Preparation of a Porous Tri-n-decylamine Modified Adsorbent for the Efficient Removal of Uranium and Iron from Rare Earth
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
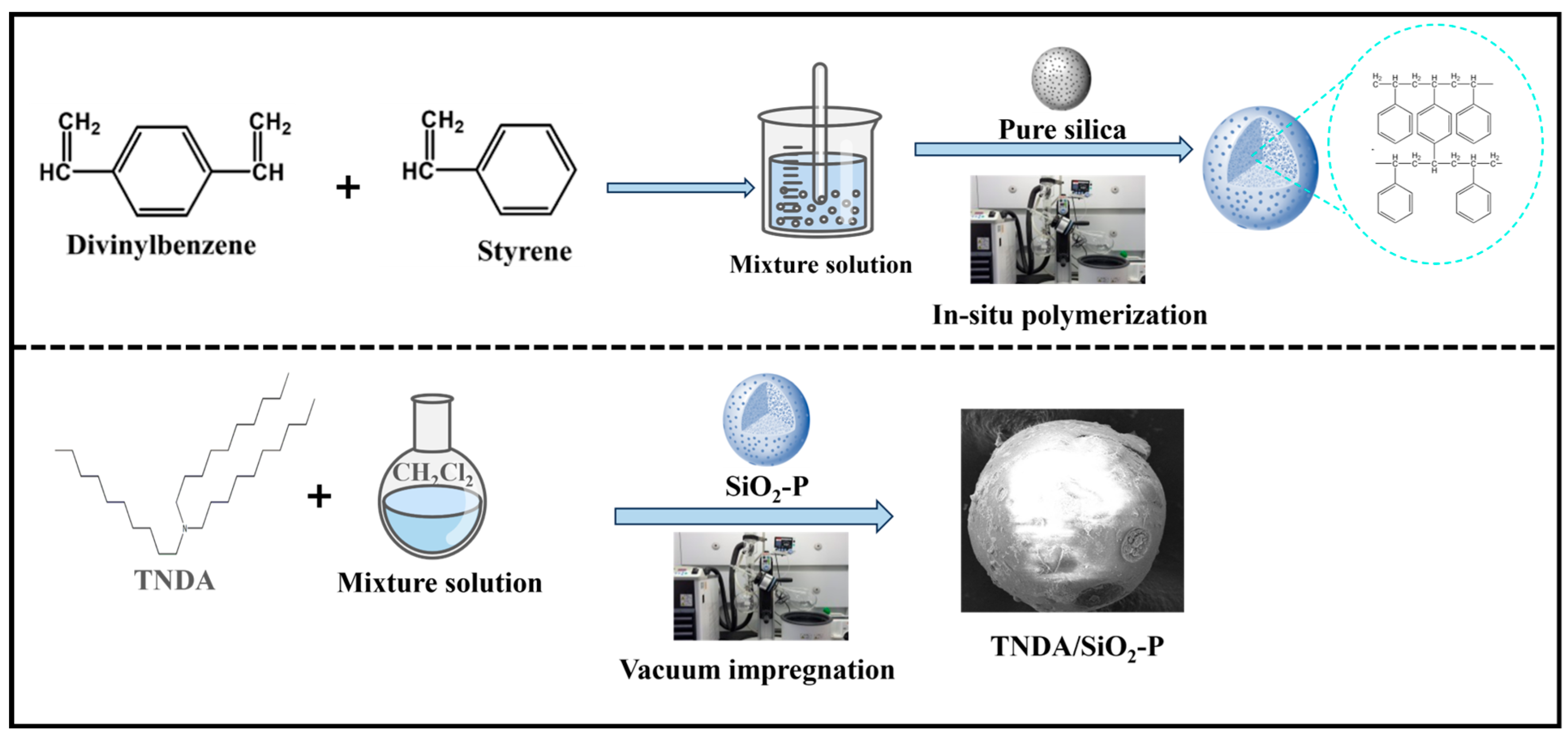
2.2. Preparation and Characterization of TNDA/SiO2-P
2.3. Batch Experiments
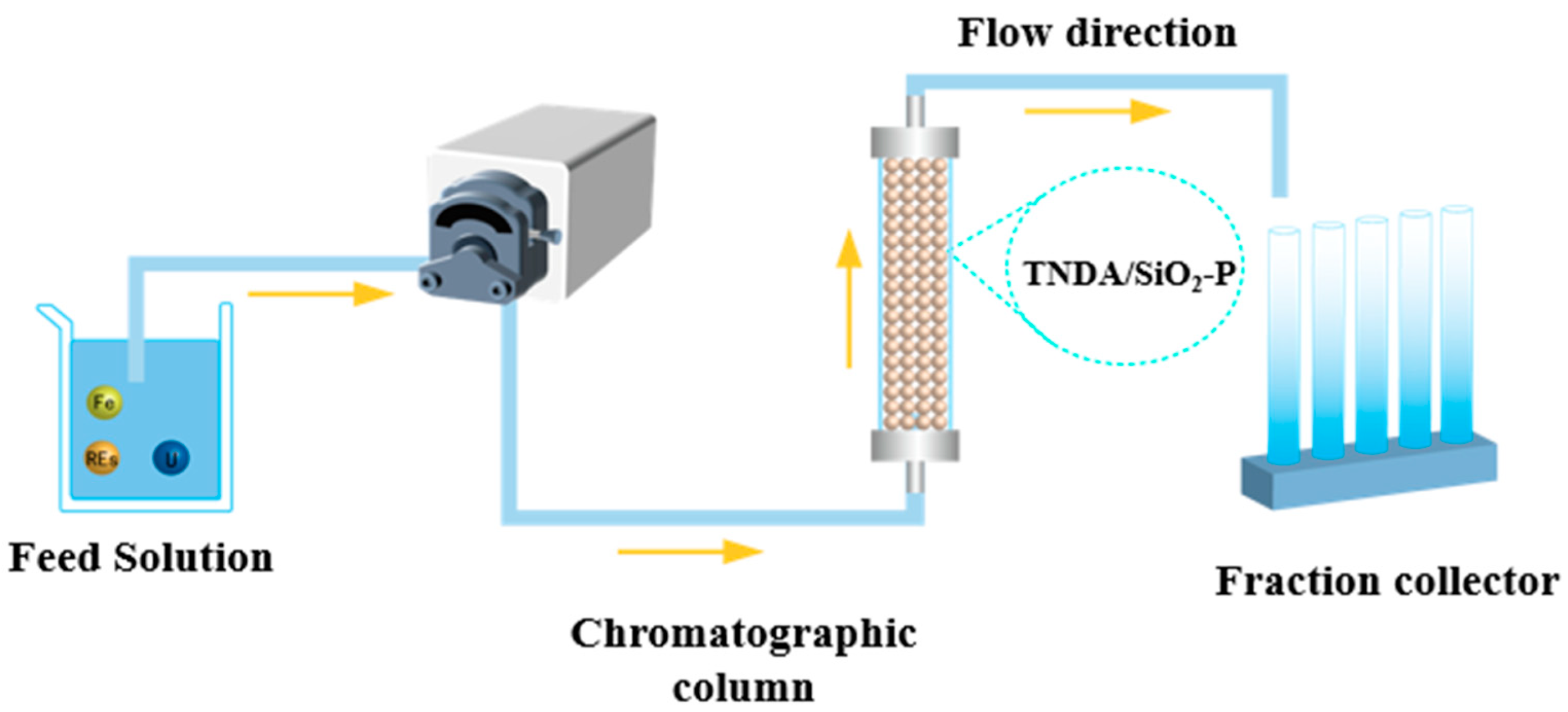
2.4. Column Experiment
2.5. DFT Calculation
3. Results and Discussion
3.1. Characterization of SiO2, SiO2-P and TNDA/SiO2-P
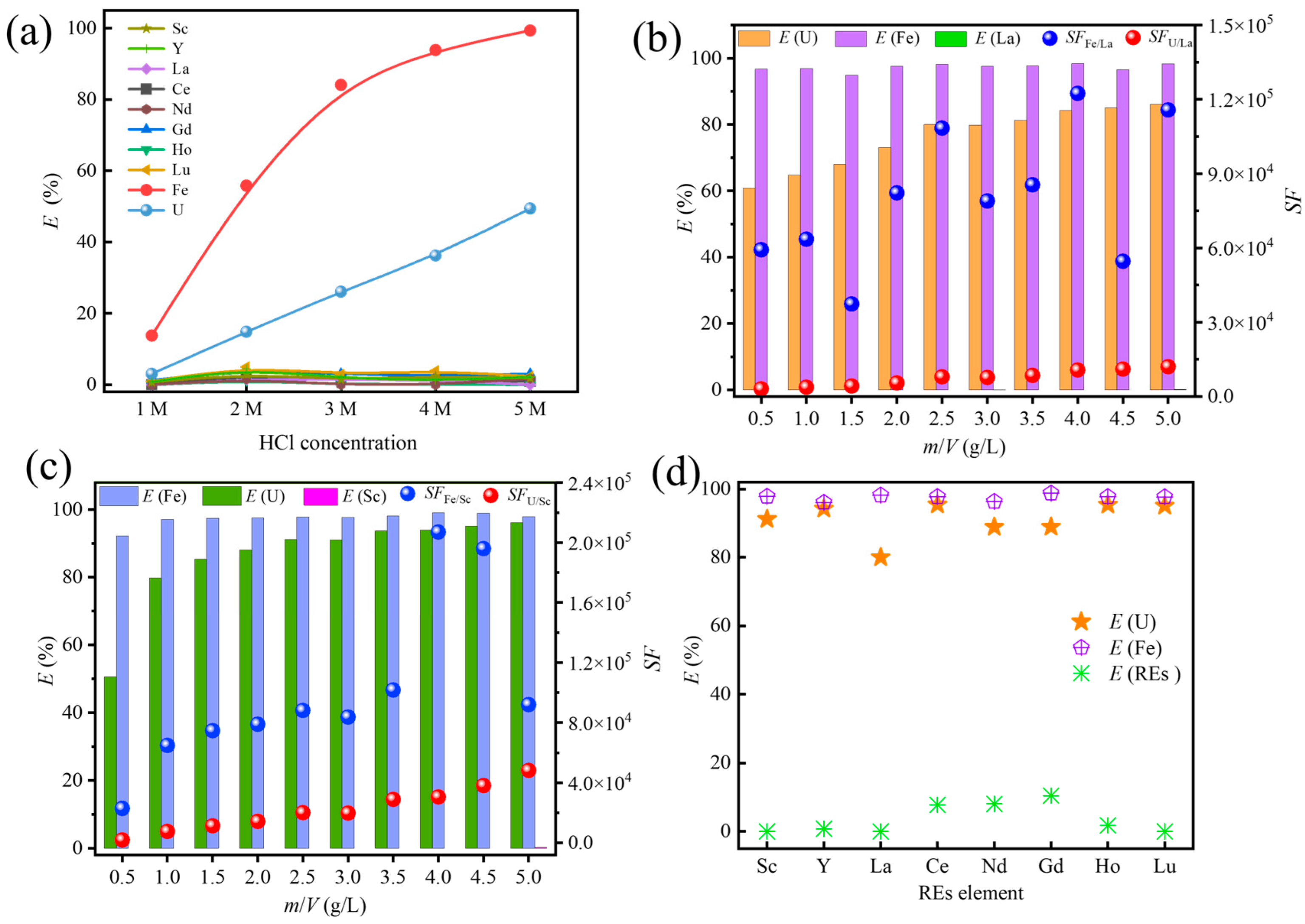
3.2. Effect of Acid Medium and Concentration
3.3. Effect of the Solid–Liquid Ratio
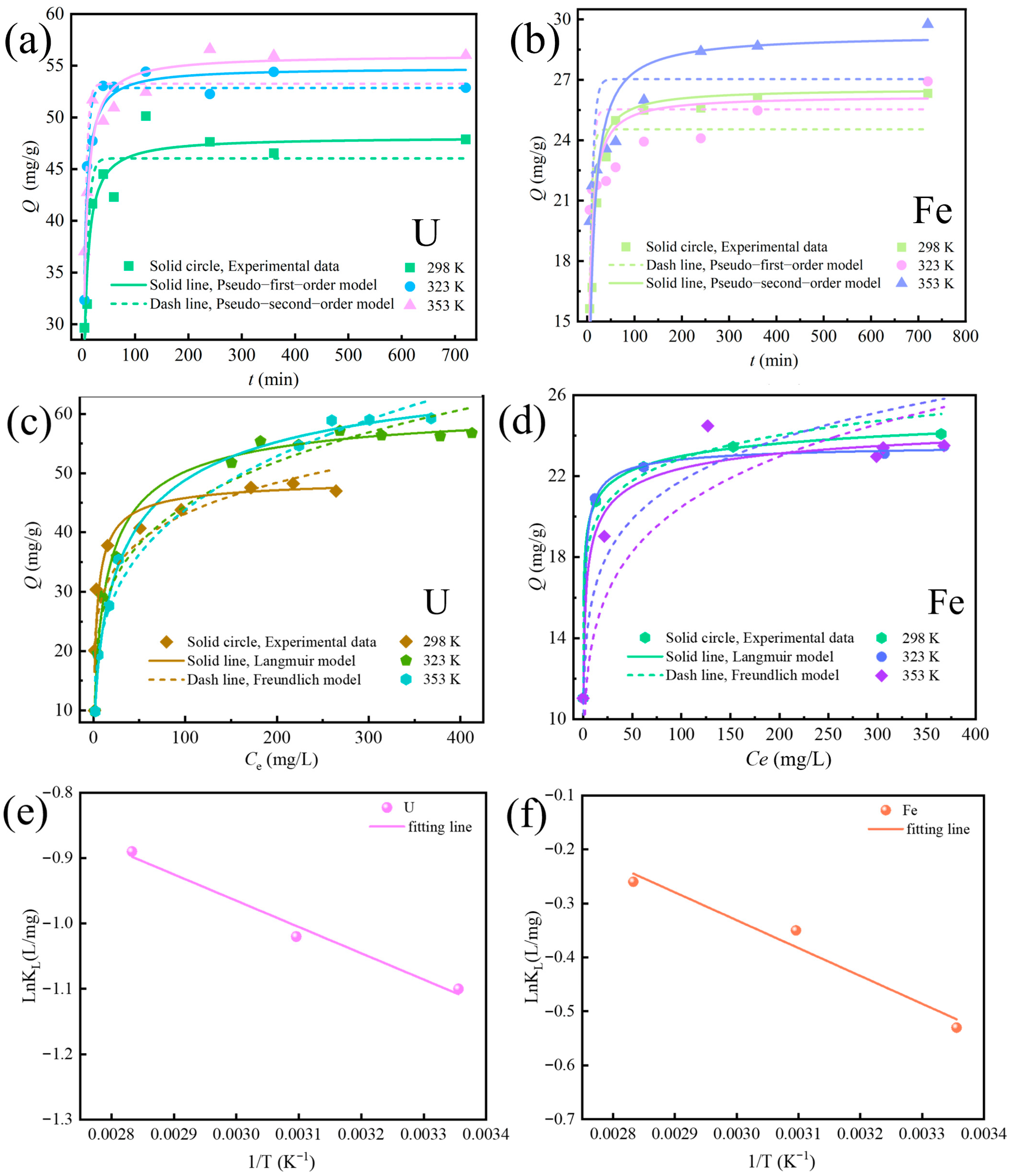
3.4. Adsorption Kinetics Towards U and Fe
3.5. Adsorption Isotherm Towards U and Fe
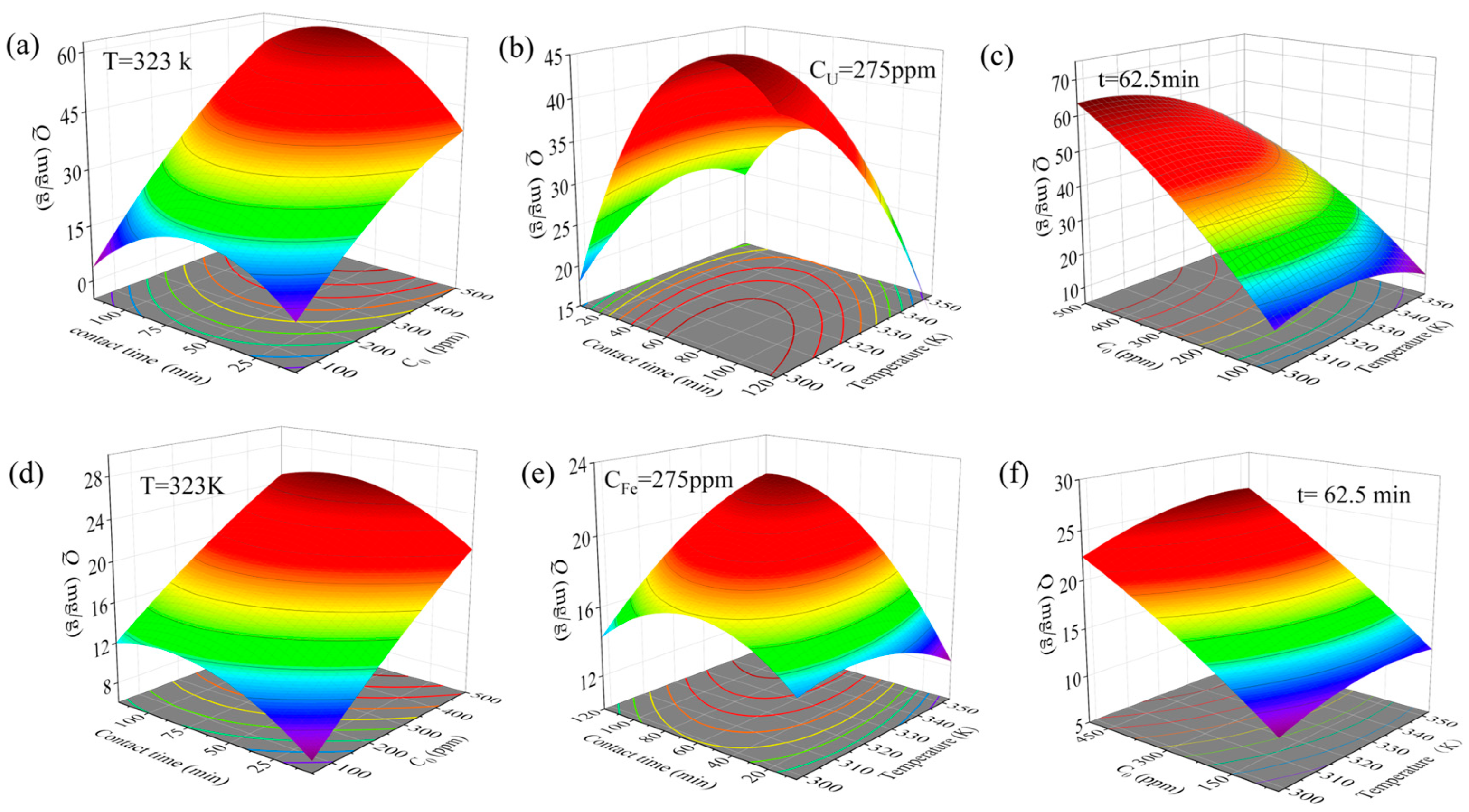
3.6. Box–Behnken Statistical Analysis
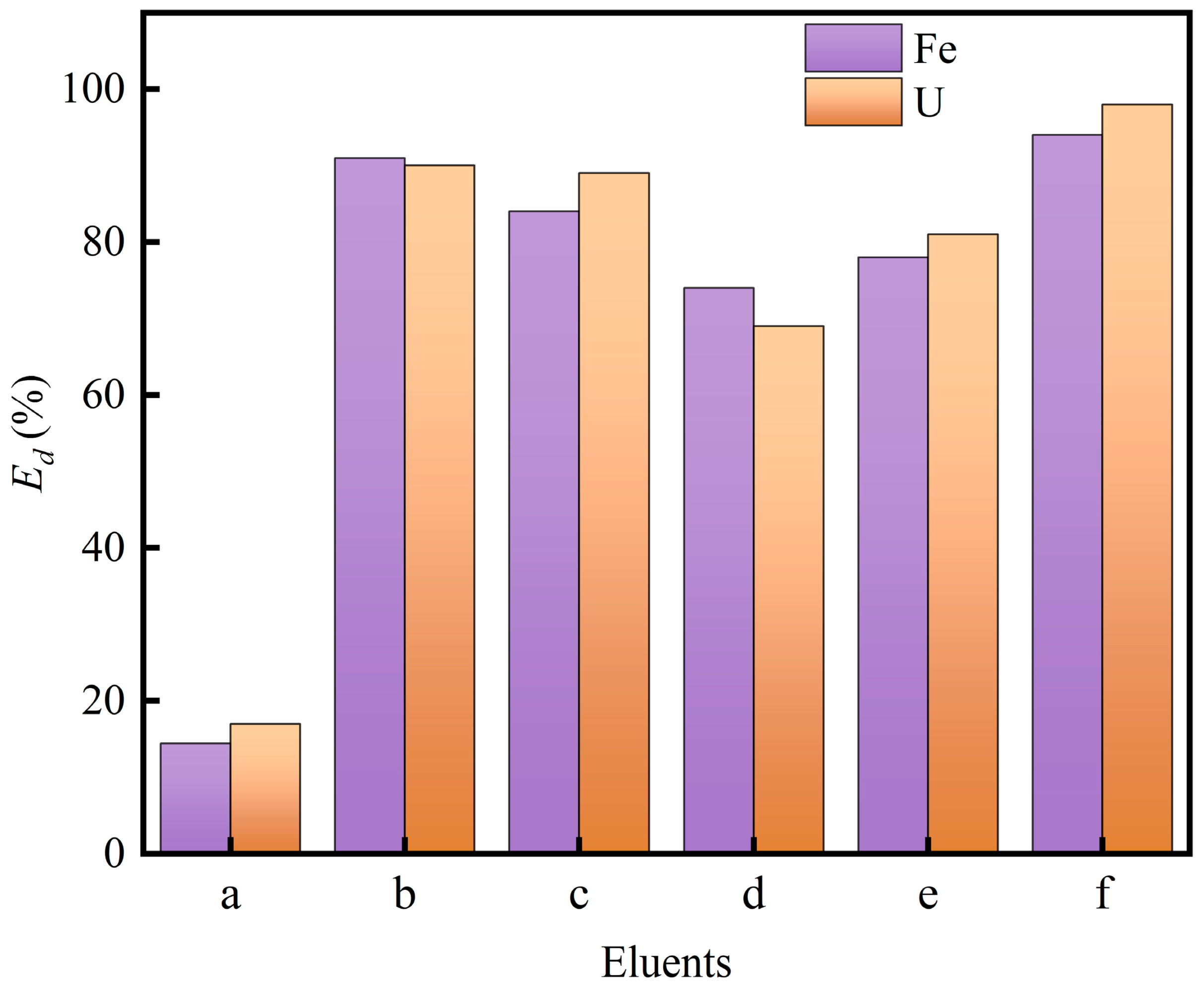
3.7. Desorption Performance
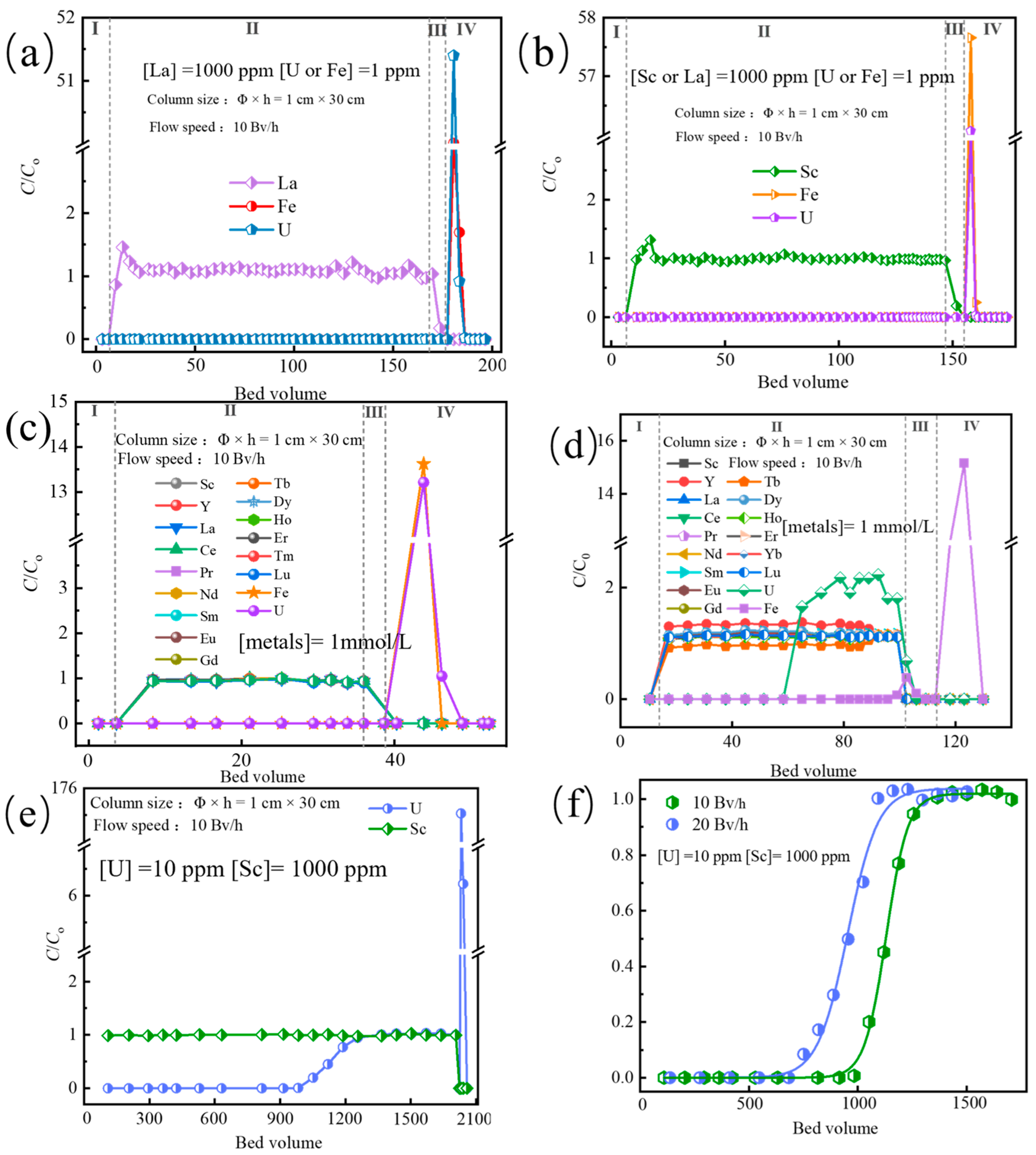
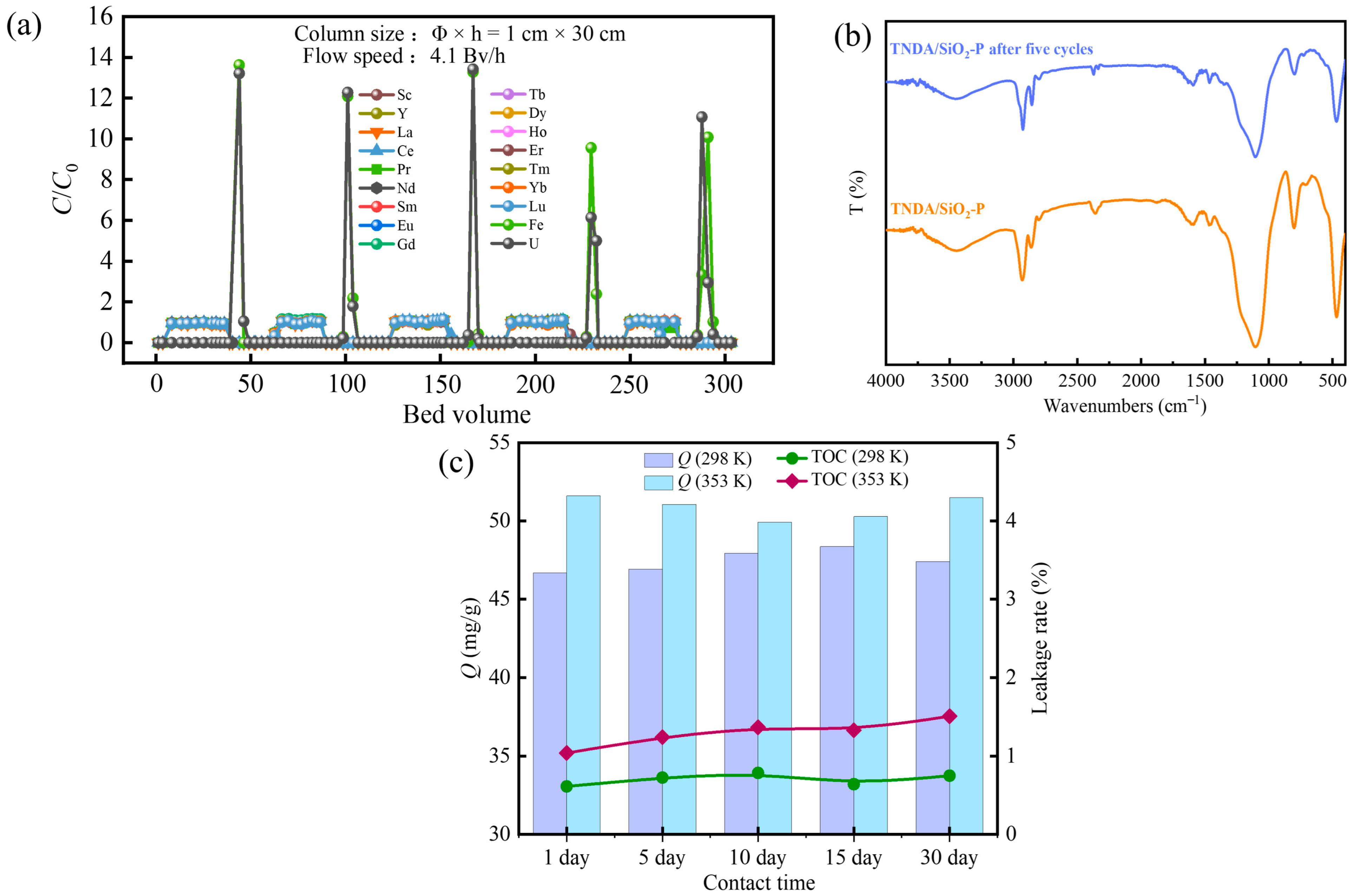
3.8. Column Experiments
3.9. Reusability Evaluation and Stability of TNDA/SiO2-P
3.10. Adsorption and Separation Mechanisms
3.10.1. FT-IR and XPS Analysis
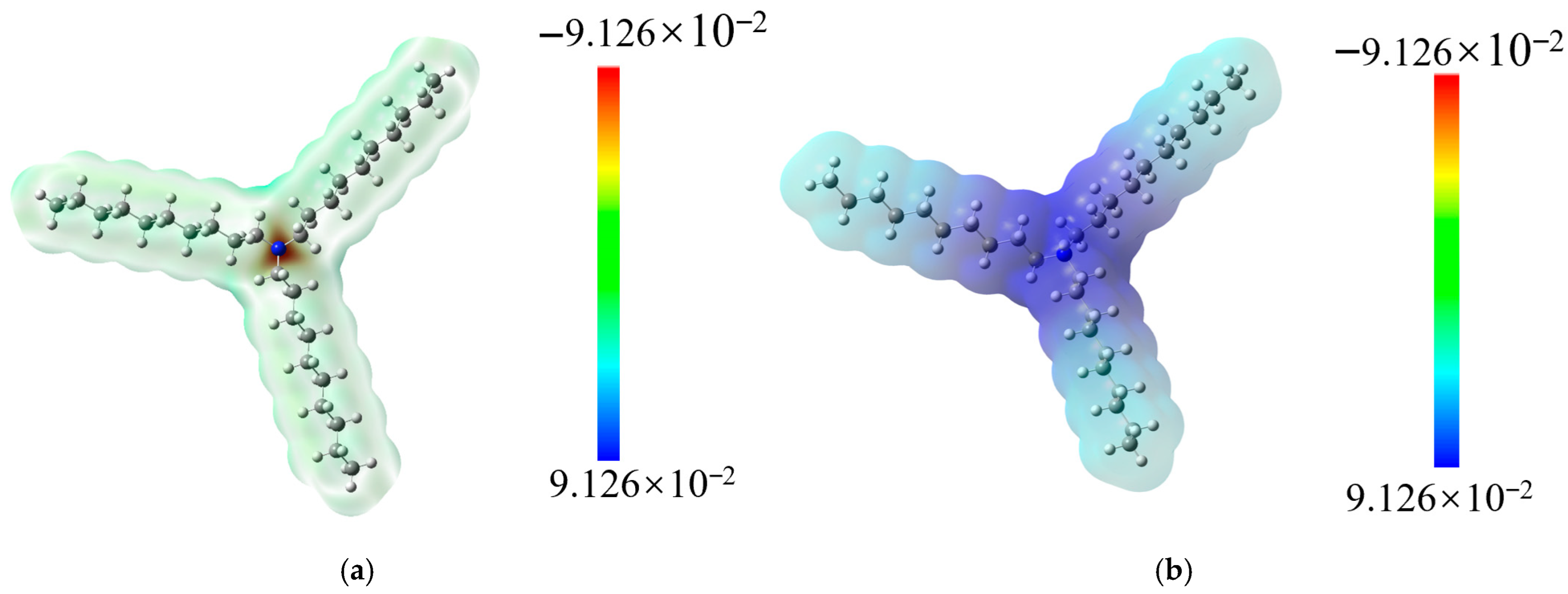
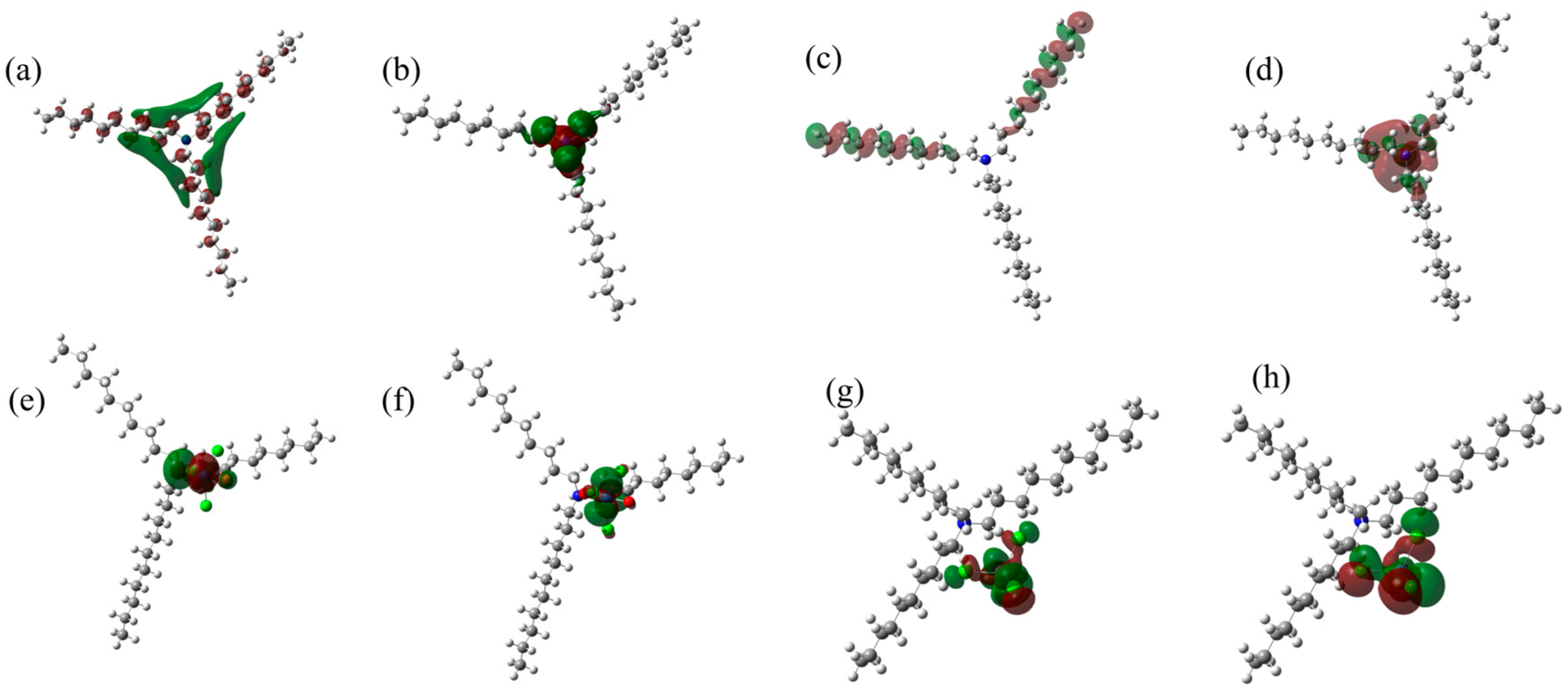
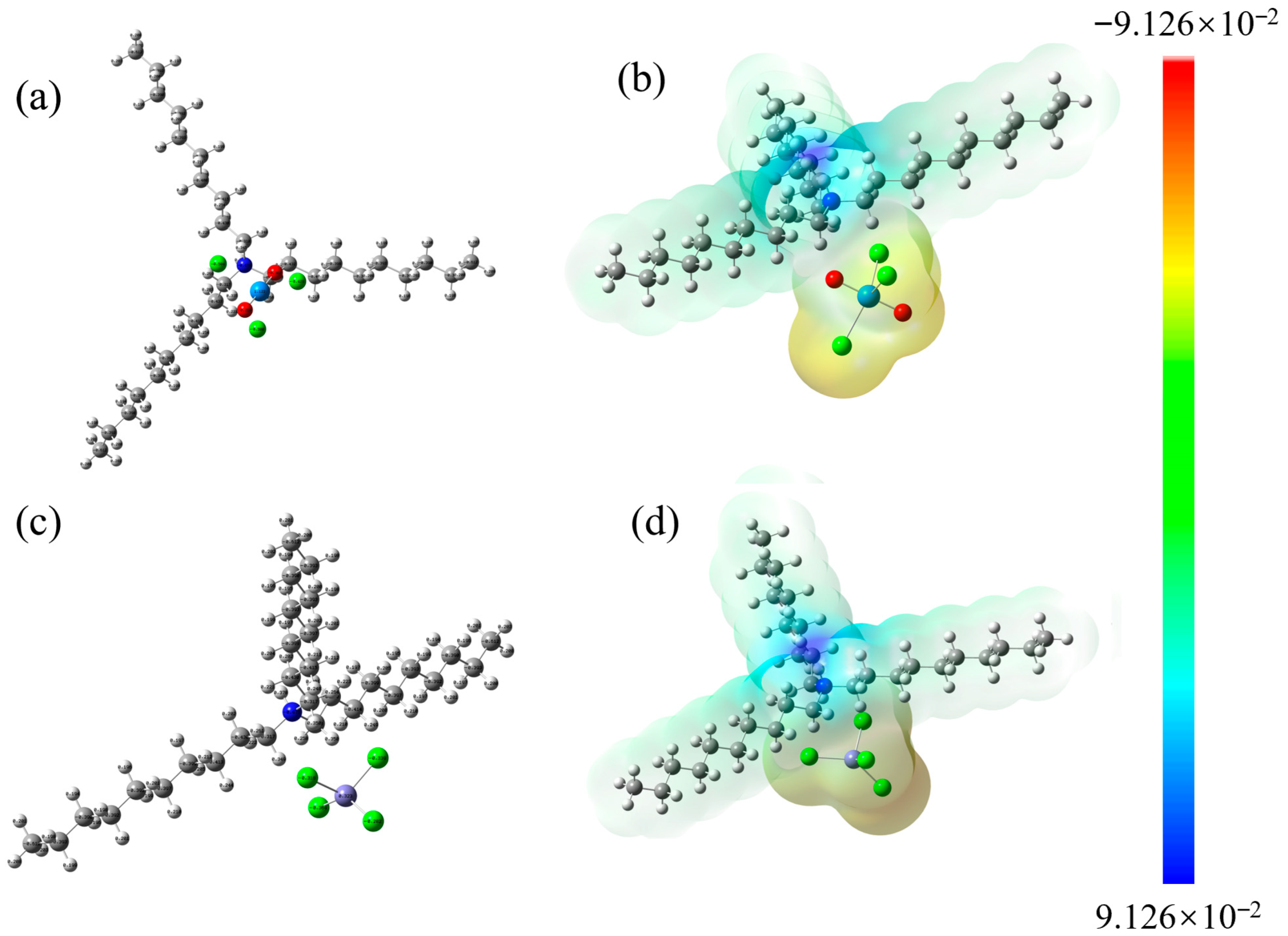
3.10.2. DFT Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Praneeth, S.; Sakr, A.K.; Roy, P.K.; Dittrich, T.M. Efficient recovery of gadolinium from magnetic resonance imaging patient urine using a diglycolamide ligand-functionalized sorbent system. Environ. Chem. Lett. 2025, 23, 937–942. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.; Yu, X.; Kang, J.; Qiu, G.; Zhao, H.; Shen, L. Effective extraction of rare earth elements from ion-adsorption type rare earth ore by three bioleaching methods. Sep. Purif. Technol. 2024, 330, 125305. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, C.; Zhang, X.; Lei, L.; Xiao, C. Selective Dissolution and Separation of Rare Earths Using Guanidine-Based Deep Eutectic Solvents. Acs Sustain. Chem. Eng. 2021, 9, 8507–8514. [Google Scholar] [CrossRef]
- Hong, J.; Tian, H.; Zhang, L.; Zhou, X.; del Rosal, I.; Weng, L.; Maron, L. Reversing Conventional Reactivity of Mixed Oxo/Alkyl Rare-Earth Complexes: Non-Redox Oxygen Atom Transfer. Angew. Chem.-Int. Ed. 2018, 57, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Yang, X.; Fang, D.; Ni, S.; Wang, S.; Ding, A.; Cen, P.; Xiao, C. Selective separation of radioactive thorium and uranium from scandium using N-heterocyclic carboxamide ligands. Sep. Purif. Technol. 2024, 328, 125028. [Google Scholar] [CrossRef]
- He, D.; Ning, S.; Liu, J.; Zhang, S.; Chen, L.; Xu, Y.; Feng, Z.; Hamza, M.F.; Wei, Y. Efficiently selective removal of radioactive thorium and uranium from rare earths by trialkyl phosphine oxide modified porous silica-polymer based adsorbent. Sep. Purif. Technol. 2025, 364, 132426. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Lei, X.; Wen, X.; Li, D.; Liu, F.; Zhou, W.; Xu, S. Separation and Recovery of Rare Earths and Iron from NdFeB Magnet Scraps. Processes 2023, 11, 2895. [Google Scholar] [CrossRef]
