An Assessment of TiN Formation on NiTi Alloy and the Corrosion Resistance of TiN/NiTi Alloy Using First-Principles Calculation
Abstract
1. Introduction
2. Calculation Model and Methods
3. Results and Discussion
3.1. Study of the Interfacial Properties of the Nitride Layer
3.2. Corrosion Resistance
4. Conclusions
- (1)
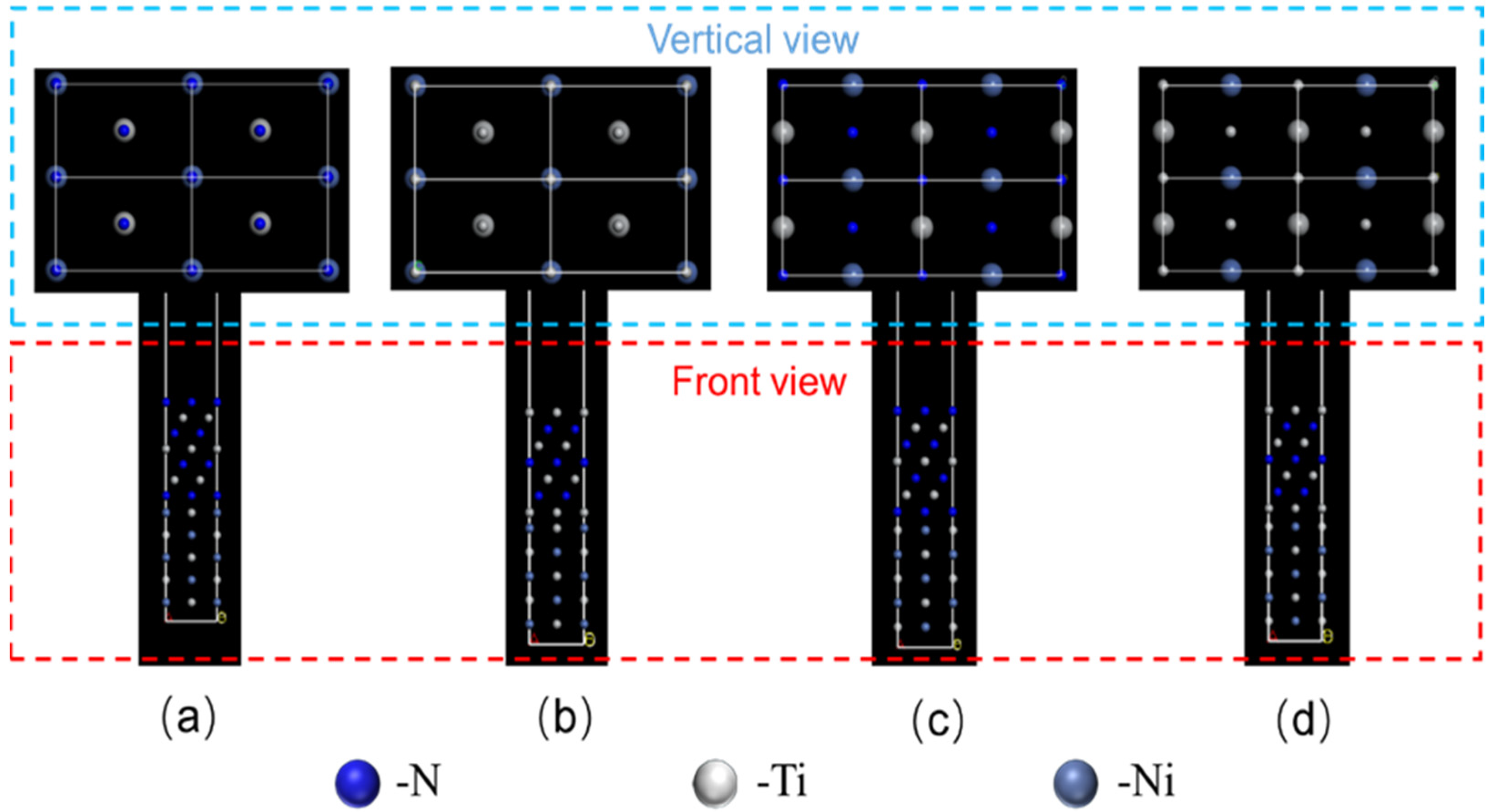
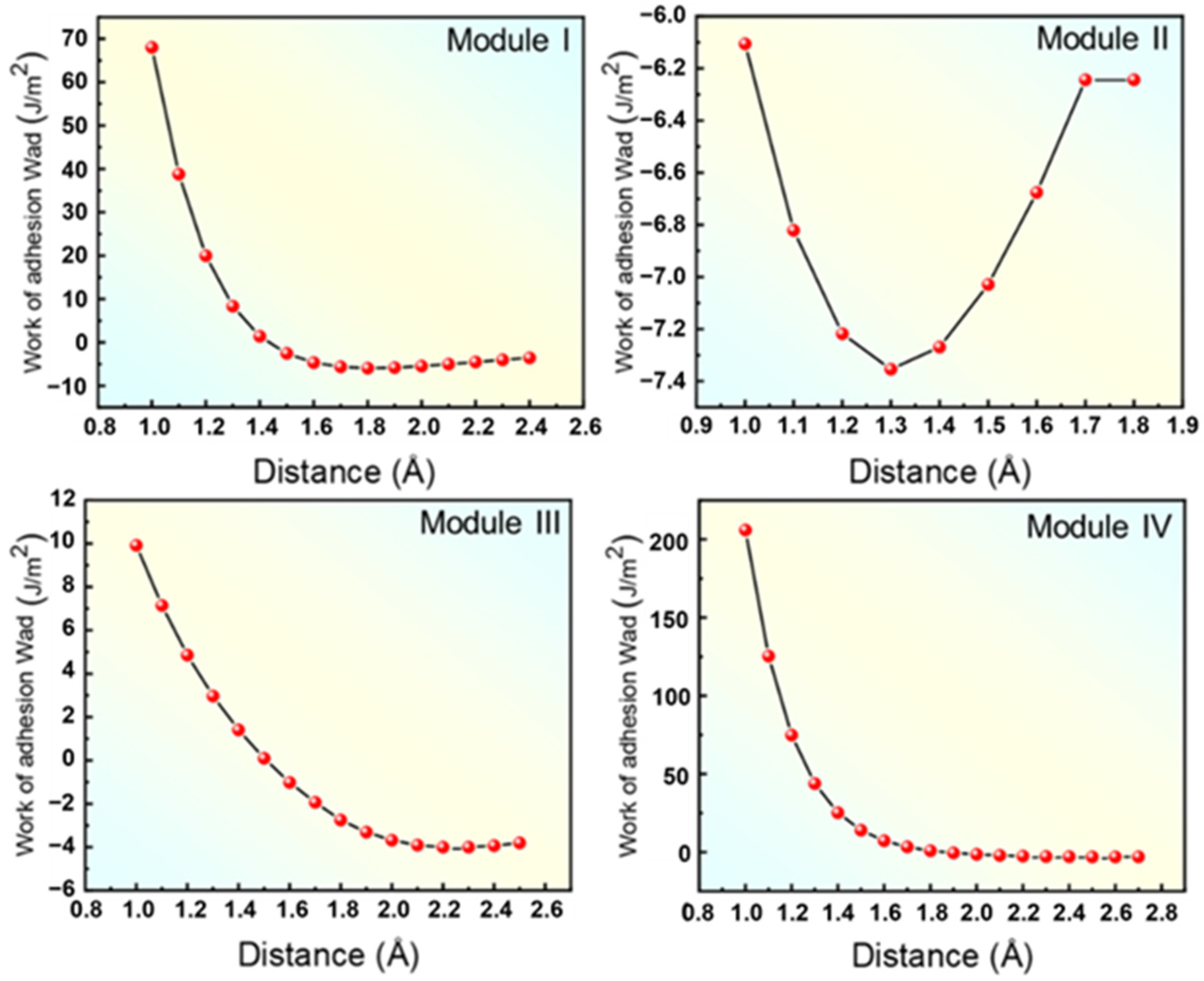
- Four kinds of interface models between (110)NiTi and (200)TiN with different atomic layer arrangements were constructed. In addition, all four interface models had a positive work of adhesion (Wad), indicating stable interface bonds between (110)NiTi and (200)TiN. In contrast, Model III had the highest Wad of 9.773 J/m2, attributed to the strong N-Ti bonds formed at the interface after relaxation.
