Green Synthesis of Cobalt–Zinc Ferrites and Their Activity in Dye Elimination via Adsorption and Catalytic Wet Peroxide Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Green Synthesis
2.1.1. Preparation of Black Grape Extract
2.1.2. Synthesis of Co1−xZnxFe2O4 Nanoparticles
2.2. Characterization Techniques
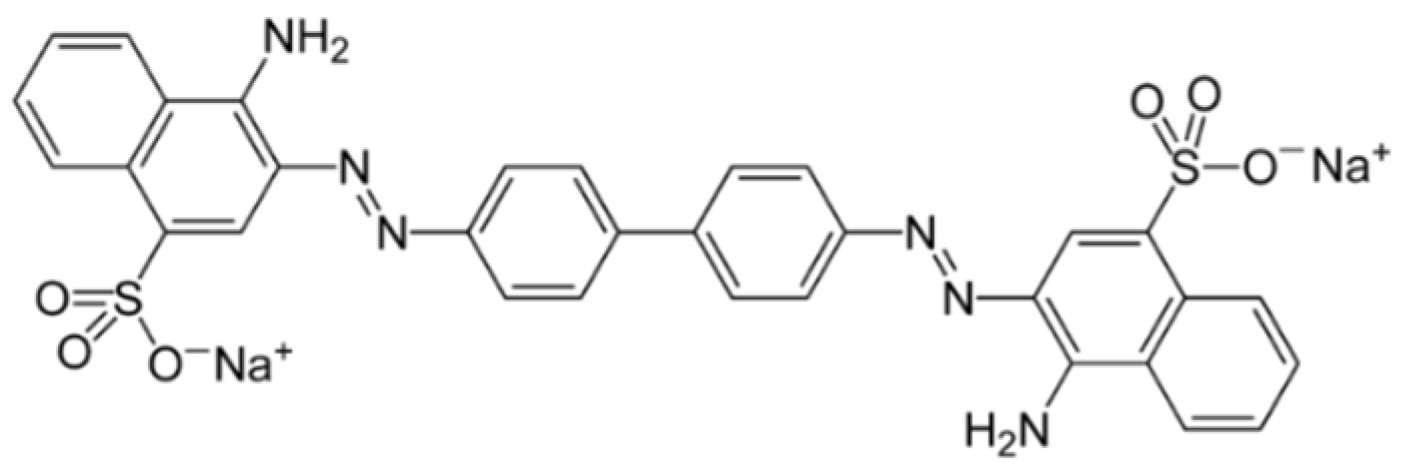
2.3. Congo Red Dye Elimination from Water
2.3.1. Adsorption Experiments
2.3.2. Catalytic Wet Peroxide Oxidation Experiments
2.3.3. Electrochemical Tests
3. Results
3.1. XRD Analysis
3.2. Scanning Electron Microscopy and Energy-Dispersive Spectroscopy
3.3. Mössbauer Spectroscopy
3.4. FTIR Spectroscopy
3.5. Surface Area and Pore Volume
3.6. Adsorption Properties
3.7. Catalytic Wet Peroxide Oxidation of Congo Red Dye
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reddy, D.H.K.; Yun, Y.-S. Spinel Ferrite Magnetic Adsorbents: Alternative Future Materials for Water Purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Qiu, B.; Deng, Y.; Du, M.; Xing, M.; Zhang, J. Ultradispersed Cobalt Ferrite Nanoparticles Assembled in Graphene Aerogel for Continuous Photo-Fenton Reaction and Enhanced Lithium Storage Performance. Sci. Rep. 2016, 6, 29099. [Google Scholar] [CrossRef] [PubMed]
- Hema, E.; Manikandan, A.; Karthika, P.; Antony, S.A.; Venkatraman, B.R. A Novel Synthesis of Zn2+-Doped CoFe2O4 Spinel Nanoparticles: Structural, Morphological, Opto-Magnetic and Catalytic Properties. J. Supercond. Nov. Magn. 2015, 28, 2539–2552. [Google Scholar] [CrossRef]
- Tatarchuk, T. Studying the Defects in Spinel Compounds: Discovery, Formation Mechanisms, Classification, and Influence on Catalytic Properties. Nanomaterials 2024, 14, 1640. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Wang, Y.; Zhao, L.; Jiang, Q. Adsorption Capability for Congo Red on Nanocrystalline MFe2O4 (M=Mn, Fe, Co, Ni) Spinel Ferrites. Chem. Eng. J. 2012, 181–182, 72–79. [Google Scholar] [CrossRef]
- Ibiyemi, A.A.; Akinrinola, O.; Yusuf, G.T. Photoelectric and Optoelectronic Effects of Hard Ferromagnetic Manganese Cobalt (Mn–Co) Ferrite Nanoparticles for High-Frequency Device Application. Appl. Phys. A Mater. Sci. Process. 2022, 128, 792. [Google Scholar] [CrossRef]
- Liu, H.; Li, A.; Ding, X.; Yang, F.; Sun, K. Magnetic Induction Heating Properties of Mg1-XZnxFe2O4 Ferrites Synthesized by Co-Precipitation Method. Solid State Sci. 2019, 93, 101–108. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K. Biological Synthesis of Cobalt Ferrite Nanoparticles. Nanotechnol. Dev. 2012, 2, 9. [Google Scholar] [CrossRef]
- Mishra, S.; Sahoo, S.S.; Debnath, A.K.; Muthe, K.P.; Das, N.; Parhi, P. Cobalt Ferrite Nanoparticles Prepared by Microwave Hydrothermal Synthesis and Adsorption Efficiency for Organic Dyes: Isotherms, Thermodynamics and Kinetic Studies. Adv. Powder Technol. 2020, 31, 4552–4562. [Google Scholar] [CrossRef]
