Research on the Solidification Structure, Properties and Composition Segregation of GCr15 Bearing Steel Under Double-Electrode Regulation
Abstract
1. Introduction
2. Experimental Methods and Numerical Simulation
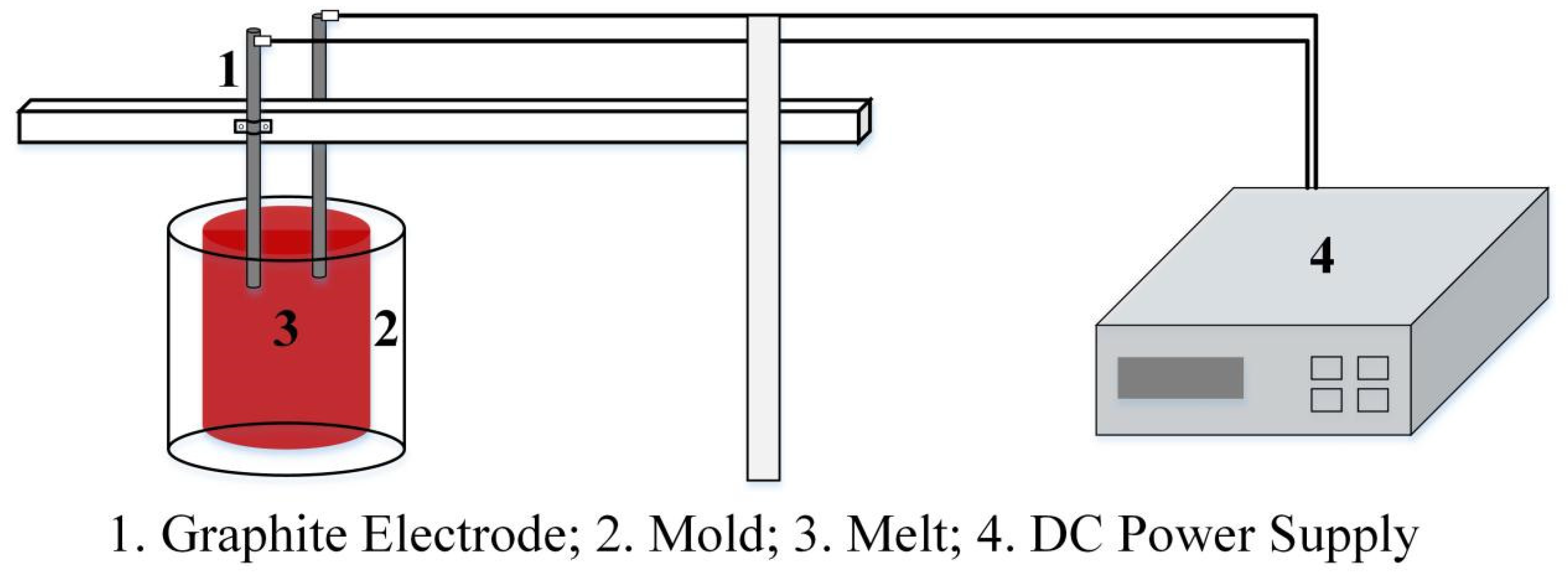
2.1. Experimental Materials and Methods
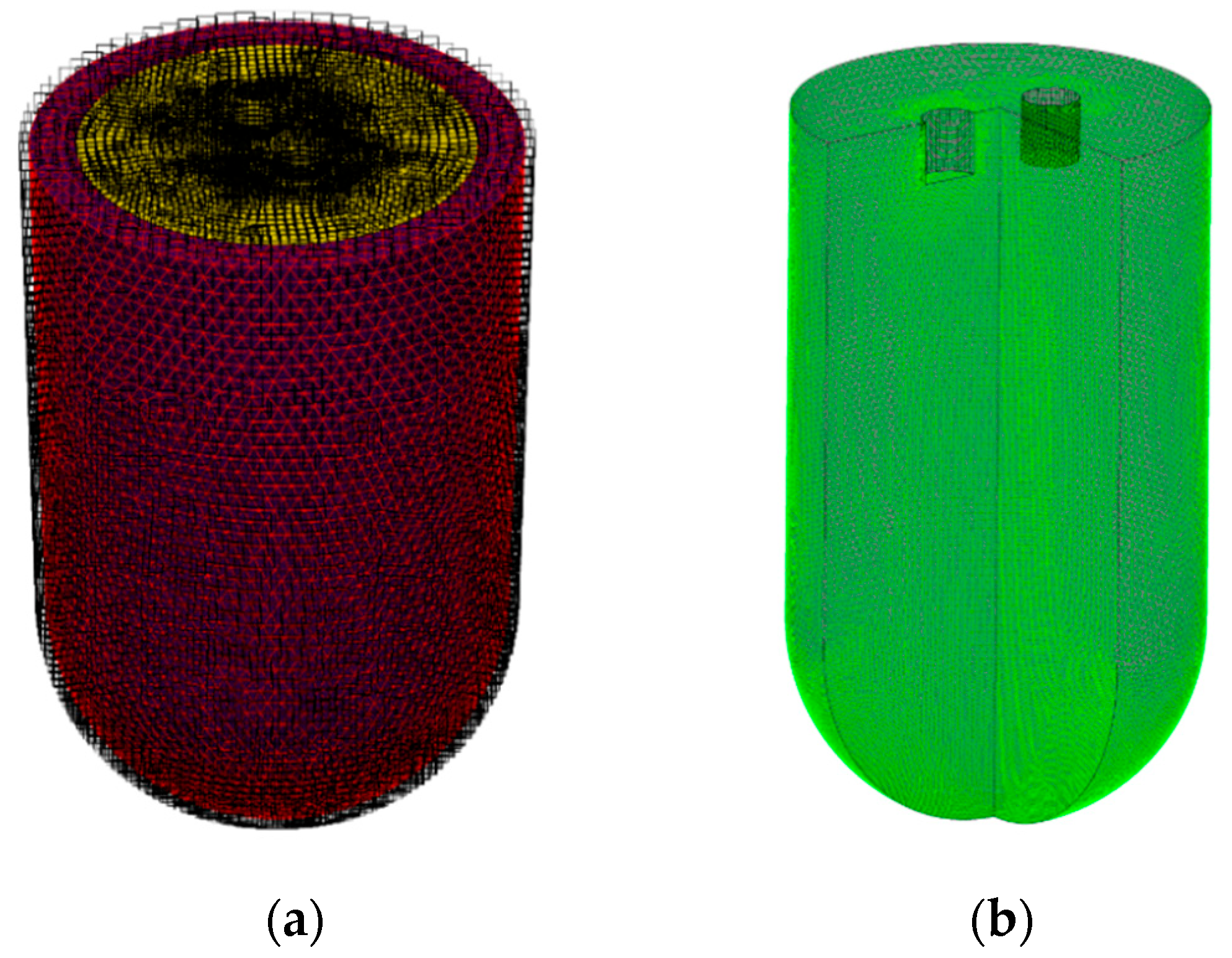
2.2. Numerical Simulation
- Conservation of flux is assumed at the phase interface;
- During the simulation, the alloy melt is assumed to remain a single-phase, stable, and continuous incompressible fluid;
- The influence of the Joule heating effect on electrode conductivity is neglected, and the heat transfer process is not considered.
