Effect of High-Temperature Isothermal Annealing on the Structure and Properties of Multicomponent Compact Ti-Al(Nb,Mo,B)-Based Materials Fabricated via Free SHS-Compression
Abstract
1. Introduction
2. Materials and Methods
2.1. Objects
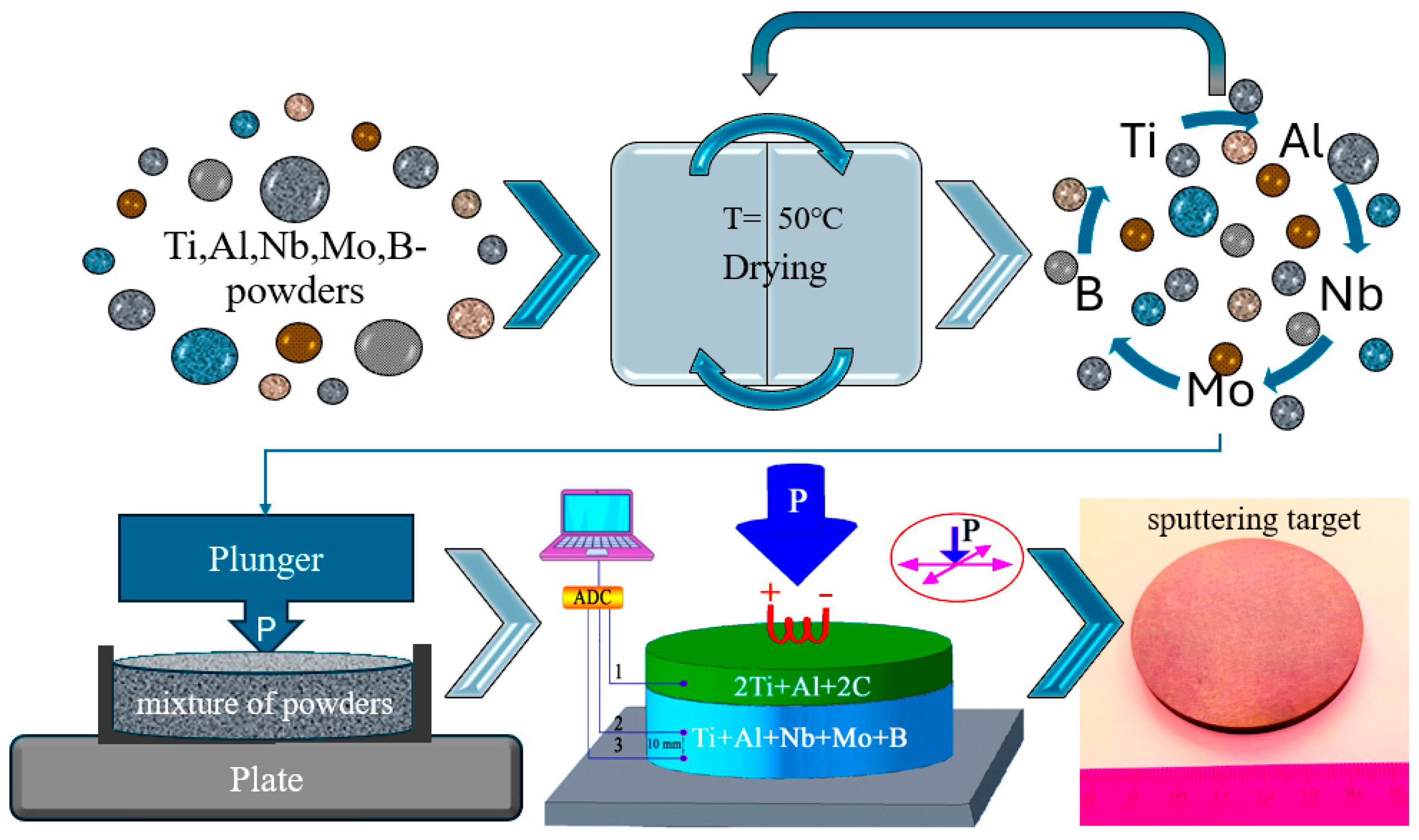
2.2. Method of Preparation
2.3. Research Techniques
3. Results and Discussion
4. Conclusions
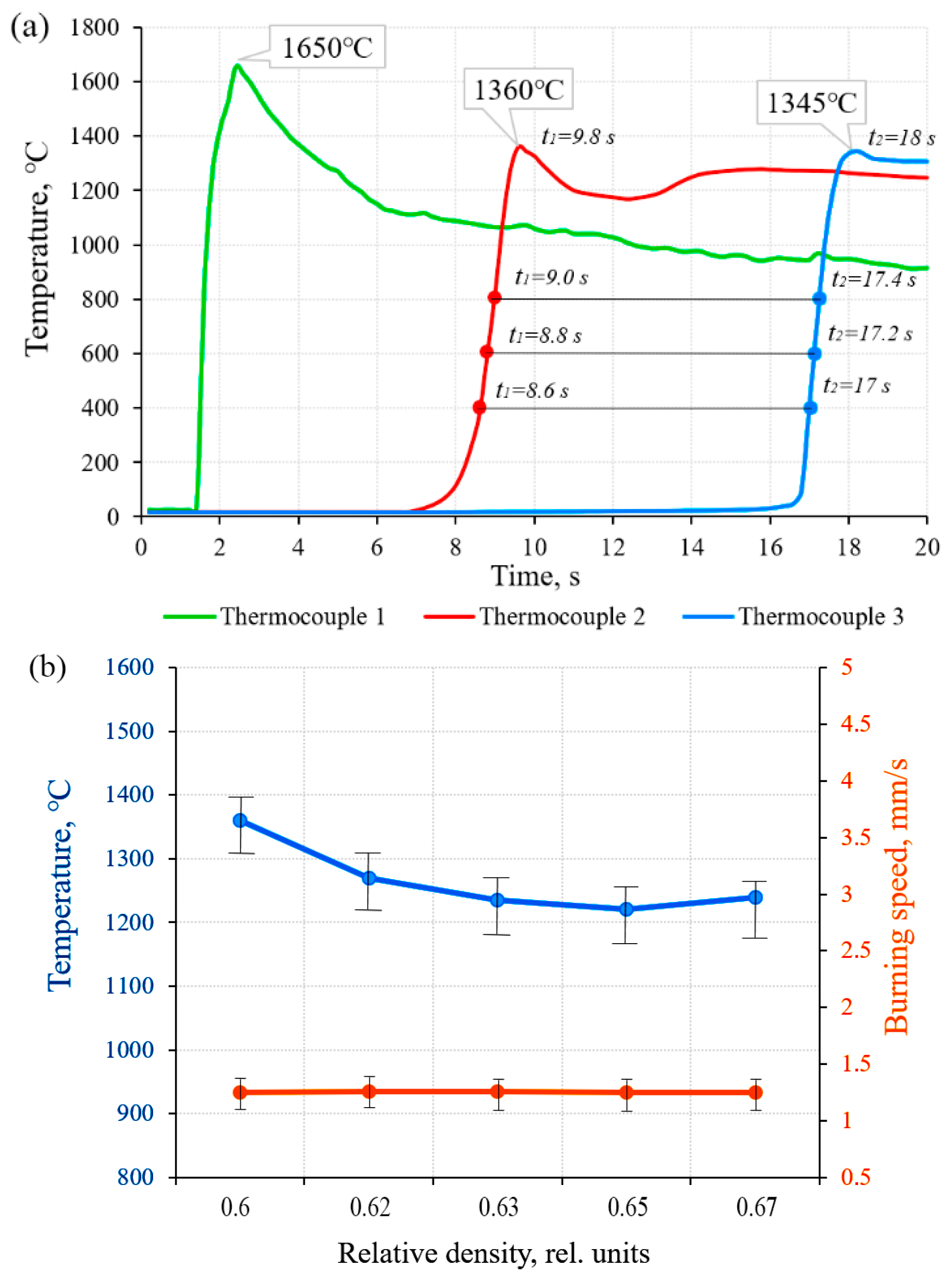
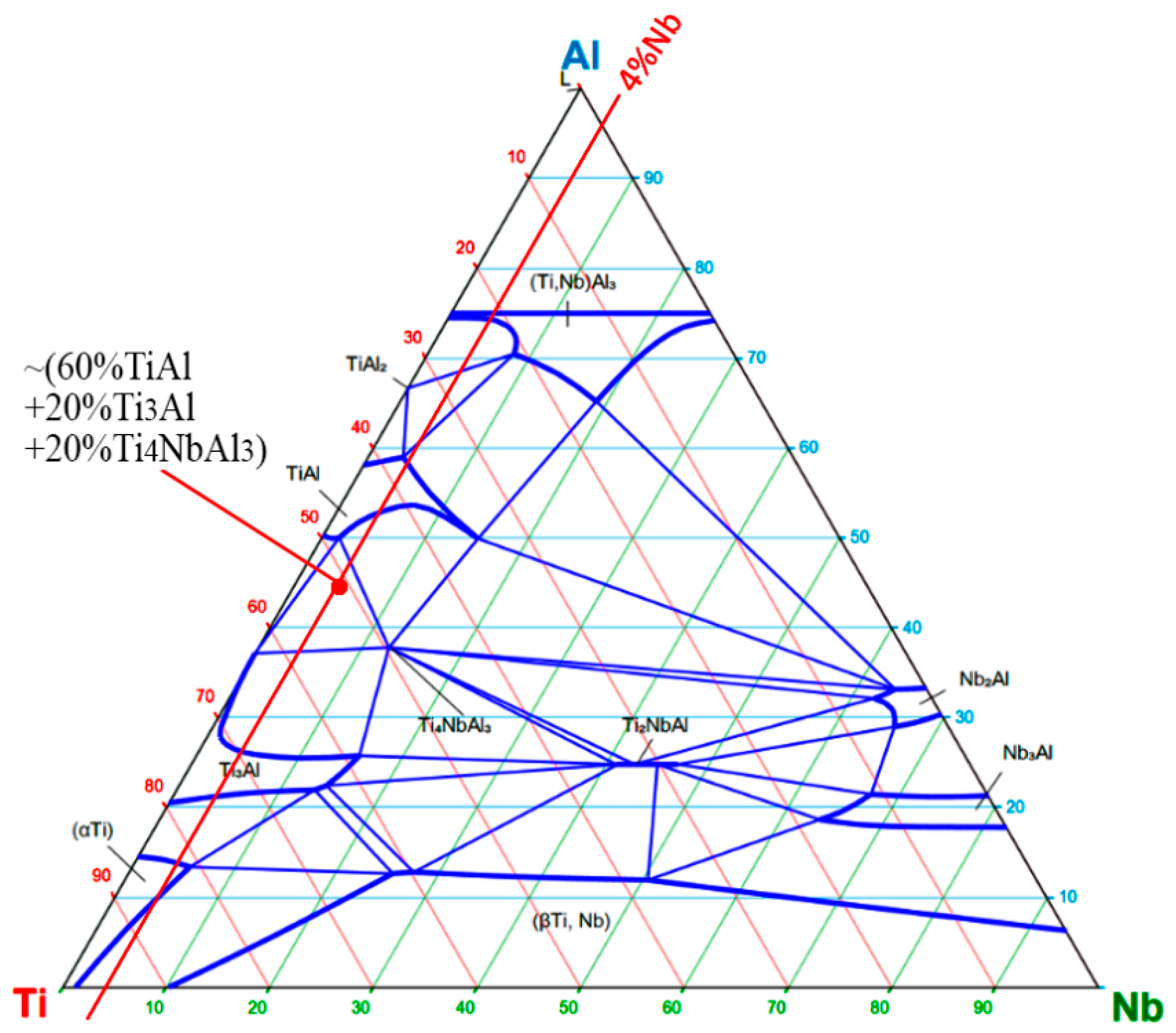
