Corrosion Behavior of S235JR Carbon Steel in 0.5 M HCl Solution During 24 Weeks
Abstract
1. Introduction
2. Materials and Methods
2.1. S235JR Carbon Steel
2.2. Gravimetric Corrosion Measurements
2.3. Electrochemical Measurements
2.4. Surface Analysis and Elemental Composition
2.5. Roughness and Vickers Hardness Measurements
3. Results and Discussion
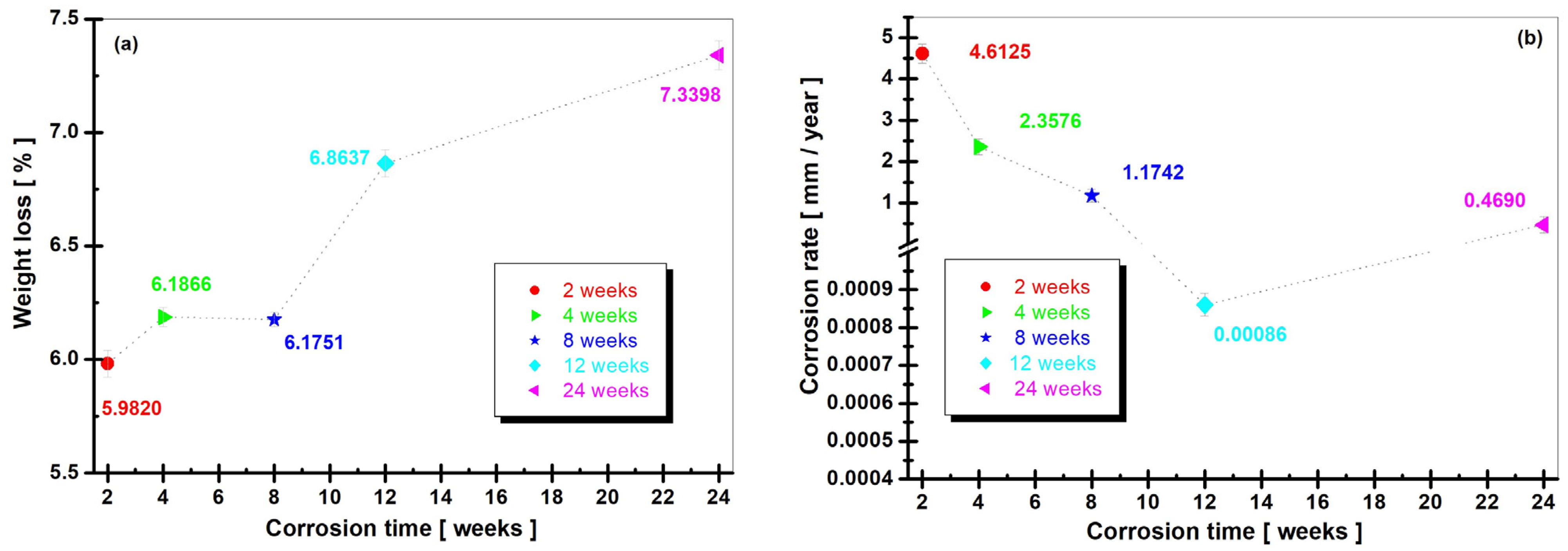
3.1. Weight Loss Results
3.2. Electrochemical Measurement Results
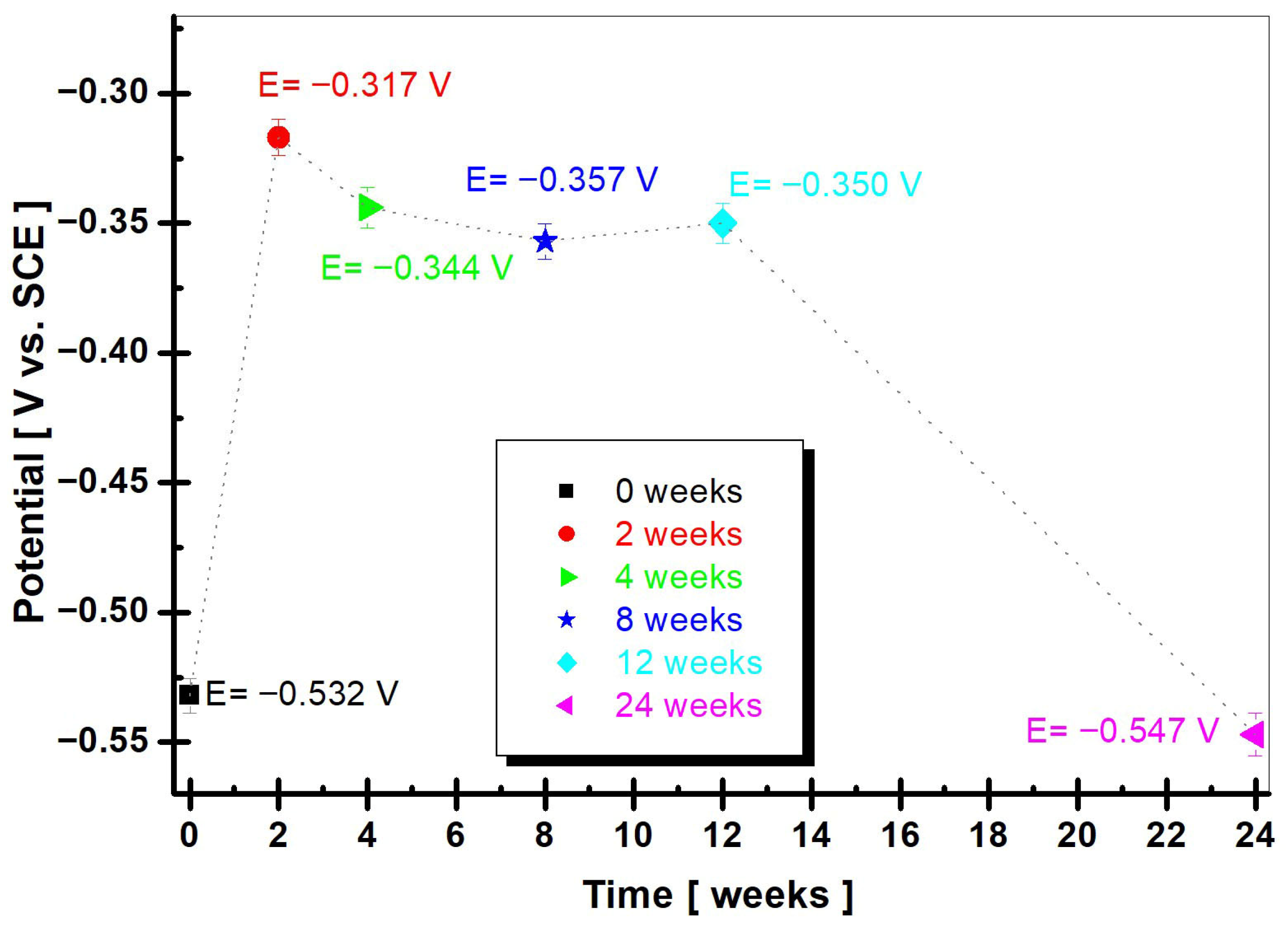
3.2.1. OCP (Open Circuit Potential)
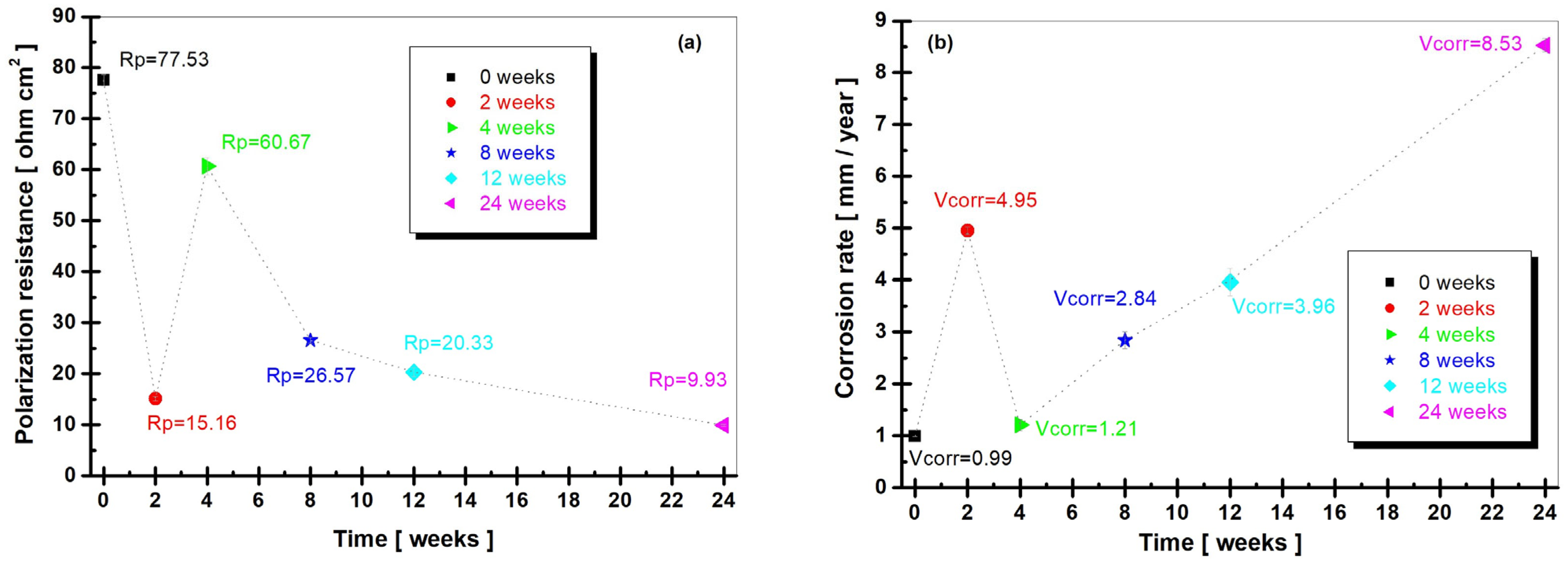
3.2.2. Polarization Resistance (Rp) and Corrosion Rate (Vcorr)
3.3. Surface Characterization and Elemental Analysis
3.4. Roughness and Vickers Hardness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wan, S.; Zhang, T.; Chen, H.; Liao, B.; Guo, X. Kapok leaves extract and synergistic iodide as novel effective corrosion inhibitors for Q235 carbon steel in H2SO4 medium. Ind. Crops Prod. 2022, 178, 114649. [Google Scholar] [CrossRef]
- Mao, T.; Huang, H.; Liu, D.; Shang, X.; Wang, W.; Wang, L. Novel cationic Gemini ester surfactant as an efficient and eco-friendly corrosion inhibitor for carbon steel in HCl solution. J. Mol. Liq. 2021, 339, 117174. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Aslam, J.; Hassane, L. Sugar based N,N′-didodecyl-N,N′digluconamideethylenediamine gemini surfactant as corrosion inhibitor for mild steel in 3.5% NaCl solution-effect of synergistic KI additive. Sci. Rep. 2018, 8, 3690. [Google Scholar] [CrossRef]
- Bentrah, H.; Rahali, Y.; Chala, A. Gum Arabic as an eco-friendly inhibitor for API 5L X42 pipeline steel in HCl medium. Corros. Sci. 2014, 82, 426–431. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Shalabi, K.; Arab, A.M.; Abdallah, Y.M. Corrosion mitigation performance of N80 steel in 5% sulfamic acid medium by applying novel tetrahydro-1,2,4-triazines including triazene moieties: Electrochemical and theoretical approaches. ACS Omega 2022, 7, 23380–23392. