Review on Environmentally Assisted Static and Fatigue Cracking of Al-Mg-Si-(Cu) Alloys
Abstract
1. Introduction
2. Key Characteristics of Heat-Treatable Al-Mg-Si-(Cu) Alloys
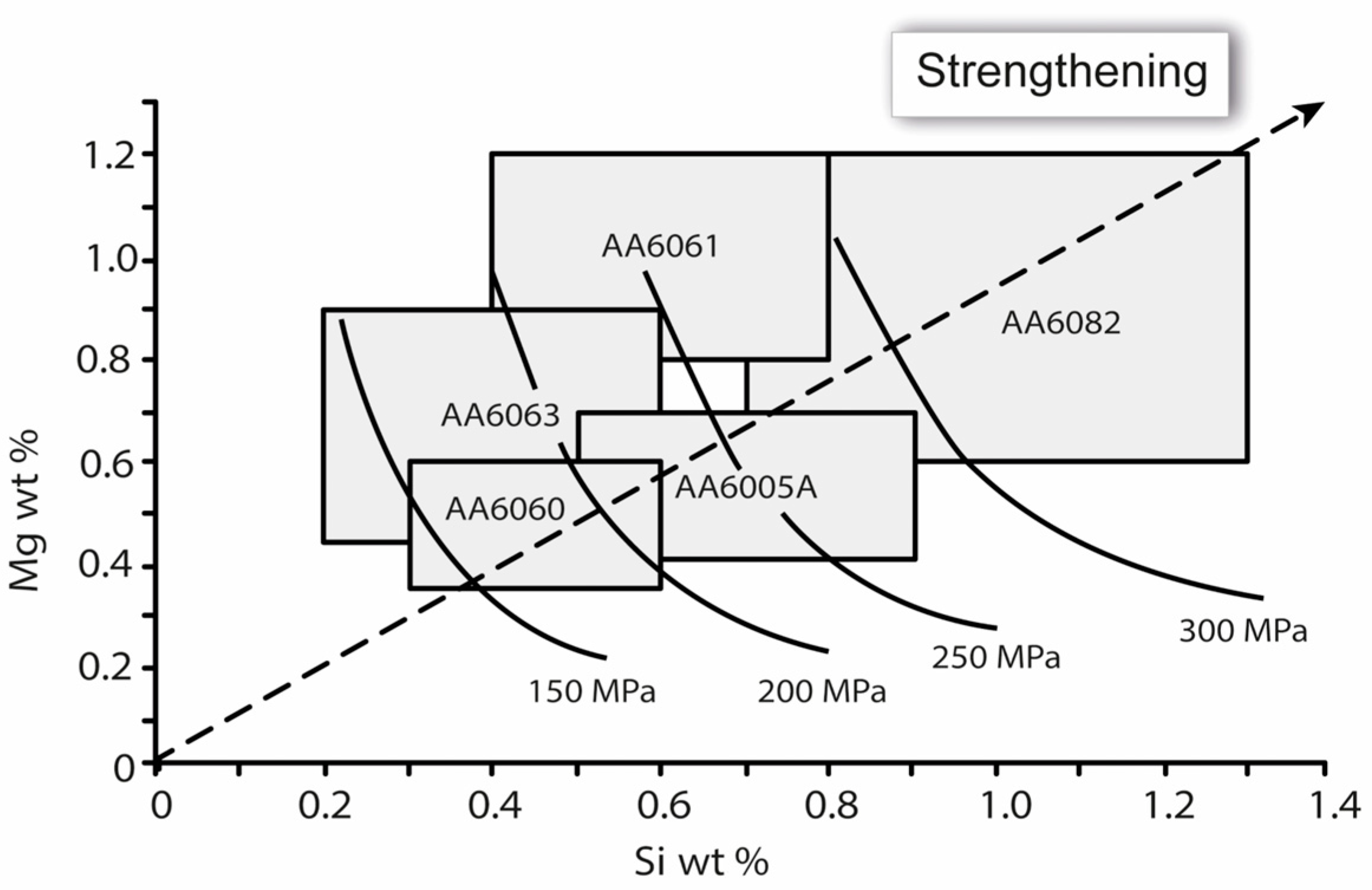
2.1. Effect of Composition and Heat Treatment on Mechanical Properties of Al-Mg-Si Alloys
2.2. Microstructure and Precipitates Sequence of Al-Mg-Si-(Cu) Alloys
3. Main Aspects Relating to Corrosion of Al-Mg-Si-(Cu) Alloys
3.1. Passivation of Aluminum
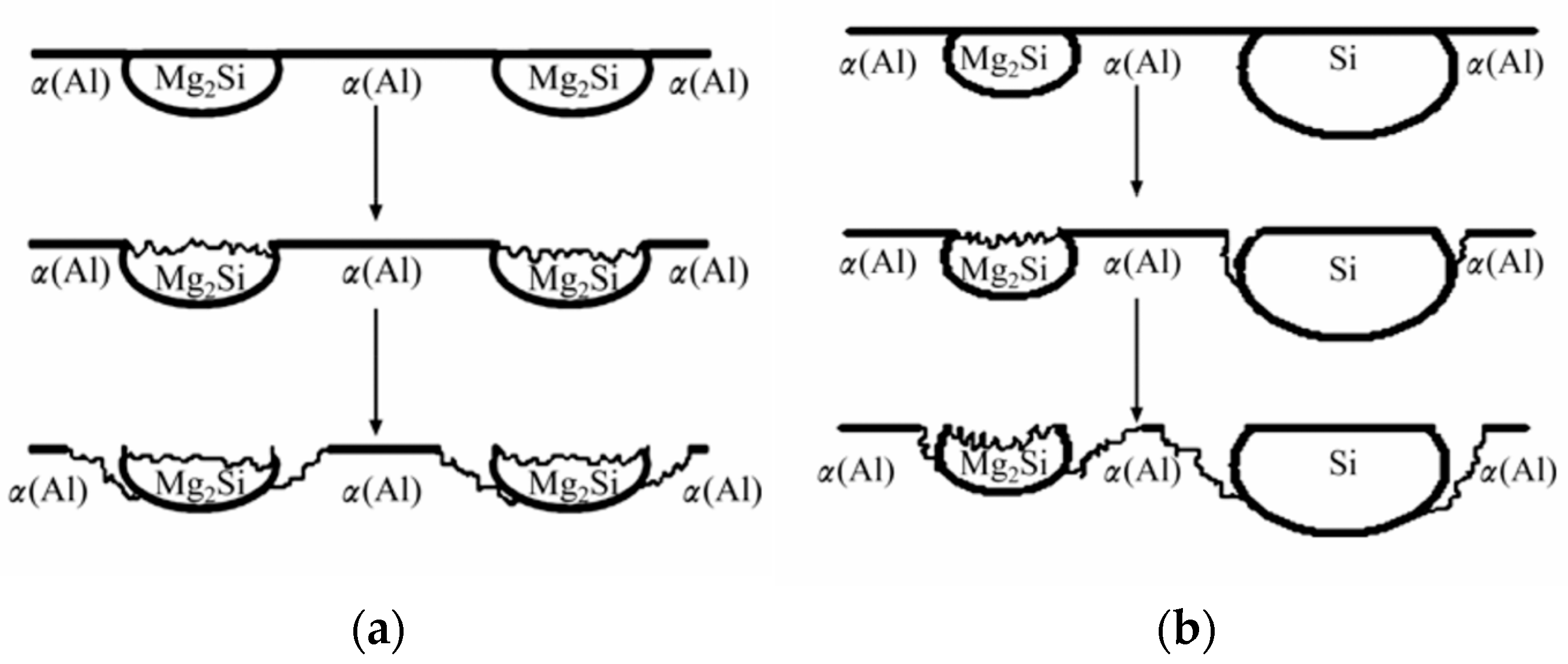
3.2. Localized Corrosion in Al-Mg-Si-(Cu) Alloys
3.3. Crack Tip Environment
4. Mechanisms of Stress Corrosion and Fatigue Corrosion Cracking
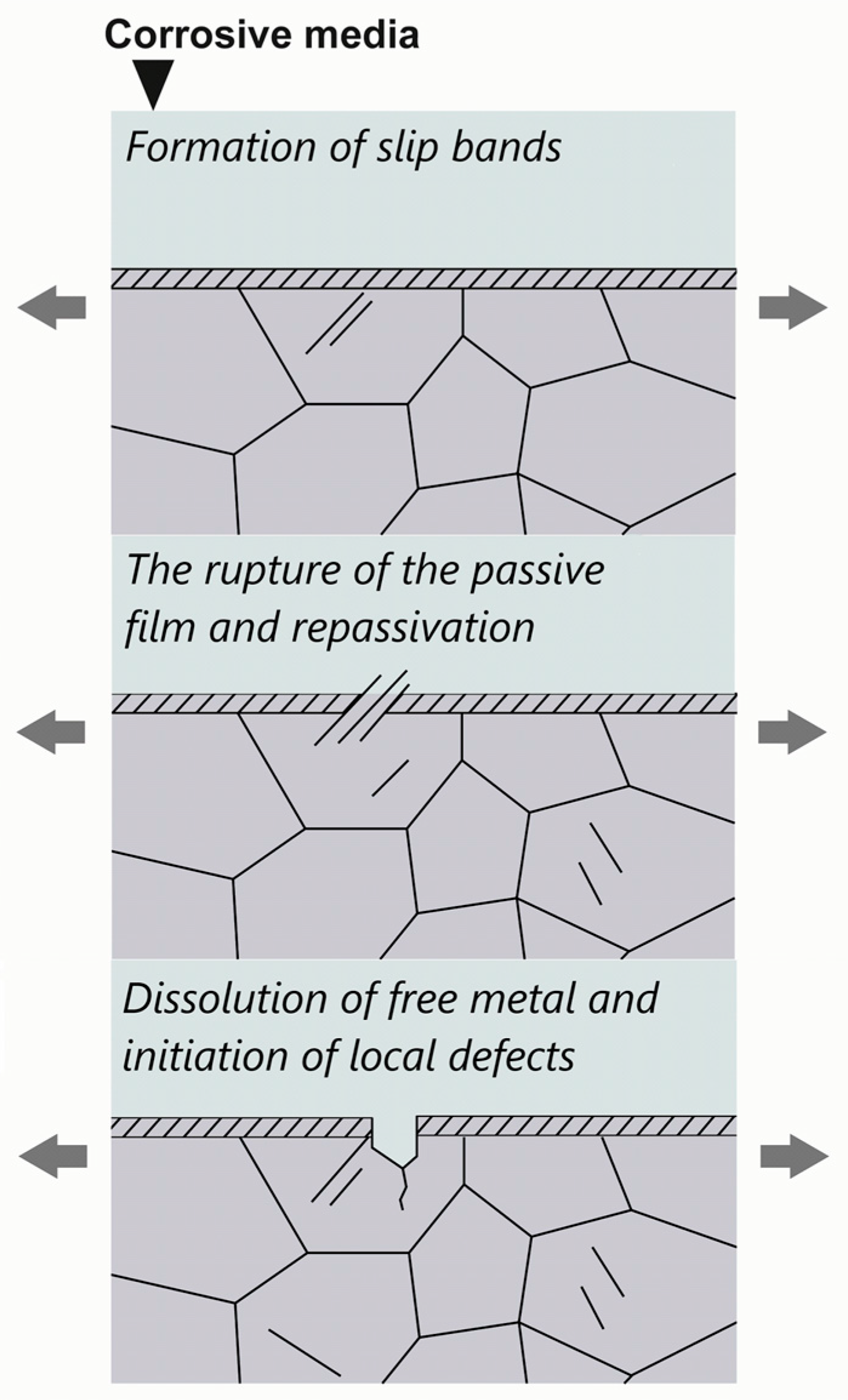
4.1. Stress-Related Corrosion Cracking
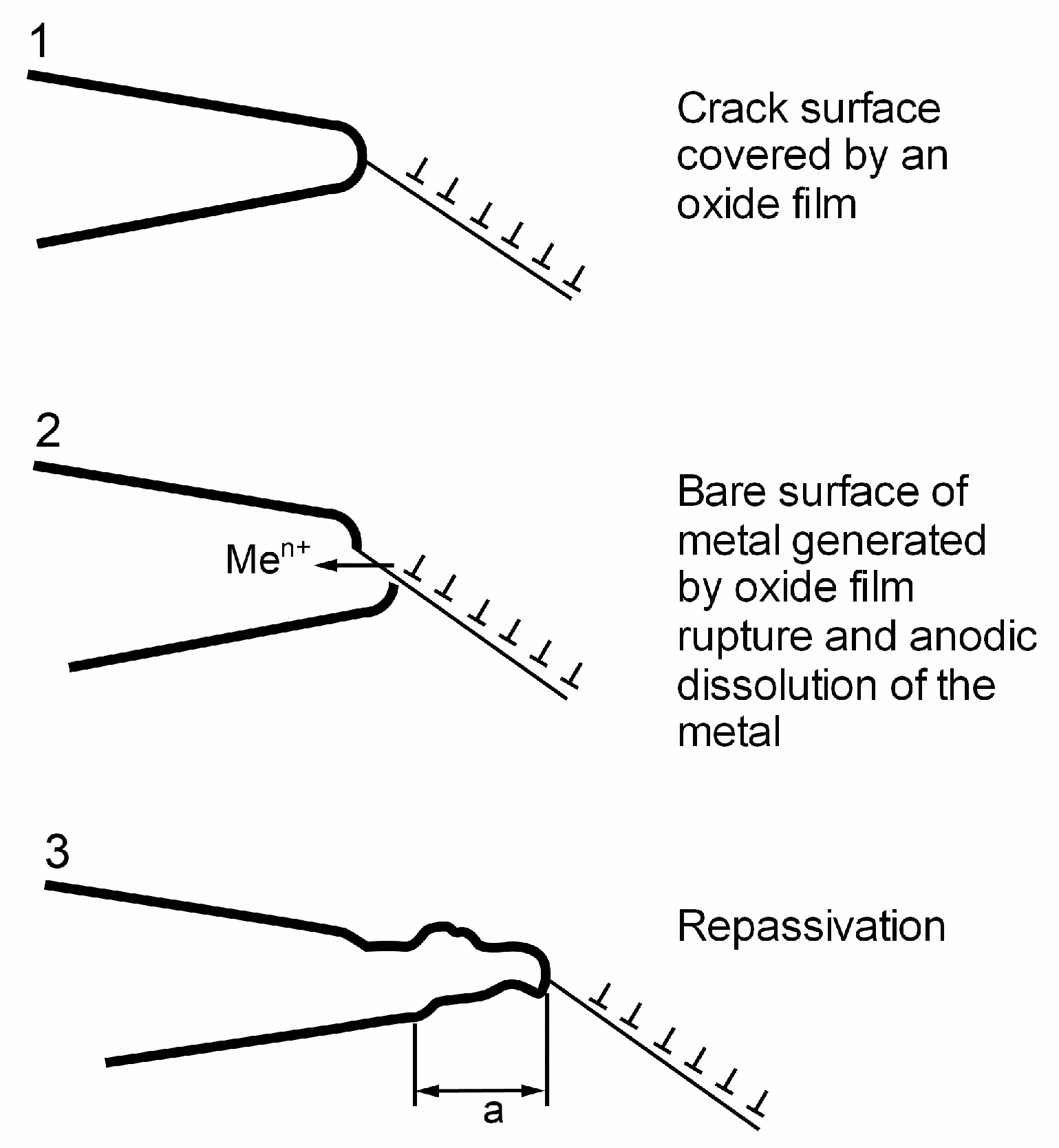
4.2. Anodic Dissolution
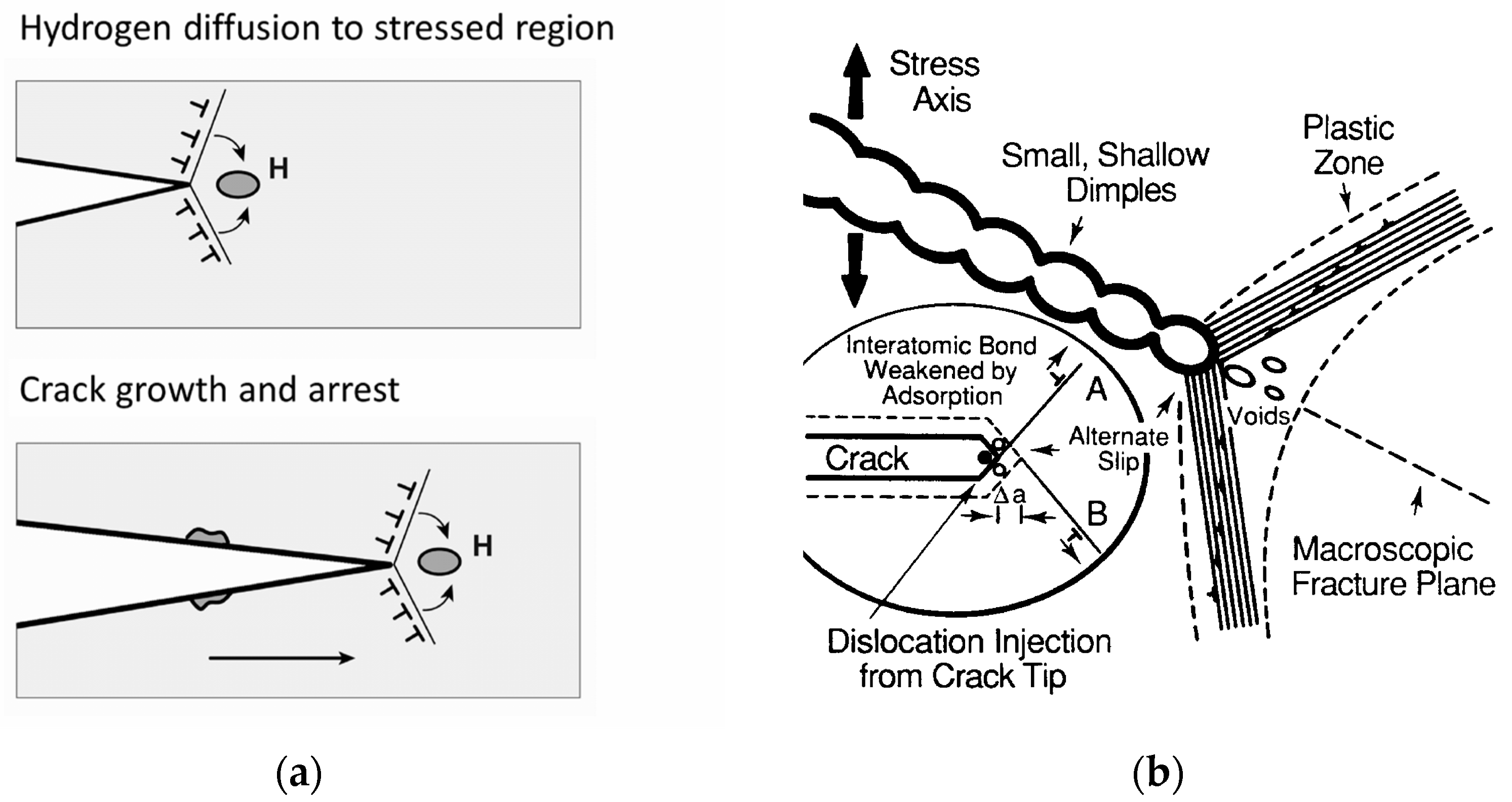
4.3. Hydrogen-Assisted Cracking
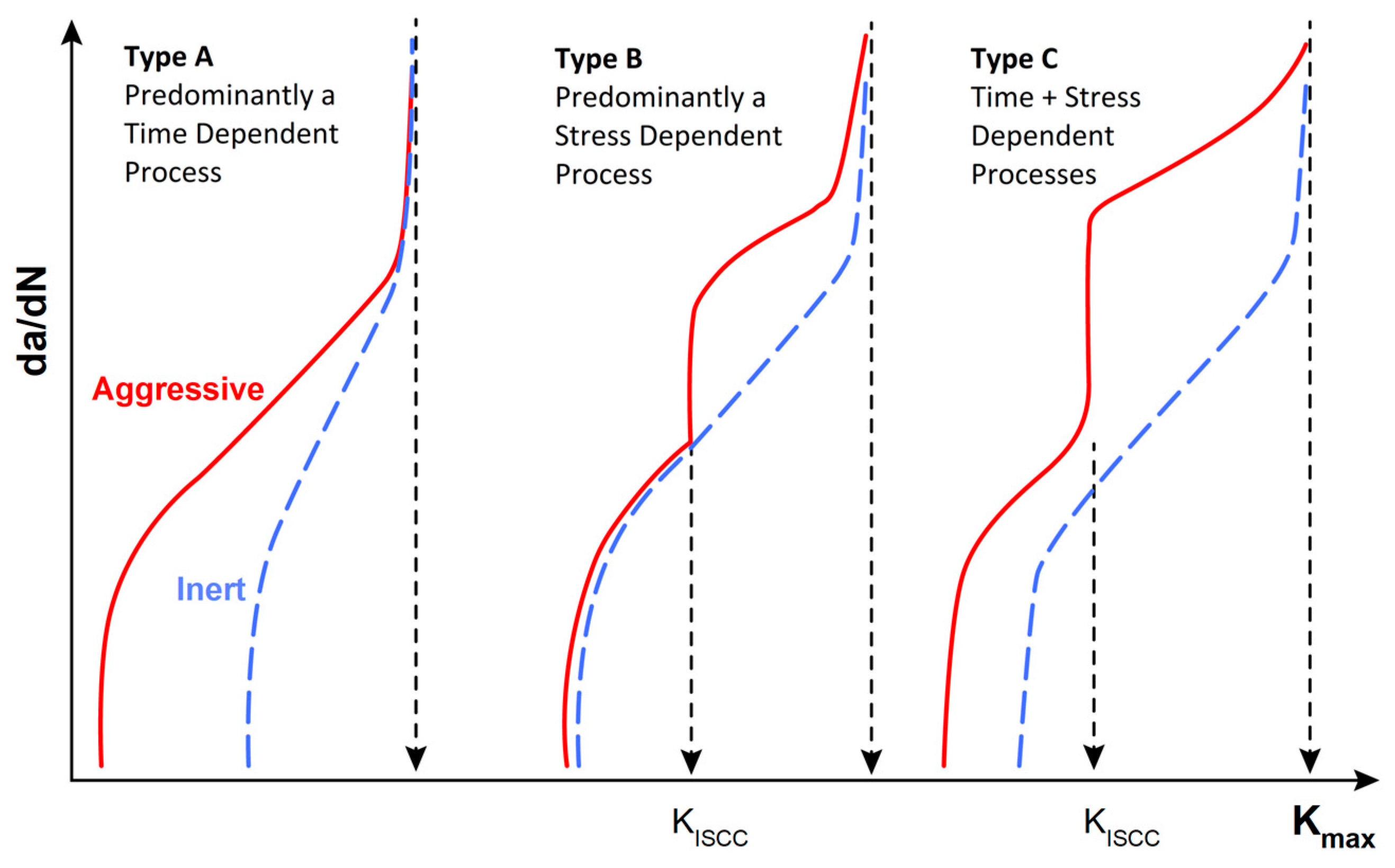
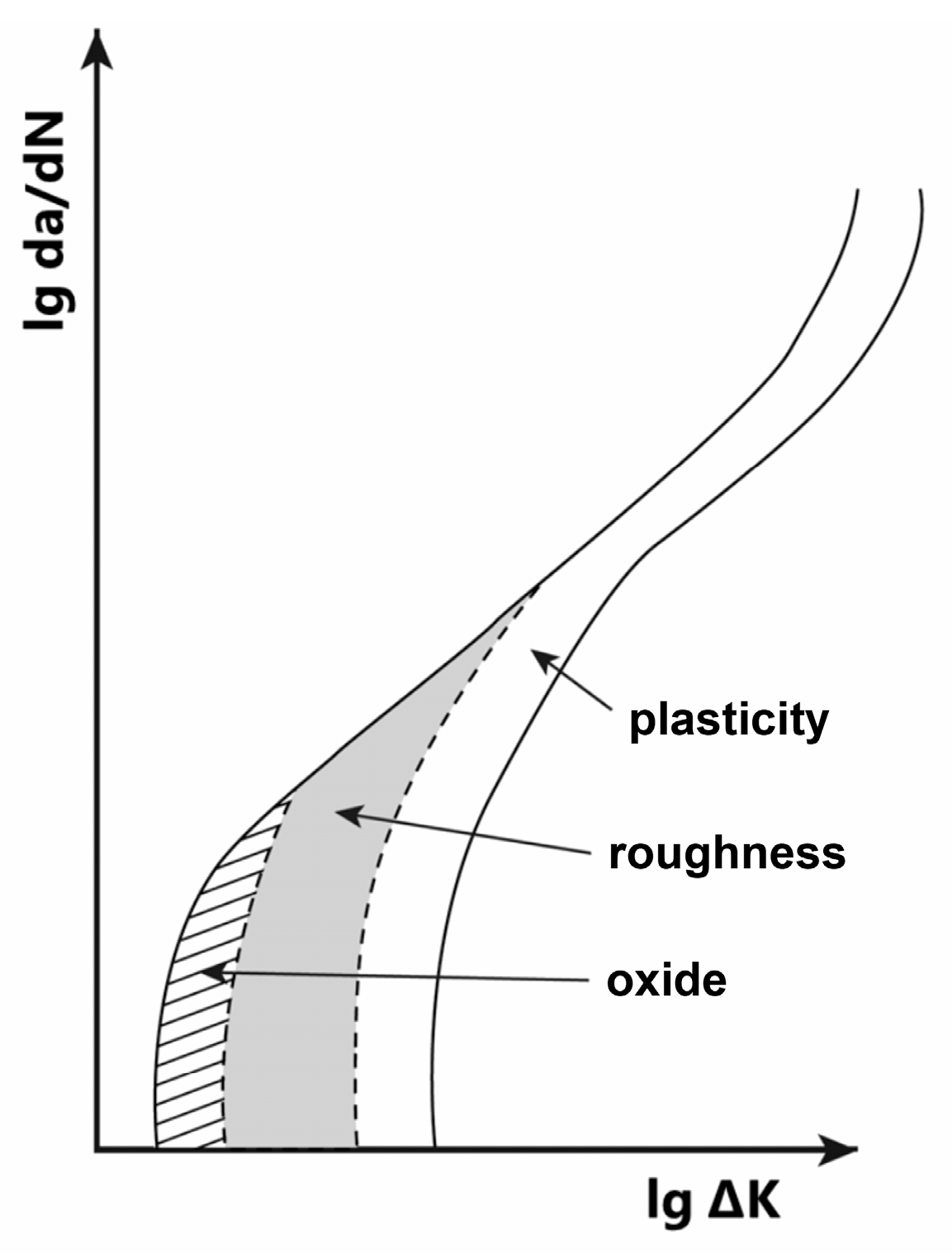
4.4. Environmentally Assisted Fatigue Crack Propagation
5. Susceptibility of Al-Mg-Si-(Cu) Alloys to Environmentally Assisted Cracking
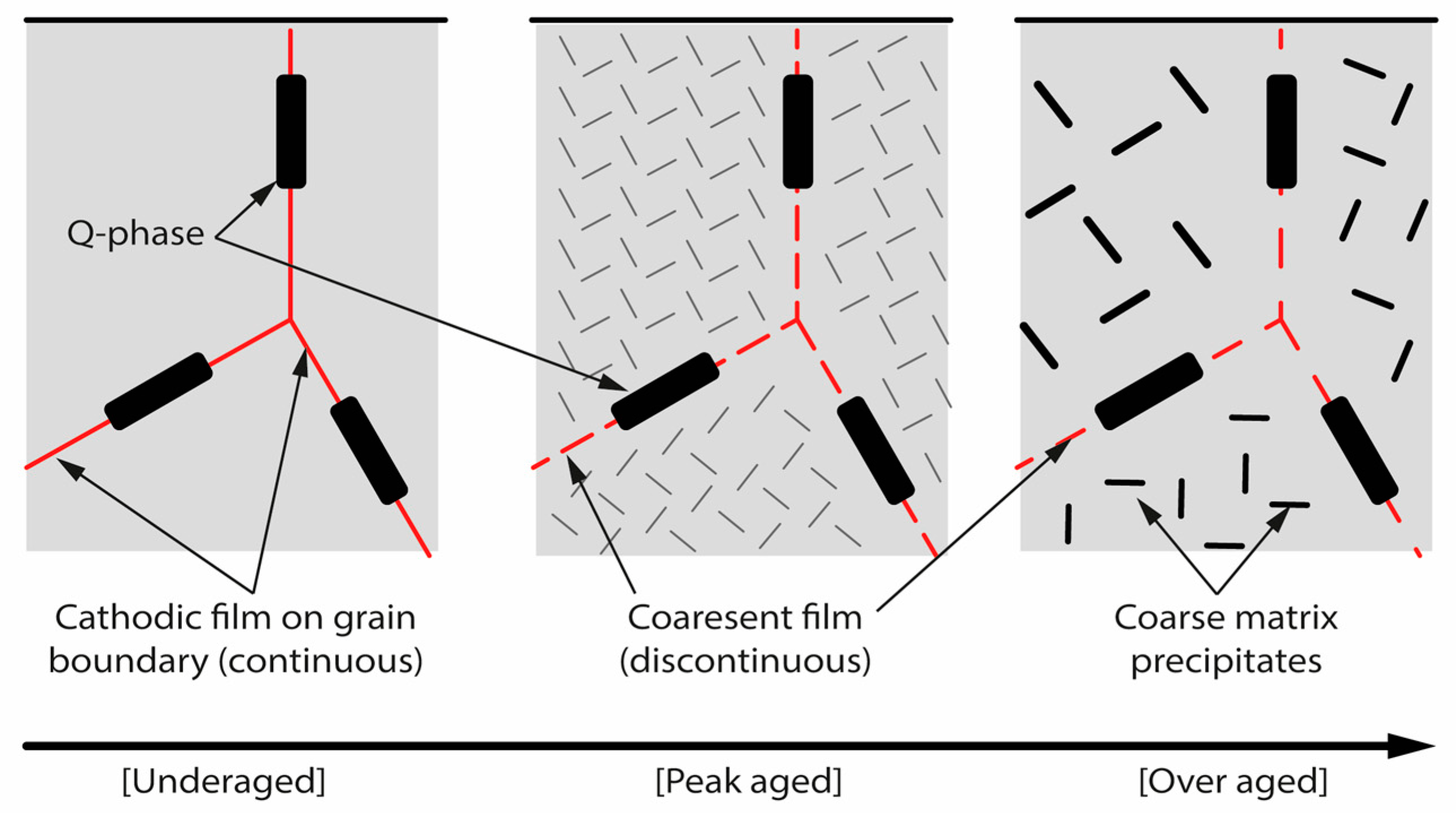
5.1. On Stress Corrosion Cracking of 6xxx Series Aluminum Alloys
5.2. Hydrogen Effect
5.3. Corrosion Fatigue of Al-Mg-Si-(Cu) Alloys
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gilbert, P.T. Corrosion-Fatigue. Metall. Rev. 1956, 1, 379–417. [Google Scholar] [CrossRef]
- Petrović, Z.C. Catastrophes Caused by Corrosion. Vojnoteh. Glas. 2016, 64, 1048–1064. [Google Scholar] [CrossRef]
- Skillingberg, M.; Green, J.; Consulting, J. Aluminum Applications in the Rail Industry. Light Met. Age 2007, 65, 8. [Google Scholar]
- Poznak, A.; Freiberg, D.; Sanders, P. Chapter 10—Automotive Wrought Aluminium Alloys. In Fundamentals of Aluminium Metallurgy; Lumley, R.N., Ed.; Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Sawston, UK, 2018; pp. 333–386. ISBN 978-0-08-102063-0. [Google Scholar]
- Sun, X.; Han, X.; Dong, C.; Li, X.; Sun, X.; Han, X.; Dong, C.; Li, X. Applications of Aluminum Alloys in Rail Transportation. In Advanced Aluminium Composites and Alloys; IntechOpen: London, UK, 2021; ISBN 978-1-83880-451-0. [Google Scholar]
- El-Hameed, A.M.A.; Abdel-Aziz, Y.A. Aluminium Alloys in Space Applications: A Short Report. J. Adv. Res. Appl. Sci. Eng. Technol. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Staley, J.T.; Lege, D.J. Advances in Aluminium Alloy Products for Structural Applications in Transportation. J. Phys. IV France 1993, 3, C7-179–C7-190. [Google Scholar] [CrossRef]
- Burger, G.B.; Gupta, A.K.; Jeffrey, P.W.; Lloyd, D.J. Microstructural Control of Aluminum Sheet Used in Automotive Applications. Mater. Charact. 1995, 35, 23–39. [Google Scholar] [CrossRef]
- Guillaumin, V.; Mankowski, G. Localized Corrosion of 6056 T6 Aluminium Alloy in Chloride Media. Corros. Sci. 2000, 42, 105–125. [Google Scholar] [CrossRef]
- Braun, R. On the Stress Corrosion Cracking Behaviour of 6XXX Series Aluminium Alloys. Int. J. Mater. Res. 2010, 101, 657–668. [Google Scholar] [CrossRef]
- Braun, R. Environmentally Assisted Cracking of Aluminium Alloys. Materialwiss. Werkst. 2007, 38, 674–689. [Google Scholar] [CrossRef]
- Siddesh Kumar, N.M.; Dhruthi; Pramod, G.K.; Samrat, P.; Sadashiva, M. A Critical Review on Heat Treatment of Aluminium Alloys. Mater. Today Proc. 2022, 58, 71–79. [Google Scholar] [CrossRef]
- Vargel, C. Chapter A.7—Selection Criteria. In Corrosion of Aluminium, 2nd ed.; Vargel, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–38. ISBN 978-0-08-099925-8. [Google Scholar]
- Zupanič, F.; Steinacher, M.; Žist, S.; Bončina, T. Microstructure and Properties of a Novel Al-Mg-Si Alloy AA 6086. Metals 2021, 11, 368. [Google Scholar] [CrossRef]