2.3. Microstructure Observation
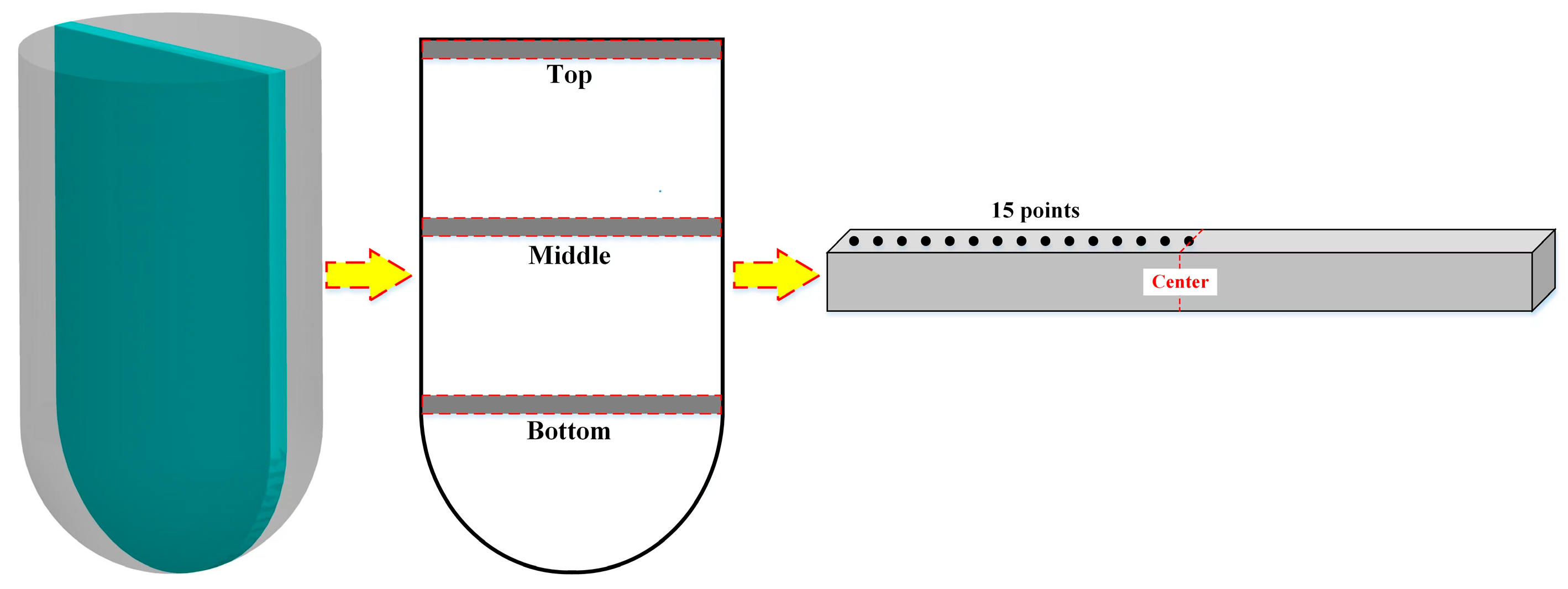
2.4. Composition Segregation Test
2.5. Mechanical Property Testing
3. Experimental Results
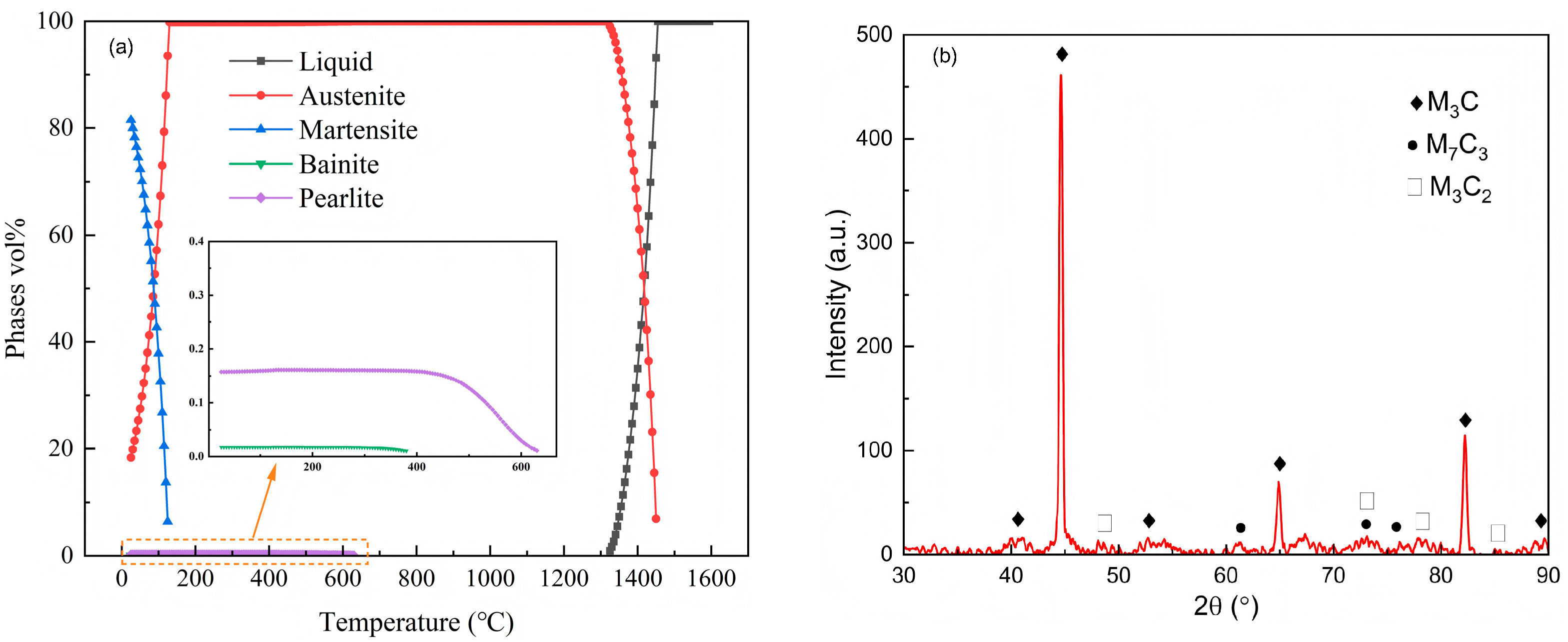
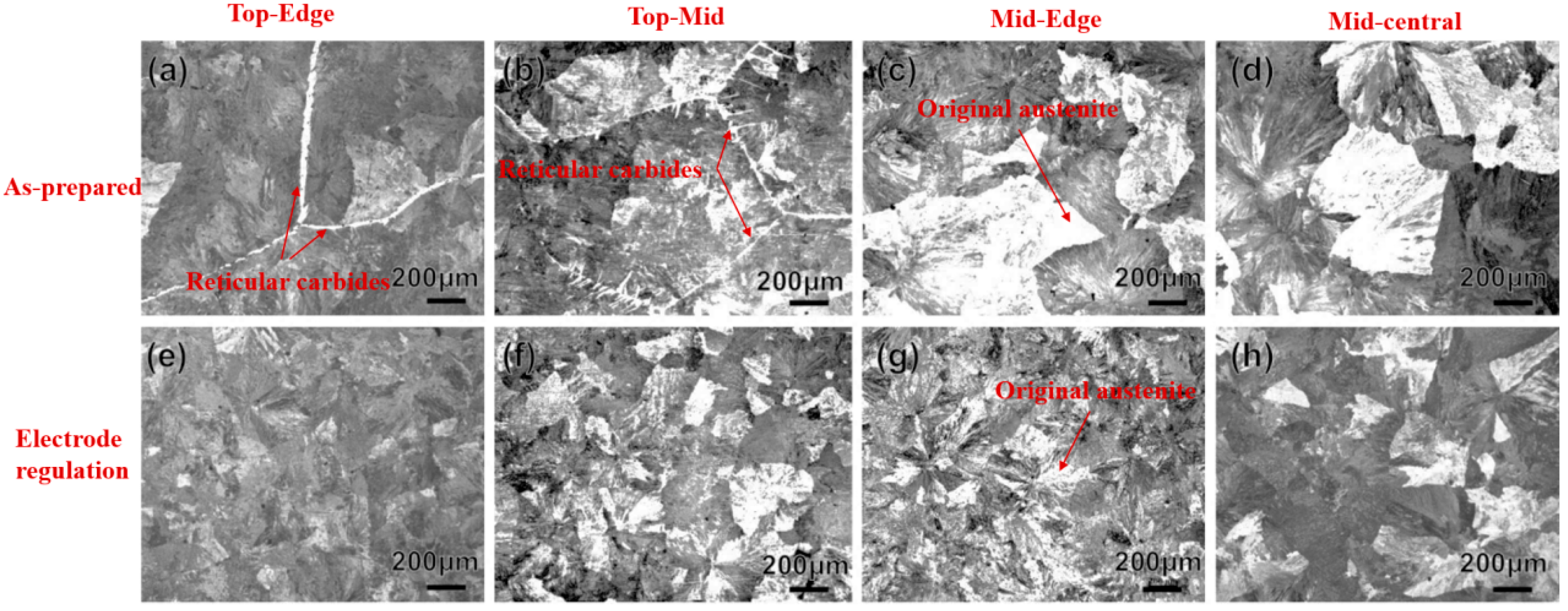
3.1. Phase Analysis
3.2. Microstructure Observation
3.3. Mechanical Properties and Hardness
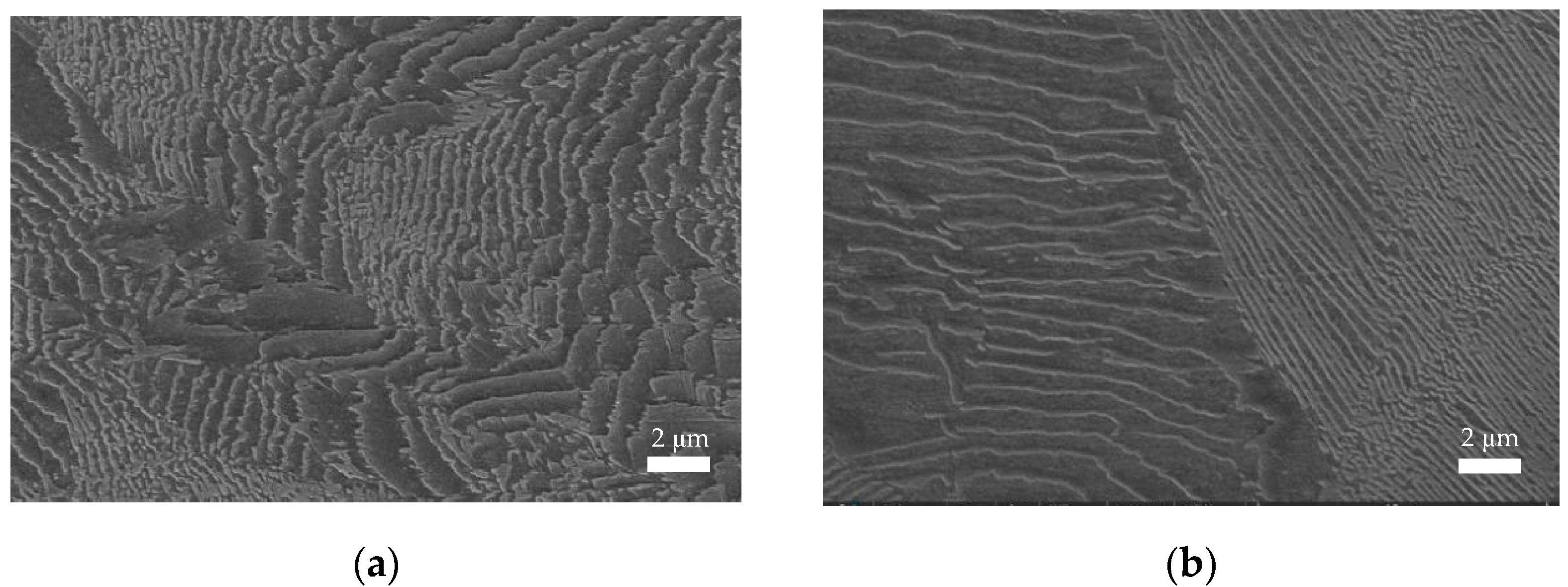
3.4. Fractographic Characterization
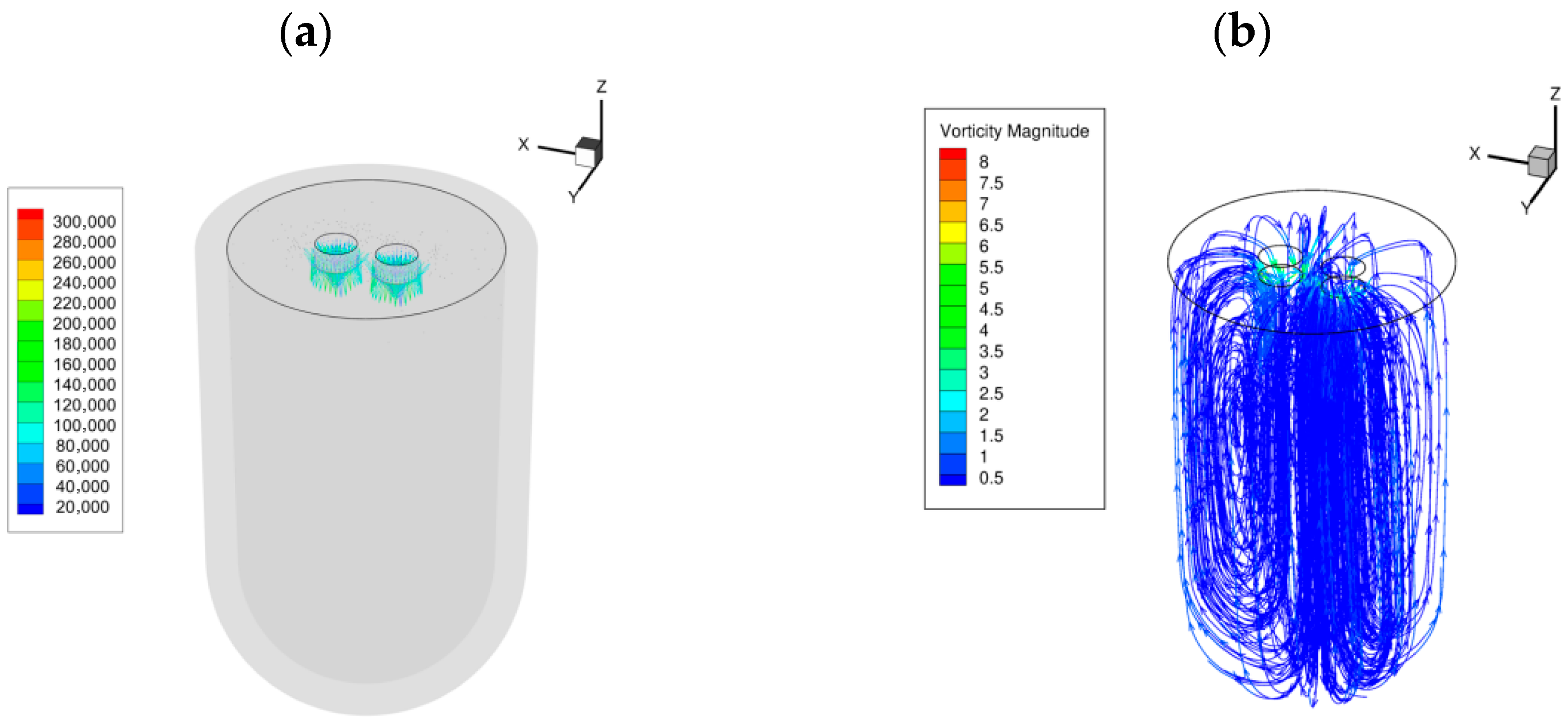
4. Numerical Results
Lorentz Force and Liquid Metal Flow
5. Discussion
5.1. Influence of Electrodes on Microstructure
5.2. Influence of Electric Field on Internal Flow of Steel Ingots
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beswick, J.M. The effect of chromium in high carbon bearing steels. Metall. Trans. A 1987, 18, 1897–1906. [Google Scholar]
- Shi, C.-B.; Huang, Y.; Zhang, J.-X.; Li, J.; Zheng, X. Review on desulfurization in electroslag remelting. Int. J. Miner. Metall. Mater. 2021, 26, 18–29. [Google Scholar]
- Schlautmann, M.; Koster, M.; Krieger, W.; Groll, M.; Schuster, R.; Bellmann, J.; Frittella, P. Measurement and Model-Based Control of Solidification in Continuous Casting of Steel Billets. Appl. Sci. 2023, 13, 885. [Google Scholar] [CrossRef]