- Optimal synthesis parameters for initiating combustion and achieving maximum performance for the 51.85Ti–43Al–4Nb–1Mo–0.15B (wt%) composition were established. These include fabricating powder compacts with a relative density of 0.42 and employing a highly exothermic 3Ti-Al-2C (molar ratio) chemical furnace. This configuration ensures a maximum combustion initiation temperature of 1650 °C, which is critical for sustaining the reaction in this low-exothermicity system.
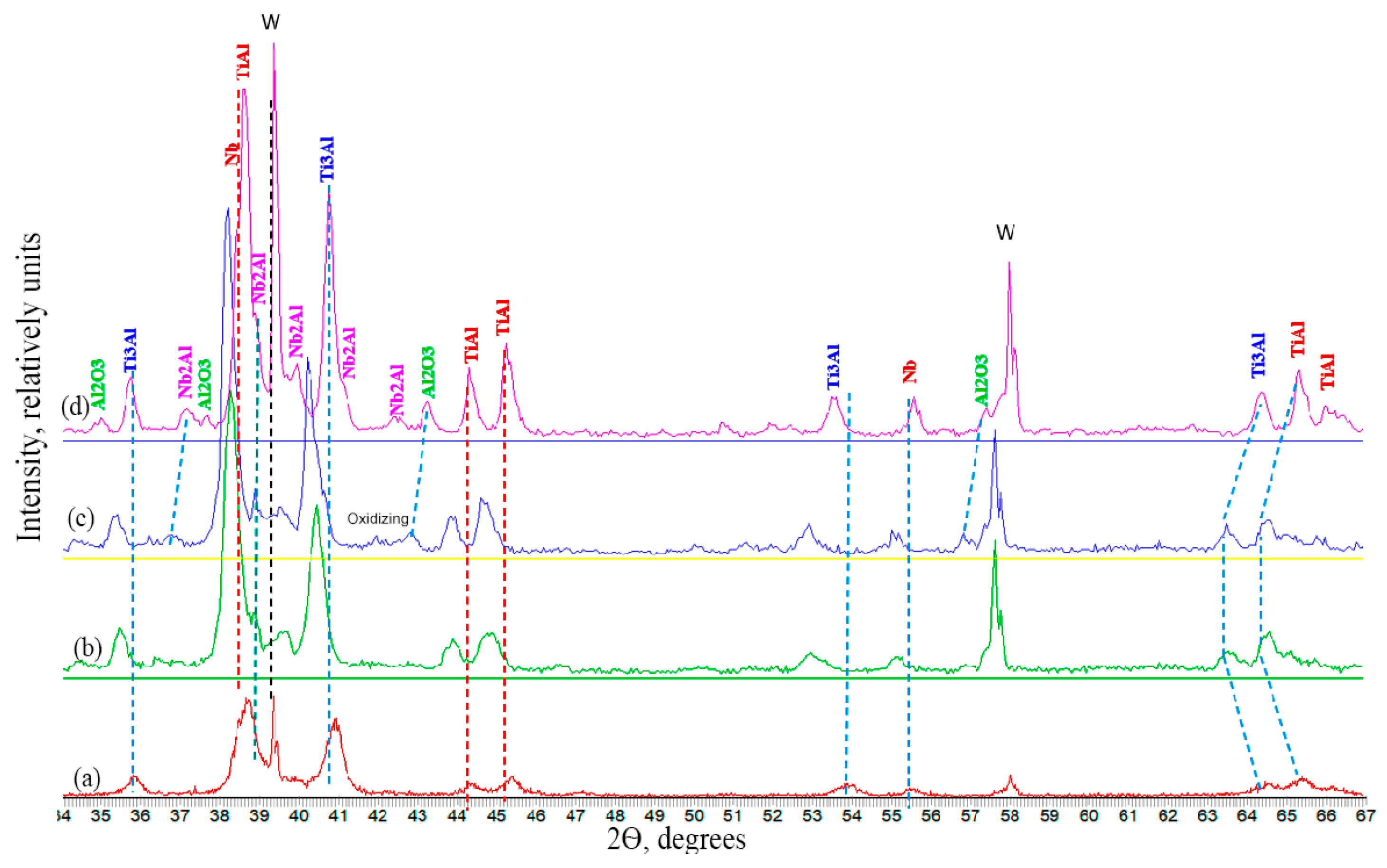
- The materials synthesized via free SHS compression exhibit a characteristic layered macrostructure with a wavy morphology. The layers are aligned parallel to the material flow direction during compression and perpendicular to the applied load. The microstructure consists of alternating intermetallic phases (TiAl (γ) and Ti3Al (α2)) with a minor amount of TiAl3, and uniformly distributed niobium particles throughout the volume. Interdiffusion of titanium and aluminum occurred at the boundaries of molybdenum and niobium grains, leading to the formation of binary (e.g., MoAl2) and ternary (Ti-Al-Mo, Ti-Al-Nb) compounds.