- Zupanič, F.; Klemenc, J.; Steinacher, M.; Glodež, S. Microstructure, Mechanical Properties and Fatigue Behaviour of a New High-Strength Aluminium Alloy AA 6086. J. Alloys Compd. 2023, 941, 168976. [Google Scholar] [CrossRef]
- Bray, J.W. Aluminum Mill and Engineered Wrought Products. In Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM Handbook Committee, Ed.; ASM International: Almere, The Netherlands, 1990; Volume 2, pp. 29–61. ISBN 978-1-62708-162-7. [Google Scholar]
- Tiryakioḡlu, M.; Staley, J. Physical Metallurgy and the Effect of the Alloying Additions in Aluminum Alloys. In Handbook of Aluminum: Vol. 1: Physical Metallurgy and Processes; Totten, G.E., MacKenzie, D.S., Eds.; CRC Press: Boca Raton, FL, USA, 2003; Volume 1, pp. 81–211. [Google Scholar]
- Polmear, I.; StJohn, D.; Nie, J.-F.; Qian, M. 4—Wrought Aluminium Alloys. In Light Alloys; Polmear, I., StJohn, D., Nie, J.-F., Qian, M., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2017; pp. 157–263. ISBN 978-0-08-099431-4. [Google Scholar]
- Lei, G.; Wang, B.; Lu, J.; Wang, C.; Li, Y.; Luo, F. Microstructure, Mechanical Properties, and Corrosion Resistance of Continuous Heating Aging 6013 Aluminum Alloy. J. Mater. Res. Technol. 2022, 18, 370–383. [Google Scholar] [CrossRef]
- Mrówka-Nowotnik, G. Influence of Chemical Composition Variation and Heat Treatment on Microstructure and Mechanical Properties of 6xxx Alloys. Arch. Mater. Sci. Eng. 2010, 46, 98–107. [Google Scholar]
- Kairy, S.K.; Rometsch, P.A.; Davies, C.H.J.; Birbilis, N. On the Intergranular Corrosion and Hardness Evolution of 6xxx Series Al Alloys as a Function of Si:Mg Ratio, Cu Content, and Aging Condition. Corrosion 2017, 73, 1280–1295. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Stiller, K.; Dunlop, G.; Couper, M. The Precipitation Sequence in Al–Mg–Si Alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- He, T.; Shi, W.; Xiang, S.; Huang, C.; Ballinger, R.G. Influence of Aging on Corrosion Behaviour of the 6061 Cast Aluminium Alloy. Materials 2021, 14, 1821. [Google Scholar] [CrossRef]
- Shishido, H.; Aruga, Y.; Murata, Y.; Marioara, C.D.; Engler, O. Evaluation of Precipitates and Clusters during Artificial Aging of Two Model Al–Mg–Si Alloys with Different Mg/Si Ratios. J. Alloys Compd. 2022, 927, 166978. [Google Scholar] [CrossRef]
- Kahlenberg, R.; Wojcik, T.; Falkinger, G.; Krejci, A.L.; Milkereit, B.; Kozeschnik, E. On the Precipitation Mechanisms of β-Mg2Si during Continuous Heating of AA6061. Acta Mater. 2023, 261, 119345. [Google Scholar] [CrossRef]
- Marioara, C.D.; Børvik, T.; Hopperstad, O.-S. The Relation between Grain Boundary Precipitate Formation and Adjacent Grain Orientations in Al-Mg-Si(-Cu) Alloys. Philos. Mag. Lett. 2021, 101, 370–379. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, Y.; Hashimoto, T.; Nilsson, J.-O.; Zhou, X. Intergranular Corrosion of AA6082 Al–Mg–Si Alloy Extrusion: The Influence of Trace Cu and Grain Boundary Misorientation. J. Alloys Compd. 2021, 853, 157228. [Google Scholar] [CrossRef]
- Zhang, Z.; Parson, N.C.; Poole, W.J. Precipitation on Grain Boundaries in Al-Mg-Si Alloys: The Role of Grain Boundary Misorientation. Scr. Mater. 2022, 211, 114494. [Google Scholar] [CrossRef]
- Zhao, Y.; Niverty, S.; Ma, X.; Chawla, N. Correlation between Corrosion Behavior and Grain Boundary Characteristics of a 6061 Al Alloy by Lab-Scale X-Ray Diffraction Contrast Tomography (DCT). Mater. Charact. 2022, 193, 112325. [Google Scholar] [CrossRef]
- Andersen, S.J.; Zandbergen, H.W.; Jansen, J.; TrÆholt, C.; Tundal, U.; Reiso, O. The Crystal Structure of the Β″ Phase in Al–Mg–Si Alloys. Acta Mater. 1998, 46, 3283–3298. [Google Scholar] [CrossRef]