- Shi, C.-B.; Wang, S.-J.; Li, J.; Cho, J.-W. Non-metallic inclusions in electroslag remelting: A review. J. Iron Steel Res. Int. 2021, 28, 1483–1503. [Google Scholar] [CrossRef]
- Shen, W.-L.; Zhang, Z.-C.; Luo, X.-Y. A Study on Behavior of Oxide Inclusions in Bearing Steel GCr15 at Refining End of a 60 t LF. Spec. Steel 2018, 39, 18–23. [Google Scholar]
- Cho, J.; Joshi, M.-S.; Sun, C.-T. Effect of inclusion size on mechanical properties of polymeric composites with micro and nano particles. Compos. Sci. Technol. 2006, 66, 1941–1952. [Google Scholar] [CrossRef]
- Agarwal, N.; Kahn, H.; Avishai, A.; Michal, G.; Ernst, F.; Heuer, A.-H. Enhanced fatigue resistance in 316l austenitic stainless steel due to low-temperature paraequilibrium carburization. Acta Mater. 2007, 55, 5572–5580. [Google Scholar] [CrossRef]
- Zhou, D.-G.; Fu, J.; Wang, P.; Xu, J.-H.; Xie, Y.-Q.; Li, Z. Formation Mechanism of Carbon Segregation for Bearing Steel Concasting Billet and Its Influencing Factors. J. Univ. Sci. Technol. B 1999, 21, 131–138. [Google Scholar]
