The Role and Modeling of Ultrafast Heating in Isothermal Austenite Formation Kinetics in Quenching and Partitioning Steel
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
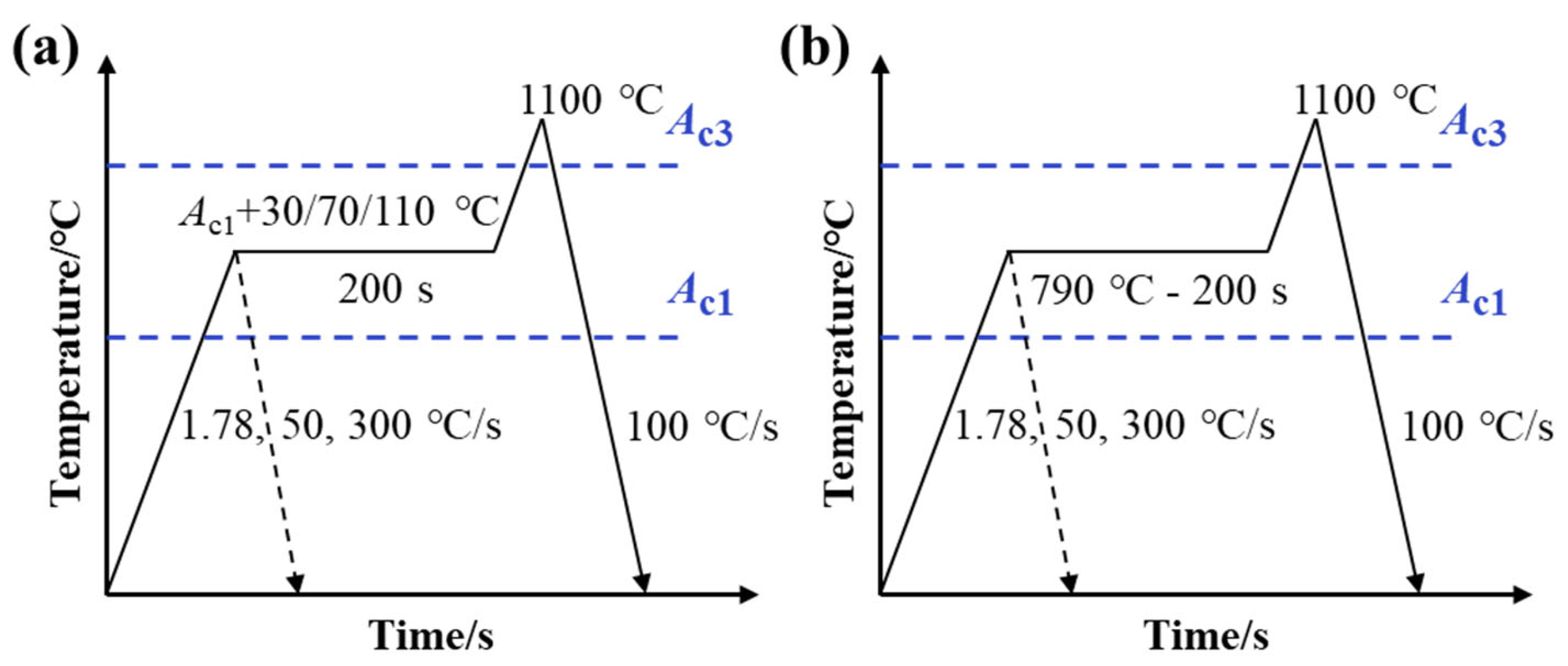
2.2. Thermal Treatments
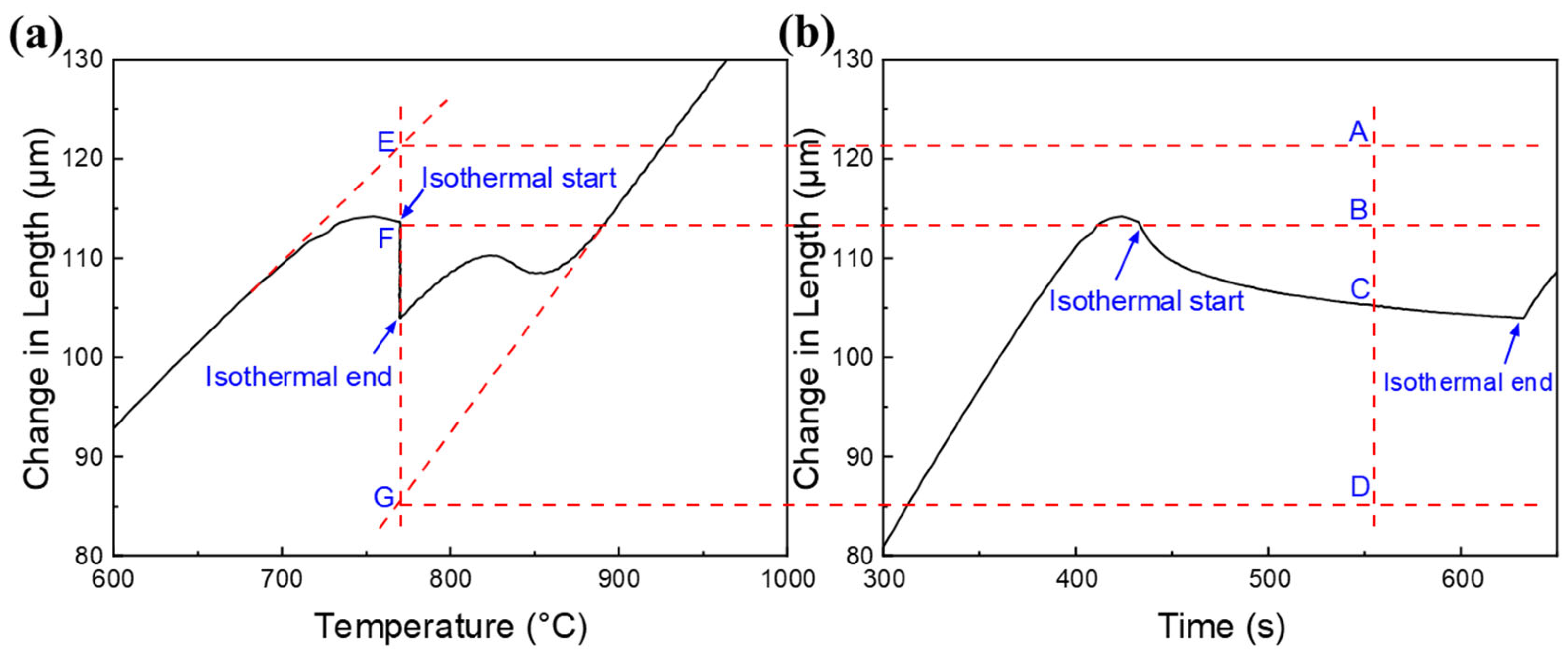
2.3. Calculation of Experimental Isothermal Austenite Fraction
2.4. Microstructure Characterization
3. Results and Discussion
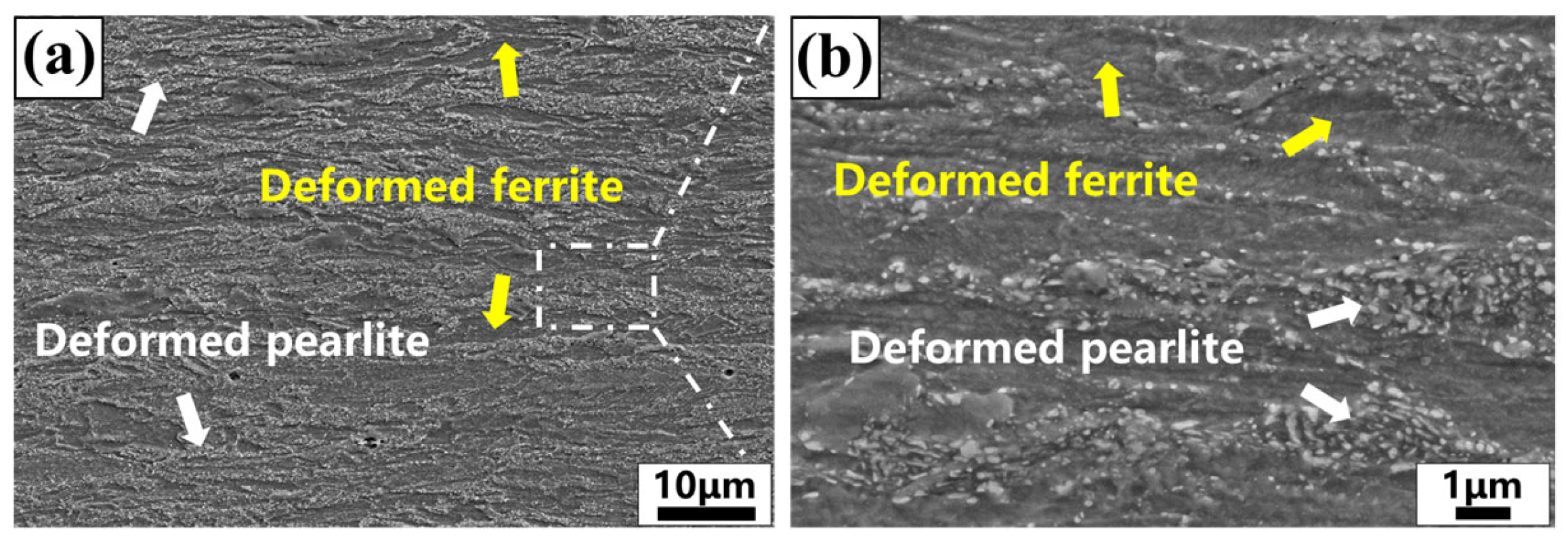
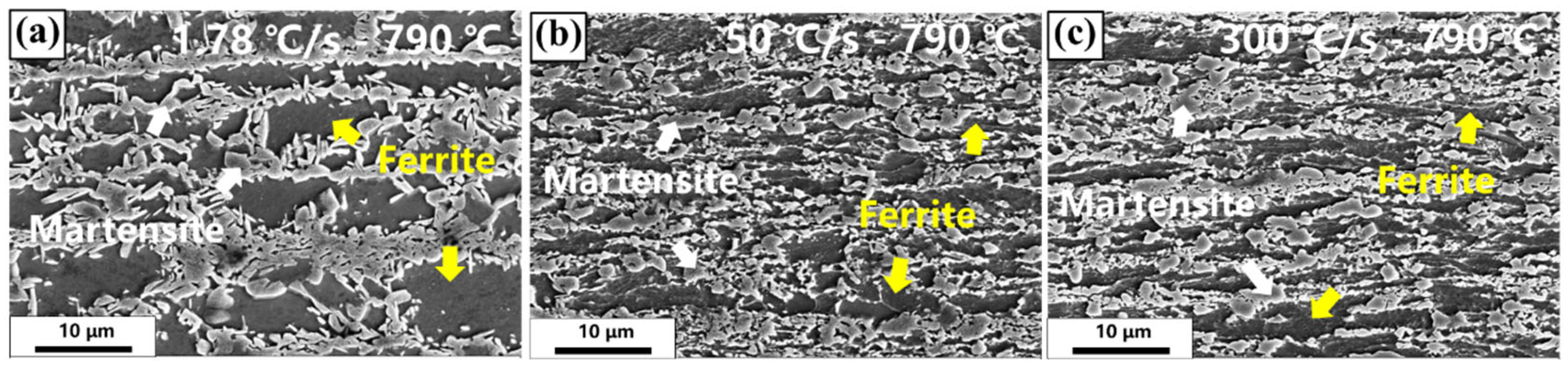
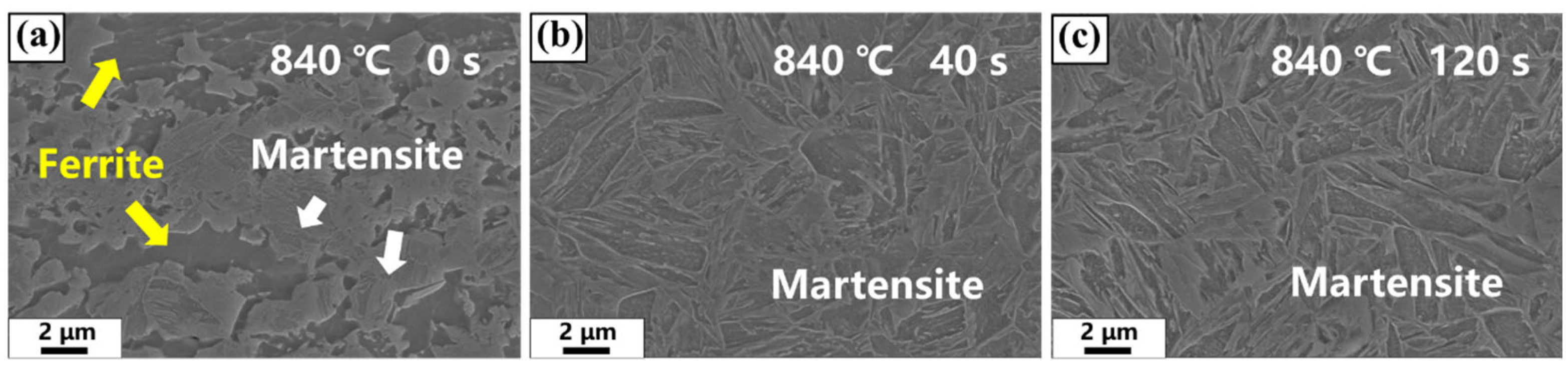
3.1. Transient Microstructure Before Isothermal Holding Process
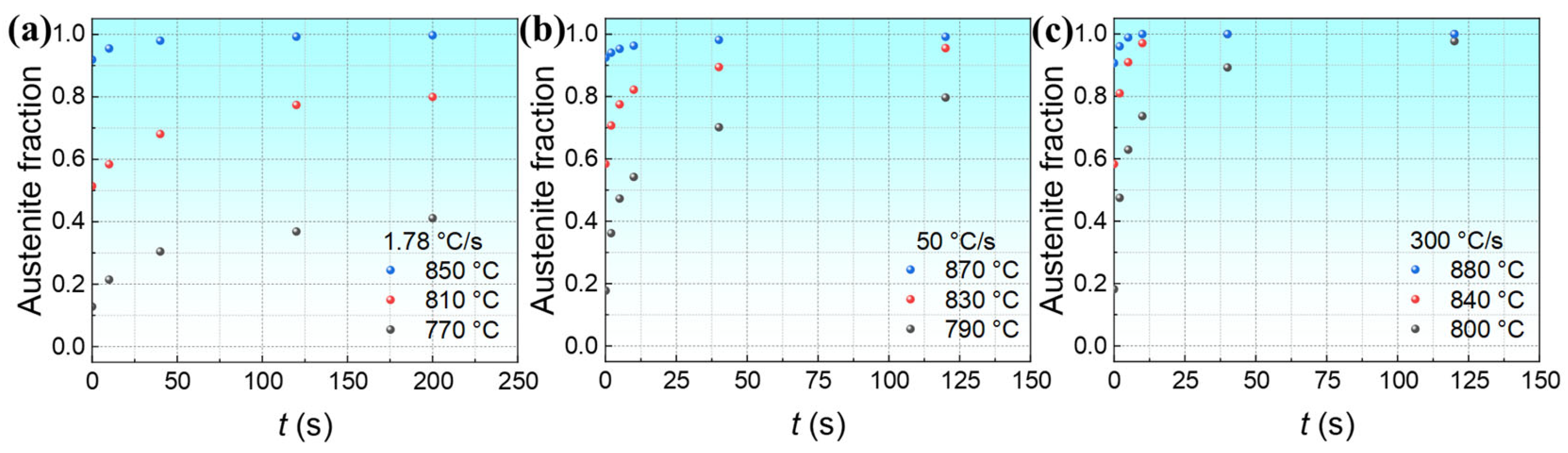
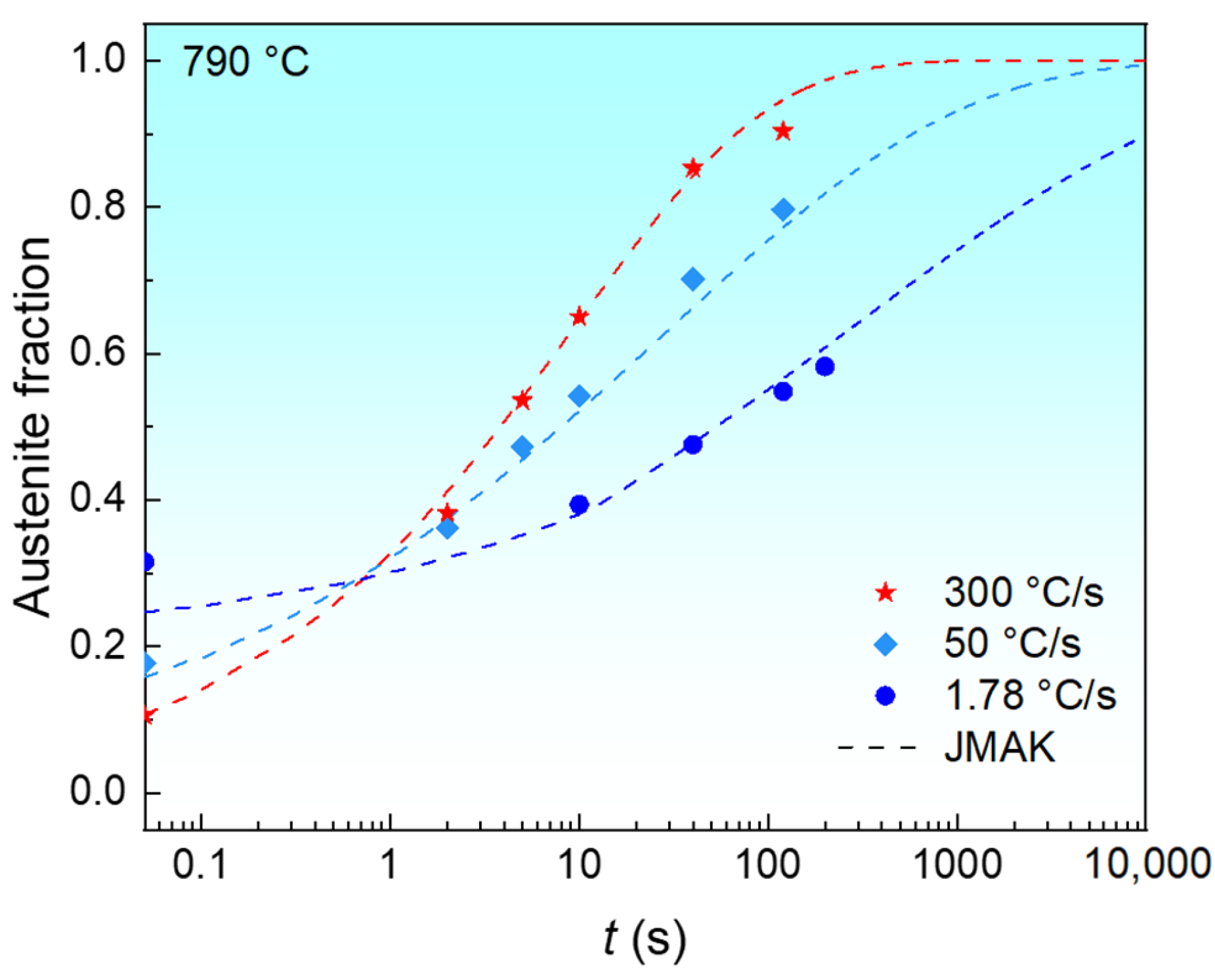
3.2. Isothermal Austenite Formation Kinetics
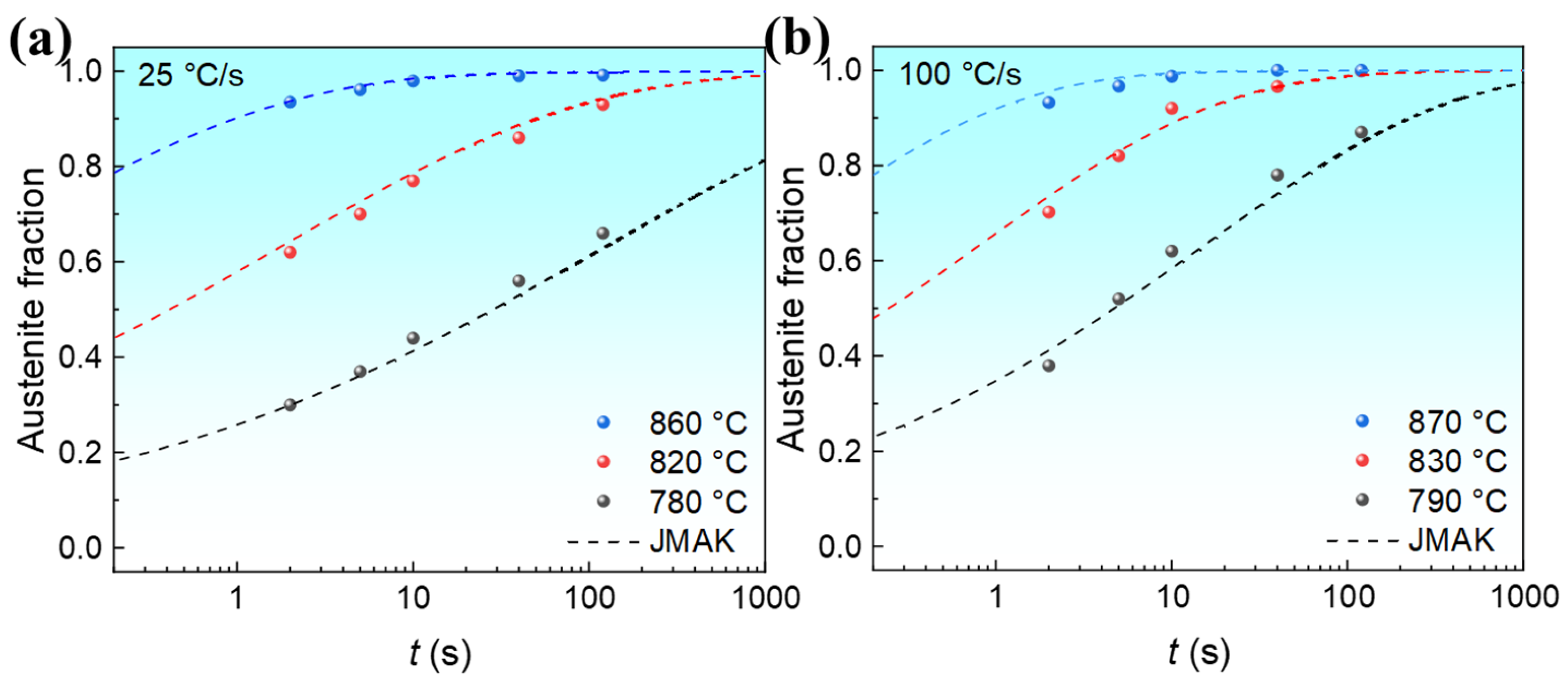
3.2.1. Effect of Austenitization Temperature on Isothermal Austenite Formation Kinetics
3.2.2. Effect of Heating Rates on Isothermal Austenite Formation Kinetics
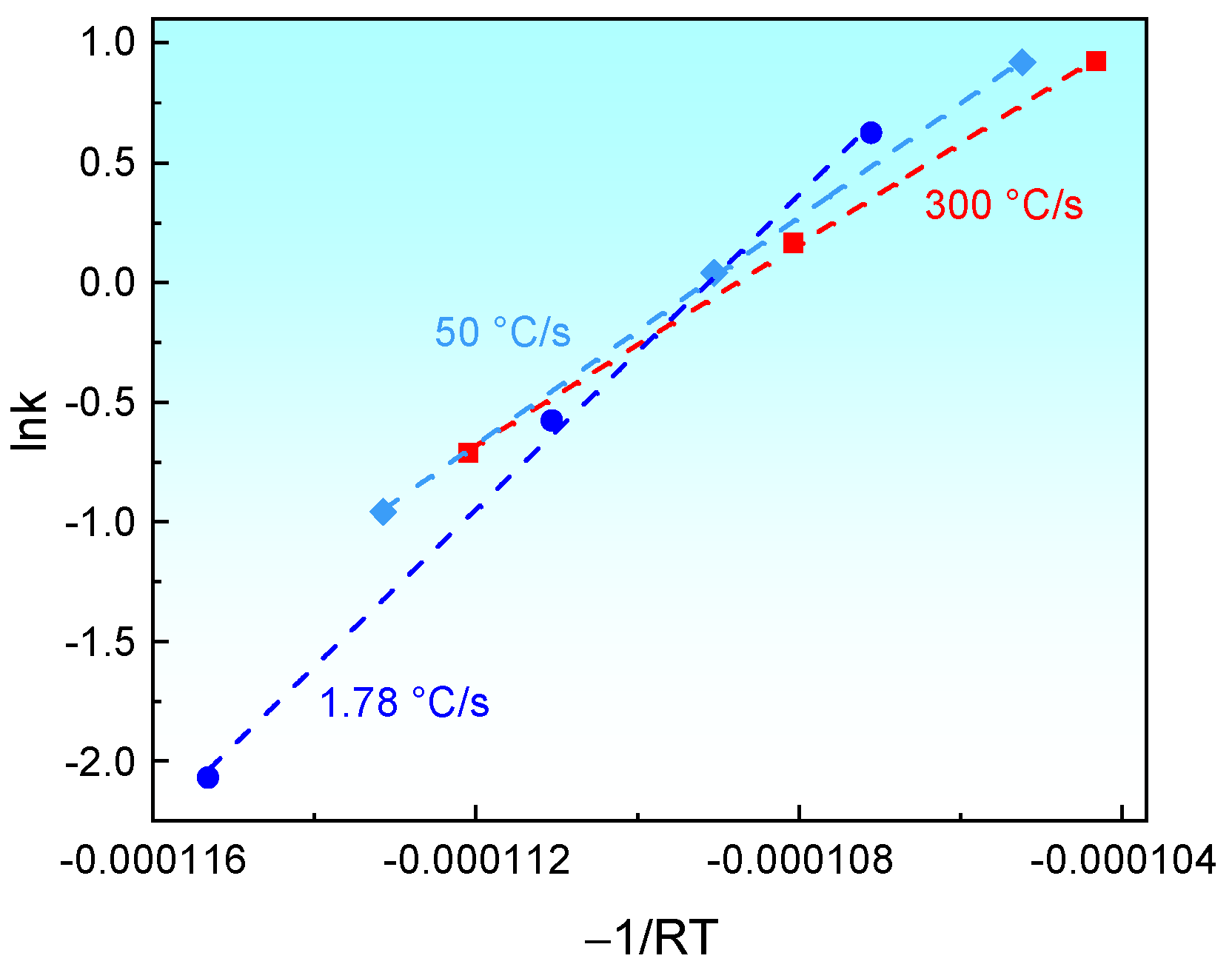
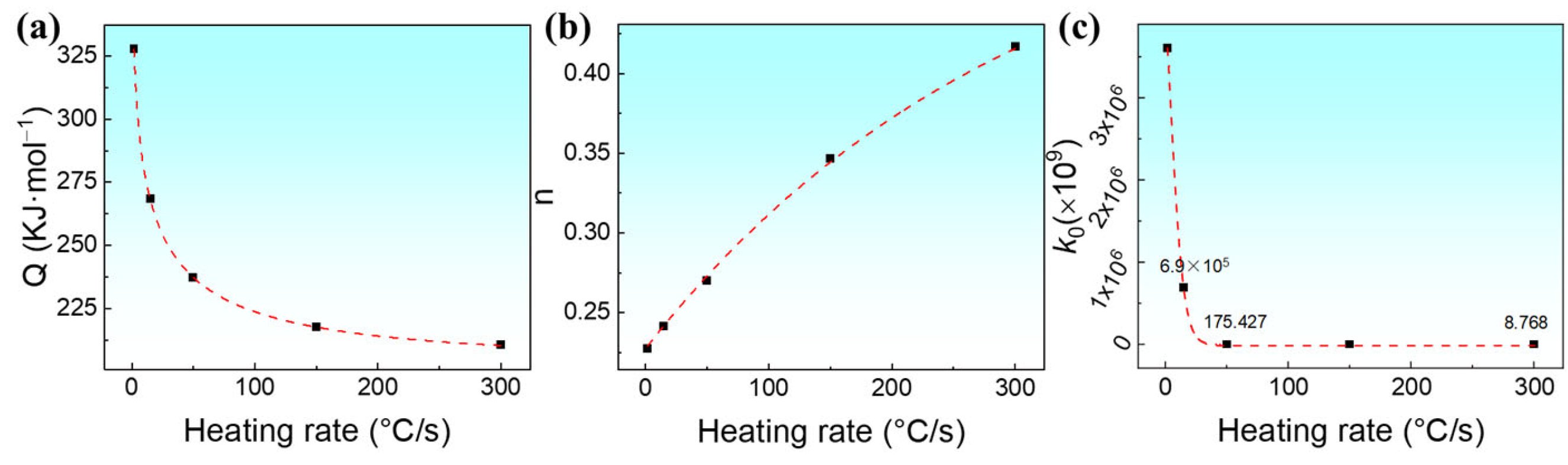
3.3. Isothermal Austenite Formation Kinetics Model Incorporating the Heating Rates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Celada-Casero, C.; Sietsma, J.; Santofimia, M.J. The role of the austenite grain size in the martensitic transformation in low carbon steels. Mater. Des. 2019, 167, 107625. [Google Scholar] [CrossRef]
