Investigation of Corrosion Resistance in Powder-Coated 6060 Aluminum Alloy: Effects of Powder Coating and Pre-Anodizing Followed by Powder Coating
Abstract
1. Introduction
2. Materials and Methods
3. Results
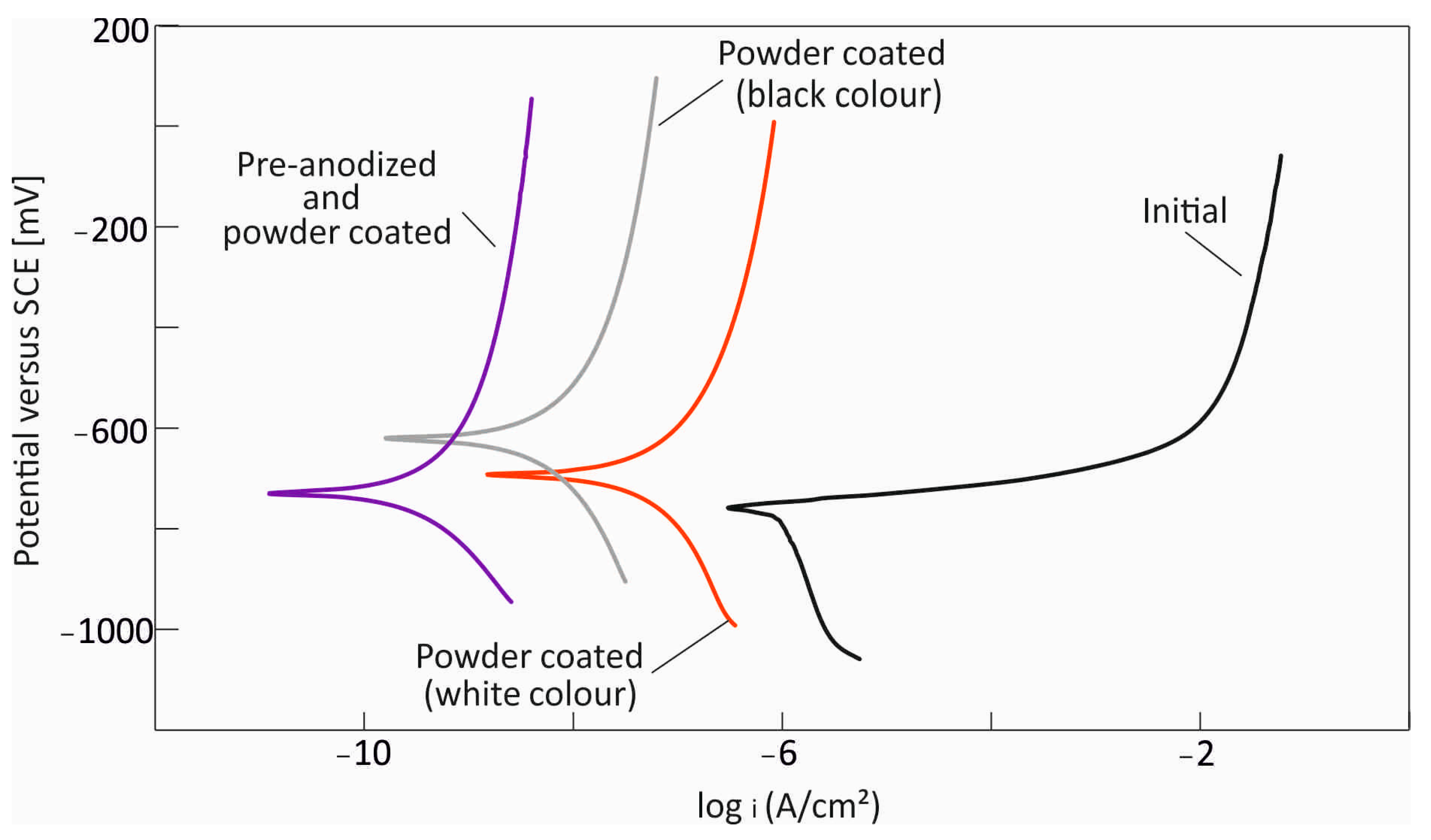
3.1. Electrochemical Characterization
3.2. Surface Morphology and Corresponding Electrical Circuit Models
4. Conclusions
- Even though the pre-anodized layer has some tiny holes and gaps, the powder coating helps considerably by covering those weak spots and making the surface tougher against corrosion. Together, these two layers provide strong protection and help the material last longer.
