar] [CrossRef]
- Kim, S.H.; Moon, H.K.; Kang, T.; Lee, C.S. Dissolution Kinetics of Delta Ferrite in AISI 304 Stainless Steel Produced by Strip Casting Process. Mater. Sci. Eng. A 2003, 356, 390–398. [Google Scholar] [CrossRef]
- Saied, M. Experimental and Numerical Modeling of the Dissolution of Delta Ferrite in the Fe-Cr-Ni System: Application to the Austenitic Stainless Steels. Ph.D. Dissertation, Université Grenoble Alpes, Grenoble, France, 2016. [Google Scholar]
- Zhu, H.C.; Li, H.B.; Zhang, S.C.; Li, K.B.; Liu, G.H.; Jiang, Z.H.; Geng, X.; Han, P.D. Numerical Simulation of Mo Macrosegregation during Ingot Casting of High-Mo Austenitic Stainless Steel. Ironmak. Steelmak. 2015, 42, 748–755. [Google Scholar] [CrossRef]
- Liang, Z.; Li, H.; Wang, Q.; Sun, X.; Gao, X.; Yuan, S.; Yang, Z.; Zhang, F. Enhancing the Resistance to Hydrogen Embrittlement in Bainitic Steel via Grain Refinement, Dislocation Density Reduction, and Retained Austenite Stability Improvement. J. Mater. Sci. Technol. 2026, 247, 214–225. [Google Scholar] [CrossRef]
- Seol, J.-B.; Raabe, D.; Choi, P.-P.; Im, Y.-R.; Park, C.-G. Atomic Scale Effects of Alloying, Partitioning, Solute Drag and Austempering on the Mechanical Properties of High-Carbon Bainitic–Austenitic TRIP Steels. Acta Mater. 2012, 60, 6183–6199. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Yang, Y.; Luo, Z.; Song, C.; Zhai, Q. A Simple Method to Produce Austenite-Based Low-Density Fe–20Mn–9Al–0.75C Steel by a near-Rapid Solidification Process. Mater. Sci. Eng. A 2017, 679, 282–291. [Google Scholar] [CrossRef]
- Yamashita, S.; Ike, K.; Yamasaki, K.; Wei, F.-G.; Wang, K.; Ogura, T.; Saida, K. Relationship between Ferrite–Austenite Phase Transformation and Precipitation Behavior of Sigma Phase in Super Duplex Stainless Steel Weldment. Weld World 2022, 66, 351–362. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Tewari, R. Microstructural and Electrochemical Characterisation of Heat-Treated 347 Stainless Steel with Different Phases. Corros. Sci. 2013, 67, 118–129. [Google Scholar] [CrossRef]
- Li, X.; Gao, F.; Jiao, J.; Cao, G.; Wang, Y.; Liu, Z. Influences of Cooling Rates on Delta Ferrite of Nuclear Power 316H Austenitic Stainless Steel. Mater. Charact. 2021, 174, 111029. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Wu, W. Overview of Intermetallic Sigma (σ) Phase Precipitation in Stainless Steels. ISRN Met. 2012, 2012, 732471. [Google Scholar] [CrossRef]
- Waanders, F.B.; Vorster, S.W.; Pollak, H. The Influence of Temperature on σ-Phase Formation and the Resulting Hardening of Fe–Cr–Mo-Alloys. Hyperfine Interact. 1999, 120, 751–755. [Google Scholar] [CrossRef]
- Li, Y.; Zou, D.; Li, M.; Tong, L.; Zhang, Y.; Zhang, W. Effect of Cooling Rate on δ-Ferrite Formation and Sigma Precipitation Behavior of 254SMO Super-Austenitic Stainless Steel During Solidification. Met. Mater. Trans. B 2023, 54, 3497–3507. [Google Scholar] [CrossRef]
- Ben Rhouma, A.; Amadou, T.; Sidhom, H.; Braham, C. Correlation between Microstructure and Intergranular Corrosion Behavior of Low Delta-Ferrite Content AISI 316L Aged in the Range 550–700 °C. J. Alloys Compd. 2017, 708, 871–886. [Google Scholar] [CrossRef]
- Perron, A.; Toffolon-Masclet, C.; Ledoux, X.; Buy, F.; Guilbert, T.; Urvoy, S.; Bosonnet, S.; Marini, B.; Cortial, F.; Texier, G.; et al. Understanding Sigma-Phase Precipitation in a Stabilized Austenitic Stainless Steel (316Nb) through Complementary CALPHAD-Based and Experimental Investigations. Acta Mater. 2014, 79, 16–29. [Google Scholar] [CrossRef]
- Qian, J.; Chen, C.; Yu, H.; Liu, F.; Yang, H.; Zhang, Z. The Influence and the Mechanism of the Precipitate/Austenite Interfacial C-Enrichment on the Intergranular Corrosion Sensitivity in 310 S Stainless Steel. Corros. Sci. 2016, 111, 352–361. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, D.; Wang, X.; Li, Y.; Li, M.; Wang, F.; Zhang, W.; Tong, L. Effect of Al Content on the Δ→γ Transformation and M23C6 Precipitation Behavior in 18Cr-xAl-Si Ferritic Heat-Resistant Stainless Steel during Solidification Process. J. Mater. Res. Technol. 2022, 18, 3104–3114. [Google Scholar] [CrossRef]
- Sasikala, G.; Ray, S.K.; Mannan, S.L. Kinetics of Transformation of Delta Ferrite during Creep in a Type 316(N) Stainless Steel Weld Metal. Mater. Sci. Eng. A 2003, 359, 86–90. [Google Scholar] [CrossRef]
- Tseng, C.C.; Shen, Y.; Thompson, S.W.; Mataya, M.C.; Krauss, G. Fracture and the Formation of Sigma Phase, M2306, and Austenite from Delta-Ferrite in an AISI 304L Stainless Steel. Metall. Mater. Trans. A 1994, 25, 1147–1158. [Google Scholar]
- Facco, A.; Couvrat, M.; Magné, D.; Roussel, M.; Guillet, A.; Pareige, C. Microstructure Influence on Creep Properties of Heat-Resistant Austenitic Alloys with High Aluminum Content. Mater. Sci. Eng. A 2020, 783, 139276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).