- (2)
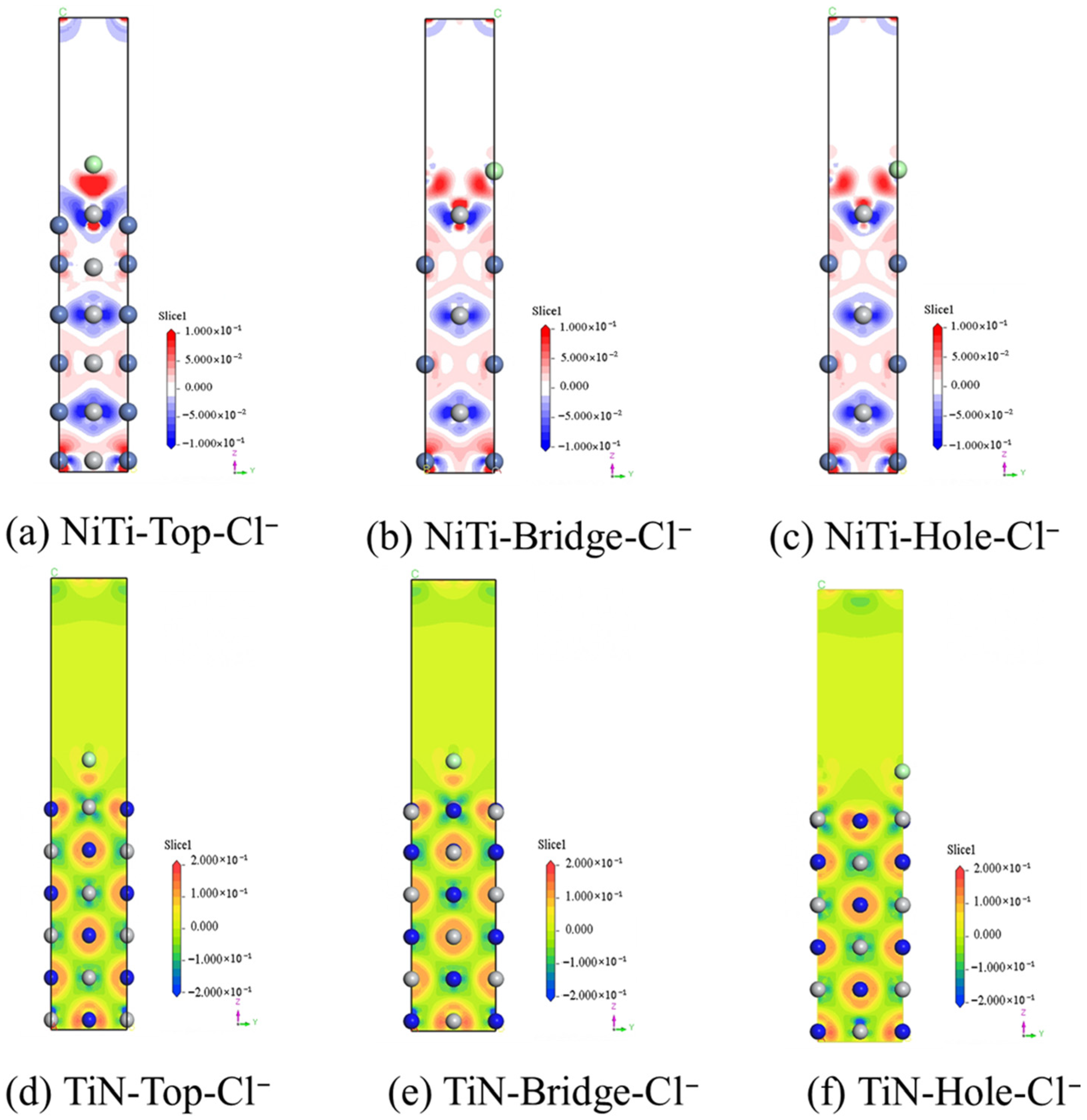
- A significant negative density difference around Ti atoms and a significant positive density difference around N atoms were observed at the interface between (110)NiTi and (200)TiN, indicating strong interface bonding stability between (110)NiTi and (200)TiN. The Ti-d orbitals of (110)NiTi and the N-p orbitals of (200)TiN overlapped at approximately −3 eV, indicating orbital hybridization and bonding formation of Ti-N.