- Naidu, K.C.B.; Madhuri, W. Hydrothermal Synthesis of NiFe2O4 Nano-Particles: Structural, Morphological, Optical, Electrical and Magnetic Properties. Bull. Mater. Sci. 2017, 40, 417–425. [Google Scholar] [CrossRef]
- Sun, W.; Pan, W.; Wang, F.; Xu, N. Removal of Se(IV) and Se(VI) by MFe2O4 Nanoparticles from Aqueous Solution. Chem. Eng. J. 2015, 273, 353–362. [Google Scholar] [CrossRef]
- Jasso-Terán, R.; Cortés-Hernández, D.; Sánchez-Fuentes, H.; Reyes-Rodríguez, P.; de-León-Prado, L.; Escobedo-Bocardo, J.; Almanza-Robles, J. Synthesis, Characterization and Hemolysis Studies of Zn(1−x)CaxFe2O4 Ferrites Synthesized by Sol-Gel for Hyperthermia Treatment Applications. J. Magn. Magn. Mater. 2017, 427, 241–244. [Google Scholar] [CrossRef]
- Jadhav, S. Sol-Gel Auto Combustion Synthesis and Structural Analysis of Cobalt Ferrite Nanoparticles. Int. Res. J. Sci. Eng. 2018, A5, 116–118. [Google Scholar]
- Nesheva, D.; Dzhurkov, V.; Stambolova, I.; Blaskov, V.; Bineva, I.; Calderon Moreno, J.M.; Preda, S.; Gartner, M.; Hristova-Vasileva, T.; Shipochka, M. Surface Modification and Chemical Sensitivity of Sol Gel Deposited Nanocrystalline ZnO Films. Mater. Chem. Phys. 2018, 209, 165–171. [Google Scholar] [CrossRef]
- Shreyash, N.; Bajpai, S.; Khan, M.A.; Vijay, Y.; Tiwary, S.K.; Sonker, M. Green Synthesis of Nanoparticles and Their Biomedical Applications: A Review. ACS Appl. Nano Mater. 2021, 4, 11428–11457. [Google Scholar] [CrossRef]
- Zhou, D.D.; Li, J.; Xiong, R.G.; Saimaiti, A.; Huang, S.Y.; Wu, S.X.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; et al. Bioactive Compounds, Health Benefits and Food Applications of Grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef]
- Serra, M.; Casas, A.; Teixeira, J.A.; Barros, A.N. Revealing the Beauty Potential of Grape Stems: Harnessing Phenolic Compounds for Cosmetics. Int. J. Mol. Sci. 2023, 24, 11751. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape Bioactive Molecules, and the Potential Health Benefits in Reducing the Risk of Heart Diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Vali, H.; Orsat, V. Value-Adding to Grape Waste: Green Synthesis of Gold Nanoparticles. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Farokhi, G.; Saidi, M. Catalytic Activity of Bimetallic Spinel Magnetic Catalysts (NiZnFe2O4, CoZnFe2O4 and CuZnFe2O4) in Biodiesel Production Process from Neem Oil: Process Evaluation and Optimization. Chem. Eng. Process.—Process Intensif. 2022, 181, 109170. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Danyliuk, N.; Shyichuk, A.; Kotsyubynsky, V.; Lapchuk, I.; Mandzyuk, V. Green Synthesis of Cobalt Ferrite Using Grape Extract: The Impact of Cation Distribution and Inversion Degree on the Catalytic Activity in the Decomposition of Hydrogen Peroxide. Emergent Mater. 2022, 5, 89–103. [Google Scholar] [CrossRef]