- The high values of resistance indicate that the powder coating process was highly effective.
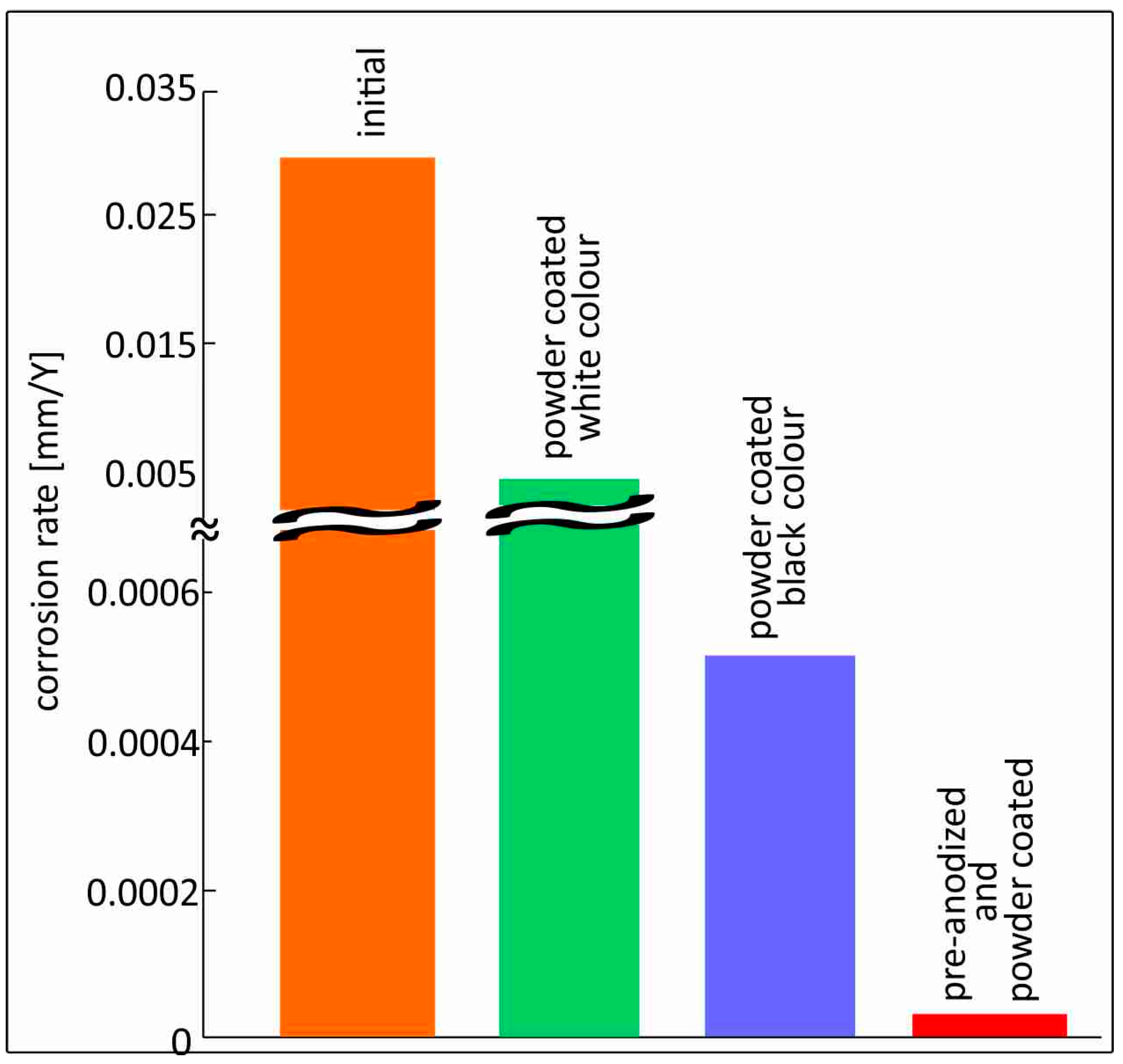
- Black powder-coated samples showed better corrosion protection than white powder-coated ones, indicating that the coating forms a compact and uniform barrier that effectively inhibits corrosion.
