Deformation–Induced Mechanical Synthesis of U and Fe
Abstract
:1. Introduction
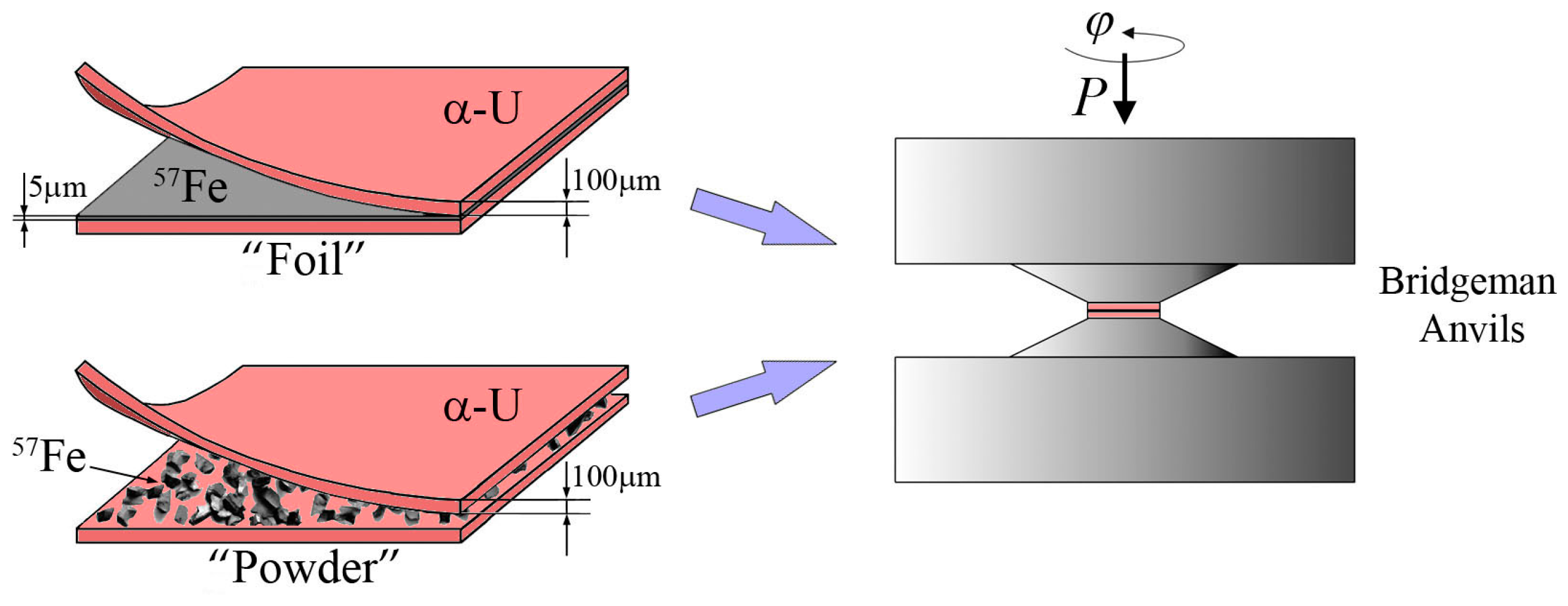
2. Materials and Methods
3. Experimental Results
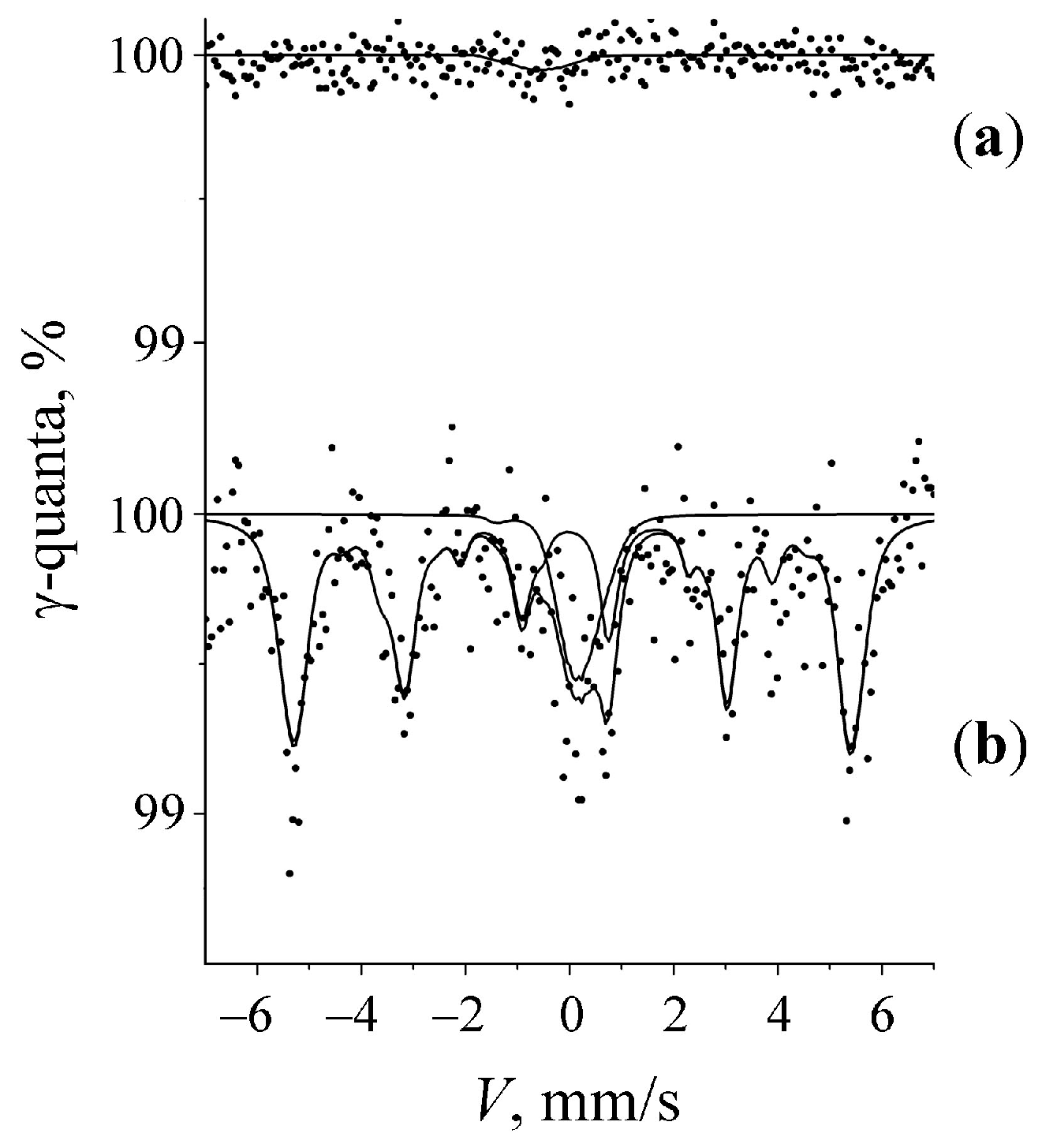
3.1. Mössbauer Spectra of Initial α-U Sample with a Natural Fe Impurity
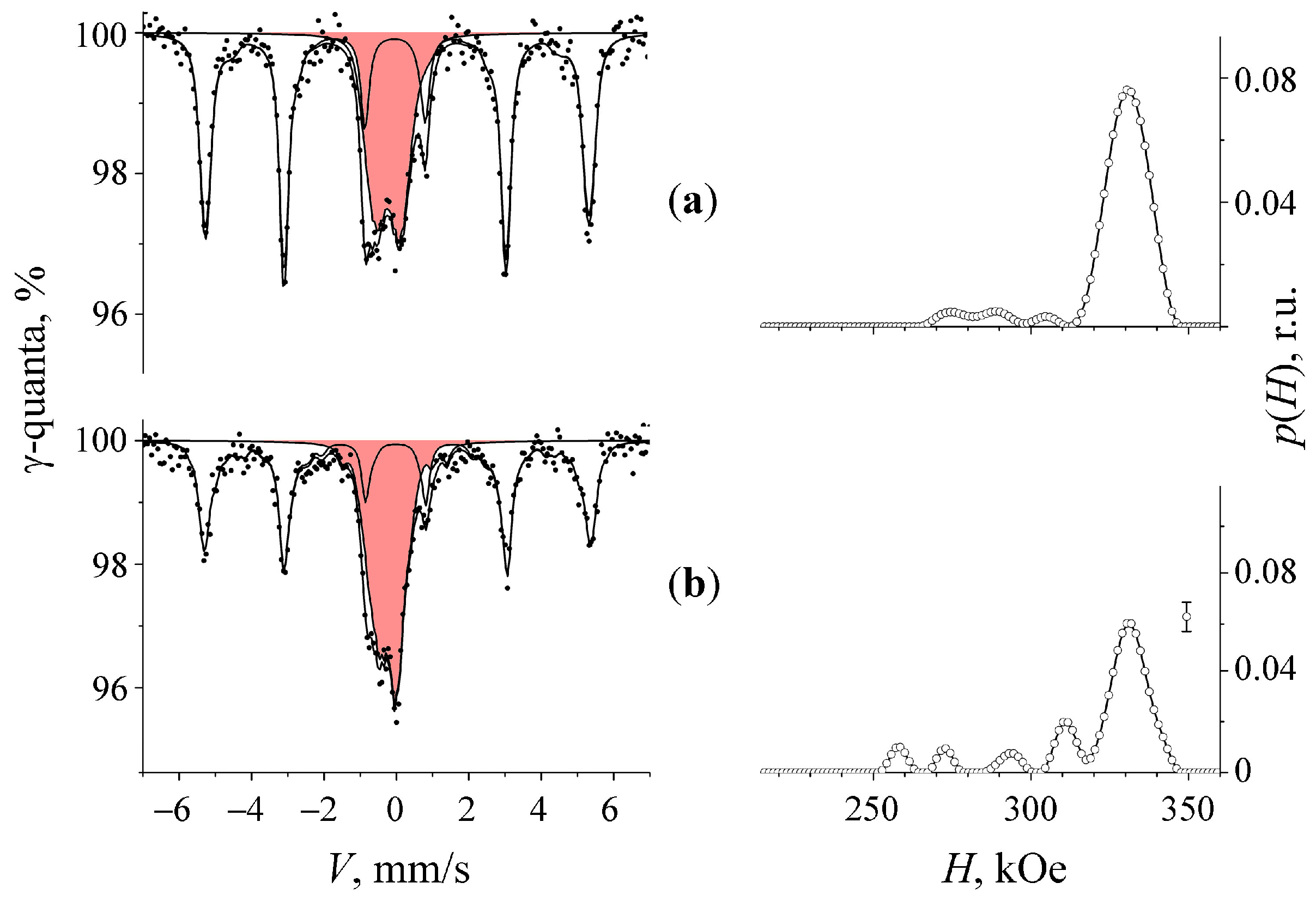
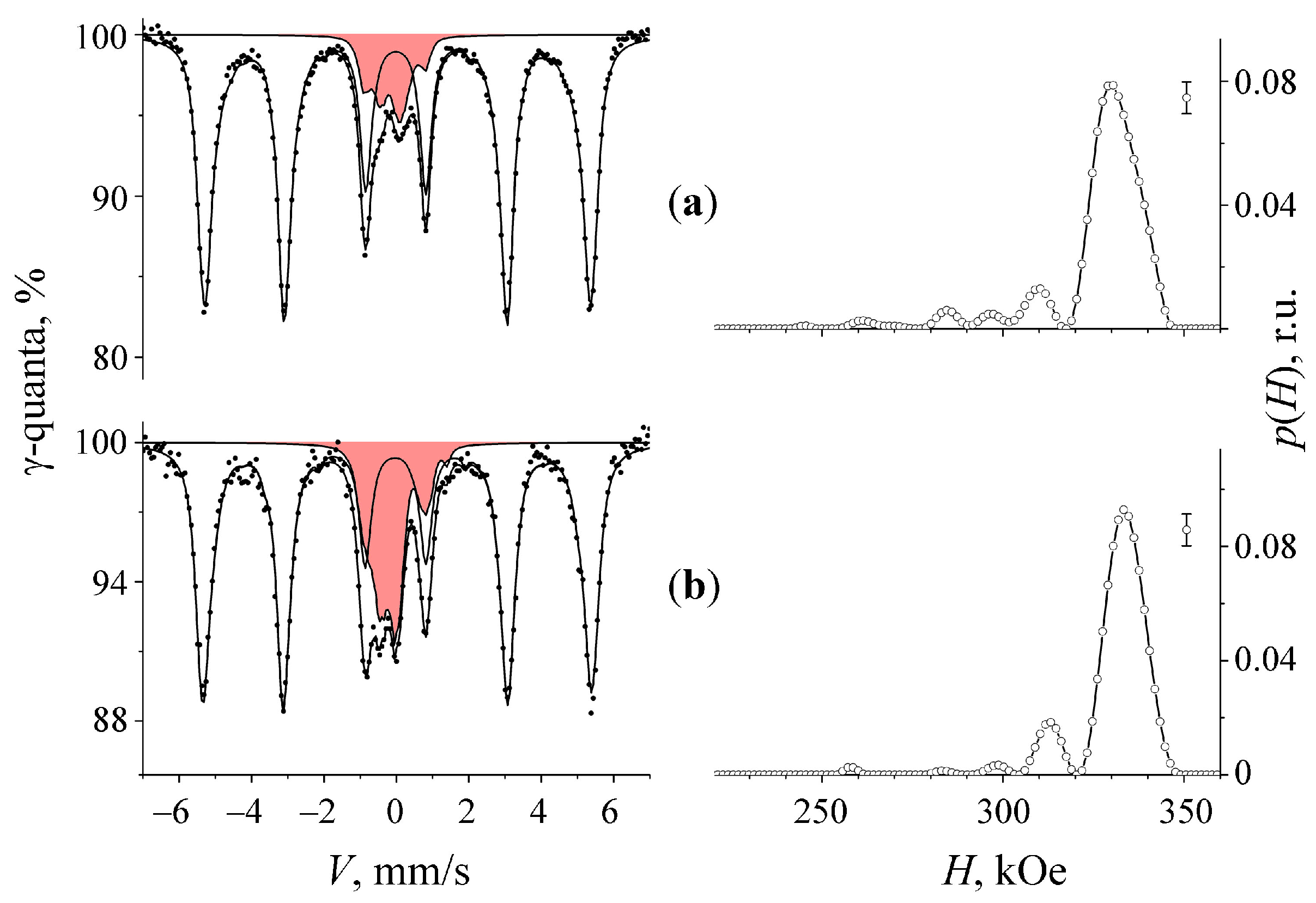
3.2. Mössbauer Spectra of α-U + 57Fe Sample after Mechanical Synthesis