- Andersen, S.J.; Marioara, C.D.; Frøseth, A.; Vissers, R.; Zandbergen, H.W. Crystal Structure of the Orthorhombic U2-Al4Mg4Si4 Precipitate in the Al–Mg–Si Alloy System and Its Relation to the Β′ and Β″ Phases. Mater. Sci. Eng. A. 2005, 390, 127–138. [Google Scholar] [CrossRef]
- Fang, X.; Song, M.; Li, K.; Du, Y. Precipitation Sequence of an Aged Al-Mg-Si Alloy. J. Min. Metall. B Metall 2010, 46, 171–180. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Hell, C.; Frafjord, J.; Friis, J.; Bjørge, R.; Ringdalen, I.G.; Engler, O.; Holmestad, R. Atomic Structure of Clusters and GP-Zones in an Al-Mg-Si Alloy. Acta Mater. 2024, 269, 119811. [Google Scholar] [CrossRef]
- Buha, J.; Lumley, R.N.; Crosky, A.G. Microstructural Development and Mechanical Properties of Interrupted Aged Al-Mg-Si-Cu Alloy. Metall. Mater. Trans. A 2006, 37, 3119–3130. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Jansen, J.; Zandbergen, H.W. Atomic Model for GP-Zones in a 6082 Al–Mg–Si System. Acta Mater. 2001, 49, 321–328. [Google Scholar] [CrossRef]
- Frøseth, A.G.; Høier, R.; Derlet, P.M.; Andersen, S.J.; Marioara, C.D. Bonding in MgSi and Al-Mg-Si Compounds Relevant to Al-Mg-Si Alloys. Phys. Rev. B 2003, 67, 224106. [Google Scholar] [CrossRef]
- Vissers, R.; van Huis, M.A.; Jansen, J.; Zandbergen, H.W.; Marioara, C.D.; Andersen, S.J. The Crystal Structure of the Β′ Phase in Al–Mg–Si Alloys. Acta Mater. 2007, 55, 3815–3823. [Google Scholar] [CrossRef]
- Chakrabarti, D.J.; Laughlin, D.E. Phase Relations and Precipitation in Al–Mg–Si Alloys with Cu Additions. Prog. Mater. Sci. 2004, 49, 389–410. [Google Scholar] [CrossRef]
- Zeng, F.-L.; Wei, Z.-L.; Li, J.; Li, C.-X.; Tan, X.; Zhang, Z.; Zheng, Z.-Q. Corrosion Mechanism Associated with Mg2Si and Si Particles in Al–Mg–Si Alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]
- Esmaeili, S.; Wang, X.; Lloyd, D.J.; Poole, W.J. On the Precipitation-Hardening Behavior of the Al−Mg−Si−Cu Alloy AA6111. Metall. Mater. Trans A 2003, 34, 751–763. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Stene, T.N.; Hasting, H.; Walmsley, J.; Van Helvoort, A.T.J.; Holmestad, R. The Effect of Cu on Precipitation in Al–Mg–Si Alloys. Philos. Mag. 2007, 87, 3385–3413. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Røyset, J.; Reiso, O.; Gulbrandsen-Dahl, S.; Nicolaisen, T.-E.; Opheim, I.-E.; Helgaker, J.F.; Holmestad, R. Improving Thermal Stability in Cu-Containing Al-Mg-Si Alloys by Precipitate Optimization. Metall. Mater. Trans. A 2014, 45, 2938–2949. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Nordlien, J.H.; Nisancioglu, K. Effect of High Temperature Heat Treatment on Intergranular Corrosion of AlMgSi(Cu) Model Alloy. Corros. Sci. 2006, 48, 258–272. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Walmsley, J.C.; Nordlien, J.H.; Nisancioglu, K. Effect of Artificial Aging on Intergranular Corrosion of Extruded AlMgSi Alloy with Small Cu Content. Corros. Sci. 2006, 48, 1528–1543. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Lein, J.-E.; Nordlien, J.-H.; Nisancioglu, K. Intergranular Corrosion of Extruded AA6000-Series Model Alloys. In Proceedings of the 9th International Conference on Aluminium Alloys (ICAA9), Brisbane, Australia, 2–5 August 2004; The Institute of Materials Engineering Australasia Ltd.: Brisbane, Australia, 2004; pp. 818–824. [Google Scholar]
- Hunter, M.S.; Fowle, P. Natural and Thermally Formed Oxide Films on Aluminum. J. Electrochem. Soc. 1956, 103, 482. [Google Scholar] [CrossRef]
- Kaufman, J.G. Corrosion of Aluminum and Aluminum Alloys. In Properties and Selection of Aluminum Alloys; Anderson, K., Weritz, J., Kaufman, J.G., Eds.; ASM International: Almere, The Netherlands, 2019; Volume 2B, pp. 95–124. ISBN 978-1-62708-210-5. [Google Scholar]