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Obot, I.B.; Oguzie, E.E. Anticorrosion performance of 1–benzylimidazole and synergistic iodide additives on C1018 carbon steel in 15% HCl solution. Mater. Today Commun. 2023, 36, 106407. [Google Scholar] [CrossRef]
- Pang, X.; Gong, M.; Zhang, Y.; Wei, Q.; Hou, B. Corrosion inhibition and mechanism of mild steel in hydrochloric acid by ceftriaxone and amoxicillin. Sci. China Chem. 2011, 54, 1529–1536. [Google Scholar] [CrossRef]
- Bogatu, N.; Buruiana, D.L.; Muresan, A.C.; Ghisman, V.; Lupu, A.; Mardare, L.; Herbei, E.E.; Basliu, V.; Ceoromila, A.; Florescu, S. Assessment of the effectiveness of protective coatings in preventing steel corrosion in the marine environment. Polymers 2025, 17, 378. [Google Scholar] [CrossRef]
- Buruiană, D.L.; Mureşan, A.C.; Bogatu, N.; Ghisman, V.; Herbei, E.E.; Başliu, V. Corrosion tendency of S235 steel in 3.5% NaCl solution and drinking water during six months of exposure. Materials 2024, 17, 5979. [Google Scholar] [CrossRef]
- Fingar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Jamal, D.A. Dianils: New and effective corrosion inhibitors for oil-well steel (N-80) and mild steel in boiling hydrochloric acid. Corrosion 2000, 56, 156–160. [Google Scholar] [CrossRef]
- Zheng, Z.; Hu, J.; Eliaz, N.; Zhou, L.; Yuan, X.; Zhong, X. Mercaptopropionic acid-modified oleic imidazoline as a highly efficient corrosion inhibitor for carbon steel in CO2-saturated formation water. Corros. Sci. 2022, 194, 109930. [Google Scholar] [CrossRef]
- Upadhyay, N.; Pujar, M.G.; George, R.P.; Philip, J. Development of a sulfamic acid-based chemical formulation for effective cleaning of modified 9Cr–1Mo steel steam generator tubes. Trans. Indian Inst. Met. 2020, 73, 343–352. [Google Scholar] [CrossRef]