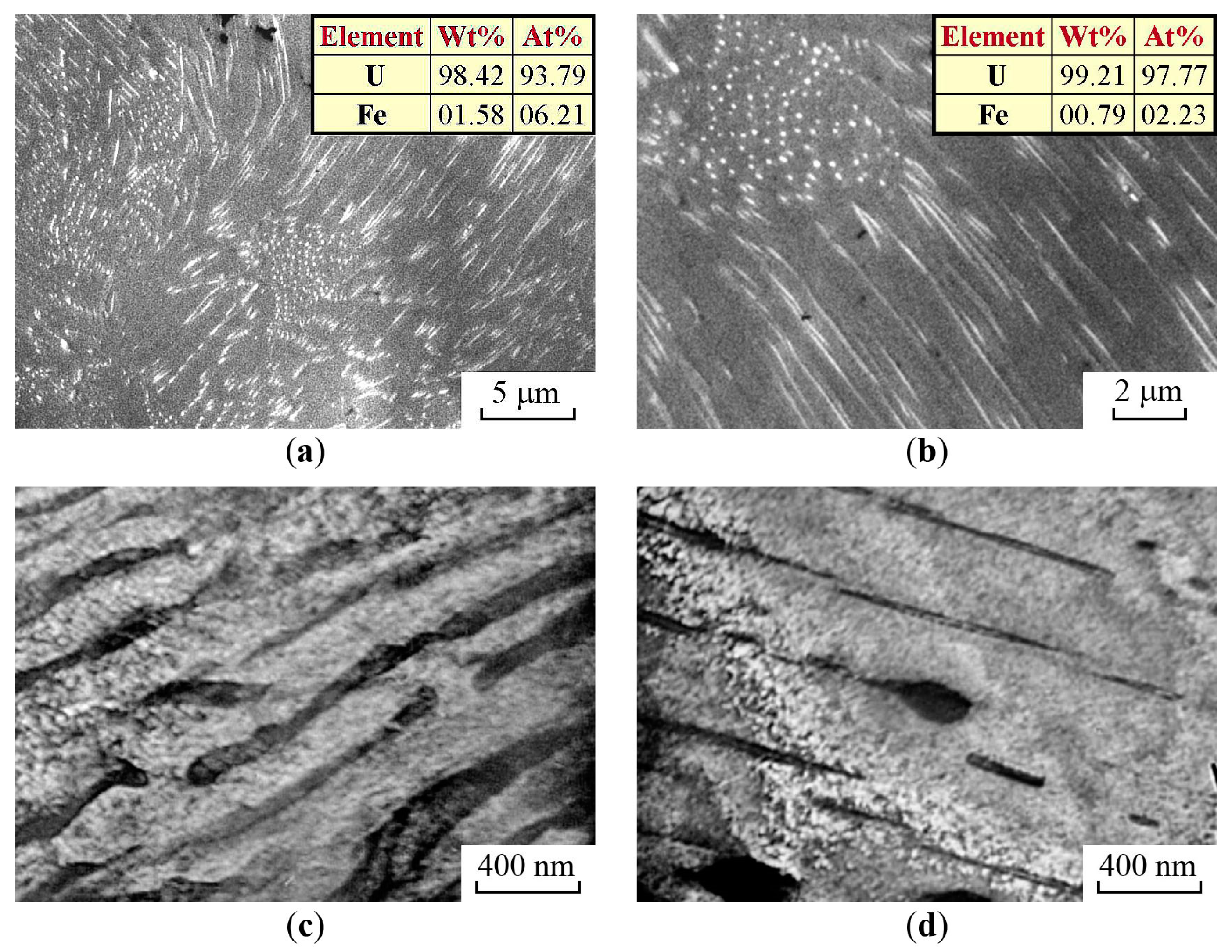
3.3. The Structure of U–Fe Alloy
- -
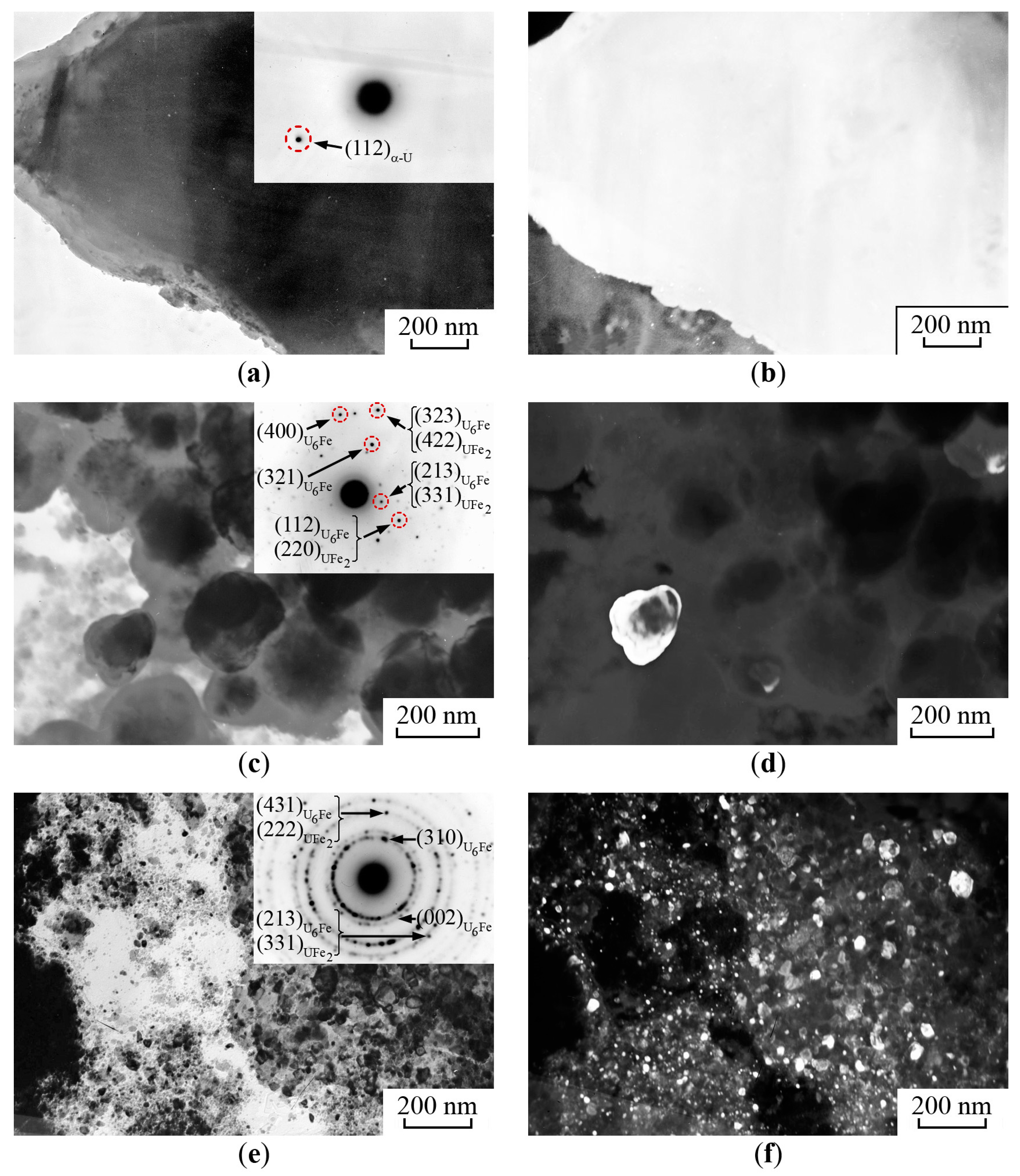
- The first type. There are coarse particles with irregular forms (Figure 10a,b) in the structure of the synthesized sample of α-U with 57Fe. The size of these particles reaches several microns. Microdiffraction of this region revealed individual reflections with an interplanar distance corresponding to α-U [28] (Figure 10a).
- -
- The second type is represented by rounded particles with a size of 100–200 nm (Figure 10c,d). The microdiffraction pattern of these particles revealed many irregular reflections (Figure 10c). The interpretation of the microdiffraction data showed that interplanar distances from individual reflections could correspond to those of the U6Fe and UFe2 phases [28]. The dark-field image (Figure 10d) was obtained in the reflection associated with the UFe6 and U2Fe phases.
- -
- The third type. The regions with nanocrystalline structures were also observed in the mechanically synthesized alloy of α-U with 57Fe, Figure 10e,f. The peculiar electron diffraction pattern contained ring reflections from heavy disordered crystallites of the α-U, U6Fe and UFe2 phases. The size of intermetallic particles in these regions was 10–50 nm.
