A Comparison of Experimental and Ab Initio Structural Data on Fe under Extreme Conditions
Abstract
1. Introduction
2. Method
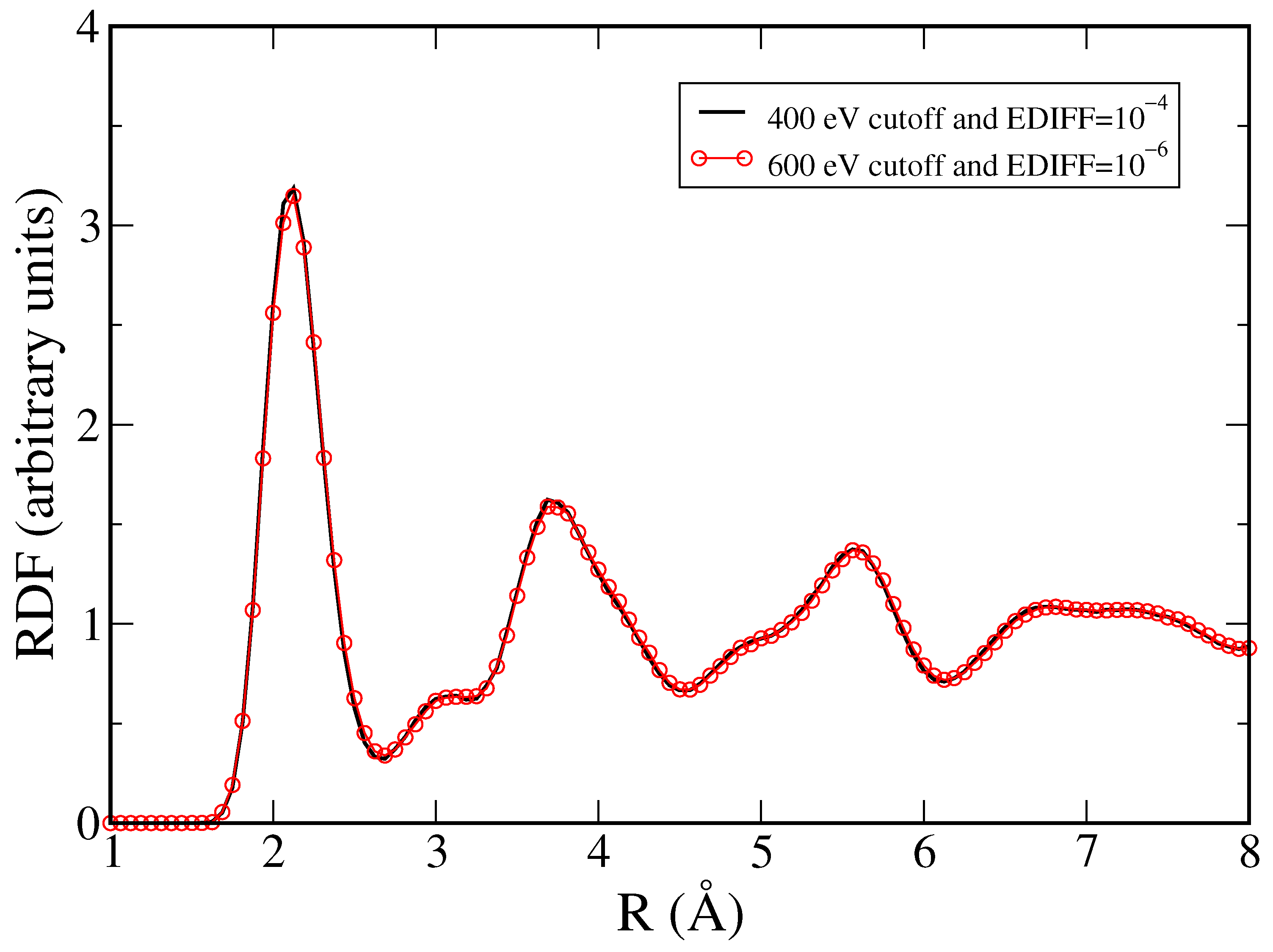