- Cai, S.; Ji, H.; Zhu, F.; Pei, W.; Xiao, W.; Tang, X. Research on the corrosion behavior of Q235 pipeline steel in an atmospheric environment through experiment. Materials 2022, 15, 6502. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Katayama, H. Corrosion simulation of carbon steels in atmospheric environment. Corr. Sci. 2005, 47, 2599–2606. [Google Scholar] [CrossRef]
- Slaimana, Q.J.M.; Hasan, B.O. Study on corrosion rate of carbon steel pipe under turbulent flow conditions. Can. J. Chem. Eng. 2010, 88, 1114–1120. [Google Scholar] [CrossRef]
- Liu, X.Y.; Sheng, K.; Zhao, T. Microstructure and texture evolution during the direct extrusion and bending–Shear deformation of AZ31 magnesium alloy. Acta Metall. Sin. Engl. Lett. 2019, 32, 710–718. [Google Scholar] [CrossRef]
- Liao, B.; Ma, S.; Zhang, S.; Li, X.; Quan, R.; Wan, S.; Guo, X. Fructus cannabis protein extract powder as a green and high effective corrosion inhibitor for Q235 carbon steel in 1 M HCl solution. Int. J. Biol. Macromol. 2023, 239, 124358. [Google Scholar] [CrossRef]
- Fang, Z.; Fu, S.; Peng, Y.; Yang, X.; Sun, Q.; Li, P.; Ma, H.; Zhang, R.; Liang, Z.; Li, J. Developing a novel imine derivative as a corrosion inhibitor for Q235 carbon steel in 1 M HCl solution: Experiments and theoretical calculations. Mater. Today Commun. 2024, 40, 109693. [Google Scholar] [CrossRef]
- Peng, M.; He, S.; Wu, C.; Chen, Z.; Cen, H. Corrosion inhibition of extracts from the expired traditional Chinese patent medicines for Q235 carbon steel in hydrochloric acid. Sustain. Chem. Pharm. 2023, 36, 101326. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Ali, I.H.; Alanazi, A.K.; Younas, M.; Lin, Y. Long chain imidazole derivative as a novel corrosion inhibitor for Q235 steel in 15 % HCl medium under hydrodynamic condition: Experimental and theoretical examinations. Mater. Chem. Phys. 2024, 313, 128798. [Google Scholar] [CrossRef]