- Liu, F.; Porvali, A.; Wang, J.; Wang, H.; Peng, C.; Wilson, B.P.; Lundstrom, M. Recovery and separation of rare earths and boron from spent Nd-Fe-B magnets. Miner. Eng. 2020, 145, 106097. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Asare, S.; Adu-Boahene, F.; Schafer, A.I. Uranium adsorption by iron modified zeolite and zeolite composite membranes. Chemosphere 2024, 368, 143711. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhang, S.; Tan, J.; Dai, Y.; Wang, Y.; He, Y.; Liu, Y.; Zhao, X.; Zhang, M.; Zhang, Q. Highly efficacious entrapment of Th (IV) and U (VI) from rare earth elements in concentrated nitric acid solution using a phosphonic acid functionalized porous organic polymer adsorbent. Sep. Purif. Technol. 2020, 237, 116379. [Google Scholar] [CrossRef]
- Tuzen, M.; Sari, A.; Saleh, T.A. Synthesis, characterization and evaluation of carbon nanofiber modified-polymer for ultra-removal of thorium ions from aquatic media. Chem. Eng. Res. Des. 2020, 163, 76–84. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Zhang, H.-D.; Li, Z.-J.; Wang, S.; Liu, S.-Y.; Xiu, T.-Y.; Yu, J.-P.; Wang, L.; Yuan, L.-Y.; Wu, W.-S.; et al. Synthesis of phosphorylated covalent organic frameworks with different linkages-Critical factors controlling the extraction of uranium from highly acidic media. Chem. Eng. J. 2025, 507, 160226. [Google Scholar] [CrossRef]
- Jiao, R.; Chen, Z.; Zeng, S.; Wang, D.; Li, J. Electrosorption of uranium (VI) by sulfonic acid-decorated FeOOH nanorods. J. Environ. Chem. Eng. 2023, 11, 111275. [Google Scholar] [CrossRef]