- Altowayti, W.A.; Salem, A.A.; Al-Fakih, A.M.; Bafaqeer, A.; Shahir, S.; Tajarudin, H.A. Optimization of As(V) Removal by Dried Bacterial Biomass: Nonlinear and Linear Regression Analysis for Isotherm and Kinetic Modelling. Metals 2022, 12, 1664. [Google Scholar] [CrossRef]
- Milutinović, A.; Lazarević, Z.Ž.; Šuljagić, M.; Andjelković, L. Synthesis-Dependent Structural and Magnetic Properties of Monodomain Cobalt Ferrite Nanoparticles. Metals 2024, 14, 833. [Google Scholar] [CrossRef]
- Torres, T.E.; Lima Jr, E.; Mayoral, A.; Ibarra, A.; Marquina, C.; Ibarra, M.R.; Goya, G.F. Validity of the Néel-Arrhenius Model for Highly Anisotropic CoxFe3−xO4 Nanoparticles. J. Appl. Phys. 2015, 118, 183902. [Google Scholar] [CrossRef]
- Muscas, G.; Cobianchi, M.; Lascialfari, A.; Cannas, C.; Musinu, A.; Omelyanchik, A.; Peddis, D. Magnetic Interactions vs. Magnetic Anisotropy in Spinel Ferrite Nanoparticles. IEEE Magn. Lett. 2019, 10, 6110305. [Google Scholar] [CrossRef]
- Liu, B.H.; Ding, J.; Dong, Z.L.; Boothroyd, C.B.; Yin, J.H.; Yi, J.B. Microstructural Evolution and Its Influence on the Magnetic Properties of CoFe2O4 Powders during Mechanical Milling. Phys. Rev. B 2006, 74, 184427. [Google Scholar] [CrossRef]
- Hasz, K.; Ijiri, Y.; Krycka, K.L.; Borchers, J.A.; Booth, R.A.; Oberdick, S.; Majetich, S.A. Particle Moment Canting in CoFe2O4 Nanoparticles. Phys. Rev. B 2014, 90, 180405. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Simple Spinels; Crystallographic Parameters, Cation Radii, Lattice Energies, and Cation Distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Rao, K.; Choudary, G.; Rao, K.; Sujatha, C. Structural and Magnetic Properties of Ultrafine CoFe2O4 Nanoparticles. Procedia Mater. Sci 2015, 10, 19–27. [Google Scholar] [CrossRef]
- Maiti, D.; Mukhopadhyay, S.; Devi, P.S. Evaluation of Mechanism on Selective, Rapid, and Superior Adsorption of Congo Red by Reusable Mesoporous α-Fe2O3 Nanorods. ACS Sustain. Chem. Eng. 2017, 5, 11255–11267. [Google Scholar] [CrossRef]
- Ben Mbarek, W.; Daza, J.; Escoda, L.; Fiol, N.; Pineda, E.; Khitouni, M.; Suñol, J.-J. Removal of Reactive Black 5 Azo Dye from Aqueous Solutions by a Combination of Reduction and Natural Adsorbents Processes. Metals 2023, 13, 474. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Aoopngan, C.; Nonkumwong, J.; Phumying, S.; Promjantuek, W.; Maensiri, S.; Noisa, P.; Pinitsoontorn, S.; Ananta, S.; Srisombat, L. Amine-Functionalized and Hydroxyl-Functionalized Magnesium Ferrite Nanoparticles for Congo Red Adsorption. ACS Appl. Nano Mater. 2019, 2, 5329–5341. [Google Scholar] [CrossRef]