- Du, S.; Chen, S.; Zhang, Z.; Ye, Z.; Mao, H.; Yang, H.; Lian, C.; Bao, C. Corrosion inhibition behavior of hydroxyl-terminated hyperbranched poly (amine-ester) for Q235 steel in HCl solution. Mater. Chem. Phys. 2022, 292, 126831. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.M.; Wang, S.S.; Wang, Y.H.; Li, H.J.; Wu, Y.C. Environmentally benign cinchonain IIa from Uncaria laevigata for corrosion inhibition of Q235 steel in HCl corrosive medium: Experimental and theoretical investigation. Environ. Res. 2022, 215, 114376. [Google Scholar] [CrossRef]
- Huang, L.; Yang, K.P.; Zhao, Q.; Li, H.J.; Wang, J.Y.; Wu, Y.C. Corrosion resistance and antibacterial activity of procyanidin B2 as a novel environment-friendly inhibitor for Q235 steel in 1 M HCl solution. Bioelectrochemistry 2022, 143, 107969. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Feng, H.; Xia, H.; Xia, W.H. Carboxymethyl cellulose -polyaniline composites as efficient corrosion inhibitor for Q235 steel in 1 M HCl solution. Int. J. Electrochem. Sci. 2022, 17, 221180. [Google Scholar] [CrossRef]
- Saad, A.J.; Ahmad, A.A.; Jasim, I.H. Corrosion behavior of carbon steel in 1 M, 2 M, and 3 M HCl solutions. Mater. Today Proc. 2022, 57, 412–417. [Google Scholar] [CrossRef]
- ASTM NACE/ASTMG31-12a; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: Houston, TX, USA, 2012.
- ISO 11845:2020; Corrosion of Metals and Alloys-General Principles for Corrosion Testing. British Standards Institution: Herndon, VA, USA, 2020.
- Bogatu, N.; Muresan, A.C.; Mardare, L.; Ghisman, V.; Ravoiu, A.; Dima, F.M.; Buruiana, D.L. The Influence of Different Type Materials of Grit Blasting on the Corrosion Resistance of S235JR Carbon Steel. Inventions 2023, 8, 39. [Google Scholar] [CrossRef]
- Hu, E.; Xu, Y.; Hu, X.; Pan, L.; Jiang, S. Corrosion behaviors of metals in biodiesel from rapeseed oil and methanol. Renew. Energy 2012, 37, 371–378. [Google Scholar] [CrossRef]