- Jeurgens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E.J. Growth Kinetics and Mechanisms of Aluminum-Oxide Films Formed by Thermal Oxidation of Aluminum. J. Appl. Phys. 2002, 92, 1649–1656. [Google Scholar] [CrossRef]
- Cabrera, N.; Mott, N.F. Theory of the Oxidation of Metals. Rep. Prog. Phys. 1949, 12, 163. [Google Scholar] [CrossRef]
- Baran, J.D.; Grönbeck, H.; Hellman, A. Mechanism for Limiting Thickness of Thin Oxide Films on Aluminum. Phys. Rev. Lett. 2014, 112, 146103. [Google Scholar] [CrossRef]
- Evertsson, J.; Bertram, F.; Zhang, F.; Rullik, L.; Merte, L.R.; Shipilin, M.; Soldemo, M.; Ahmadi, S.; Vinogradov, N.; Carlà, F.; et al. The Thickness of Native Oxides on Aluminum Alloys and Single Crystals. Appl. Surf. Sci. 2015, 349, 826–832. [Google Scholar] [CrossRef]
- Zähr, J.; Oswald, S.; Türpe, M.; Ullrich, H.J.; Füssel, U. Characterisation of Oxide and Hydroxide Layers on Technical Aluminum Materials Using XPS. Vacuum 2012, 86, 1216–1219. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting Corrosion of Aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Böhni, H. Breakdown of Passivity and Localized Corrosion Processes. Langmuir 1987, 3, 924–930. [Google Scholar] [CrossRef]
- McCafferty, E. Sequence of Steps in the Pitting of Aluminum by Chloride Ions. Corros. Sci. 2003, 45, 1421–1438. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, B.; Bai, Z.; Wang, J.; Yin, Y. Study of the Precipitation Hardening Behaviour and Intergranular Corrosion of Al-Mg-Si Alloys with Differing Si Contents. Metals 2017, 7, 387. [Google Scholar] [CrossRef]
- Svenningsen, G.; Lein, J.E.; Bjørgum, A.; Nordlien, J.H.; Yu, Y.; Nisancioglu, K. Effect of Low Copper Content and Heat Treatment on Intergranular Corrosion of Model AlMgSi Alloys. Corros. Sci. 2006, 48, 226–242. [Google Scholar] [CrossRef]
- Bartawi, E.H.; Mishin, O.V.; Shaban, G.; Nordlien, J.H.; Ambat, R. Electron Microscopy Analysis of Grain Boundaries and Intergranular Corrosion in Aged Al-Mg-Si Alloy Doped with 0.05 Wt% Cu. Corros. Sci. 2022, 209, 110758. [Google Scholar] [CrossRef]
- Bartawi, E.H.; Mishin, O.V.; Shaban, G.; Grumsen, F.; Nordlien, J.H.; Ambat, R. The Effect of Trace Level Copper Content on Intergranular Corrosion of Extruded AA6082-T6 Alloys. Mater. Chem. Phys. 2023, 309, 128303. [Google Scholar] [CrossRef]
- Liang, W.J.; Rometsch, P.A.; Cao, L.F.; Birbilis, N. General Aspects Related to the Corrosion of 6xxx Series Aluminium Alloys: Exploring the Influence of Mg/Si Ratio and Cu. Corros. Sci. 2013, 76, 119–128. [Google Scholar] [CrossRef]
- Bland, L.G.; Locke, J.S. (Warner) Chemical and Electrochemical Conditions within Stress Corrosion and Corrosion Fatigue Cracks. npj Mater. Degrad. 2017, 1, 12. [Google Scholar] [CrossRef]
- Cooper, K.R.; Kelly, R.G. Crack Tip Chemistry and Electrochemistry of Environmental Cracks in AA 7050. Corros. Sci. 2007, 49, 2636–2662. [Google Scholar] [CrossRef]
- Cooper, K.R.; Kelly, R.G. Using Capillary Electrophoresis to Study the Chemical Conditions within Cracks in Aluminum Alloys. J. Chromatogr. A 1999, 850, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.; Ferriss, D.H. Mathematical Modelling of the Electrochemistry in Corrosion Fatigue Cracks in Structural Steel Cathodically Protected in Sea Water. Corros. Sci. 1986, 26, 601–628. [Google Scholar] [CrossRef]
- Turnbull, A. Theoretical Analysis of Influence of Crack Dimensions and Geometry on Mass Transport in Corrosion–Fatigue Cracks. Mater. Sci. Technol. 1985, 1, 700–710. [Google Scholar] [CrossRef]
- Jones, R.H. Mechanisms of Stress-Corrosion Cracking[1]. In Stress-Corrosion Cracking: Materials Performance and Evaluation; Jones, R.H., Ed.; ASM International: Almere, The Netherlands, 2017; pp. 1–42. ISBN 978-1-62708-266-2. [Google Scholar]