- Isothermal annealing at 1000 °C for 3 h promotes significant phase evolution, resulting in an increased volume fraction of the (Al0.86Nb0.14)(Ti2.85Nb0.15) ternary solid solution and the formation of the Nb2Al intermetallic phase. This phase transformation confirms a solid-state diffusion mechanism as the primary driver for the formation of these compounds during the annealing process.
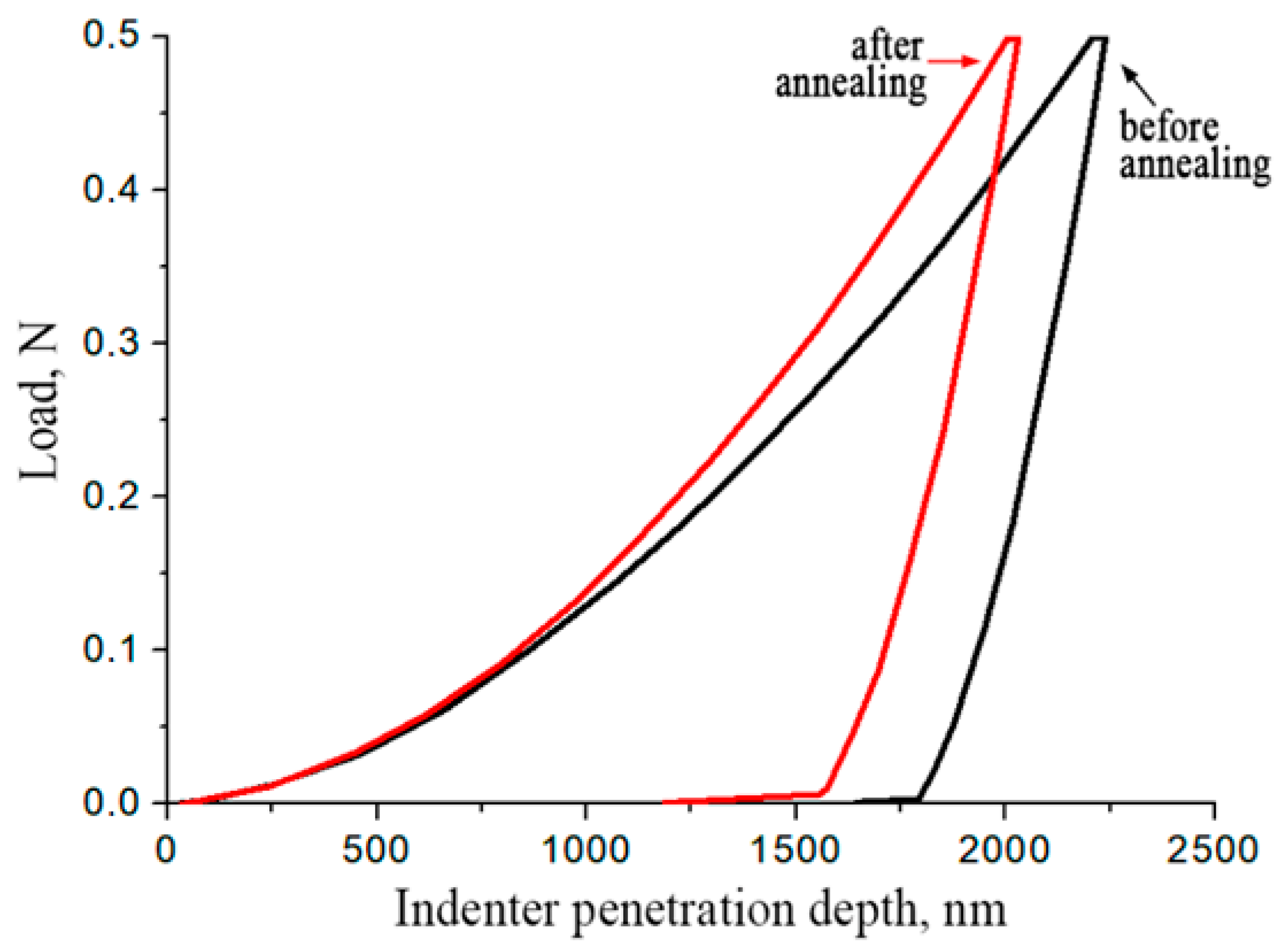
- The phase and structural changes induced by isothermal annealing directly lead to an enhancement of mechanical properties: (i) microhardness and elastic recovery increased by a factor of 1.2, and (ii) Young’s modulus exhibited a modest increase of ~10 GPa. The maximum values achieved for the annealed material were: 7.4 GPa (microhardness), 31.8% (elastic recovery), and 200 GPa (Young’s modulus). This improvement is attributed to the formation of a harder and more stable phase assemblage.