- Cui, J.; Chen, L.; Lou, Z.; Shan, W.; Xiong, Y. Noncovalent functionalization of graphene via perylene bisimide-amidoxime for highly efficient capture of uranium. Sep. Purif. Technol. 2025, 361, 131434. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, X.; Tan, S.; Wang, H.; Kuang, S.; Liu, X.; Liao, W. Ultra-selective removal of thorium from rare earths by aminophosphonic acid-modified porous silica. Sep. Purif. Technol. 2024, 341, 126952. [Google Scholar] [CrossRef]
- Wang, Z.; Su, D.; Luo, T.; Yu, Q.; Wang, Y.; Liu, X.; Huang, X.; Ding, S. Highly Selective Separation of Thorium Using an Extraction Resin by Encapsulating an Amine-Based Ionic Liquid In Situ within a Porous Silica-Polymer Matrix Instead of Conventional Impregnation Method. Acs Sustain. Chem. Eng. 2025, 13, 5269–5281. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Wang, W.; Wu, X.; Wang, H.; Li, J. Efficient and Selective Removal of Palladium from Simulated High-Level Liquid Waste Using a Silica-Based Adsorbent NTAamide(C8)/SiO2-P. Nanomaterials 2024, 14, 544. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Q.; Chen, L.; Zhong, Y.; Hu, F.; Wu, K.; Yin, X.; Wei, Y.; Ning, S. Phosphination of amino-modified mesoporous silica for the selective separation of strontium. J. Hazard. Mater. 2024, 467, 133741. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhang, S.; Huang, Q.; Chen, L.; Zhang, W.; Yin, X.; Wei, Y.; Ning, S. Efficient separation of strontium in different environments with novel acid-resistant silica-based ion exchanger. Sep. Purif. Technol. 2023, 322, 124347. [Google Scholar] [CrossRef]