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, R.S.; Hudson, J.A.; Mazey, D.J. The stability of precipitates in an irradiation environment. J. Nucl. Mater. 1972, 44, 318–330. [Google Scholar] [CrossRef]
- Sagaradze, V.V.; Shabashov, V.A.; Litvinov, A.V.; Koloskov, V.M.; Parkhomenko, V.D. Radiation-induced dissolution of ni3m intermetallic particles (M = Ti, Al, Zr) in displacement cascades in Fe-Ni-M alloys irradiated by neutrons at 340 K. Phys. Met. Metall. 2010, 109, 475–482. [Google Scholar] [CrossRef]
- Shabashov, V.; Sagaradze, V.; Kozlov, K.; Ustyugov, Y. Atomic order and submicrostructure in iron alloys at megaplastic deformation. Metals 2018, 8, 995. [Google Scholar] [CrossRef]
- Glezer, A.M.; Metlov, L.S. Physics of megaplastic (severe) deformation in solids. Phys. Sol. State 2010, 52, 1162–1169. [Google Scholar] [CrossRef]
- Shabashov, V.; Lyashkov, K.; Kozlov, K.; Zavalishin, A.; Zamatovskii, A.; Kataeva, N.; Sagaradze, V.; Ustyugov, Y. Critical redistribution of nitrogen in the austenitic Cr–Mn steel under severe plastic deformation. Materials 2021, 14, 7116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Umemoto, M.; Todaka, Y.; Fujisaku, K.; Tsuchiya, K. The dynamic phase transformation and formation of nanocrystalline structure in SUS304 austenitic stainless steel subjected to high pressure torsion. Rev. Adv. Mater. Sci. 2008, 18, 577–582. [Google Scholar]
- Shabashov, V.; Lyashkov, K.; Zamatovskii, A.; Kozlov, K.; Kataeva, N.; Novikov, E.; Ustyugov, Y. Mechanosynthesis of high-nitrogen steels strengthened by secondary titanium nitrides. Materials 2022, 15, 5038. [Google Scholar] [CrossRef]
- Tagawa, H. Phase relations and thermodynamic properties of the uranium-nitrogen system. J. Nucl. Mater. 1974, 51, 78–89. [Google Scholar] [CrossRef]
- Fink, J.K. Thermophysical properties of uranium dioxide. J. Nucl. Mater. 2000, 279, 1–18. [Google Scholar] [CrossRef]
- Kittel, J.H.; Ayer, J.E.; Beck, W.N.; Brodsky, M.B.; O’Boyle, D.R.; Zegler, S.T.; Ellinger, F.H.; Miner, W.N.; Schonfeld, F.W.; Nelson, R.D. Plutonium and plutonium alloys as nuclear fuel materials. Nucl. Eng. Des. 1971, 15, 373–440. [Google Scholar] [CrossRef]
- Ogata, T. Metal Fuel. Compr. Nucl. Mater. 2012, 3, 1–40. [Google Scholar] [CrossRef]
- Sandratskii, L.M.; Silkin, V.M.; Havela, L. Entangled origins of the nonmagnetic states of U and Fe atoms in hydrogenated UFeGe. Phys. Rev. Materials 2023, 7, 024414. [Google Scholar] [CrossRef]
- Geiger, T.; Fizzotti, C. Studien an Uran-Eisen-Legierungen. Schweizer Arch. Angew. Wiss. Technol. 1958, 24, 27–32. [Google Scholar]
- Okamoto, H. Phase Diagrams of Binary Iron Alloys; ASM International, The Material Information Society: Geauga County, OH, USA, 1993. [Google Scholar]
- Levitas, V.I.; Zarechnyy, O.M. Numerical study of stress and plastic strain evolution under compression and shear of a sample in a rotational anvil cell. High Press Res. 2010, 30, 653–669. [Google Scholar] [CrossRef]
- Rusakov, V.S.; Kadyrzhanov, K.K. Mössbauer spectroscopy of locally inhomogeneous systems. Hyperfine Interact. 2005, 164, 87–97. [Google Scholar] [CrossRef]
- Frauenfelder, H.; Nagle, D.E.; Taylor, R.D.; Cochran, D.R.F.; Visscher, W.M. Elliptical polarization of Fe57 gamma rays. Phys. Rev. 1962, 126, 1065–1075. [Google Scholar] [CrossRef]
- Lemon, G.; Boolchand, P.; Stevens, M.; Marcuso, M.; DeLong, L.E.; Huber, J.G. 57Fe Mossbauer spectroscopy of U6Fe. J. Less Common Metals 1987, 127, 329–334. [Google Scholar] [CrossRef]
- Blow, S. A Mössbauer effect study of the metallic compounds U6Fe and Pu6Fe, and the relevance of the results to theories of the behaviour of actinide metals. J. Phys. Chem. Sol. 1969, 30, 1549–1559. [Google Scholar] [CrossRef]
- McGuire, T.K.; Herber, R.H. 57Fe Mössbauer effect evidence for magnetostriction in UFe2. Solid State Commun. 1983, 48, 393–395. [Google Scholar] [CrossRef]
- Tsutsui, S.; Nakada, M.; Kobayashi, Y.; Nasu, S.; Haga, Y.; Ōnuki, Y. 238U and 57Fe Mössbauer Spectroscopic Study of UFe2. Hyperfine Interact. 2001, 133, 17–21. [Google Scholar] [CrossRef]
- Paolasini, L.; Lander, G.H.; Shapiro, S.M.; Caciuffo, R.; Lebech, B.; Regnault, L.-P.; Roessli, B.; Fournier, J.-M. Magnetic excitations in the itinerant ferromagnet UFe2. Phys. Rev. B 1996, 54, 7222–7232. [Google Scholar] [CrossRef] [PubMed]
- Havela, L.; Miliyanchuk, K.; Gonçalves, A.P.; Waerenborgh, J.C.; Pereira, L.C.J.; Gaczynski, P.; Lopes, E.B.; Pešička, J. Increase of TC in UFe2+x synthesized by ultrafast cooling. Intermetallics 2011, 19, 113–120. [Google Scholar] [CrossRef]
- Vincze, I.; Campbell, I.A. Mossbauer measurements in iron based alloys with transition metals. J. Phys. F Met. Phys. 1973, 3, 647–663. [Google Scholar] [CrossRef]
- Van der Woude, F.; Sawatzky, G.A. Mossbauer effect in iron and dilute iron based alloys. Phys. Rep. 1974, 12, 335–374. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Ovchinnikov, V.V.; Mulyukov, R.R.; Valiev, R.Z.; Filippova, N.P. Deformation-induced nonequilibrium grain-boundary phase in submicrocrystalline iron. Nanostruct. Mater. 1999, 11, 1017–1029. [Google Scholar] [CrossRef]
- Birringer, R.; Gleiter, H. Nanocrystalline Materials. In Encyclopaedia of Materials Science and Engineering; Suppl. 1; Pergamon Press: Oxford, UK, 1988. [Google Scholar]
- Output Generated by SingleCrystal(TM) for Windows 2.3.2 Copyright © 2023–2015 CrystalMaker Software Ltd. Available online: http://www.crystalmaker.com (accessed on 10 August 2023).