- The authors first demonstrated the feasibility of synthesizing multicomponent materials based on the Ti-Al-(Nb,Mo) system under conditions that combine combustion processes and high-temperature shear deformation. The results of the measured physico-mechanical properties of the obtained materials indicate their promising potential for use both as structural and functional components, e.g., as targets for depositing protective coatings via PVD methods.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, Q.; Bu, L.; Li, Y.; Guan, Y.; Wang, P.; Lou, Z.; Luo, Y. Effects of Ti3SiC2 on Microstructure and Properties of TiC0.4 Enhanced TiAl Matrix Composites. Mater. Chem. Phys. 2023, 297, 127330. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl Alloys in Commercial Aircraft Engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Ye, X.-C.; Xiao, K.-Q.; Cao, R.-X.; Wu, H.; Zhao, G.; Li, B. Microstructure Evolution and Microhardness of TiAl Based Alloy Blade by Vacuum Suction Casting. Vacuum 2019, 163, 186–193. [Google Scholar] [CrossRef]
- Wei, D.-B.; Zhou, X.; Li, F.-K.; Li, M.-F.; Li, S.-Q.; Zhang, P.-Z. Effects of Plasma Surface Ta Alloying on the Tribology Behavior of γ-TiAl. Trans. Inst. Min. Metall. Sect. B 2021, 57, 97–104. [Google Scholar] [CrossRef]
- Kagerer, S.; Hudak, O.E.; Wojcik, T.; Hahn, R.; Davydok, A.; Schloffer, M. Oxidation protection of TNM alloys with Al-rich γ-TiAl-based coatings. J. Alloys Compd. 2023, 969, 172343. [Google Scholar] [CrossRef]
- Nochovnaya, N.A.; Shiryaev, A.A.; Pomel’nikova, A.S.; Yakovlev, A.L.; Alekseev, E.B. Structural-Phase Composition and Mechanical Properties of Experimental Compositions of High-Strength Pseudo-β-Titanium Alloy Containing Rare-Earth Elements. Met. Sci. Heat Treat. 2020, 62, 152–160. [Google Scholar] [CrossRef]
- Mosleh, A.O.; Kotov, A.D.; Vidal, V.; Mochugovskiy, A.G.; Velay, V.; Mikhaylovskaya, A.V. Initial Microstructure Influence on Ti–Al–Mo–v Alloy’s Superplastic Deformation Behavior and Deformation Mechanisms. Mater. Sci. Eng. A 2020, 802, 140626. [Google Scholar] [CrossRef]
- Brotzu, A.; Felli, F.; Pilone, D. Effect of Alloying Elements on the Behaviour of TiAl-Based Alloys. Intermetallics 2014, 54, 176–180. [Google Scholar] [CrossRef]
- Soyama, J.; Limberg, W.; Ebel, T.; Pyczak, F. Sintering and Creep Resistance of Powder-Metallurgy-Processed Ti-(43–47)Al-5Nb-0.2B-0.2C. Adv. Eng. Mater. 2020, 22, 8. [Google Scholar] [CrossRef]
- Szkliniarz, W.; Szkliniarz, A. The Characteristics of TiAl-Based Alloys Melted in Graphite Crucibles. Mater. Sci. Technol. 2017, 35, 297–305. [Google Scholar] [CrossRef]
- Shaaban, A.; Hayashi, S.; Takeyama, M. A Comparative Study on the Oxidation Behaviours of a TNM Alloy in Argon and Oxygen Atmospheres at 650 °C. Corros. Sci. 2021, 185, 109415. [Google Scholar] [CrossRef]
- Toshimitsu, T.; Taketo, F.; Kazuhiro, M. Comparison of the impact resistance of TiAl4822 and TNM alloy under expected service conditions of jet engine blades. Intermetallics. 2025, 183, 108793. [Google Scholar] [CrossRef]
- Fang, H.; Chen, R.; Liu, Y.; Tan, Y.; Su, Y.; Ding, H.; Guo, J. Effects of Niobium on Phase Composition and Improving Mechanical Properties in TiAl Alloy Reinforced by Ti2AlC. Intermetallics 2019, 115, 106630. [Google Scholar] [CrossRef]