- Xiao, C.; Zhang, J.-M.; Luo, Y.-Z.; Wei, X.-D.; Wu, L.; Wang, S.-X. Control of Macrosegregation Behavior by Applying Final Electromagnetic Stirring for Continuously Cast High Carbon Steel Billet. J. Iron Steel Res. Int. 2013, 20, 13–20. [Google Scholar] [CrossRef]
- Yang, Z.-G.; Wang, B.; Zhang, X.-F.; Wang, Y.-T.; Dong, H.-B.; Liu, Q.J. Effect of Electromagnetic Stirring on Molten Steel Flow and Solidification in Bloom Mold. Iron Steel Res. Int. 2014, 21, 1095–1113. [Google Scholar]
- Zhou, S.-C.; Bai, C.-G.; Lei, Y.; Ren, Z.-D.; Cao, P.-J.; Yang, Z.-L. Effect of low-frequency rotary electromagnetic-field on solidification structure of continuous casting austenitic stainless steel. J. Cent. South Univ. Technol. 2009, 16, 360–364. [Google Scholar] [CrossRef]
- Tomar, N.; Pradyumna, S.; Jain, J. Analysis of microscopic images using image processing to measure homogeneity of metal powder mixture samples. Powder Technol. 2024, 444, 119980. [Google Scholar] [CrossRef]
- Cha, P.R.; Hwang, Y.S.; Nam, H.S.; Chung, S.-H.; Yoon, J.-K. 3D numerical analysis on electromagnetic and fluid dynamic pheonomena in a soft contact electromagnetic slab caster. ISIJ Int. 1998, 38, 403–410. [Google Scholar] [CrossRef]