- Surface modifications greatly enhance corrosion resistance. Black powder-coated specimens exhibit corrosion rates about 60 times lower, whereas samples that are both pre-anodized and powder-coated have the greatest protection, reducing corrosion rates by nearly 1000 times compared to untreated aluminum.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sukiman, N.L.; Zhou, X.; Birbilis, N.; Hughes, A.E.; Mol, J.M.C.; Garcia, S.J.; Zhou, X.; Thompson, G.E. Durability and corrosion of aluminium and its alloys: Overview, property space, techniques and developments. In Aluminium Alloys—New Trends in Fabrication and Applications; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Whelan, M.; Barton, K.; Cassidy, J.; Colreavy, J.; Duffy, B. Corrosion inhibitors for anodised aluminium. Surf. Coat. Technol. 2013, 227, 75–83. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.V.; Dautov, S.S.; Shornikov, P.G.; Akhatov, I.S. Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings 2021, 11, 4. [Google Scholar] [CrossRef]
- Khoshnaw, F.; Gubner, R. Part I: General aspects of corrosion, corrosion control, and corrosion prevention. In Corrosion Atlas Case Studies; Elsevier: Amsterdam, The Netherlands, 2020; pp. xxv–xli. [Google Scholar] [CrossRef]
- Loto, R.T.; Oladipupo, S.; Folarin, T.; Okosun, E. Impact of chloride concentrations on the electrochemical performance and corrosion resistance of austenitic and ferritic stainless steels in acidic chloride media. Discov. Appl. Sci. 2025, 7, 672. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Karbasi, M.; Chaharmahali, R.; Fardosi, A.; Kaseem, M. An overview of electrochemical, non-electrochemical and analytical approaches for studying corrosion in magnesium and its alloys. J. Magnes. Alloys 2024, 12, 3516–3542. [Google Scholar] [CrossRef]