- Shabashov, V.A.; Brodova, I.G.; Mukoseev, A.G.; Sagaradze, V.V.; Litvinov, A.V. Deformation-induced phase transformations in the Al–Fe system under intensive plastic deformation. J. Phys. Condens. Matter. 2007, 19, 386222. [Google Scholar] [CrossRef]
- Preston, R.S.; Gerlach, R. Mössbauer effect in dilute alloys of iron in aluminum. Phys. Rev. B 1971, 3, 1519–1526. [Google Scholar] [CrossRef]
- Preston, R.S.; Nasu, S.; Gonser, U. Association of defects with iron impurities in FCC aluminium. J. Phys. Colloques 1979, 40, C2-564–C2-565. [Google Scholar] [CrossRef]
- Sassa, K.; Goto, H.; Ishida, Y.; Kato, M. Mössbauer analysis of 57Co associated with quenched-in lattice defects in aluminium. Scr. Metall. 1977, 11, 1029–1032. [Google Scholar] [CrossRef]
- Sassa, K.; Ishida, Y.; Kaneko, K. Mössbauer spectroscopy of 57Fe implanted to aluminium at liquid nitrogen temperature. J. Phys. Colloques 1979, 40, C2-556–C2-558. [Google Scholar] [CrossRef]
- Young, R.M.K.; Clyne, T.W. An Al–Fe intermetallic phase formed during controlled solidification. Scr. Metall. 1981, 15, 1211–1216. [Google Scholar] [CrossRef]