- Tang, G.-P.; Luo, B.-Y.; Sun, Z.; Feng, M.-L.; Lin, W.-H.; Xia, Z.-B.; Lin, Z.-Z.; Zheng, T.-X.; Zhou, B.-F.; Shi, P.-J.; et al. Evolution of Solid-Liquid Interface Influenced by Fluid Flow During Transverse Static Magnetic Field-Electric Current-Assisted Directional Solidification. Metall. Mater. Trans. B 2024, 55B, 4200–4215. [Google Scholar] [CrossRef]
- Cai, B.; Kao, A.; Boller, E.; Magdysyuk, O.-V.; Atwood, R.-C.; Vo, N.-T.; Pericleous, K.; Lee, P.-D. Revealing the mechanisms by which magneto-hydrodynamics disrupts solidification microstructures. Acta Mater. 2020, 196, 200–209. [Google Scholar] [CrossRef]
- Bao, X.-Y.; Ma, Y.-L.; Xing, S.-Q.; Liu, Y.-Z.; Shi, W.-W. Effects of Pulsed Magnetic Field Melt Treatment on Grain Refinement of Al-Si-Mg-Cu-Ni Alloy Direct-Chill Casting Billet. Metals 2022, 12, 1080. [Google Scholar] [CrossRef]
- Li, X.; Ren, Z.-M. Thermoelectric Magnetic Effect and Its Effect on the Solidification Structure under Static Magnetic Fields. Mater. China 2014, 33, 349–354. [Google Scholar]
- Annalisa, P.; Marcello, G.; Giovina, L.-V. Comprehensive Numerical Simulation of Filling and Solidification of Steel Ingots. Materials 2016, 9, 769. [Google Scholar] [CrossRef]