- (3)
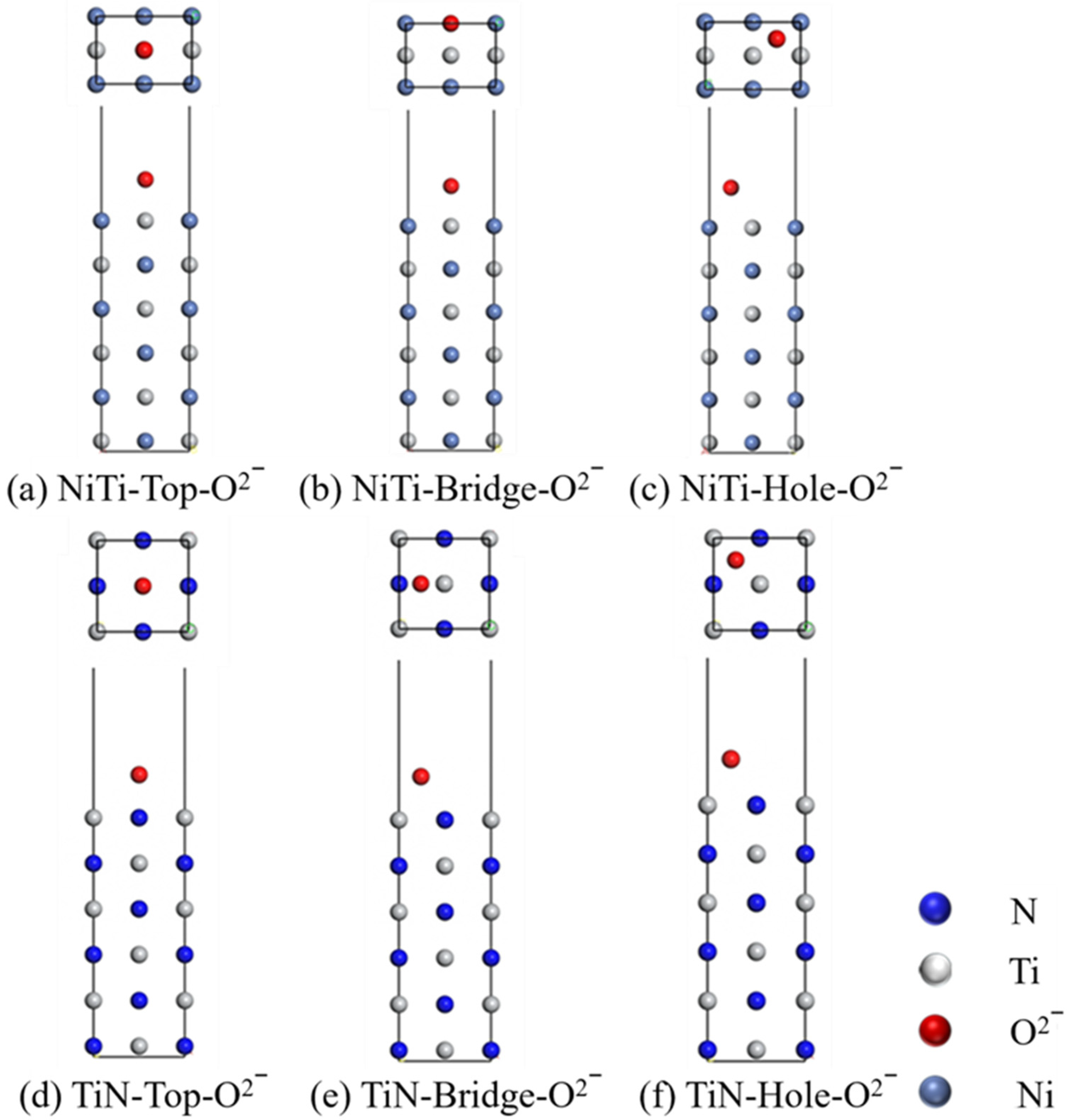
- The adsorption capacities of Cl− on the surface of (110)NiTi and (200)TiN were investigated. The adsorption energies of Cl− on the surface of (110)NiTi are lower than that of (200)TiN, regardless of the Cl− adsorption sites. This suggested that (110)NiTi was more prone to reacting and forming bonds with Cl−, indicating a higher thermodynamic driving force for corrosion initiation. Therefore, the introduction of a TiN layer on the surface of NiTi alloy can effectively enhance its corrosion resistance properties by acting as a more inert barrier layer.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alvarez, K.; Nakajima, H. Metallic Scaffolds for Bone Regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Chung, C.Y.; Chu, C.L.; Wang, S.D. Porous TiNi shape memory alloy with high strength fabricated by self-propagating high-temperature synthesis. Mater. Lett. 2004, 58, 1683–1686. [Google Scholar] [CrossRef]
- Elahinia, M.; Shayesteh-Moghaddam, N.; Taheri-Andani, M.; Amerinatanzi, A.; Bimber, B.A.; Hamilton, R.F. Fabrication of NiTi through additive manufacturing: A review. Prog. Mater. Sci. 2016, 83, 630–663. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, D.; Chen, Y.; Xie, L.; Wang, L.; Zhang, L.C.; Lu, W.; Chen, G. Non-negligible role of gradient porous structure in superelasticity deterioration and improvement of NiTi shape memory alloys. J. Mater. Sci. Technol. 2024, 186, 48–63. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Gao, Z.; Cai, W. Martensitic transformation and microstructure of dual-phase Ti44Ni47Nb9 shape memory alloy after high-velocity impact. Mater. Charact. 2016, 122, 162–169. [Google Scholar] [CrossRef]
- Kabla, M.; Seiner, H.; Musilova, M.; Landa, M.; Shilo, D. The relationships between sputter deposition conditions, grain size, and phase transformation temperatures in NiTi thin films. Acta Mater. 2014, 70, 79–91. [Google Scholar] [CrossRef]
- Khanlari, K.; Ramezani, M.; Kelly, P. 60NiTi: A Review of Recent Research Findings, Potential for Structural and Mechanical Applications, and Areas of Continued Investigations. Trans. Indian Inst. Met. 2018, 71, 781–799. [Google Scholar] [CrossRef]