- Soderholm, L.; Skanthakumar, S.; Wilson, R.E. Structural Correspondence between Uranyl Chloride Complexes in Solution and Their Stability Constants. J. Phys. Chem. A 2011, 115, 4959–4967. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, S.; Yan, Z.C.; Li, D.-S.; Wang, Y.; An, W.; Yang, H.Y. Effect of protonation and deprotonation on oxygen-containing groups functionalized graphene for boron adsorption removal. Desalination 2024, 583, 117692. [Google Scholar] [CrossRef]
- Isik, B.; Kurtoglu, A.E.; Gurdag, G.; Keceli, G. Radioactive cesium ion removal from wastewater using polymer metal oxide composites. J. Hazard. Mater. 2021, 403, 123652. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Khalafalla, M.S.; Abed, N.S.; Fouda, A.; Elwakeel, K.Z.; Guibal, E.; Hamad, N.A. U(VI) and Th(IV) recovery using silica beads functionalized with urea- or thiourea-based polymers—Application to ore leachate. Sci. Total Environ. 2022, 821, 153184. [Google Scholar] [CrossRef]
- Tangde, V.M.; Prajapati, S.S.; Mandal, B.B.; Kulkarni, N.P. Study of Kinetics and Thermodynamics of Removal of Phosphate from Aqueous Solution using Activated Red Mud. Int. J. Environ. Res. 2017, 11, 39–47. [Google Scholar] [CrossRef]
- Xu, S.; Ning, S.; Wang, Y.; Wang, X.; Dong, H.; Chen, L.; Yin, X.; Fujita, T.; Wei, Y. Precise separation and efficient enrichment of palladium from wastewater by amino-functionalized silica adsorbent. J. Clean. Prod. 2023, 396, 136479. [Google Scholar] [CrossRef]