- Kumar, S. Quenching and partitioning (Q&P) process: A critical review of the competing reactions. Mater. Sci. Technol. 2022, 38, 663–675. [Google Scholar] [CrossRef]
- Gaudez, S.; Macchi, J.; Geandier, G.; Denis, S.; Allain, S.Y. Thermodynamic Approach to Describe the Martensite Phase Transformation Kinetics via the Stabilization of Austenite. Metall. Mater. Trans. A 2024, 55, 812–826. [Google Scholar]
- Kim, J.H.; Gu, G.; Kwon, M.; Koo, M.; Kim, E.; Kim, J.; Lee, J.S.; Suh, D. Microstructure and tensile properties of chemically heterogeneous steel consisting of martensite and austenite. Acta Mater. 2022, 223, 117506. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.B. Microstructure-property relationship in the quenching and partitioning (Q&P) steel. Mater. Charact. 2023, 196, 112561. [Google Scholar]
- Mao, W.; Gao, S.; Gong, W.; Bai, Y.; Harjo, S.; Park, M.; Shibata, A.; Tsuji, N. Quantitatively evaluating respective contribution of austenite and deformation-induced martensite to flow stress, plastic strain, and strain hardening rate in tensile deformed TRIP steel. Acta Mater. 2023, 256, 119139. [Google Scholar] [CrossRef]
- Park, J.; Hou, Y.; Min, J.; Hou, Z.; Han, H.N.; He, B.; Lee, M. Understanding plasticity in multiphase quenching & partitioning steels: Insights from crystal plasticity with stress state-dependent martensitic transformation. Int. J. Plast. 2024, 180, 104075. [Google Scholar] [CrossRef]