- El May, M.; Palin-Luc, T.; Saintier, N.; Devos, O. Effect of Corrosion on the High Cycle Fatigue Strength of Martensitic Stainless Steel X12CrNiMoV12-3. Int. J. Fatigue 2013, 47, 330–339. [Google Scholar] [CrossRef]
- El May, M.; Saintier, N.; Palin-Luc, T.; Devos, O.; Brucelle, O. Modelling of Corrosion Fatigue Crack Initiation on Martensitic Stainless Steel in High Cycle Fatigue Regime. Corros. Sci. 2018, 133, 397–405. [Google Scholar] [CrossRef]
- Akid, R. 2.12—Corrosion Fatigue*. In Shreir’s Corrosion; Cottis, B., Graham, M., Lindsay, R., Lyon, S., Richardson, T., Scantlebury, D., Stott, H., Eds.; Elsevier: Oxford, UK, 2010; pp. 928–953. ISBN 978-0-444-52787-5. [Google Scholar]
- Brown, B.F. Stress Corrosion Cracking Control Measures; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1977. [Google Scholar]
- Parkins, R.N. Current Understanding of Stress-Corrosion Cracking. JOM 1992, 44, 12–19. [Google Scholar] [CrossRef]
- Cheng, Y. Fundamentals of Stress Corrosion Cracking. In Stress Corrosion Cracking of Pipelines; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 7–41. ISBN 978-1-118-53702-2. [Google Scholar]
- Burleigh, T.D. The Postulated Mechanisms for Stress Corrosion Cracking of Aluminum Alloys: A Review of the Literature 1980–1989. Corrosion 1991, 47, 89–98. [Google Scholar] [CrossRef]
- Pao, P.S. Mechanisms of Corrosion Fatigue; ASM International: Almere, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Magnin, T. Recent Advances for Corrosion Fatigue Mechanisms. ISIJ International 1995, 35, 223–233. [Google Scholar] [CrossRef]
- Komai, K. 4.13—Corrosion Fatigue. In Comprehensive Structural Integrity; Milne, I., Ritchie, R.O., Karihaloo, B., Eds.; Pergamon: Oxford, UK, 2003; pp. 345–358. ISBN 978-0-08-043749-1. [Google Scholar]
- Fong, C.; Tromans, D. High Frequency Stage I Corrosion Fatigue of Austenitic Stainless Steel (316L). Metall. Mater. Trans. A 1988, 19, 2753–2764. [Google Scholar] [CrossRef]
- Tirbonod, B. A Model for an Anodic Dissolution Cell in Connection to Its Dimensions for Stress Corrosion Cracking. Corros. Sci. 2004, 46, 2715–2741. [Google Scholar] [CrossRef]
- Lynch, S.P. Environmentally Assisted Cracking: Overview of Evidence for an Adsorption-Induced Localised-Slip Process. Acta Metall. 1988, 36, 2639–2661. [Google Scholar] [CrossRef]
- Lynch, S.P. Metallographic Contributions to Understanding Mechanisms of Environmentally Assisted Cracking. Metallography 1989, 23, 147–171. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen Embrittlement Phenomena and Mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Lynch, S.P. Mechanisms and Kinetics of Environmentally Assisted Cracking: Current Status, Issues, and Suggestions for Further Work. Metall. Mat. Trans. A 2013, 44, 1209–1229. [Google Scholar] [CrossRef]
- Lynch, S.P. Mechanisms of Environmentally Assisted Cracking in Al-Zn-Mg Single Crystals. Corros. Sci. 1982, 22, 925–937. [Google Scholar] [CrossRef]
- McEvily, A.J.; Wei, R.P. Fracture Mechanics and Corrosion Fatigue; University of Connecticut: Storrs, CT, USA, 1972; pp. 381–395. [Google Scholar]
- Vasudevan, A.K.; Sadananda, K. Classification of Environmentally Assisted Fatigue Crack Growth Behavior. Int. J. Fatigue 2009, 31, 1696–1708. [Google Scholar] [CrossRef]
- King, J.E. Role of Oxides in Fatigue Crack Propagation. Mater. Sci. Technol. 1990, 6, 19–31. [Google Scholar] [CrossRef]
- Vasudévan, A.K.; Suresh, S. Influence of Corrosion Deposits on Near-Threshold Fatigue Crack Growth Behavior in 2xxx and 7xxx Series Aluminum Alloys. Metall. Mater. Trans. A 1982, 13, 2271–2280. [Google Scholar] [CrossRef]
- Pippan, R.; Hohenwarter, A. Fatigue Crack Closure: A Review of the Physical Phenomena. Fatigue Fract. Eng. Mater. Struct. 2017, 40, 471–495. [Google Scholar] [CrossRef]