- Castro, L.; Ayala, L.A.; Vardanyan, A.; Zhang, R.; Muñoz, J.Á. Arsenate and Arsenite Sorption Using Biogenic Iron Compounds: Treatment of Real Polluted Waters in Batch and Continuous Systems. Metals 2021, 11, 1608. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, X.; Wang, G.; Qian, B.; Liu, Y.; Hu, X.; He, B. Modulating Mesoporous Co3O4 Hollow Nanospheres with Oxygen Vacancies for Highly e Ffi Cient Peroxymonosulfate Activation. Chem. Eng. J. 2020, 400, 125869. [Google Scholar] [CrossRef]














| Sample | % | Element Content | |||
|---|---|---|---|---|---|
| Fe | Co | Zn | O | ||
| CoFe2O4 | at. | 28.29 | 14.57 | – | 57.14 |
| wt. | 44.60 | 28.50 | – | 26.90 | |
| Zn0.2Co0.8Fe2O4 | at. | 28.30 | 11.48 | 3.13 | 57.09 |
| wt. | 45.00 | 21.50 | 6.70 | 26.80 | |
| Zn0.4Co0.6Fe2O4 | at. | 28.24 | 8.80 | 5.80 | 57.16 |
| wt. | 44.70 | 16.60 | 12.10 | 26.60 | |
| Zn0.6Co0.4Fe2O4 | at. | 28.32 | 5.49 | 9.03 | 57.16 |
| wt. | 45.40 | 10.20 | 17.80 | 26.60 | |
| Zn0.8Co0.2Fe2O4 | at. | 28.26 | 2.30 | 12.34 | 57.10 |
| wt. | 44.50 | 5.30 | 23.80 | 26.40 | |
| ZnFe2O4 | at. | 28.16 | – | 14.76 | 57.08 |
| wt. | 43.70 | – | 30.10 | 26.20 | |
| Site | Is, mm/s | Qs, mm/s | H, kOe | S, % | G, mm/s | |
|---|---|---|---|---|---|---|
| CoFe2O4 | ||||||
| 1 | A1 | 0.39 | −0.006 | 504.3 | 45.2 | 0.46 |
| 2 | B1 | 0.48 | −0.001 | 533.6 | 29.9 | 0.53 |
| 3 | B2 | 0.43 | −0.035 | 474.7 | 24.9 | 0.66 |
| Zn0.2Co0.8Fe2O4 | ||||||
| 1 | A1 | 0.39 | −0.005 | 502.8 | 35.9 | 0.49 |
| 2 | B1 | 0.51 | 0.041 | 522.6 | 29.8 | 0.73 |
| 3 | B2 | 0.44 | −0.05 | 472.3 | 34.4 | 0.80 |
| Zn0.4Co0.6Fe2O4 | ||||||
| 1 | A1 | 0.37 | −0.041 | 498.9 | 25.3 | 0.56 |
| 2 | B1 | 0.45 | −0.052 | 471.0 | 16.5 | 0.55 |
| 3 | B2 | 0.42 | 0.008 | 442.9 | 36.3 | 1.77 |
| 4 | B3 | 0.46 | 0.073 | 512.8 | 21.9 | 0.69 |
| Zn0.6Co0.4Fe2O4 | ||||||
| 1 | A1 | 0.35 | −0.051 | 499.8 | 12.5 | 0.47 |
| 2 | B1 | 0.41 | −0.064 | 473.6 | 38.0 | 0.83 |
| 3 | B2 | 0.45 | 0.006 | 446.7 | 8.8 | 0.53 |
| 4 | B3 | 0.43 | −0.054 | 397.4 | 21.9 | 1.18 |
| 5 | B4 | 0.46 | 0.079 | 497.2 | 18.8 | 0.58 |
| Chemical Composition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn2+ Content (Predicted) | Predicted | Experimental | |||||||||
| Co, wt. % | Zn, wt. % | Fe, wt. % | Co/Fe, Molar Ratio | Zn/Fe, Molar Ratio | Co, wt. % | Zn, wt. % | Fe, wt. % | Co/Fe, Molar Ratio (K1) | Zn/Fe, Molar Ratio (K2) | FeT/FeO, Molar Ratio (K3) | |
| 0.0 | 25.2 | – | 47.6 | 0.50 | – | 28.5 | – | 44.6 | 0.61 | – | 0.82 |
| 0.2 | 19.9 | 5.6 | 47.3 | 0.40 | 0.14 | 21.5 | 6.7 | 45.0 | 0.45 | 0.14 | 0.59 |