- Gubicza, J.; Dobatkin, S.V.; Khosravi, E. Reduction of vacancy concentration during storage of severely deformed Cu. Mater. Sci. Eng. A 2010, 527, 6102–6104. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Kozlov, K.; Ustyugov, Y.; Zamatovskii, A.; Tolmachev, T.; Novikov, E. Mössbauer Analysis of Deformation–Induced Acceleration of Short-Range Concentration Separation in Fe-Cr Alloys—Effect of the Substitution Impurity: Sb and Au. Metals 2020, 10, 725. [Google Scholar] [CrossRef]
- Hume-Rothery, W. Elements of Structural Metallurgy. In Monograph and Report; Series No. 26; Institute of Metals: London, UK, 1961. [Google Scholar]
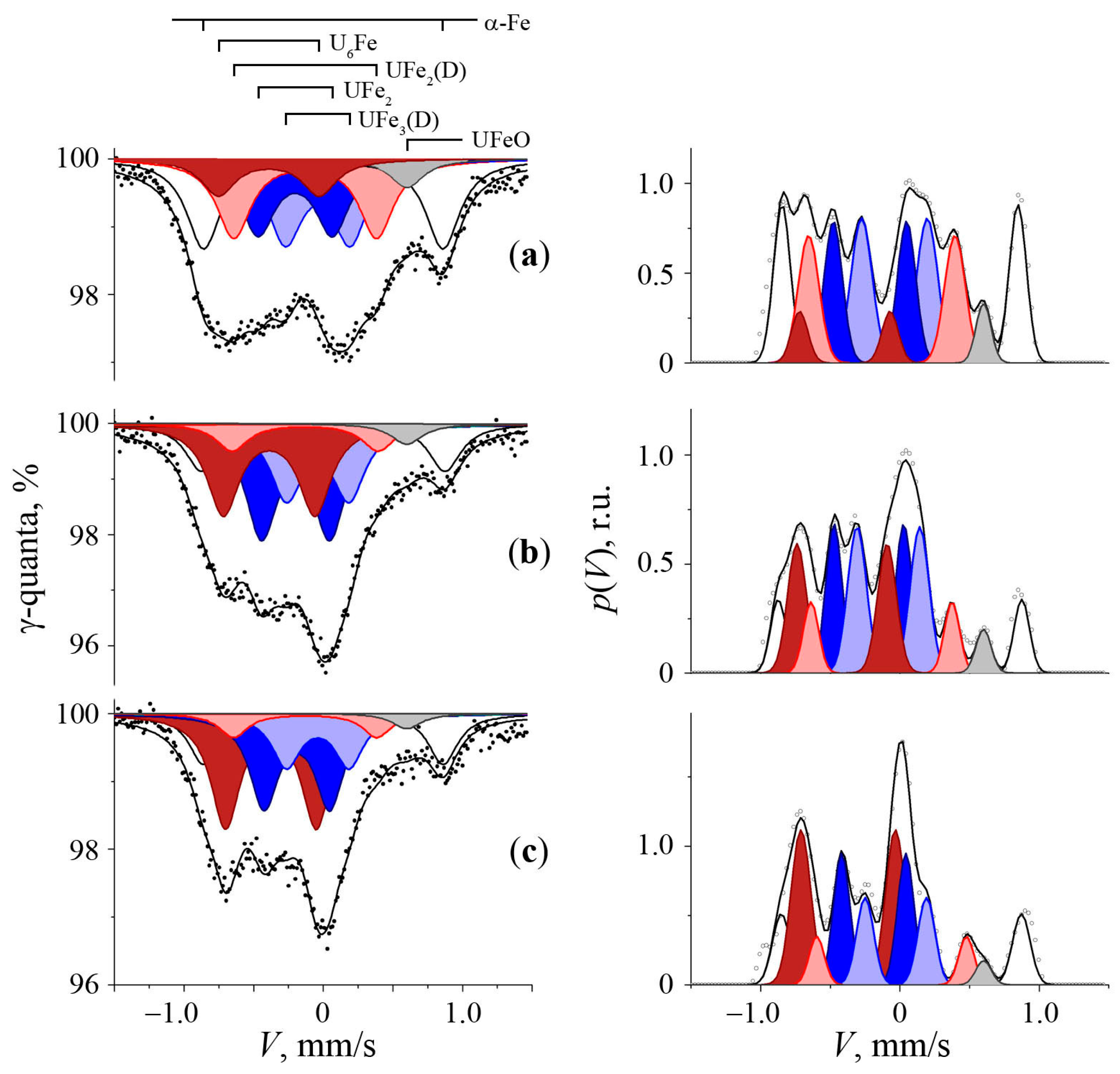
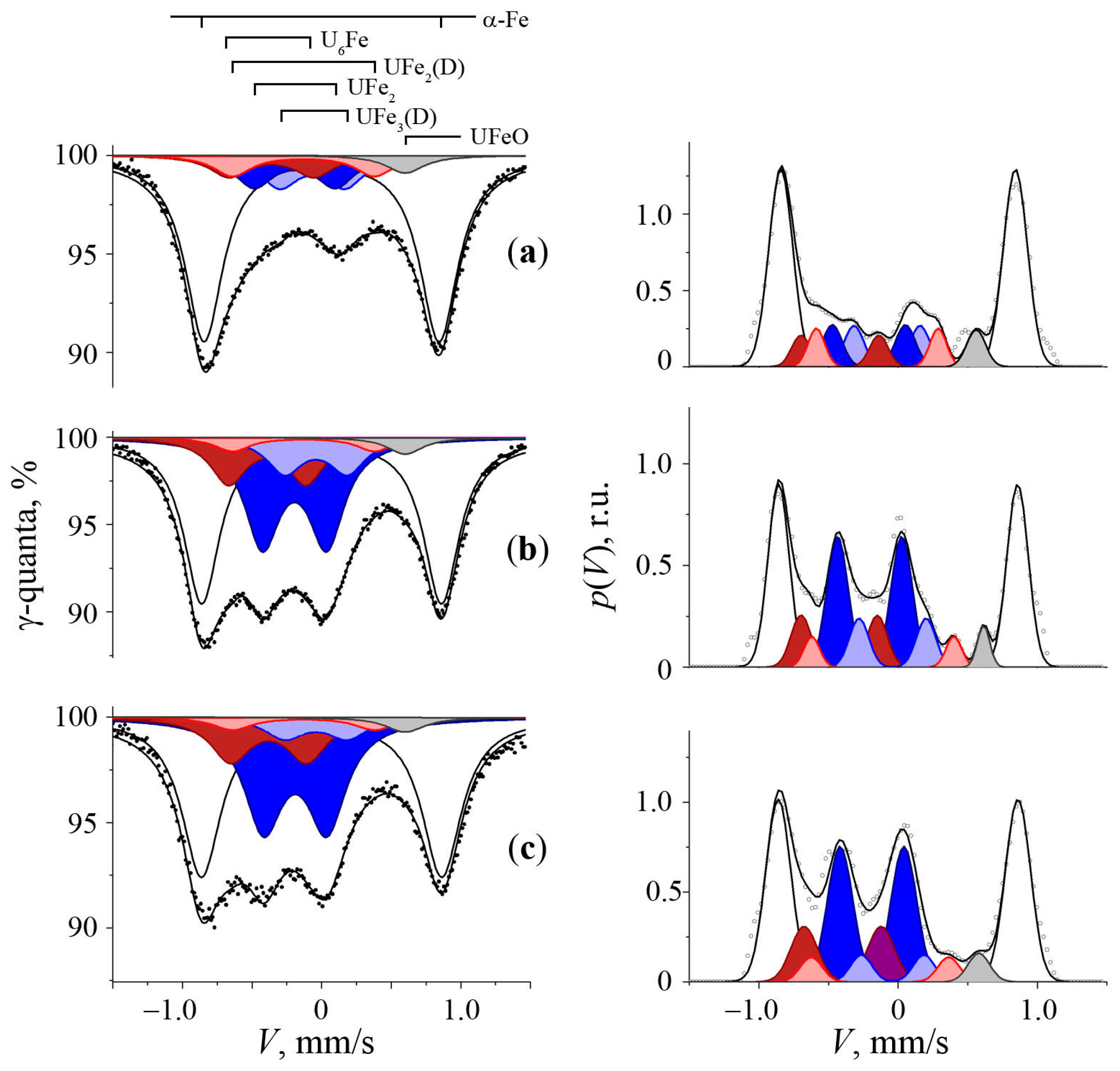
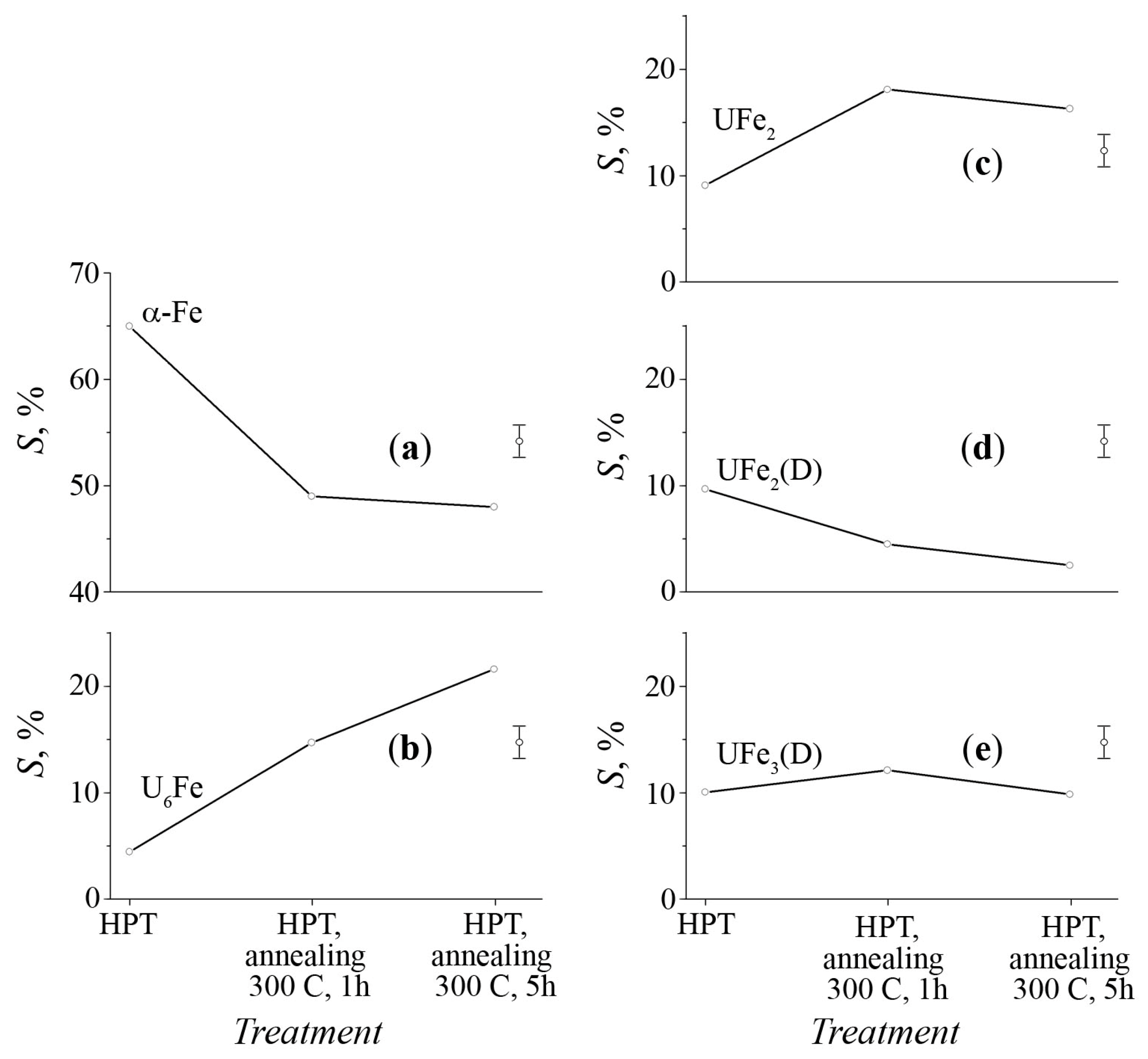
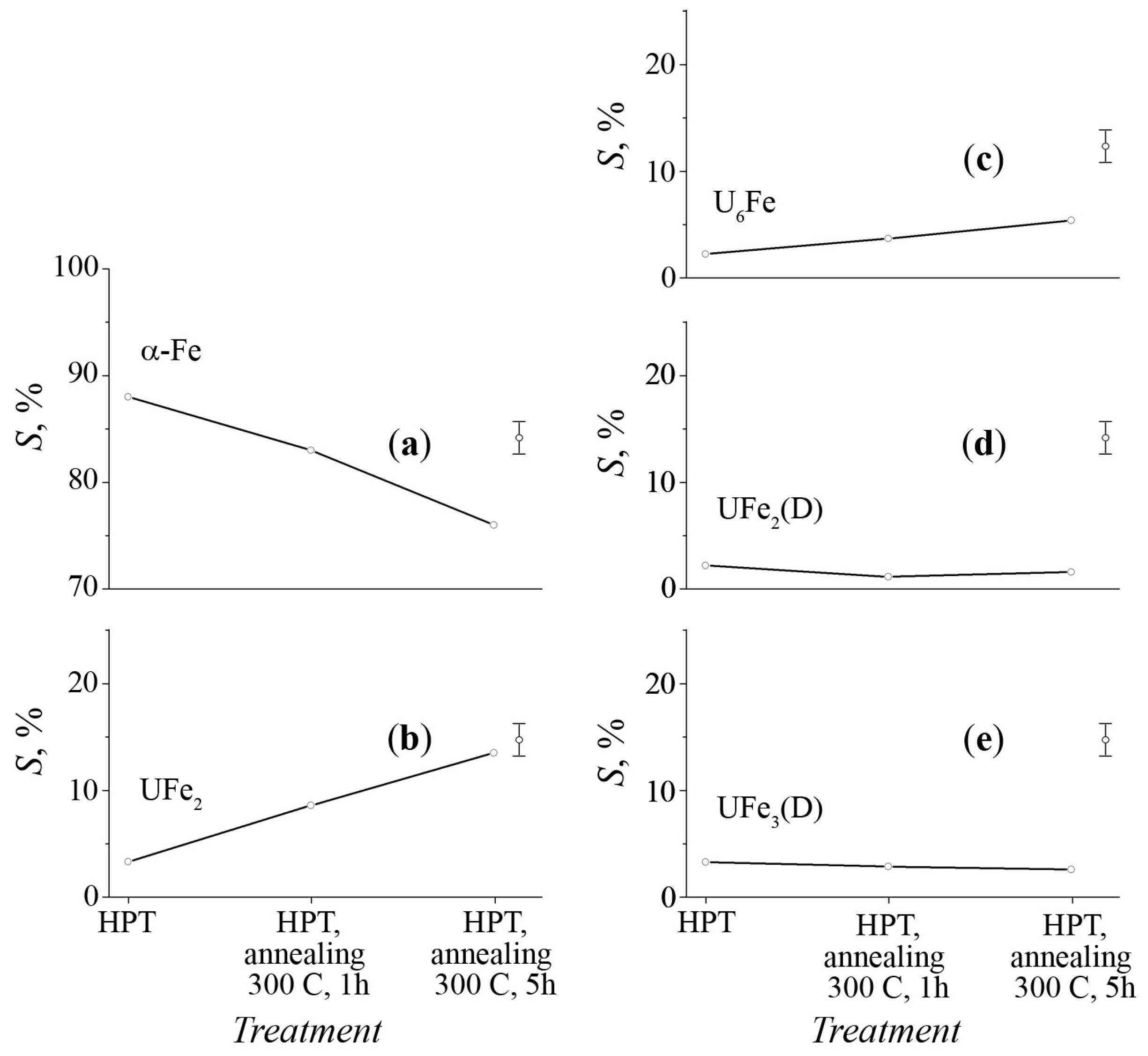
| Phase | IS, mm/s ±0.01 | QS, mm/s ±0.02 | S, % (Foil) ±2.0 | S, % (Powder) ±2.0 | Reference |
|---|---|---|---|---|---|
| U6Fe | −0.37 | 0.69 | 4.5 | 2.2 | Current work |
| UFe2 | −0.19 | 0.48 | 9.1 | 3.3 | |
| UFe2(D) | −0.13 | 1.02 | 9.7 | 2.2 | |
| UFe3(D) | −0.04 | 0.46 | 10.0 | 3.3 | |