- Cobbinah, P.V.; Matizamhuka, W.; Machaka, R.; Shongwe, M.B.; Yamabe-Mitarai, Y. The Effect of Ta Additions on the Oxidation Resistance of SPS-Produced TiAl Alloys. Int. J. Adv. Manuf. Technol. 2020, 106, 3203–3215. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, H.; Chen, R.; Su, Y.; Ding, H.; Guo, J. A Comparative Study on Microstructure and Mechanical Properties of Ti--43/46Al–5Nb–0.1B Alloys Modified by Mo. Adv. Eng. Mater. 2019, 22, 1901075. [Google Scholar] [CrossRef]
- Ostrovskaya, O.; Badini, C.; Baudana, G.; Padovano, E.; Biamino, S. Thermogravimetric Investigation on Oxidation Kinetics of Complex Ti-Al Alloys. Intermetallics 2018, 93, 244–250. [Google Scholar] [CrossRef]
- Hao, Y.; Quan, Z.; Jiabo, F.; Yanzhen, H.; Jingjing, L.; Jinguo, L.; Wei, X. The design of oxidation resistant Ni superalloys for additive manufacturing. Addit. Manuf. 2025, 97, 104616. [Google Scholar] [CrossRef]
- Feng, L.; Li, B.; Li, Q.; Gao, Y.; Pei, Z.; Liang, C. Enhancement of Mechanical Properties and Oxidation Resistance of TiAl Alloy with Addition of Nb and Mo Alloying Elements. Mater. Chem. Phys. 2024, 316, 129148. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Bai, Q.; Xie, G. Correlation of Microstructure and Mechanical Properties of Ti2AlNb Manufactured by SLM and Heat Treatment. Intermetallics 2021, 139, 107367. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, R.; Fang, H.; Liu, Y.; Cui, H.; Su, Y.; Guo, J.; Fu, H. Enhanced Strength and Ductility in Ti46Al4Nb1Mo Alloys via Boron Addition. J. Mater. Sci. Technol. 2021, 102, 16–23. [Google Scholar] [CrossRef]
- Banerjee, T.; Banumathy, S.; Shekhar, S.; Gupta, D.K.; Bhattacharjee, A.; Kar, S.K. Exploring an Alloy Space in a New Generation γ-TiAl System Ti–XAl–YNb–1Cr–1Mo–0.2C–0.2B (At.%)): Aspects of Phase Transformation, Microstructure and Texture. Mater. Charact. 2024, 217, 114332. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, Y.; Li, M.; Xu, L.; Tian, J.; Zhang, D.; Yang, J. The Improved Properties and Microstructure of β-Solidify TiAl Alloys by Boron Addition and Multi Steps Forging Process. Sci. Rep. 2019, 9, 12393. [Google Scholar] [CrossRef]
- Loginov, P.A.; Kaplanskii, Y.Y.; Markov, G.M.; Patsera, E.I.; Vorotilo, K.V.; Korotitskiy, A.V.; Shvyndina, N.V.; Levashov, E.A. Structural and Mechanical Properties of Ti–Al–Nb–Mo–B Alloy Produced from the SHS Powder Subjected to High-Energy Ball Milling. Mater. Sci. Eng. A 2021, 814, 141153. [Google Scholar] [CrossRef]
- Decker, S.; Lindemann, J.; Kruger, L. Metal Matrix Composites Based on Ti-6242 Synthesized by Spark Plasma Sintering. Mater. Sci. Eng. A 2018, 732, 35–40. [Google Scholar] [CrossRef]
- Bernal, D.; Chamorro, X.; Hurtado, I.; Madariaga, I. Evolution of Lamellar Microstructures in a Cast TNM Alloy Modified with Boron through Single-Step Heat Treatments. Intermetallics 2020, 124, 106842. [Google Scholar] [CrossRef]
- Pilone, D.; Felli, F. Isothermal Oxidation Behaviour of TiAl–Cr–Nb–B Alloys Produced by Induction Melting. Intermetallics 2012, 26, 36–39. [Google Scholar] [CrossRef]
- Galetz, M.C.; Ulrich, A.S.; Oskay, C.; Fahsing, D.; Laska, N.; Schulz, U.; Schutze, M. Oxidation-Induced Microstructural Changes of the TiAl TNM-B1 Alloy after Exposure at 900 °C in Air. Intermetallics 2020, 123, 106830. [Google Scholar] [CrossRef]