- Valcarce, M.B.; Vasquez, M.; Sanchez, S.R. Determination of the speed of corrosion of aluminum brass in drinking water. In Proceedings of the CONGRESO CONAMET/SAM, La Serena, Chile, 3–5 November 2004; pp. 1–6. [Google Scholar]
- Wang, D.; Li, Y.; Chen, B.; Zhang, L. Novel surfactants as green corrosion inhibitors for mild steel in 15% HCl: Experimental and theoretical studies. Chem. Eng. J. 2020, 402, 126219. [Google Scholar] [CrossRef]
- Fajobi, M.A.; Loto, R.T.; Oluwole, O.O. Steel corrosion behaviour in acidic solution for application in petrochemical distillation systems. IOP Conf. Ser. Mater. Sci. Eng. 2020, 811, 012030. [Google Scholar] [CrossRef]
- Musab, A.; Othman, H.Z.; Almahdi, A. Study of corrosion products induced under different environmental conditions. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1090, 012050. [Google Scholar] [CrossRef]
- Evgeny, B.; Hughes, T.; Eskin, D. Effect of surface roughness on corrosion behaviour of low carbon steel in inhibited 4 M hydrochloric acid under laminar and turbulent flow conditions. Corros. Sci. 2016, 103, 196–205. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, G.; Chen, Y.; Peng, Z.; Yimei, T. Effects of chloride ions on corrosion of ductile iron and carbon steel in soil environments. Sci. Rep. 2017, 7, 6865. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Wang, B.; Gong, P.-X.; Liu, Y.; Li, H.-J.; Wu, Y.-C. Lentinan as an eco-friendly corrosion inhibitor for Q235 steel in acid medium: Experimental and theoretical studies. J. Mol. Liq. 2022, 360, 119513. [Google Scholar] [CrossRef]
- Muresan, A.C.; Istrate, G.G. Elemente de Electrochimie si Coroziune; Note de curs; Editura Galati, University Press: Galati, Romania, 2021; pp. 170–171. [Google Scholar]
- Stratmann, M.; Müller, J. The mechanism of the oxygen reduction on rust covered metal substrates. Corros. Sci. 1994, 36, 327–359. [Google Scholar] [CrossRef]