| UFeO | 0.9 | 0.83 | 3.0 | 1.0 | |
| U6Fe | −0.37 | 0.69 | – | – | [18] |
| UFe2 | −0.19 | 0.46 | – | – | [23] |
| UFe2.3 | −0.14 | 0.94 | – | – | [23] |
| UFe3 | −0.04 | 1.15 | – | – | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, K.; Shabashov, V.; Kataeva, N.; Sagaradze, V.; Pilyugin, V.; Zamatovskii, A. Deformation–Induced Mechanical Synthesis of U and Fe. Metals 2024, 14, 55. https://doi.org/10.3390/met14010055
Kozlov K, Shabashov V, Kataeva N, Sagaradze V, Pilyugin V, Zamatovskii A. Deformation–Induced Mechanical Synthesis of U and Fe. Metals. 2024; 14(1):55. https://doi.org/10.3390/met14010055
Chicago/Turabian StyleKozlov, Kirill, Valery Shabashov, Natalya Kataeva, Victor Sagaradze, Vitalii Pilyugin, and Andrey Zamatovskii. 2024. "Deformation–Induced Mechanical Synthesis of U and Fe" Metals 14, no. 1: 55. https://doi.org/10.3390/met14010055
APA StyleKozlov, K., Shabashov, V., Kataeva, N., Sagaradze, V., Pilyugin, V., & Zamatovskii, A. (2024). Deformation–Induced Mechanical Synthesis of U and Fe. Metals, 14(1), 55. https://doi.org/10.3390/met14010055