- Guo, Q.; Yen, H.; Luo, H.; Ringer, S.P. On the mechanism of Mn partitioning during intercritical annealing in medium Mn steels. Acta Mater. 2022, 225, 117601. [Google Scholar] [CrossRef]
- Umasankar, C.; Prasad, K.; Choi, Y.T.; Lee, D.W.; Kim, H.S.; Sankaran, S.; Chakkingal, U. Role of direct quenching and partitioning processes in improving austenite stability and stretch flangeability in a low c steel. Met. Mater. Int. 2025, 31, 84–99. [Google Scholar]
- Gui, X.; Gao, G.; Guo, H.; Zhao, F.; Tan, Z.; Bai, B. Effect of bainitic transformation during BQ&P process on the mechanical properties in an ultrahigh strength Mn-Si-Cr-C steel. Mater. Sci. Eng. A 2017, 684, 598–605. [Google Scholar]
- Zhang, C.; Xiong, Z.; Li, Z.; Cao, Y.; Yang, D.; Cheng, X. On the role of chemical heterogeneity in carbon diffusion during quenching and partitioning. Acta Mater. 2024, 271, 119902. [Google Scholar] [CrossRef]
- Yan, S.; Liu, X.; Liu, W.J.; Lan, H.; Wu, H. Comparison on mechanical properties and microstructure of a C–Mn–Si steel treated by quenching and partitioning (Q&P) and quenching and tempering (Q&T) processes. Mater. Sci. Eng. A 2015, 620, 58–66. [Google Scholar]