- Nazeri, A.R.; Calvin, J.; Allene, A.L.; Badrul, H.M.J.; Parabi, A.; Ming, C.K.; Parabi, A.S.L.; James, A.; Shamsol, N.S.; Belun, S.J.; et al. Corrosion resistance and electrochemical adaptation of aluminium in brackish peat water sources under seawater intrusion in the rural tropical peatlands of Borneo. Sustain. Chem. Clim. Action 2025, 6, 100074. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Chen, W.; Hong, H.; Zhang, T. Long-Term Corrosion Behavior of 434 Stainless Steel Coatings on T6061 Aluminum Alloy in Chloride Environments. Coatings 2025, 15, 144. [Google Scholar] [CrossRef]
- Ress, J.; Martin, U.; Bosch, J.; Gupta, R.K.; Bastidas, D.M. Intergranular to Intragranular Pitting Corrosion Transition Mechanism of Sensitized AA5083 at 150 °C. Metals 2020, 10, 1082. [Google Scholar] [CrossRef]
- Zhou, Z.; Ge, X.; Fan, W.; Shan, B.; Yang, J.; Zhao, X. Corrosion behavior of 7B04 aluminum alloy under the synergistic effect of Lacticaseibacillus paracasei and Acinetobacter lwoffi. Arab. J. Chem. 2023, 16, 1878–5352. [Google Scholar] [CrossRef]
- Malaret, F. Exact calculation of corrosion rates by the weight-loss method. Exp. Results 2022, 3, e13. [Google Scholar] [CrossRef]
- Laurent, C.; Scenini, F.; Monetta, T.; Bellucci, F.; Curioni, M. The contribution of hydrogen evolution processes during corrosion of aluminium and aluminium alloys investigated by potentiodynamic polarisation coupled with real-time hydrogen measurement. npj Mater. Degrad. 2017, 1, 6. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Wang, H.; Li, B.; Hu, Q.; Shao, T.; Yang, R.; Wang, B.; Wan, Q.; Li, Z.; et al. Experimental Study on Neutral Salt Spray Accelerated Corrosion of Metal Protective Coatings for Power-Transmission and Transformation Equipment. Coatings 2023, 13, 480. [Google Scholar] [CrossRef]
- Wang, G.; Tuo, X.; Kou, L.; Zhao, W.; Zhu, X. Research on corrosion performance of 6061aluminum alloy in salt spray environment. Mater. Werkst. 2020, 51, 1686–1699. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I. Electrochemical and Salt Spray Testing of Hybrid Coatings Based on Si and Zr Deposited on Aluminum and Its Alloys. J. Electrochem. Soc. 2015, 162, C592–C600. [Google Scholar] [CrossRef]