- Zhang, C.-P.; Loucif, A.; Jahazi, M.; Morin, J.-B. FE Modelling and Prediction of Macrosegregation Patterns in Large Size Steel Ingots: Influence of Filling Rate. Metals 2022, 12, 29. [Google Scholar] [CrossRef]
- Li, X.; Ren, Z.-M.; Wang, H.; Li, W.-X.; Deng, K.; Zhuang, Y.-Q. Ring--Like Solidification Structure Of Mnbi Phase In Bi—Mn Alloy Under A High Magnetic Field. Acta Metall. Sin. 2004, 40, 40–45. [Google Scholar]
- Wang, F.; Sun, B.-Y.; Liu, Z.-Q.; Li, B.-K.; Huang, S.; Zhang, B.-J. Numerical Simulation on Motion Behavior of Inclusions in the Lab-Scale Electroslag Remelting Process with a Vibrating Electrode. Metals 2021, 11, 1784. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Z.-G.; Zhang, X.-F.; Wang, Y.-T.; Nie, C.-P.; Liu, Q.; Dong, H.-B. Analysis of the effects of electromagnetic stirring on solidification structure of bearing steel. Metalurgija 2015, 54, 327–330. [Google Scholar]




| C | Si | Mn | P | S | Cr | Ni | Al | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 1.03 | 0.24 | 0.29 | 0.18 | 0.04 | 1.44 | 0.02 | 0.01 | 0.0027 | Balance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Q.; Li, S.; Liu, S.; Zhao, J.; Ai, X.; Zhou, Y.; Miao, X.; Wang, M. Research on the Solidification Structure, Properties and Composition Segregation of GCr15 Bearing Steel Under Double-Electrode Regulation. Metals 2025, 15, 1086. https://doi.org/10.3390/met15101086
Xiao Q, Li S, Liu S, Zhao J, Ai X, Zhou Y, Miao X, Wang M. Research on the Solidification Structure, Properties and Composition Segregation of GCr15 Bearing Steel Under Double-Electrode Regulation. Metals. 2025; 15(10):1086. https://doi.org/10.3390/met15101086
Chicago/Turabian StyleXiao, Qinghe, Shengli Li, Siyao Liu, Jiyu Zhao, Xingang Ai, Ye Zhou, Xincheng Miao, and Min Wang. 2025. "Research on the Solidification Structure, Properties and Composition Segregation of GCr15 Bearing Steel Under Double-Electrode Regulation" Metals 15, no. 10: 1086. https://doi.org/10.3390/met15101086
APA StyleXiao, Q., Li, S., Liu, S., Zhao, J., Ai, X., Zhou, Y., Miao, X., & Wang, M. (2025). Research on the Solidification Structure, Properties and Composition Segregation of GCr15 Bearing Steel Under Double-Electrode Regulation. Metals, 15(10), 1086. https://doi.org/10.3390/met15101086