- Liu, Y.; Gan, X.; Wang, S.; Liang, L.; Xu, Y.; Xu, G.; Liu, M. Effect of partitioning treatment on the strengthening and plasticising mechanism of one-step quenching and partitioning steels. J. Mater. Res. Technol. 2024, 31, 1091–1103. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Petrov, R.; Kwakernaak, C.; Sloof, W.G.; Sietsma, J. Microstructural development during the quenching and partitioning process in a newly designed low-carbon steel. Acta Mater. 2011, 59, 6059–6068. [Google Scholar] [CrossRef]
- Navarro-López, A.; Sietsma, J.; Santofimia, M.J. Effect of prior athermal martensite on the isothermal transformation kinetics below M s in a low-C high-Si steel. Metall. Mater. Trans. A 2016, 47, 1028–1039. [Google Scholar]
- Lesch, C.; Alvarez, P.; Bleck, W.; Gil Sevillano, J. Rapid transformation annealing: A novel method for grain refinement of cold-rolled low-carbon steels. Metall. Mater. Trans. A 2007, 38, 1882–1890. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Meng, Q.; Hu, W.; Xu, D. Effect of heating rate on ferrite recrystallization and austenite formation of cold-roll dual phase steel. J. Alloys Compd. 2013, 578, 320–327. [Google Scholar] [CrossRef]
- Liu, G.; Li, T.; Yang, Z.; Zhang, C.; Li, J.; Chen, H. On the role of chemical heterogeneity in phase transformations and mechanical behavior of flash annealed quenching & partitioning steels. Acta Mater. 2020, 201, 266–277. [Google Scholar] [CrossRef]