- Michel, S.A.; Kieselbach, R.; Figliolino, M. Environmental and Frequency Effects on Fatigue Crack Growth Rate and Paths in Aluminium Alloy. Fatigue Fract. Eng. Mater. Struct. 2005, 28, 205–219. [Google Scholar] [CrossRef]
- Russell, H. Jones Stress-Corrosion Cracking of Aluminum Alloys. In Stress-Corrosion Cracking: Materials Performance and Evaluation; ASM International: Almere, The Netherlands, 1992; p. 448. [Google Scholar]
- Brown, B.F. Stress-Corrosion Cracking in High Strength Steels and in Titanium and Aluminum Alloys; Naval Research Laboratory; [for sale by the Supeuintandent of Document, U.S. Government Printing Office]: Washington, DC, USA, 1972. [Google Scholar]
- Reboul, M.C.; Magnin, T.; Warner, T.J. Stress Corrosion Cracking of High Strength Aluminium Alloys. In Proceedings of the 3rd International Conference on Aluminum Alloys. Their Physical and Mechanical Properties (ICAA3), Trondheim, Norway, 22–26 June 1992; Volume II, pp. 453–460. [Google Scholar]
- Braun, R. Investigation on Microstructure and Corrosion Behaviour of 6XXX Series Aluminium Alloys. Mater. Sci. Forum 2006, 519–521, 735–740. [Google Scholar]
- Ogawa, T.; Hasunuma, S.; Shirawachi, T.; Fukada, N. Effect of Chemical Composition and Relative Humidity on the Humid Gas Stress Corrosion Cracking of Aluminum Alloys. J. High Pres. Inst. Jpn. 2019, 57, 24–33. [Google Scholar] [CrossRef]
- Fujii, T.; Sawada, T.; Shimamura, Y. Nucleation of Stress Corrosion Cracking in Aluminum Alloy 6061 in Sodium Chloride Solution: Mechanical and Microstructural Aspects. J. Alloys Compd. 2023, 938, 168583. [Google Scholar] [CrossRef]
- Chandler, W.T.; Walter, R.J. Effects of High Pressure Hydrogen on Metals at Ambient Temperature Final Report; National Aeronautics and Space Administration: Huntsville, AL, USA, 1969; p. 290. [Google Scholar]
- Yamabe, J.; Awane, T.; Murakami, Y. Hydrogen Trapped at Intermetallic Particles in Aluminum Alloy 6061-T6 Exposed to High-Pressure Hydrogen Gas and the Reason for High Resistance against Hydrogen Embrittlement. Int. J. Hydrogen Energy 2017, 42, 24560–24568. [Google Scholar] [CrossRef]
- Ratke, L.; Gruhl, W. Modellversuch zum Mechanismus der Spannungsrißkorrosion von AlZnMg-Legierungen. Mater. Corros 1980, 31, 768–773. [Google Scholar] [CrossRef]
- Safyari, M.; Moshtaghi, M.; Hojo, T.; Akiyama, E. Mechanisms of Hydrogen Embrittlement in High-Strength Aluminum Alloys Containing Coherent or Incoherent Dispersoids. Corros. Sci. 2022, 194, 109895. [Google Scholar] [CrossRef]
- Zhao, H.; Chakraborty, P.; Ponge, D.; Hickel, T.; Sun, B.; Wu, C.-H.; Gault, B.; Raabe, D. Hydrogen Trapping and Embrittlement in High-Strength Al Alloys. Nature 2022, 602, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Z.; Zhang, C.; Peng, W.; Ma, K.; Oleksandr, M. Effects of Hydrogen on the Dynamic Mechanical Properties and Microstructure of 7055 and 7A52 Aluminum Alloys. Mater. Charact. 2023, 203, 113151. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Safyari, M.; Hojo, T. Effect of Solution Treatment Temperature on Grain Boundary Composition and Environmental Hydrogen Embrittlement of an Al–Zn–Mg–Cu Alloy. Vacuum 2021, 184, 109937. [Google Scholar] [CrossRef]
- Safyari, M.; Moshtaghi, M.; Kuramoto, S. On the Role of Traps in the Microstructural Control of Environmental Hydrogen Embrittlement of a 7xxx Series Aluminum Alloy. J. Alloys Compd. 2021, 855, 157300. [Google Scholar] [CrossRef]
- Osaki, S.; Harano, T.; Ikeda, J.; Ichitani, K.; Zhao, P.; Takeshima, Y. Effect of Grain Size on Hydrogen Embrittlement Properties of 6061 Aluminum Alloys. J. Jpn. Inst. Light Metals 2008, 58, 139–145. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.; Giannakopoulos, K.I.; Orfanos, P.G. Effect of pH on Stress Corrosion Cracking of 6082 Al Alloy. Metals 2018, 8, 578. [Google Scholar] [CrossRef]