- Comas, C.; Huet, F.; Ngo, K.; Fregonese, M.; Idrissi, H.; Normand, B. Corrosion propagation monitoring using electrochemical noise measurements on carbon steel in hydrogenocarbonated solution containing chloride ions. Corros. Sci. 2021, 193, 109885. [Google Scholar] [CrossRef]
- Morcillo, E.M.; Veleva, L.; Wipf, D.O. Initial corrosion study of magnesium alloys in simulated body fluid by SECM. ECS Meet. Abstr. 2017, 1, 932. [Google Scholar] [CrossRef]
- Cui, J.; Yu, D.; Long, Z.; Xi, B.; He, X.; Pei, Y. Application of electrochemical noise (EN) technology to evaluate the passivation performances of adsorption and film-forming type corrosion inhibitors. J. Electroanal. Chem. 2019, 855, 113584. [Google Scholar] [CrossRef]
- Zhu, H.; Li, J. Advancements in corrosion protection for aerospace aluminum alloys through surface treatment. Int. J. Electrochem. Sci. 2024, 19, 100487. [Google Scholar] [CrossRef]
- Meddings, N.; Heinrich, M.; Overney, F.; Lee, J.-S.; Ruiz, V.; Napolitano, E.; Seitz, S.; Hinds, G.; Raccichini, R.; Gaberšček, M.; et al. Application of electrochemical impedance spectroscopy to commercial Li-ion cells: A review. J. Power Sources 2020, 480, 228742. [Google Scholar] [CrossRef]
- Padha, B.; Verma, S.; Mahajan, P.; Arya, S. Electrochemical impedance spectroscopy (EIS) performance analysis and challenges in fuel cell applications. J. Electrochem. Sci. Technol. 2022, 13, 167–176. [Google Scholar] [CrossRef]
- Hernández, H.H.; Reynoso, A.M.R.; González, J.C.T.; Morán, C.O.G.; Hernández, J.G.M.; Ruiz, A.M.; Hernández, J.M.; Cruz, R.O. Electrochemical impedance spectroscopy (EIS): A review study of basic aspects of the corrosion mechanism applied to steels. In Electrochemical Impedance Spectroscopy; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Feliu, S., Jr. Electrochemical Impedance Spectroscopy for the Measurement of the Corrosion Rate of Magnesium Alloys: Brief Review and Challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. A review of electrochemical impedance spectroscopy for bioanalytical sensors. Anal. Methods 2022, 14, 4602–4624. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Li, Y.; Zhang, Y.X.; Chen, G.Y. A method to select the optimal equivalent electrical circuit applied to study corrosion system of composite coating on magnesium alloy. Phys. Lett. A 2020, 384, 126452. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical impedance spectroscopy—A tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Santoni, F.; De Angelis, A.; Moschitta, A.; Carbone, P.; Galeotti, M.; Cinà, L.; Giammanco, C.; Carlo, A. A guide to equivalent circuit fitting for impedance analysis and battery state estimation. J. Energy Storage 2024, 82, 110389. [Google Scholar] [CrossRef]
- Werle, P.; Graf, H.; Walter, E. Process for the Production of Carbon Black Containing Pigment-Synthetic Resin Concentrates. U.S. Patent No. 865,968, 30 December 1977. [Google Scholar]
- Kamal Akhtar, M.; Banerjee, S. Process for the Production of Coated Titanium Dioxide Pigments. U.S. Patent 20090148605A1, 5 December 2007. [Google Scholar]
- Šolić, T.; Marić, D.; Peko, I.; Samardžić, I. Optimization of Coating Process Parameters by Analysis of Target Powder Thickness and Regression Modeling. Appl. Sci. 2025, 15, 673. [Google Scholar] [CrossRef]
- González, J.; López, V.; Bautista, A.; Otero, E.; Nóvoa, X. Characterization of porous aluminium oxide films from a.c. im-pedance measurements. J. Appl. Electrochem. 1999, 29, 229–238. [Google Scholar] [CrossRef]
- Margono; Darmadi, D.B.; Gapsari, F.; Widodo, T.D.; Kartika, B.M. Enhanced Corrosion Resistance of Aluminum 6061 Alloy Using Ti-Based Thin Films and Plasma Nitriding. JCIS Open 2025, 18, 100139. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, G.; Meng, G.; Cheng, Y. Electronic structure and pitting behavior of 3003 aluminum alloy passivated under various conditions. Electrochim. Acta 2009, 54, 4155–4163. [Google Scholar] [CrossRef]