- Tang, W.; Huang, H.; Gao, Y.; Liu, X.; Yang, X.; Ni, H.; Zhang, J. Preparation of a novel porous adsorption material from coal slag and its adsorption properties of phenol from aqueous solution. Mater. Des. 2015, 88, 1191–1200. [Google Scholar] [CrossRef]
- Kenawy, I.M.M.; Eldefrawy, M.M.; Eltabey, R.M.; Zaki, E.G. Melamine grafted chitosan-montmorillonite nanocomposite for ferric ions adsorption: Central composite design optimization study. J. Clean. Prod. 2019, 241, 118189. [Google Scholar] [CrossRef]
- Lefévre, G.; Noinville, S.; Fédoroff, M. Study of uranyl sorption onto hematite by in situ attenuated total reflection-infrared spectroscopy. J. Colloid Interface Sci. 2006, 296, 608–613. [Google Scholar] [CrossRef]
- Wen, C.; Yao, Y.; Meng, L.; Duan, E.; Wang, M.; Chen, Z.; Wang, X. Photocatalytic and Electrocatalytic Extraction of Uranium by COFs: A Review. Ind. Eng. Chem. Res. 2023, 62, 18230–18250. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Wang, C.; Xi, W.; Bai, Y.; Lu, W.; Wang, J. Elimination of radionuclide uranium (VI) from aqueous solutions using an α-MnO2@CTS composite adsorbent. J. Mol. Liq. 2022, 360, 119448. [Google Scholar] [CrossRef]
- He, X.; Feng, W.; Wang, Z.; Ning, S.; Lv, L.; Chen, L.; Li, W.; Yin, X.; Wei, Y.; Watabe, H. An advanced separation method for the acquisition of 212Pb/212Bi from natural thorium. Chem. Eng. J. 2024, 502, 157971. [Google Scholar] [CrossRef]
- Reda, A.T.; Zhang, D.; Lu, X. Rapid and selective uranium adsorption by glycine functionalized europium hydroxide. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 299–308. [Google Scholar] [CrossRef]