- Stolin, A.M.; Bazhin, P.M.; Konstantinov, A.S.; Alymov, M.I. Production of Large Compact Plates from Ceramic Powder Materials by Free SHS Compaction. Dokl. Chem. 2018, 480, 136–138. [Google Scholar] [CrossRef]
- Bazhina, A.D.; Bazhin, P.M.; Chizhikov, A.P.; Konstantinov, A.S.; Stolin, A.M. Influence of High-Temperature Annealing on Structure of Titanium Aluminide Materials Obtained by Combustion and High-Temperature Shear Deformation. Intermetallics 2021, 139, 107313. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Boldyreva, E.V.; Boldyrev, V.V. Role of Mixing and Milling in Mechanochemical Synthesis (Review). Russ. J. Inorg. Chem. 2021, 66, 433–453. [Google Scholar] [CrossRef]
- Chizhikov, A.P.; Konstantinov, A.S.; Bazhin, P.M. Self-Propagating High-Temperature Synthesis of Ceramic Material Based on Aluminum-Magnesium Spinel and Titanium Diboride. Russ. J. Inorg. Chem. 2021, 66, 1115–1120. [Google Scholar] [CrossRef]
- Bazhina, A.; Konstantinov, A.; Chizhikov, A.; Bazhin, P.; Stolin, A.; Avdeeva, V. Structure and Mechanical Characteristics of a Layered Composite Material Based on TiB/TiAl/Ti. Ceram. Int. 2022, 48, 14295–14300. [Google Scholar] [CrossRef]
- Bazhin, P.; Konstantinov, A.; Chizhikov, A.; Antipov, M.; Stolin, P.; Avdeeva, V.; Antonenkova, A. Compactability Regularities Observed during Cold Uniaxial Pressing of Layered Powder Green Samples Based on Ti-Al-Nb-Mo-B and Ti-B. Metals 2023, 13, 1827. [Google Scholar] [CrossRef]
- Vardanyan, E.L.; Ramazanov, K.N.; Nagimov, R.S.; Nazarov, A.Y. Properties of Intermetallic Ti al Based Coatings Deposited on Ultrafine Grained Martensitic Steel. Surf. Coat. Technol. 2020, 389, 125657. [Google Scholar] [CrossRef]
- Tulina, A.A.; Nazarov, A.Y.; Ramazanov, K.N.; Mukhamadeev, V.R.; Khusainov, Y.G.; Nikolaev, A.A.; Maslov, A.A.; Oleinik, A.V. Investigation of Properties of TiAlCO Wear-Resistant Coatings. Russ. Phys. J. 2025, 68, 49–55. [Google Scholar] [CrossRef]
- Oleinik, A.V.; Nikolaev, A.A.; Ramazanov, K.N.; Nazarov, A.Y.; Haitkulov, A.R.; Muhamadeev, V.P.; Maslov, A.A.; Khusainov, Y.G. Investigation of Shadow Region Influence on TiN Coating Properties. Russ. Phys. J. 2025, 68, 214–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Kong, F.; Sun, L.; Chen, Y. A High-Performance β-Solidifying TiAl Alloy Sheet: Multi-Type Lamellar Microstructure and Phase Transformation. Mater. Charact. 2018, 138, 136–144. [Google Scholar] [CrossRef]
- Song, L.; Appel, F.; Wang, L.; Oehring, M.; Hu, X.; Stark, A.; He, J.; Lorenz, U.; Zhang, T.; Lin, J.; et al. New Insights into High-Temperature Deformation and Phase Transformation Mechanisms of Lamellar Structures in High Nb-Containing TiAl Alloys. Acta Mater. 2020, 186, 575–586. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, D.; Zhu, J.; Xiao, H.; Liu, C.T.; Wang, Y. Non-Conventional Transformation Pathways and Ultrafine Lamellar Structures in γ-TiAl Alloys. Acta Mater. 2020, 189, 25–34. [Google Scholar] [CrossRef]

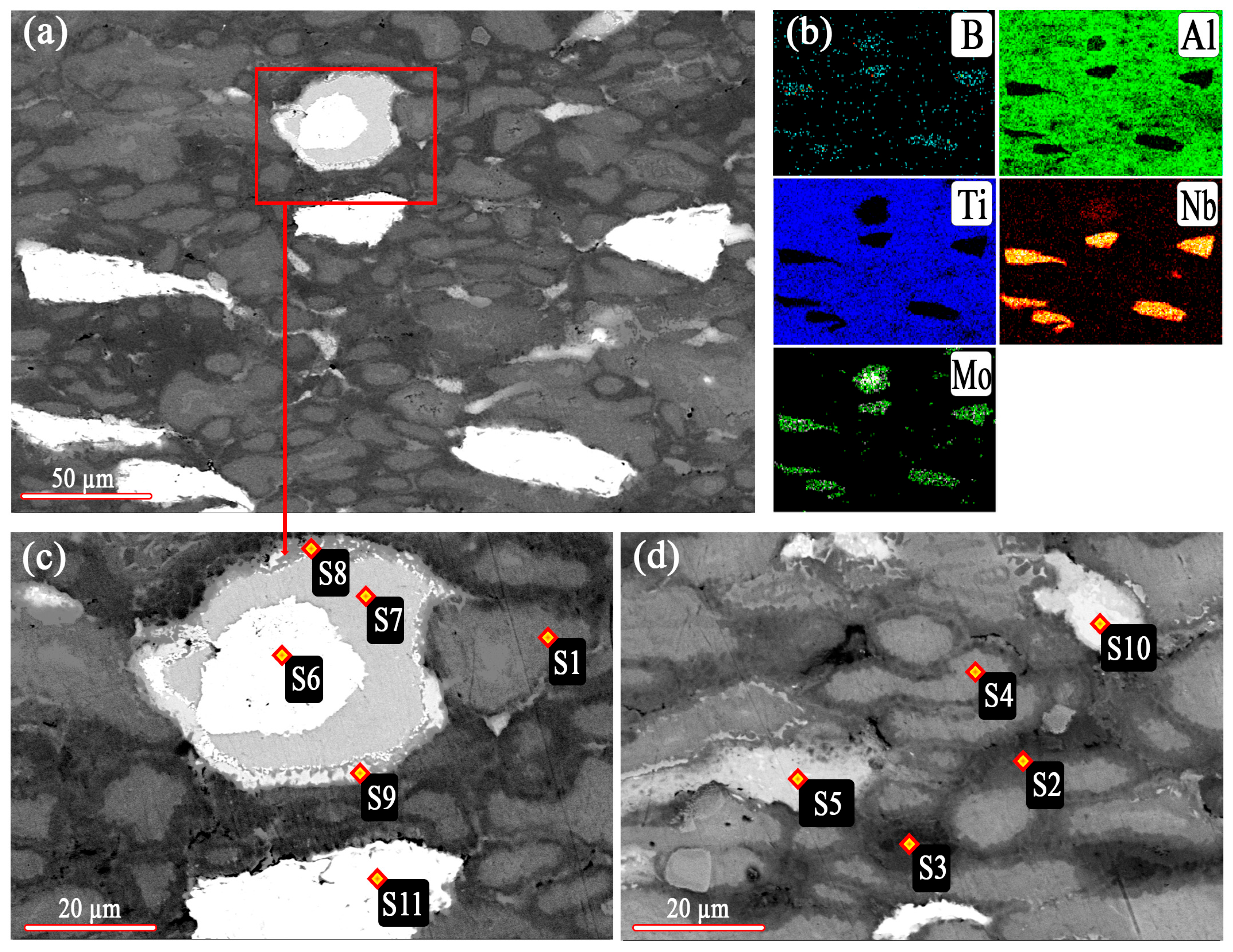


| Spectrum | Content of Chemical Elements, wt% | ||||
|---|---|---|---|---|---|
| B | Al | Ti | Nb | Mo | |
| S1 | - | 32.95 | 63.72 | - | - |
| S2 | - | 35.97 | 64.03 | - | - |
| S3 | - | 39.49 | 60.51 | - | - |