- Lolla, T.; Cola, G.; Narayanan, B.; Alexandrov, B.; Babu, S.S. Development of rapid heating and cooling (flash processing) process to produce advanced high strength steel microstructures. Mater. Sci. Technol. 2011, 27, 863–875. [Google Scholar] [CrossRef]
- Cerda, F.C.; Sabirov, I.; Goulas, C.; Sietsma, J.; Monsalve, A.; Petrov, R.H. Austenite formation in 0.2% C and 0.45% C steels under conventional and ultrafast heating. Mater. Des. 2017, 116, 448–460. [Google Scholar] [CrossRef]
- Chang, J.; Wang, M.; Yang, Y.; Wu, Y.; Mi, Z. Modeling of austenite formation kinetics overlapping recrystallization in cold-rolled Q&P steel during ultrafast heating process. J. Iron Steel Res. Int. 2025, 32, 2452–2462. [Google Scholar]
- Asadabad, M.A.; Goodarzi, M.; Kheirandish, S. Kinetics of austenite formation in dual phase steels. ISIJ Int. 2008, 48, 1251–1255. [Google Scholar] [CrossRef]
- Caseiro, J.F.; Oliveira, J.A.; Andrade-Campos, A. Thermomechanical modelling strategies for multiphase steels. Int. J. Mech. Sci. 2011, 53, 720–733. [Google Scholar] [CrossRef]
- Kulakov, M.; Poole, W.J.; Militzer, M. The effect of the initial microstructure on recrystallization and austenite formation in a DP600 steel. Metall. Mater. Trans. A 2013, 44, 3564–3576. [Google Scholar] [CrossRef]