- Miao, W.; Mi, X.; Xu, G.; Li, H. Effect of surface preparation on corrosion properties and nickel release of a NiTi alloy. Rare Met. 2006, 25, 243–245. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and technology of magnetron sputtering discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Fan, Y. Interfacial Modification of Ti3AlC2/Cu Composites by Multi-Arc Ion Plating Titanium. Coatings 2022, 12, 1754. [Google Scholar] [CrossRef]
- Li, B.; Zheng, L.J.; Chen, X.M.; Zhang, H. Microstructure evolution of Ni-rich NiTi(-Hf) alloy during thermal oxidation at different temperatures. Mater. Chem. Phys. 2023, 295, 127114. [Google Scholar] [CrossRef]
- Ng, C.H.; Chan, C.W.; Man, H.C.; Waugh, D.G.; Lawrence, J. NiTi shape memory alloy with enhanced wear performance by laser selective area nitriding for orthopaedic applications. Surf. Coat. Technol. 2017, 309, 1015–1022. [Google Scholar] [CrossRef]
- Czarnowska, E.; Borowski, T.; Sowińska, A.; Lelątko, J.; Oleksiak, J.; Kamiński, J.; Tarnowski, M.; Wierzchoń, T. Structure and properties of nitrided surface layer produced on NiTi shape memory alloy by low temperature plasma nitriding. Appl. Surf. Sci. 2015, 334, 24–31. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Shao, M.; Zhang, Z.; Wang, C.; Yan, J.; Lu, J.; Zhang, L.; Xie, B.; He, Y.; et al. Characterization and electrochemical behavior of a multilayer-structured Ti–N layer produced by plasma nitriding of electron beam melting TC4 alloy in Hank’s solution. Vacuum 2023, 208, 111737. [Google Scholar] [CrossRef]
- Li, G.F.; Zheng, H.Z.; Shu, X.Y.; Peng, P. Structural stability of characteristic interface for NiTi/Nb Nanowire: First-Principle study. Met. Mater. Int. 2016, 22, 69–74. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Z.; Gao, W.; Sun, B.; Yang, Y.; Zhao, D.; Yan, M.; Fu, Y.D. The effect of impurities on the adhesion behavior of TiN(1 1 1)/α-Ti(0 0 0 1) semi-coherent interface: A first-principles investigation. Surf. Interf. 2022, 35, 102488. [Google Scholar] [CrossRef]
- André, V.F.; Patrícia, F.R.; Daniela, S.; Ana, S.R. Exploring the Influence of the Deposition Parameters on the Properties of NiTi Shape Memory Alloy Films with High Nickel Content. Coatings 2024, 14, 138. [Google Scholar] [CrossRef]