- Peng, T.-Q.; Wang, Y.-F.; Xu, Y.-F.; Liu, Z.-C. Synthesis, characterization and uranium (VI) adsorption mechanism of novel adsorption material poly(tetraethylenepentamine–trimesoyl chloride). J. Radioanal. Nucl. Chem. 2023, 332, 409–422. [Google Scholar] [CrossRef]
- Schio, L.; Li, C.; Monti, S.; Salen, P.; Yatsyna, V.; Feifel, R.; Alagia, M.; Richter, R.; Falcinelli, S.; Stranges, S.; et al. NEXAFS and XPS studies of nitrosyl chloride. Phys. Chem. Chem. Phys. 2015, 17, 9040–9048. [Google Scholar] [CrossRef]
- Uchikoshi, M.; Akiyama, D.; Kimijima, K.I.; Shinoda, K. The Distribution and Structures of Ferric Aqua and Chloro Complexes in Hydrochloric Acid Solutions. ISIJ Int. 2022, 62, 912–921. [Google Scholar] [CrossRef]
- Yu, F.-D.; Zhao, J.-Z.; Wang, H.-N.; Wu, J.-H.; Lan, Z.; Xie, Y.-M.; Que, L.-F. Unveiling the Chemical Heterogeneity and Structural Evolution in Oxide Cathodes During Ion Exchange. Adv. Funct. Mater. 2025, 35, 2501977. [Google Scholar] [CrossRef]
- Buehl, M.; Sieffert, N.; Golubnychiy, V.; Wipff, G. Density functional theory study of uranium(VI) aquo chloro complexes in aqueous solution. J. Phys. Chem. A. 2008, 112, 2428–2436. [Google Scholar] [CrossRef]
- Zou, Q.; Wu, Y.; Shu, Q.; Ning, S.; Wang, X.; Wei, Y.; Tang, F. Separation of Palladium along with Minor Actinides by isoBu-BTP/SiO2-P Adsorbent from High-Level Liquid Waste. J. Chem. Eng. Data. 2018, 63, 2931–2939. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, C.; Ren, X.; Tan, X.; Song, G.; Chen, D.; Alsaedi, A.; Hayat, T. Coupling g-C3N4 nanosheets with metal-organic frameworks as 2D/3D composite for the synergetic removal of uranyl ions from aqueous solution. J. Colloid Interface Sci. 2019, 550, 117–127. [Google Scholar] [CrossRef]