- Peng, G.S.; Chen, K.H.; Fang, H.C.; Chao, H.; Chen, S.Y. EIS study on pitting corrosion of 7150 aluminum alloy in sodium chloride and hydrochloric acid solution. Mater. Corros. 2010, 61, 783–789. [Google Scholar] [CrossRef]
- Mahdavian, M.; Attar, M.M. Another approach in analysis of paint coatings with EIS measurement: Phase angle at high frequencies. Corros. Sci. 2006, 48, 4152–4157. [Google Scholar] [CrossRef]
- Mrówka, G.; Sieniawski, J. Analysis of intermetallic phases in 2024 aluminium alloy. Solid State Phenom. 2013, 197, 238–243. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion Resistance of Hard Coat Anodized AA 6061 in Citric–Sulfuric Solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Stergioudi, F.; Vogiatzis, C.A.; Gkrekos, K.; Michailidis, N.; Skolianos, S.M. Electrochemical corrosion evaluation of pure, carbon-coated and anodized Al foams. Corros. Sci. 2015, 91, 151–159. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Sioulas, D.; Grimanelis, D. Corrosion and wear of coatings fabricated by HVOF-spraying of nanostructured and conventional WC–10Co-4Cr powders on Al7075-T6. Int. J. Refract. Met. Hard Mater. 2023, 112, 106164. [Google Scholar] [CrossRef]
- Córdoba-Torres, P. Relationship between constant-phase element (CPE) parameters and physical properties of films with a distributed resistivity. Electrochim. Acta 2017, 225, 592–604. [Google Scholar] [CrossRef]
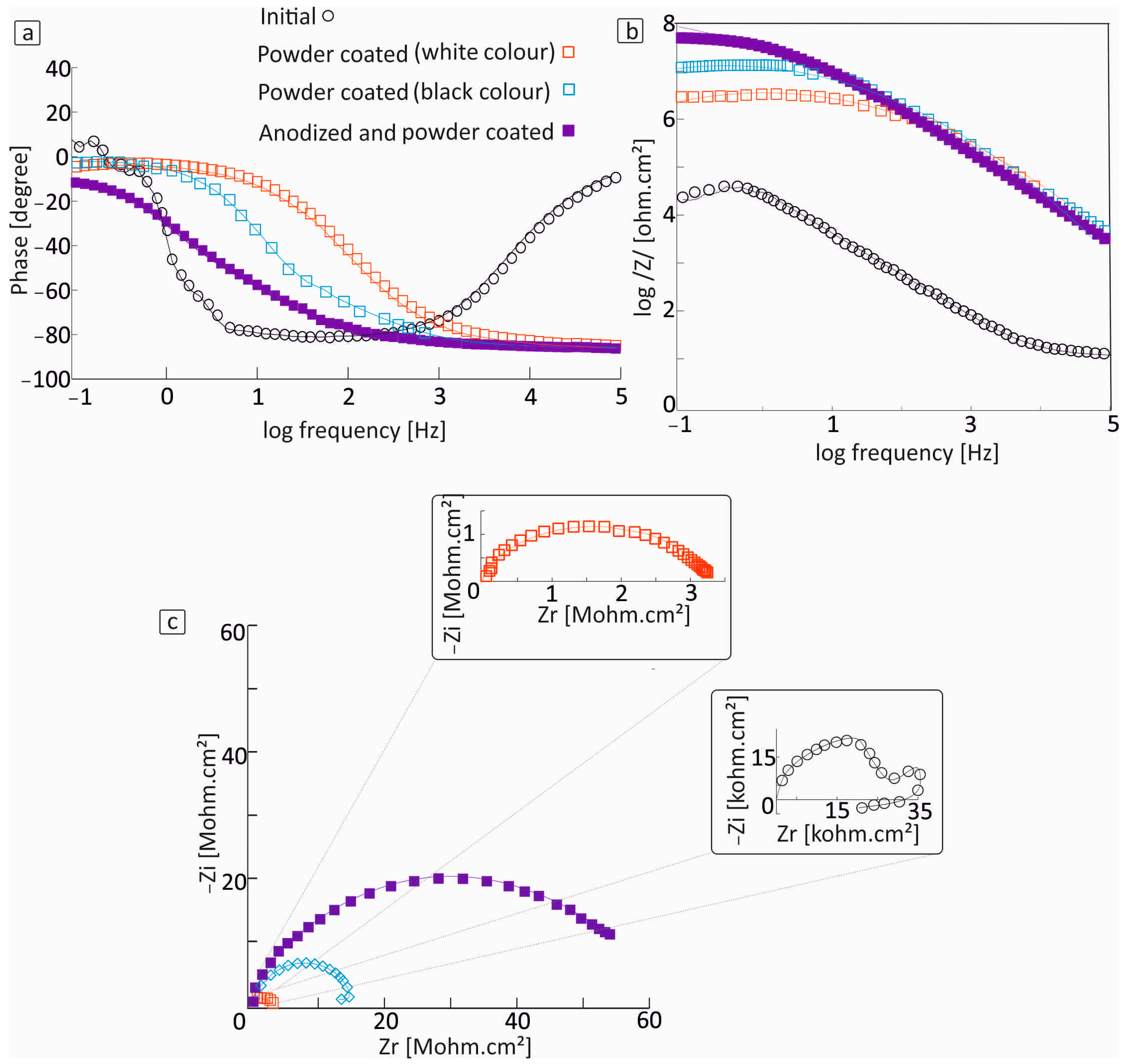

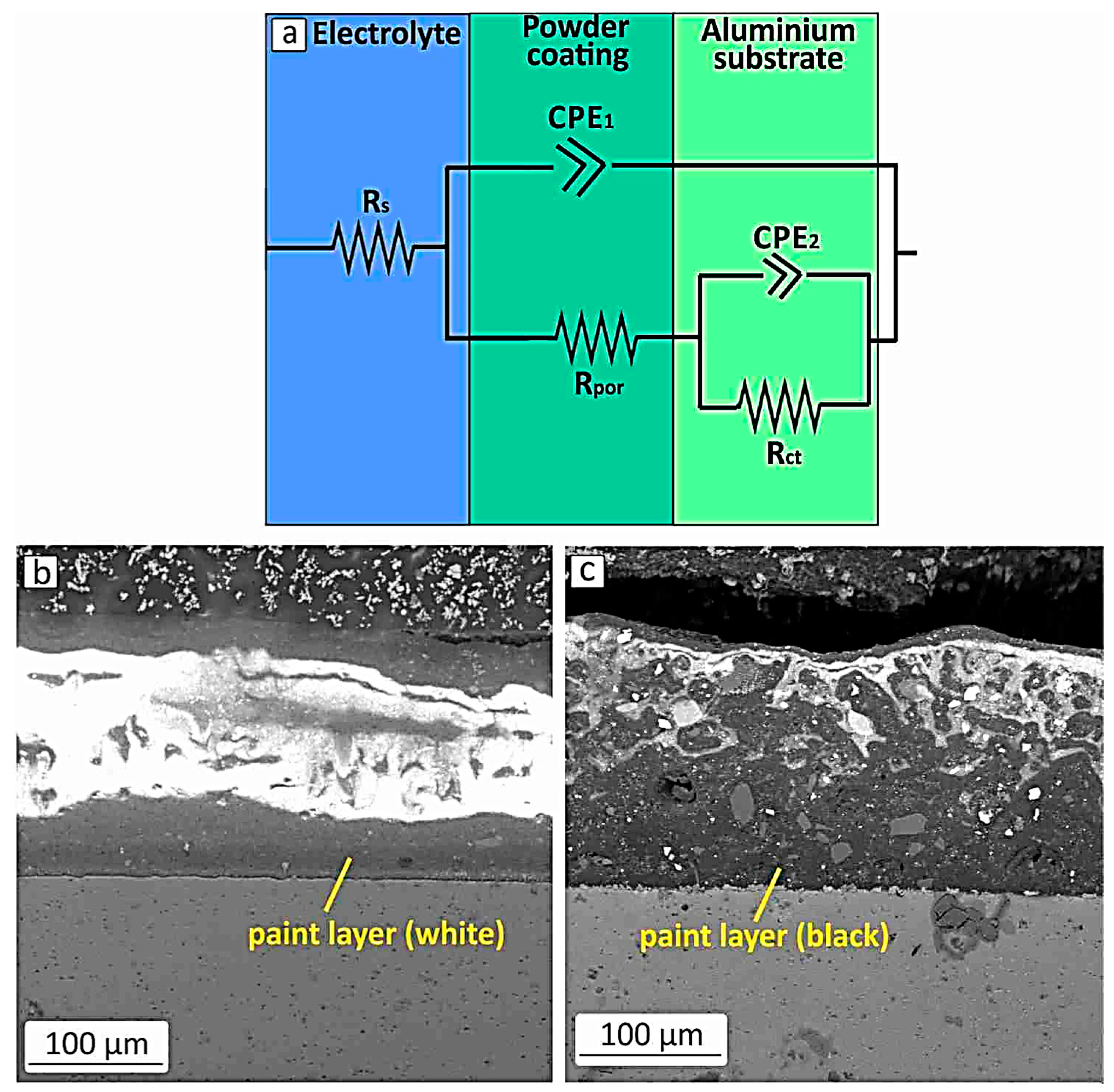
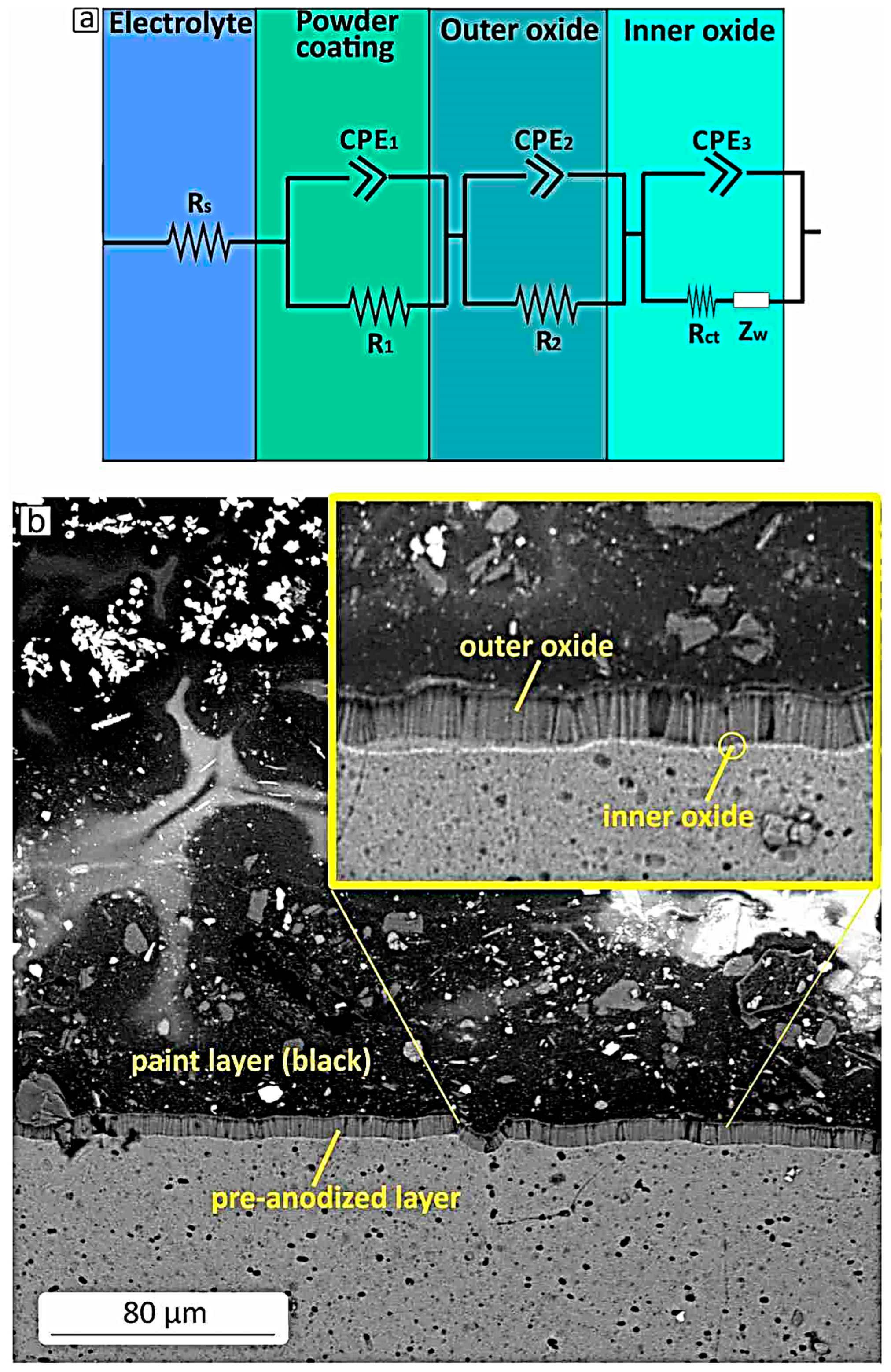

| Al Samples NaCl solution | Rs | CPE1 S × sa | α1 | CPE2 S × sa | α2 | CPE3 S × sa | α3 | Rpor | R1 | R2 | Rct | W S × s1/2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| initial | 11 Ω | 2.2 × 10−6 | 0.98 | 8.9 × 10−7 | 1 | - | - | 618 Ω | - | - | 25 kΩ | - |
| Powder coated (white colour) | 0.1 Ω | 8.9 × 10−10 | 0.92 | 1.1 × 10−8 | 0.91 | - | - | 2.4 ΜΩ | - | - | 0.7 ΜΩ | - |