- Yu, F.; Liu, Y. First-Principles Calculations of Structural, Mechanical, and Electronic Properties of the B2-Phase NiTi Shape-Memory Alloy Under High Pressure. Computation 2019, 7, 57. [Google Scholar] [CrossRef]
- Strutt, E.R.; Radetic, T.; Olevsky, E.A.; Meyers, M.A. Combustion synthesis / quasi-isostatic pressing of TiC0.7–NiTi cermets: Microstructure and transformation characteristics. J. Mater. Sci. 2008, 43, 5905–5923. [Google Scholar] [CrossRef]
- Lazar, P.; Redinger, J.; Podloucky, R. Density functional theory applied to VN/TiN multilayers. Phys. Rev. B 2007, 76, 174112. [Google Scholar] [CrossRef]
- Marlo, M.; Milman, V. Density-functional study of bulk and surface properties of titanium nitride using different exchange-correlation functionals. Phys. Rev. B 2000, 62, 2899–2907. [Google Scholar] [CrossRef]
- White, J.A.; Bird, D.M. Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations. Phys. Rev. B 1994, 50, 4954–4957. [Google Scholar] [CrossRef]
- Wei, K.M.; Hu, K.M.; Sa, B.S.; Wu, B. Pressure-induced structure, electronic, thermodynamic and mechanical properties of Ti2AlNb orthorhombic phase by first-principles calculations. Rare Met. 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Yang, W.; Yang, J.; Pang, M.; Zhan, Y. Atomic structure, stability and electronic properties of S(Al2CuMg)/Al interface: A first-principles study. Intermetallics 2018, 93, 329–337. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Yan, L.T.; Yu, J.L.; Houston, J.; Flores, N.; Luo, H.M. Biomass derived porous nitrogen doped carbon for electrochemical devices. Green Energy Environ. 2017, 2, 84. [Google Scholar] [CrossRef]
- Li, R.Y.; Duan, T.H. Electronic structures and thermodynamic properties of HfAl3 in L12, D022 and D023 structures. Trans. Nonferr. Met. Soc. China 2016, 26, 2404–2412. [Google Scholar] [CrossRef]
- Khanlari, K.; Ramezani, M.; Kelly, P.; Cao, P.; Neitzert, T. Comparison of the reciprocating sliding wear of 58Ni39Ti-3Hf alloy and baseline 60NiTi. Wear 2018, 408–409, 120–130. [Google Scholar] [CrossRef]



| Structure | Methods or References | a (Å) | b (Å) | c (Å) | |