- Zhao, X.; Kang, Y.; Han, Q. Austenite isothermal transformation kinetics of a 1000 MPa grade cold rolled dual phase steel. Trans. Mater. Heat Treat. 2013, 34, 53–59. [Google Scholar]
- Kolmogorov, A.N. A Statistical Theory for the Recrystallization of Metals. Ser. Mat. 1937, 1, 355–359. [Google Scholar]
- Johnson, W.A. Reaction kinetics in process of nucleation and growth. Trans. Trans. Am. Inst. Min. Metall. Eng. 1939, 135, 416–458. [Google Scholar]
- Avrami, M. Transformation-time relations for random distribution of nuclei kinetics of phase change II. J. Chem. Phys. 1940, 8, 212. [Google Scholar] [CrossRef]
- Fanfoni, M.; Tomellini, M. The johnson-mehl-avrami-kohnogorov model: A brief review. Il Nuovo Cimento D 1998, 20, 1171–1182. [Google Scholar] [CrossRef]
- Ollat, M.; Massardier, V.; Fabregue, D.; Buscarlet, E.; Keovilay, F.; Perez, M. Modeling of the recrystallization and austenite formation overlapping in cold-rolled dual-phase steels during intercritical treatments. Metall. Mater. Trans. A 2017, 48, 4486–4499. [Google Scholar] [CrossRef]
- Zheng, C.; Raabe, D. Interaction between recrystallization and phase transformation during intercritical annealing in a cold-rolled dual-phase steel: A cellular automaton model. Acta Mater. 2013, 61, 5504–5517. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Kermanpur, A.; Najafizadeh, A.; Kalashami, A.G. Kinetics of ferrite recrystallization and austenite formation during intercritical annealing of the cold-rolled ferrite/martensite duplex structures. Metall. Mater. Trans. A 2016, 47, 1040–1051. [Google Scholar] [CrossRef]
- Chbihi, A.; Barbier, D.; Germain, L.; Hazotte, A.; Gouné, M. Interactions between ferrite recrystallization and austenite formation in high-strength steels. J. Mater. Sci. 2014, 49, 3608–3621. [Google Scholar] [CrossRef]
- Valdes-Tabernero, M.A.; Celada-Casero, C.; Sabirov, I.; Kumar, A.; Petrov, R.H. The effect of heating rate and soaking time on microstructure of an advanced high strength steel. Mater. Charact. 2019, 155, 109822. [Google Scholar] [CrossRef]
- Liu, Z.; Olivares, R.O.; Lei, Y.; Garcia, C.I.; Wang, G. Microstructural characterization and recrystallization kinetics modeling of annealing cold-rolled vanadium microalloyed HSLA steels. J. Alloys Compd. 2016, 679, 293–301. [Google Scholar] [CrossRef]



| C | Si | Mn | P | S | Cr | Fe |
|---|---|---|---|---|---|---|
| 0.19 | 1.58 | 1.9 | 0.0088 | 0.0014 | 0.016 | Bal. |
| °C/s | Ac1 °C | Ac3 °C | AT °C | ||
|---|---|---|---|---|---|
| 1.78 | 740 | 880 | 770 | 810 | 850 |
| 50 | 760 | 910 | 790 | 830 | 870 |
| 300 | 770 | 920 | 800 | 840 | 880 |
| Heating Rate (°C/s) | (J·mol−1) | ||