| S4 | - | 20.36 | 79.64 | - | - |
| S5 | - | 42.00 | 10.59 | - | 47.41 |
| S6 | - | 5.61 | - | - | 94.39 |
| S7 | - | 37.72 | - | - | 62.28 |
| S8 | - | 19.60 | 22.34 | - | 58.06 |
| S9 | - | 37.05 | 29.29 | - | 33.66 |
| S10 | - | 37.03 | 2.31 | - | 60.66 |
| S11 | - | 4.35 | - | 88.53 | - |
| Spectrum | Content of Chemical Elements, wt% | ||||
|---|---|---|---|---|---|
| B | Al | Ti | Nb | Mo | |
| S1 | 6.85 | - | - | 82.11 | - |
| S2 | 13.11 | 15.68 | 26.96 | 40.00 | - |
| S3 | 14.42 | 18.82 | 32.02 | 30.91 | - |
| S4 | - | 21.96 | 64.11 | 13.92 | - |
| Phase | Lattice Parameters, Å | Crystal System | Space Group | ICDD PDF2 No. |
|---|---|---|---|---|
| Nb | a = 3.30332 | cubic | Im-3m | 34-0370 |
| TiAl | a = 2.844 c = 3.9447 | tetragonal | P4/mmm | 10-84-3907 |
| TiAl3 | a = 5.782 c = 4.629 | hexagonal | P63/mmc | 10-82-5277 |
| Ti3Al | a = 5.77 c = 4.62 | hexagonal | P63/mmc | 65–7534 |
| (Al0.86Nb0.14)(Ti2.85Nb0.15) | a = 5.77 c = 4.64 | hexagonal | P63/mmc | |
| Ti2AlMo | a = 3.17 | cubic | Pm-3m | 10-82-5224 |
| Nb2Al | a = 9.943 c = 5.186 | tetragonal | P42/mnm | 12-0074 |
| Spectrum | Content of Chemical Elements, wt% | ||||
|---|---|---|---|---|---|
| B | Al | Ti | Nb | Mo | |
| S1 | 8.40 | 2.16 | 1.41 | 88.03 | - |
| S2 | - | 19.55 | 48.42 | 32.04 | - |
| S3 | 14.42 | 26.18 | 42.83 | 30.98 | - |
| S4 | - | 20.83 | 55.59 | - | 23.58 |
| S5 | - | 29.86 | 70.14 | - | - |
| Type of Heat Treatment | Microhardness, GPa | Modulus of Elasticity, GPa | Elastic Recovery, % |
|---|---|---|---|
| Without annealing | 5.4 | 175 | 21.3 |
| After annealing at 1000 °C for 3 h | 6.6 (up to 7.4) | 185 (up to 200) | 26.5 (up to 31.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhin, P.; Nazarko, I.; Bazhina, A.; Chizhikov, A.; Konstantinov, A.; Ivanov, A.; Antipov, M.; Stolin, P.; Agasieva, S.; Avdeeva, V. Effect of High-Temperature Isothermal Annealing on the Structure and Properties of Multicomponent Compact Ti-Al(Nb,Mo,B)-Based Materials Fabricated via Free SHS-Compression. Metals 2025, 15, 1088. https://doi.org/10.3390/met15101088
Bazhin P, Nazarko I, Bazhina A, Chizhikov A, Konstantinov A, Ivanov A, Antipov M, Stolin P, Agasieva S, Avdeeva V. Effect of High-Temperature Isothermal Annealing on the Structure and Properties of Multicomponent Compact Ti-Al(Nb,Mo,B)-Based Materials Fabricated via Free SHS-Compression. Metals. 2025; 15(10):1088. https://doi.org/10.3390/met15101088
Chicago/Turabian StyleBazhin, Pavel, Ivan Nazarko, Arina Bazhina, Andrey Chizhikov, Alexander Konstantinov, Artem Ivanov, Mikhail Antipov, Pavel Stolin, Svetlana Agasieva, and Varvara Avdeeva. 2025. "Effect of High-Temperature Isothermal Annealing on the Structure and Properties of Multicomponent Compact Ti-Al(Nb,Mo,B)-Based Materials Fabricated via Free SHS-Compression" Metals 15, no. 10: 1088. https://doi.org/10.3390/met15101088
APA StyleBazhin, P., Nazarko, I., Bazhina, A., Chizhikov, A., Konstantinov, A., Ivanov, A., Antipov, M., Stolin, P., Agasieva, S., & Avdeeva, V. (2025). Effect of High-Temperature Isothermal Annealing on the Structure and Properties of Multicomponent Compact Ti-Al(Nb,Mo,B)-Based Materials Fabricated via Free SHS-Compression. Metals, 15(10), 1088. https://doi.org/10.3390/met15101088