|---|---|---|---|---|---|
| B2-NiTi | Current study | GGA-PBE | 3.031 | 3.031 | 3.031 |
| Other calculations | GGA-PBE | 3.005 | 3.005 | 3.005 | |
| Experiment | Strutt et al. | 2.998 | 2.998 | 2.998 | |
| TiN | Current study | GGA-PBE | 4.261 | 4.261 | 4.261 |
| Other calculations | GGA-PBE | 4.270 | 4.270 | 4.270 | |
| Experiment | Kutschej et al. | 4.240 | 4.240 | 4.240 | |
| ETiN (eV) | ENiTi (eV) | ETiN+NiTi (eV) | Area (Å) | d (Å) | Wad (J/m2) | |
|---|---|---|---|---|---|---|
| Module I | −11,796.72 | −14,787.35 | −26,592.03 | 4.75 × 3.02 | 1.72 | 8.873 |
| Module II | 14,466.10 | −14,787.35 | −29,257.93 | 4.75 × 3.02 | 2.54 | 4.988 |
| Module III | −11,796.73 | −14,787.35 | −26,592.84 | 4.75 × 3.02 | 1.28 | 9.773 |
| Module IV | −14,466.12 | −14,787.42 | −29,258.44 | 4.75 × 3.02 | 2.26 | 5.457 |
| Structure | Eint (eV) |
|---|---|
| (110)NiTi-Top | 0.618518267 |
| (110)NiTi-Bridge | 0.618599997 |
| (110)NiTi-Hole | 0.618399257 |
| (200)TiN-Top | 0.688416877 |
| (200)TiN-Bridge | 1.128183357 |
| (200)TiN-Hole | 1.362865157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; He, H.; Yang, H.; Li, W.; Gao, Z.; Wang, H.; Yi, X. An Assessment of TiN Formation on NiTi Alloy and the Corrosion Resistance of TiN/NiTi Alloy Using First-Principles Calculation. Metals 2025, 15, 1089. https://doi.org/10.3390/met15101089
Wang Y, He H, Yang H, Li W, Gao Z, Wang H, Yi X. An Assessment of TiN Formation on NiTi Alloy and the Corrosion Resistance of TiN/NiTi Alloy Using First-Principles Calculation. Metals. 2025; 15(10):1089. https://doi.org/10.3390/met15101089
Chicago/Turabian StyleWang, Yunfei, Haodong He, Huan Yang, Weijian Li, Zhiyong Gao, Haizhen Wang, and Xiaoyang Yi. 2025. "An Assessment of TiN Formation on NiTi Alloy and the Corrosion Resistance of TiN/NiTi Alloy Using First-Principles Calculation" Metals 15, no. 10: 1089. https://doi.org/10.3390/met15101089
APA StyleWang, Y., He, H., Yang, H., Li, W., Gao, Z., Wang, H., & Yi, X. (2025). An Assessment of TiN Formation on NiTi Alloy and the Corrosion Resistance of TiN/NiTi Alloy Using First-Principles Calculation. Metals, 15(10), 1089. https://doi.org/10.3390/met15101089