- Noor, E.A.; Al-Moubaraki, A.H. Corrosion behavior of mild steel in hydrochloric acid solutions. Int. J. Electrochem. Sci. 2008, 3, 806–818. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Kuznetsov, Y.I. Effect of iron (III) salts on steel corrosion in acid solutions. A review. Int. J. Corros. Scale Inhib. 2021, 10, 1069–1109. [Google Scholar] [CrossRef]

| Mn | Si | P | S | N | Cu | C | Fe |
|---|---|---|---|---|---|---|---|
| ≤1.40 | ≤0.025 | ≤0.028 | ≤0.025 | ≤0.012 | ≤0.45 | ≤0.17 | Balance |
| Parameters | Period of Immersion [Weeks] | ||||
|---|---|---|---|---|---|
| 2 (After 2 Weeks) | 4 (After 4 Weeks) | 8 (After 8 Weeks) | 12 (After 12 Weeks) | 24 (After 24 Weeks) | |
| W1 [g] | 34.9317 ± 0.002 | 34.5117 ± 0.005 | 35.0814 ± 0.002 | 34.9376 ± 0.005 | 35.0690 ± 0.002 |
| W2 [g] | 32.8421 ± 0.003 | 32.3766 ± 0.007 | 32.9151 ± 0.004 | 32.5396 ± 0.003 | 32.4950 ± 0.006 |
| A [cm2] | 15 ± 0.1 | ||||
| t [h] | 336 | 672 | 1344 | 2016 | 4032 |
| K | 87.600 | ||||
| [g/cm3] | 7.87 | ||||
| Period of Immersion | pH | Electrical Conductivity [mS/cm] | Salinity [g/L] | TDS (Total Dissolved Solids) [ppt] |
|---|---|---|---|---|
| 0 (initial) | 0.53 ± 0.006 | 155.5 ± 4.2 | 102.0 ± 6.3 | 110.7 ± 2.8 |
| 2 (after 2 weeks) | 3.35 ± 0.2 | 40.2 ± 0.9 | 25.0 ± 1.5 | 28.5 ± 1.2 |
| 4 (after 4 weeks) | 3.50 ± 0.1 | 40.3 ± 1.3 | 25.0 ± 0.6 | 28.6 ± 2.3 |
| 8 (after 8 weeks) | 4.13 ± 0.3 | 40.3 ± 0.5 | 25.1 ± 0.7 | 28.7 ± 3.2 |
| 12 (after 12 weeks) | 3.21 ± 0.1 | 41.0 ± 2.6 | 25.6 ± 1.4 | 29.1 ± 0.9 |
| 24 (after 24 weeks) | 3.14 ± 0.4 | 40.6 ± 1.8 | 25.2 ± 0.5 | 28.9 ± 1.3 |
| Chemical Elements | Period of Immersion [Weeks] | |||||
|---|---|---|---|---|---|---|
| 0 (Before Corrosion) | 2 (After 2 Weeks) | 4 (After 4 Weeks) | 8 (After 8 Weeks) | 12 (After 12 Weeks) | 24 (After 24 Weeks) | |
| Fe (wt%) | 99.4 | 77.1 | 86.3 | 83.3 | 79.1 | 70.7 |