| 0.4 | 14.9 | 11.1 | 47.1 | 0.30 | 0.26 | 16.6 | 12.1 | 44.7 | 0.33 | 0.26 | 0.34 |
| 0.6 | 9.9 | 16.5 | 46.8 | 0.20 | 0.37 | 10.2 | 17.8 | 45.4 | 0.21 | 0.37 | 0.14 |
| 0.8 | 4.9 | 21.9 | 46.6 | 0.10 | 0.51 | 5.3 | 23.8 | 44.5 | 0.11 | 0.51 | – |
| 1.0 | – | 27.2 | 46.3 | – | 0.59 | – | 30.1 | 43.7 | – | 0.59 | – |
| Theoretically Predicted Zn Content | Experimentally Obtained Zn Content (From EDS Data) | Cation Distribution |
|---|---|---|
| 0.00 | 0 | (Co2+0.13Fe3+0.87)A[Co2+Fe3+]BO4 |
| 0.20 | 0.26 | (Zn2+0.26Co2+0.04Fe3+0.70)A[Co2+0.81Fe3+1.19]BO4 |
| 0.40 | 0.48 | (Zn2+0.48Co2+0.04Fe3+0.48)A[Co2+0.61Fe3+1.39]BO4 |
| 0.60 | 0.70 | (Zn2+0.70Co2+0.07Fe3+0.23)A[Co2+0.34Fe3+1.66]BO4 |
| 0.80 * | 0.94 | (Zn2+0.94Co2+0.06)A[Co2+0.14Fe3+1.86]BO4 |
| 1.00 * | 1.02 | (Zn2+)A[Zn2+<0.1Fe3+>1.9]BO4 |
| Parameter | x(Zn) = 0.0 | x(Zn) = 0.2 | x(Zn) = 0.4 | x(Zn) = 0.6 | x(Zn) = 0.8 | x(Zn) = 1.0 |
|---|---|---|---|---|---|---|
| νT, cm−1 | 536 | 530 | 538 | 526 | 530 | 524 |
| KT × 104, dynes/cm2 | 2.64 | 2.58 | 2.66 | 2.54 | 2.58 | 2.52 |
| SBET, m2/g | Smicro, m2/g | Smeso, m2/g | V, cc/g | Vmicro, cc/g | Vmeso, cc/g | RDFT, nm |
|---|---|---|---|---|---|---|
| 60 | 9 | 51 | 0.12 | 0.03 | 0.09 | 4.4 |
| Zn Content | Adsorption Model | ||||||
|---|---|---|---|---|---|---|---|
| Langmuir | Freundlich | ||||||
| qmax | KL | RL | R2 | n | KF | R2 | |
| 0 | 32.57 | 0.06 | 0.153 | 0.99 | 2.03 | 3.51 | 0.96 |
| 0.2 | 23.75 | 0.24 | 0.040 | 0.99 | 4.81 | 9.53 | 0.99 |
| 0.4 | 45.45 | 0.18 | 0.054 | 0.99 | 2.84 | 10.87 | 0.99 |
| 0.6 | 32.15 | 0.22 | 0.043 | 0.98 | 3.34 | 9.47 | 0.95 |
| 0.8 | 46.73 | 0.40 | 0.025 | 0.99 | 3.06 | 14.36 | 0.90 |
| 1 | 54.64 | 0.29 | 0.030 | 0.99 | 2.76 | 14.49 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liaskovska, M.; Tatarchuk, T.; Kotsyubynsky, V. Green Synthesis of Cobalt–Zinc Ferrites and Their Activity in Dye Elimination via Adsorption and Catalytic Wet Peroxide Oxidation. Metals 2025, 15, 44. https://doi.org/10.3390/met15010044
Liaskovska M, Tatarchuk T, Kotsyubynsky V. Green Synthesis of Cobalt–Zinc Ferrites and Their Activity in Dye Elimination via Adsorption and Catalytic Wet Peroxide Oxidation. Metals. 2025; 15(1):44. https://doi.org/10.3390/met15010044
Chicago/Turabian StyleLiaskovska, Mariia, Tetiana Tatarchuk, and Volodymyr Kotsyubynsky. 2025. "Green Synthesis of Cobalt–Zinc Ferrites and Their Activity in Dye Elimination via Adsorption and Catalytic Wet Peroxide Oxidation" Metals 15, no. 1: 44. https://doi.org/10.3390/met15010044
APA StyleLiaskovska, M., Tatarchuk, T., & Kotsyubynsky, V. (2025). Green Synthesis of Cobalt–Zinc Ferrites and Their Activity in Dye Elimination via Adsorption and Catalytic Wet Peroxide Oxidation. Metals, 15(1), 44. https://doi.org/10.3390/met15010044