| Powder coated (black colour) | 0.1 Ω | 6.2 × 10−10 | 0.95 | 8.6 × 10−10 | 0.92 | - | - | 4.97 ΜΩ | - | - | 9.65 ΜΩ | - |
| Pre-anodized and painted | 0.1 Ω | 1.74 × 10−9 | 0.95 | 1.8 × 10−9 | 0.93 | 1.4 × 10−9 | 0.9 | - | 7.5 ΜΩ | 2 kΩ | 0.47 ΜΩ | 1.3 × 10−5 |
| Sample | Ecorr [mV] | Icorr [A/cm2] | βa | βc | Corrosion Rate [μmpy] |
|---|---|---|---|---|---|
| Initial | −0.756 | 9 × 10−7 | 0.022 | −0.52 | 30 |
| Powder-coated (white color) | −0.693 | 1.11 × 10−7 | 0.47 | −0.55 | 3.73 |
| Powder-coated (black color) | −0.622 | 1.56 × 10−8 | 0.83 | −0.68 | 0.508 |
| Pre-anodized and painted | −0.729 | 9.72 × 10−10 | 0.9 | −0.46 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baxevani, A.; Lamprou, E.; Mavropoulos, A.; Stergioudi, F.; Michailidis, N.; Tsoulfaidis, I. Investigation of Corrosion Resistance in Powder-Coated 6060 Aluminum Alloy: Effects of Powder Coating and Pre-Anodizing Followed by Powder Coating. Metals 2025, 15, 1062. https://doi.org/10.3390/met15101062
Baxevani A, Lamprou E, Mavropoulos A, Stergioudi F, Michailidis N, Tsoulfaidis I. Investigation of Corrosion Resistance in Powder-Coated 6060 Aluminum Alloy: Effects of Powder Coating and Pre-Anodizing Followed by Powder Coating. Metals. 2025; 15(10):1062. https://doi.org/10.3390/met15101062
Chicago/Turabian StyleBaxevani, Aikaterini, Eleni Lamprou, Azarias Mavropoulos, Fani Stergioudi, Nikolaos Michailidis, and Ioannis Tsoulfaidis. 2025. "Investigation of Corrosion Resistance in Powder-Coated 6060 Aluminum Alloy: Effects of Powder Coating and Pre-Anodizing Followed by Powder Coating" Metals 15, no. 10: 1062. https://doi.org/10.3390/met15101062
APA StyleBaxevani, A., Lamprou, E., Mavropoulos, A., Stergioudi, F., Michailidis, N., & Tsoulfaidis, I. (2025). Investigation of Corrosion Resistance in Powder-Coated 6060 Aluminum Alloy: Effects of Powder Coating and Pre-Anodizing Followed by Powder Coating. Metals, 15(10), 1062. https://doi.org/10.3390/met15101062