|---|---|---|---|
| 1.78 | 327,796.79 | 0.227 | 3.606 × 1015 |
| 50 | 237,209.10 | 0.270 | 1.754 × 1011 |
| 300 | 210,500.63 | 0.417 | 8.768 × 109 |
| (J·mol−1) | 428,000 | −224,000 | 0.485 | 0.350 |
| 0.277 | 0.3 | 0.003 | 1 | |
| 3,900,000 | −3,899,991 | 0.034 | 1.455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Wang, M.; Yang, X.; Yang, Y.; Wu, Y.; Mi, Z. The Role and Modeling of Ultrafast Heating in Isothermal Austenite Formation Kinetics in Quenching and Partitioning Steel. Metals 2025, 15, 1111. https://doi.org/10.3390/met15101111
Chang J, Wang M, Yang X, Yang Y, Wu Y, Mi Z. The Role and Modeling of Ultrafast Heating in Isothermal Austenite Formation Kinetics in Quenching and Partitioning Steel. Metals. 2025; 15(10):1111. https://doi.org/10.3390/met15101111
Chicago/Turabian StyleChang, Jiang, Mai Wang, Xiaoyu Yang, Yonggang Yang, Yanxin Wu, and Zhenli Mi. 2025. "The Role and Modeling of Ultrafast Heating in Isothermal Austenite Formation Kinetics in Quenching and Partitioning Steel" Metals 15, no. 10: 1111. https://doi.org/10.3390/met15101111
APA StyleChang, J., Wang, M., Yang, X., Yang, Y., Wu, Y., & Mi, Z. (2025). The Role and Modeling of Ultrafast Heating in Isothermal Austenite Formation Kinetics in Quenching and Partitioning Steel. Metals, 15(10), 1111. https://doi.org/10.3390/met15101111