| O (wt%) | 0.6 | 21.6 | 13.0 | 15.1 | 19.7 | 26.6 |
| Cl (wt%) | 0.0 | 1.4 | 0.7 | 1.7 | 1.1 | 2.8 |
| Period of Immersion | Roughness Parameters [µm] | |||
|---|---|---|---|---|
| Ra | Rz | Rq | Rt | |
| 0 (initial) | 0.91 ± 0.05 | 8.75 ± 0.61 | 1.11 ± 0.08 | 9.71 ± 0.23 |
| 2 (after 2 weeks) | 2.92 ± 0.21 | 21.24 ± 1.02 | 3.87 ± 0.21 | 30.42 ± 2.45 |
| 4 (after 4 weeks) | 8.05 ± 0.57 | 49.10 ± 3.21 | 10.64 ± 0.83 | 58.40 ± 3.12 |
| 8 (after 8 weeks) | 3.76 ± 0.24 | 25.47 ± 1.13 | 4.97 ± 0.31 | 53.16 ± 3.05 |
| 12 (after 12 weeks) | 7.57 ± 0.58 | 48.71 ± 3.17 | 10.08 ± 0.82 | 61.73 ± 4.78 |
| 24 (after 24 weeks) | 9.03 ± 0.65 | 44.09 ± 2.88 | 10.79 ± 1.16 | 66.24 ± 5.02 |
| Period of Immersion | Vickers Hardness, HV0.5 |
|---|---|
| 0 (initial) | 148.7 ± 3.2 |
| 2 (after 2 weeks) | 94.6 ± 2.6 |
| 4 (after 4 weeks) | 96.0 ± 2.8 |
| 8 (after 8 weeks) | 95.5 ± 2.8 |
| 12 (after 12 weeks) | 83.8 ± 1.9 |
| 24 (after 24 weeks) | 87.3 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureșan, A.C.; Buruiana, D.L.; Ghisman, V.; Herbei, E.E.; Bogatu, N. Corrosion Behavior of S235JR Carbon Steel in 0.5 M HCl Solution During 24 Weeks. Metals 2025, 15, 1092. https://doi.org/10.3390/met15101092
Mureșan AC, Buruiana DL, Ghisman V, Herbei EE, Bogatu N. Corrosion Behavior of S235JR Carbon Steel in 0.5 M HCl Solution During 24 Weeks. Metals. 2025; 15(10):1092. https://doi.org/10.3390/met15101092
Chicago/Turabian StyleMureșan, Alina Crina, Daniela Laura Buruiana, Viorica Ghisman, Elena Emanuela Herbei, and Nicoleta Bogatu. 2025. "Corrosion Behavior of S235JR Carbon Steel in 0.5 M HCl Solution During 24 Weeks" Metals 15, no. 10: 1092. https://doi.org/10.3390/met15101092
APA StyleMureșan, A. C., Buruiana, D. L., Ghisman, V., Herbei, E. E., & Bogatu, N. (2025). Corrosion Behavior of S235JR Carbon Steel in 0.5 M HCl Solution During 24 Weeks. Metals, 15(10), 1092. https://doi.org/10.3390/met15101092