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Adsorption of CO2 on KOH activated, N-enriched carbon derived from urea formaldehyde resin: Kinetics, isotherm and thermodynamic studies. Appl. Surf. Sci. 2018, 439, 760–771. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Z.; Chen, L.; Zhang, S.; Liu, J.; Ye, Z.; Hamza, M.F.; Wei, Y.; Ning, S. Preparation of a Porous Tri-n-decylamine Modified Adsorbent for the Efficient Removal of Uranium and Iron from Rare Earth. Metals 2025, 15, 1064. https://doi.org/10.3390/met15101064
Yi Z, Chen L, Zhang S, Liu J, Ye Z, Hamza MF, Wei Y, Ning S. Preparation of a Porous Tri-n-decylamine Modified Adsorbent for the Efficient Removal of Uranium and Iron from Rare Earth. Metals. 2025; 15(10):1064. https://doi.org/10.3390/met15101064
Chicago/Turabian StyleYi, Zihang, Lifeng Chen, Shichang Zhang, Juan Liu, Zhifu Ye, Mohammed F. Hamza, Yuezhou Wei, and Shunyan Ning. 2025. "Preparation of a Porous Tri-n-decylamine Modified Adsorbent for the Efficient Removal of Uranium and Iron from Rare Earth" Metals 15, no. 10: 1064. https://doi.org/10.3390/met15101064
APA StyleYi, Z., Chen, L., Zhang, S., Liu, J., Ye, Z., Hamza, M. F., Wei, Y., & Ning, S. (2025). Preparation of a Porous Tri-n-decylamine Modified Adsorbent for the Efficient Removal of Uranium and Iron from Rare Earth. Metals, 15(10), 1064. https://doi.org/10.3390/met15101064