- NSS 1740.16; NASA Safety Standard for Hydrogen and Hydrogen Systems. National Aeronautics and Space Administration: Washington, DC, USA, 1997.
- Osaki, S.; Ikeda, J.; Kinoshita, K.; Sasaki, Y. Hydrogen Embrittlement Properties of 7075 and 6061 Aluminum Alloys in Humid Air. J. Jpn. Inst. Light Metals 2006, 56, 721–727. [Google Scholar] [CrossRef]
- Osaki, S.; Ikeda, J.; Kinoshita, K.; Ichitani, K.; Takeshima, Y.; Sasaki, Y. Hydrogen Embrittlement Properties of Notched-Aluminum Alloy Plates in Humid Air. J. Jpn. Inst. Light Metals 2007, 57, 74–79. [Google Scholar] [CrossRef]
- Osaki, S.; Maeda, N.; Kinoshita, K.; Ichitani, K.; Iton, G.; Yabuta, H. Embrittlement Properties of Aluminum Alloys 7075 and 6061 in High−Pressure Gaseous Hydrogen. Trans. Jpn. Soc. Mech. Eng. Ser. A 2009, 75, 366–372. [Google Scholar] [CrossRef]
- Xu, Y.; Toda, H.; Shimizu, K.; Wang, Y.; Gault, B.; Li, W.; Hirayama, K.; Fujihara, H.; Jin, X.; Takeuchi, A.; et al. Suppressed Hydrogen Embrittlement of High-Strength Al Alloys by Mn-Rich Intermetallic Compound Particles. Acta Mater. 2022, 236, 118110. [Google Scholar] [CrossRef]
- Davis, J.R. Corrosion of Aluminum and Aluminum Alloys; ASM International: Almere, The Netherlands, 1999; ISBN 978-1-61503-238-9. [Google Scholar]
- Chanyathunyaroj, K.; Phetchcrai, S.; Laungsopapun, G.; Rengsomboon, A. Fatigue Characteristics of 6061 Aluminum Alloy Subject to 3.5% NaCl Environment. Int. J. Fatigue 2020, 133, 105420. [Google Scholar] [CrossRef]
- Chanyathunyaroj, K.; Moonrin, N.; Laungsopapun, G.; Phetchcrai, S. Corrosion Fatigue Study of 6061 Aluminum Alloy: The Effect of Coatings on the Fatigue Characteristics. Metall. Mater. Trans. A 2022, 53, 2874–2889. [Google Scholar] [CrossRef]
- Nguyen, N.; Li, P. Fatigue Behaviour of AA6061-T6 Alloys in the Corrosive Environment. MATEC Web Conf. 2018, 165, 03015. [Google Scholar] [CrossRef]
- Madhavi, Y.; Rama Krishna, L.; Narasaiah, N. Corrosion-Fatigue Behavior of Micro-Arc Oxidation Coated 6061-T6 Al Alloy. Int. J. Fatigue 2021, 142, 105965. [Google Scholar] [CrossRef]
- Kurumada, A.; Itoh, G.; Mochizuki, K. Effects of Test Temperature and Environment on Fatigue Properties of a 6061 Aluminum Alloy. J. Jpn. Inst. Light Metals 2017, 67, 2–7. [Google Scholar] [CrossRef]
- Weber, M.; Eason, P.D.; Özdeş, H.; Tiryakioğlu, M. The Effect of Surface Corrosion Damage on the Fatigue Life of 6061-T6 Aluminum Alloy Extrusions. Mater. Sci. Eng. A. 2017, 690, 427–432. [Google Scholar] [CrossRef]
- Dominguez Almaraz, G.M.; Mercado Lemus, V.H.; Jesús Villalon Lopez, J. Rotating Bending Fatigue Tests for Aluminum Alloy 6061-T6, Close to Elastic Limit and with Artificial Pitting Holes. Procedia Eng. 2010, 2, 805–813. [Google Scholar] [CrossRef]
- Alqahtani, I.; Starr, A.; Khan, M. Investigation of the Combined Influence of Temperature and Humidity on Fatigue Crack Growth Rate in Al6082 Alloy in a Coastal Environment. Materials 2023, 16, 6833. [Google Scholar] [CrossRef]
- Schönowitz, M.; Maier, B.; Grün, F. Influence of Corrosion Fatigue on the Stress Gradient Effect of the Aluminium Alloy EN AW-6082 T6. Int. J. Fatigue 2024, 184, 108322. [Google Scholar] [CrossRef]
- Locke, J. Comparison of Age-Hardenable Al Alloy Corrosion Fatigue Crack Growth Susceptibility and the Effect of Testing Environment. Corrosion 2016, 72, 911–926. [Google Scholar] [CrossRef]
- Tokaji, K.; Goshima, Y. Fatigue behaviour of 6063 aluminium alloy in corrosive environments. Zair. J. Soc. Mater. Sci. Jpn. 2002, 51, 1411–1416. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Tan, V.M.; Patni, K.; Eftekhari, A. Comparison of Corrosion-Fatigue Properties of 6013 Bare, Alclad 2024, and 2024 Bare Aluminum Alloy Sheet Materials. J. Mater. Eng. Perform. 1992, 1, 91–96. [Google Scholar] [CrossRef]
- Zamponi, C.; Sonneberger, S.; Haaks, M.; Müller, I.; Staab, T.; Tempus, G.; Maier, K. Investigation of Fatigue Cracks in Aluminium Alloys 2024 and 6013 in Laboratory Air and Corrosive Environment. J. Mater. Sci. 2004, 39, 6951–6956. [Google Scholar] [CrossRef]
- Ichitani, K.; Koyama, K. Effects of Constituent Elements on Hydrogen Embrittlement Resistance of 6061 Aluminum Alloy in Fatigue Tests. J. Jpn. Inst. Light Metals 2012, 62, 212–218. [Google Scholar] [CrossRef]
- Ichitani, K.; Koyama, K. Effect of Experimental Humidity on Fatigue Fracture of 6XXX-Series Aluminum Alloys. In Proceedings of the 12th International Conference on Aluminium Alloys, Yokohama, Japan, 5–9 September 2010; pp. 363–370. [Google Scholar]
- Osaki, S.; Maeda, N.; Kinoshita, K.; Yabuta, H. Fatigue crack growth properties and the effect of applied frequency on these of 7000 series aluminum alloys in high-humidity air. J. Jpn. Inst. Light Metals 2010, 60, 499–504. [Google Scholar] [CrossRef]
- Osaki, S. Fatigue Crack Growth of 6000 Series Aluminum Alloys in High−humidity Air. In Proceedings of the Conference of Chugoku-Shikoku Branch, Matsuyama, Japan, 14–15 March 2008; Volume 46, pp. 81–82. [Google Scholar]
- Engler, T.; Andersohn, G.; Oechsner, M.; de Araújo, F.D.; Kaufmann, H.; Melz, T. Electrochemical Characterization of Automotive Aluminum Alloys Regarding Their Corrosion Fatigue Behavior. Mater. Werkst. 2018, 49, 264–272. [Google Scholar] [CrossRef]

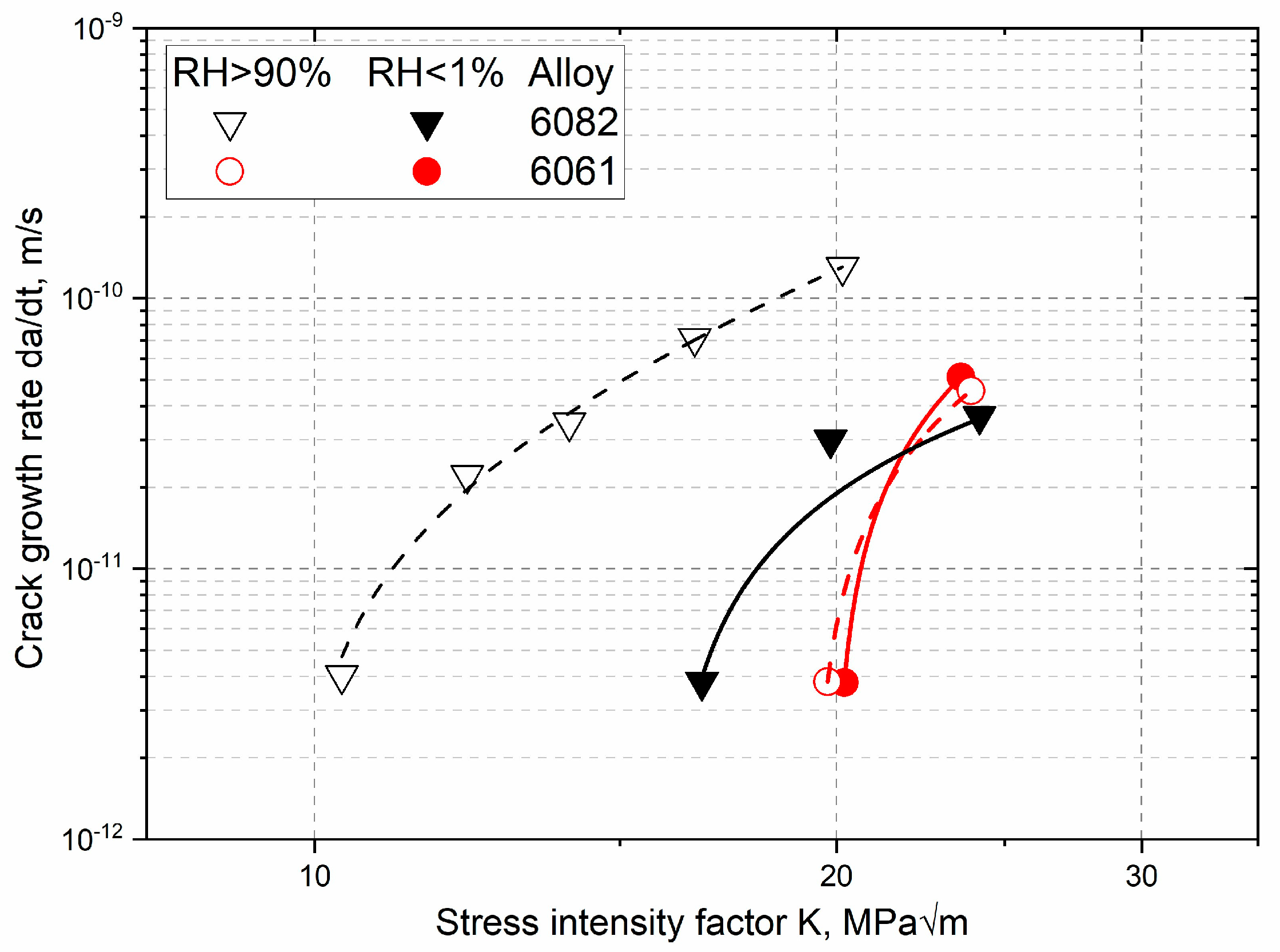
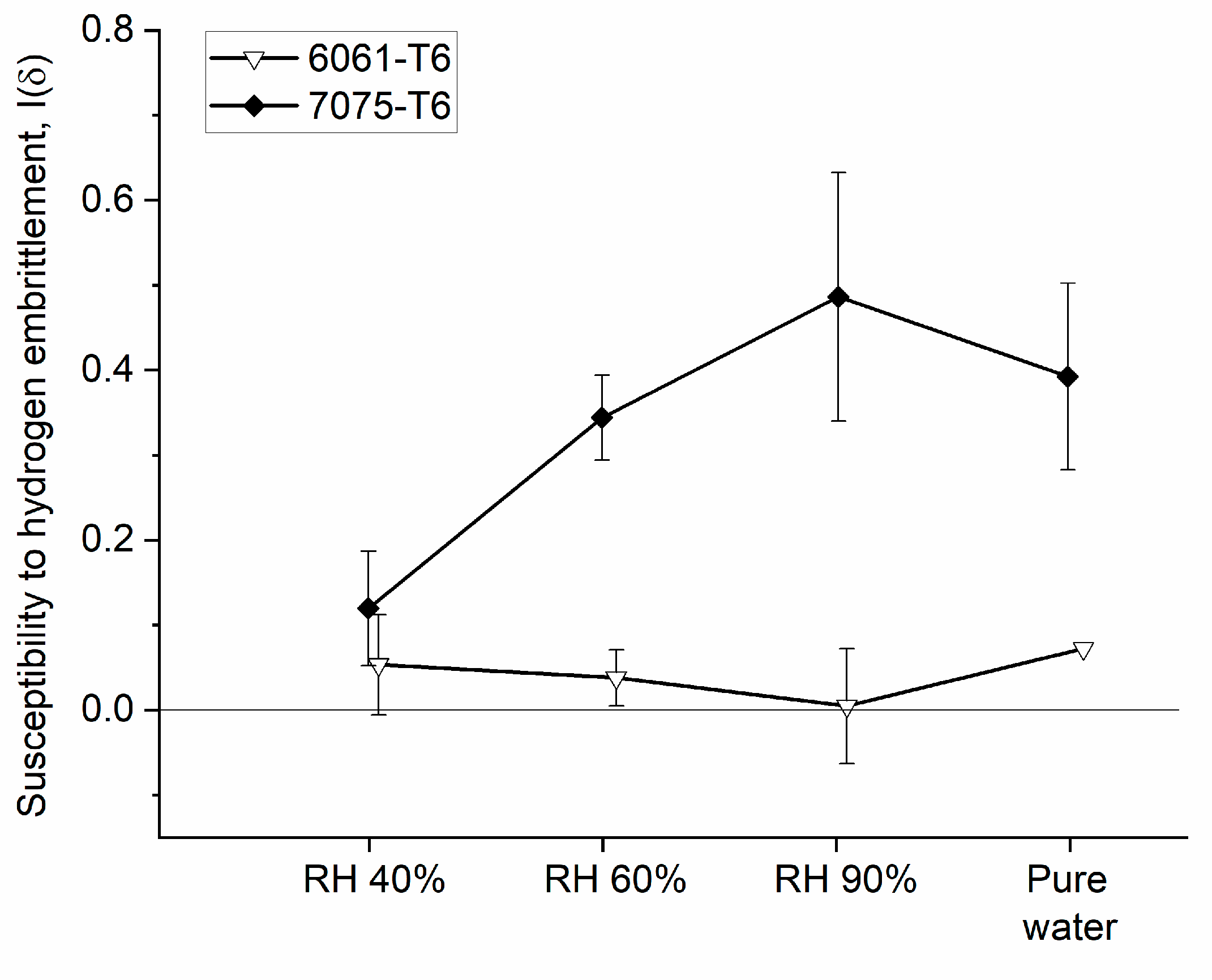
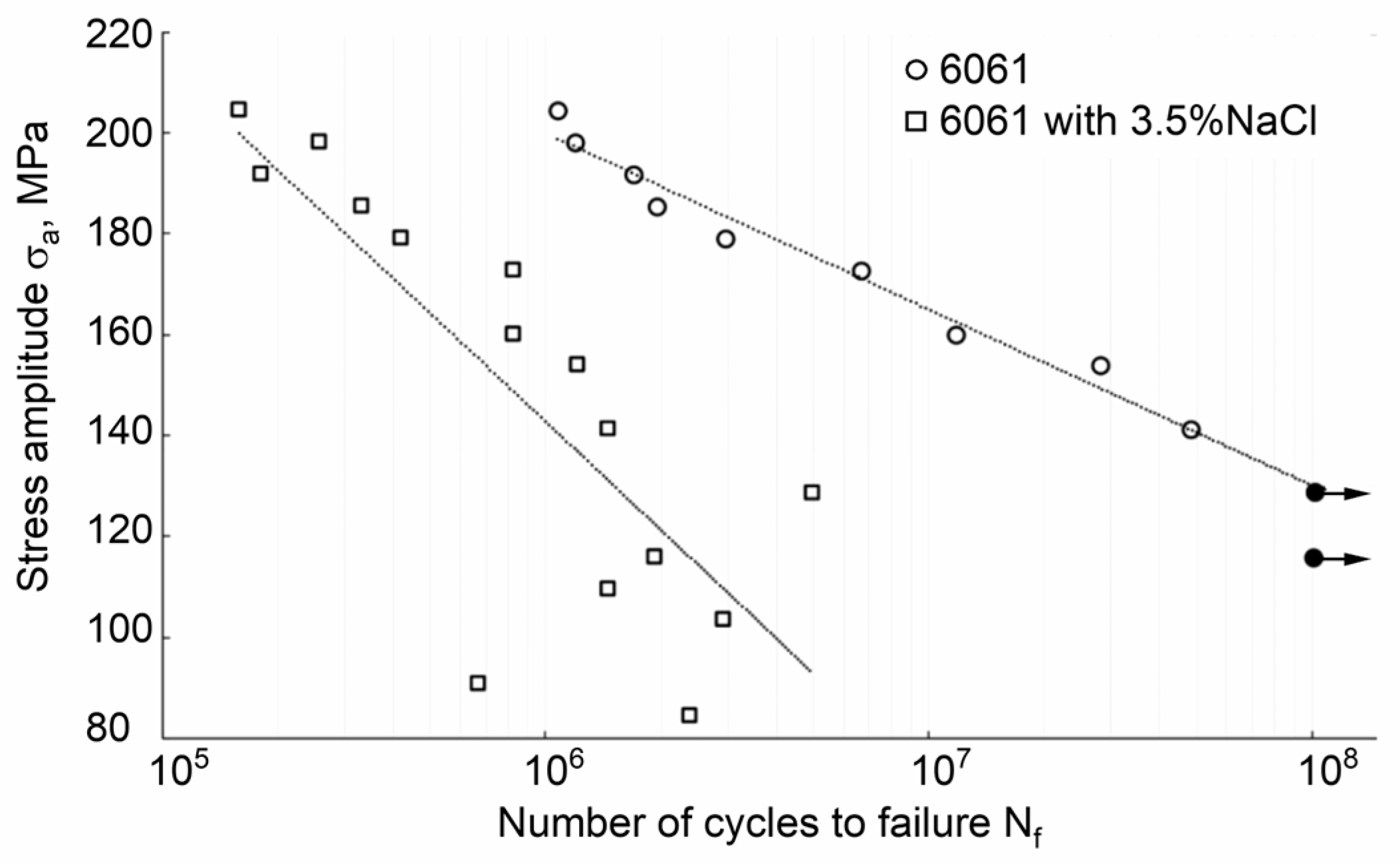
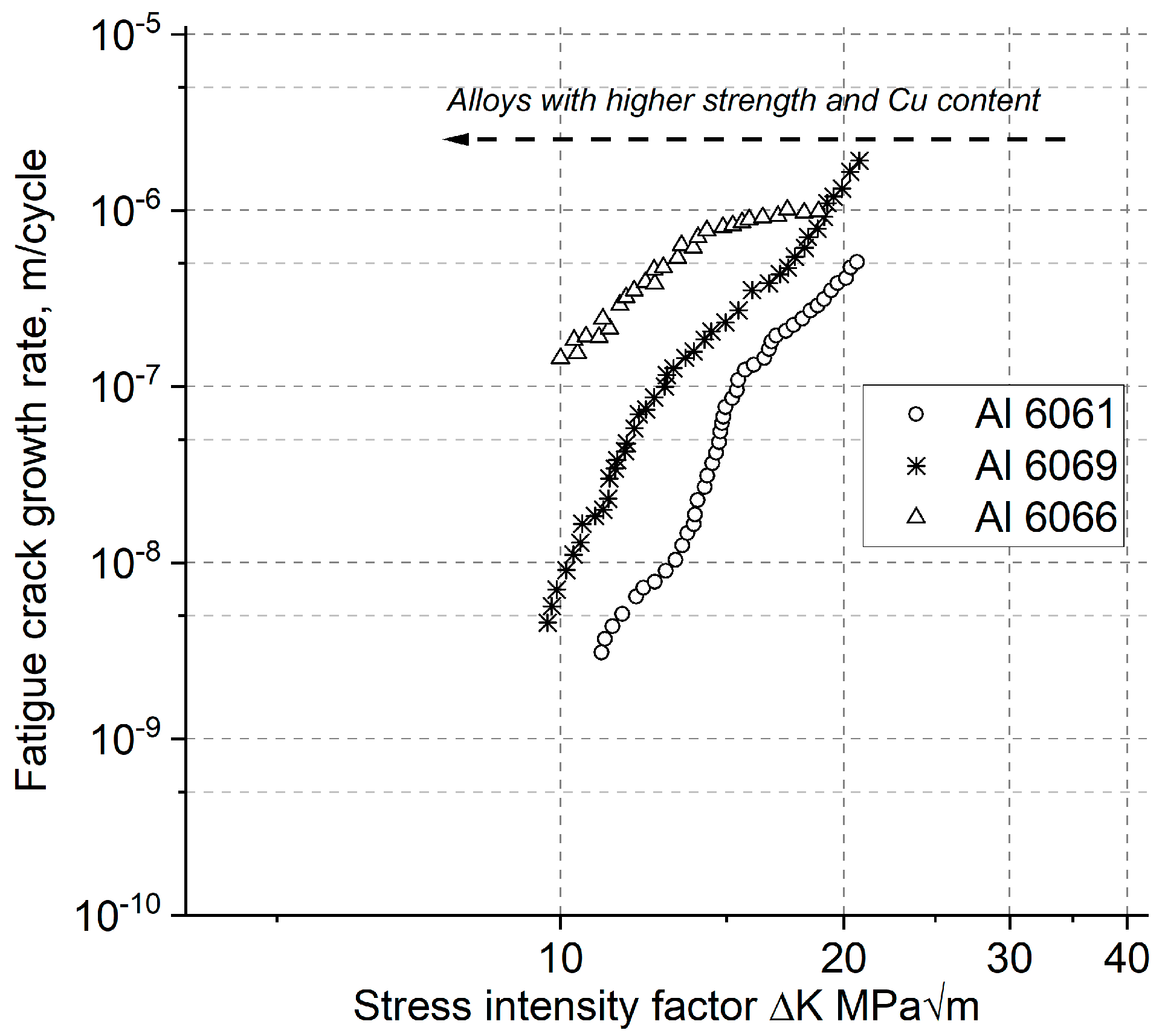
| Properties | Age–Hardenable Alloys | ||
|---|---|---|---|
| 6xxx | 2xxx | 7xxx | |
| Main alloying elements | Mg, Si | Cu | Mg, Zn |
| Strength (UTS in MPa) | 150–480 | 300–450 | 320–600 |
| Weldability | good | poor | poor |
| Extrudability/formability | good | poor | poor |
| Corrosion resistance | good | poor | poor |
| Name of Phase | Composition | Morphology/Typical Size | References |
|---|---|---|---|
| GP | Si/Mg > 1 | Spherical particles with a diameter of 1–5 nm; needles with a diameter of 2 nm and a length of 20 nm | [22,32,35] |
| β″ | Mg5Si6 | Needles with a length of 4–50 nm aligned along Al {1 0 0} | [30] |
| U1 | MgAl2Si2 | Needles of several hundred nm with a diameter of 15 nm | [36] |
| U2 | MgAlSi | ||
| β′ | Mg9Si5 | Rods several hundred nm with a diameter of 10–20 nm Ribbons up to 1 µm length | [37] |
| Q | Al4Cu2Mg8Si7 Al5Cu2Mg8Si6 | Round or oval | [21,38] |
| Q′ | Lath | ||
| β | Mg2Si | Plates or cubes, up to 20 µm, platelets along Al {1 0 0} | [22,32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramenko, T.; Michel, S.; Kollender, J.; Burda, I.; Hans, U.; Affolter, C. Review on Environmentally Assisted Static and Fatigue Cracking of Al-Mg-Si-(Cu) Alloys. Metals 2024, 14, 621. https://doi.org/10.3390/met14060621
Avramenko T, Michel S, Kollender J, Burda I, Hans U, Affolter C. Review on Environmentally Assisted Static and Fatigue Cracking of Al-Mg-Si-(Cu) Alloys. Metals. 2024; 14(6):621. https://doi.org/10.3390/met14060621
Chicago/Turabian StyleAvramenko, Tetiana, Silvain Michel, Jan Kollender, Iurii Burda, Ulrik Hans, and Christian Affolter. 2024. "Review on Environmentally Assisted Static and Fatigue Cracking of Al-Mg-Si-(Cu) Alloys" Metals 14, no. 6: 621. https://doi.org/10.3390/met14060621
APA StyleAvramenko, T., Michel, S., Kollender, J., Burda, I., Hans, U., & Affolter, C. (2024). Review on Environmentally Assisted Static and Fatigue Cracking of Al-Mg-Si-(Cu) Alloys. Metals, 14(6), 621. https://doi.org/10.3390/met14060621