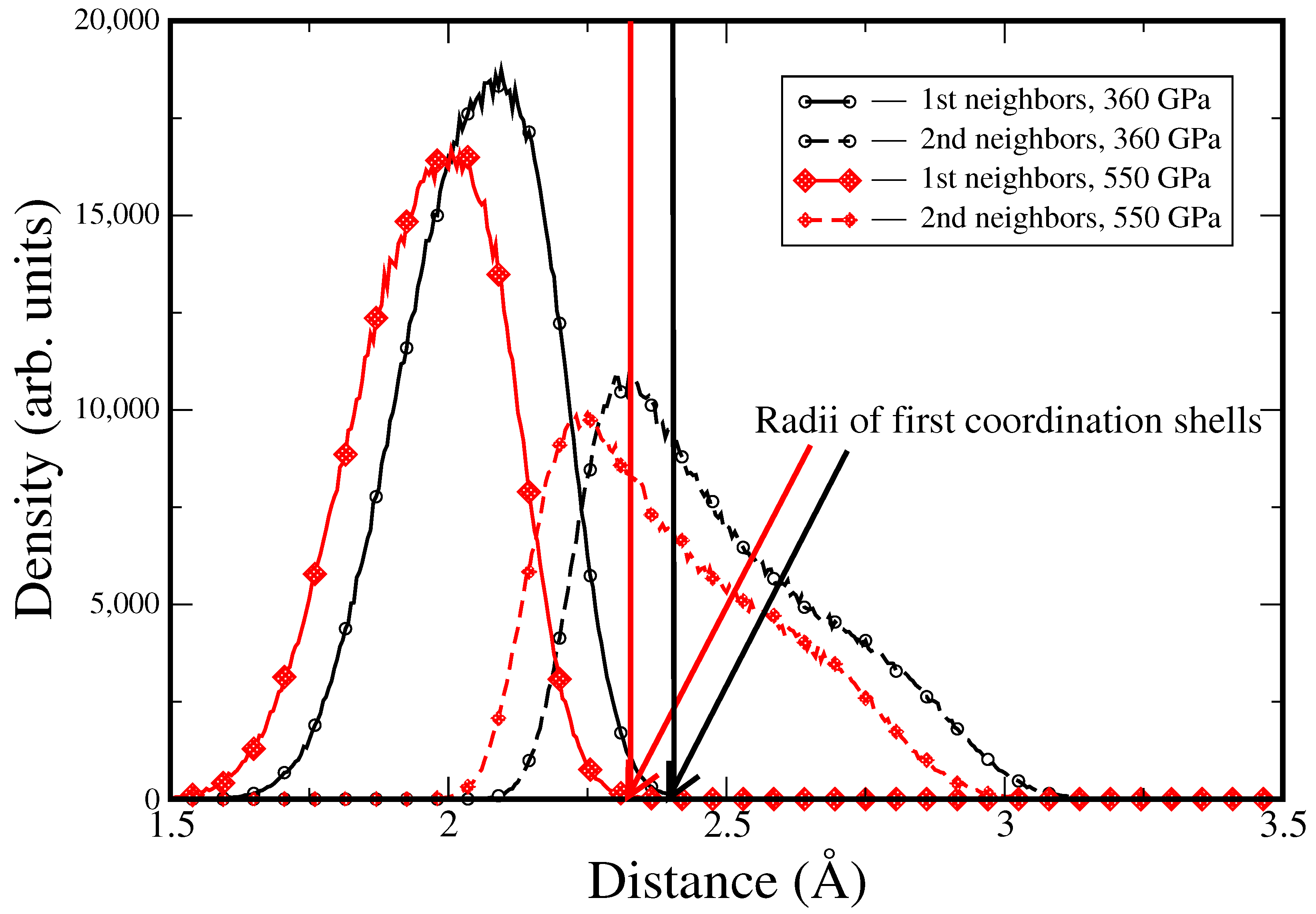
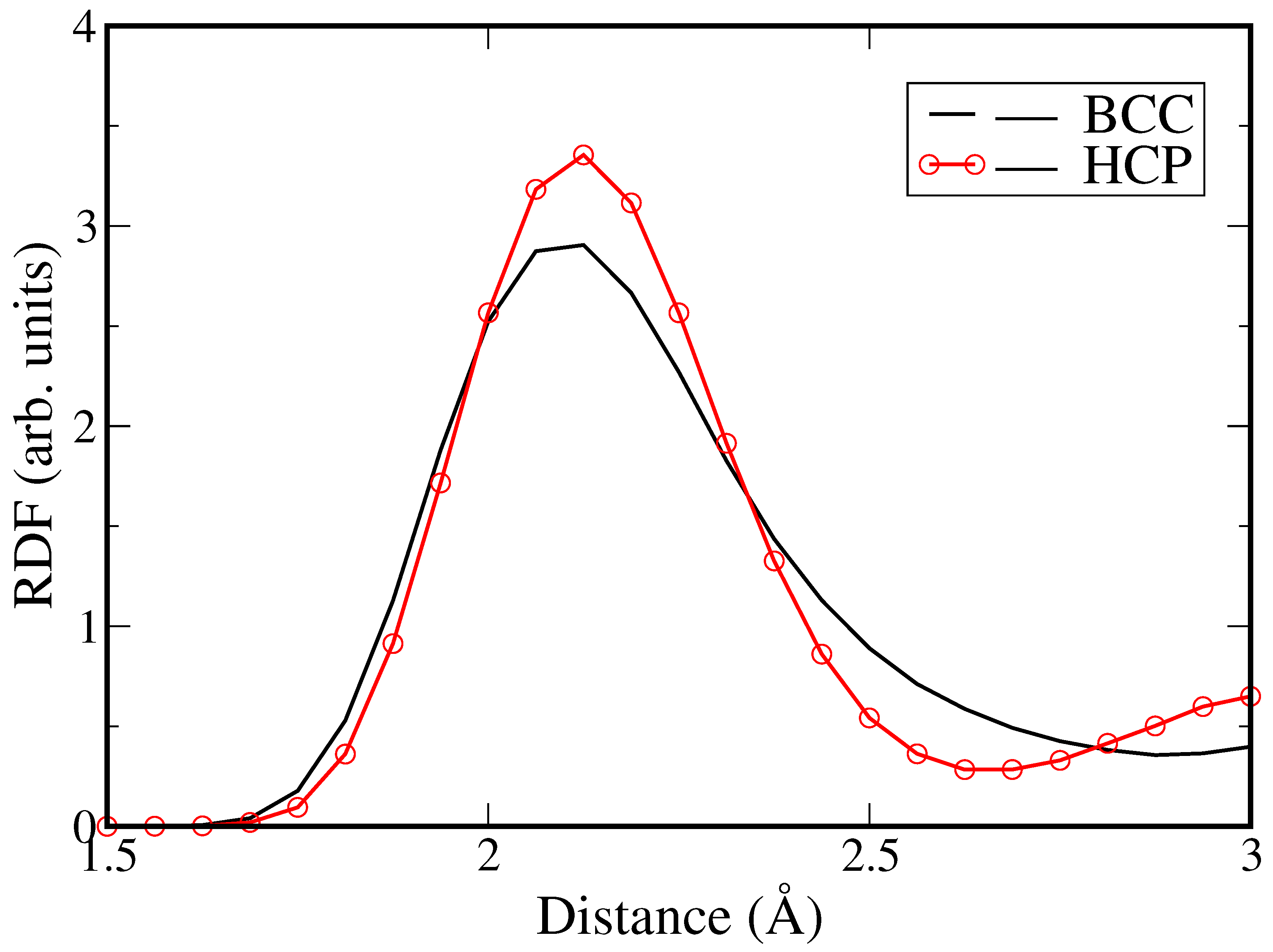
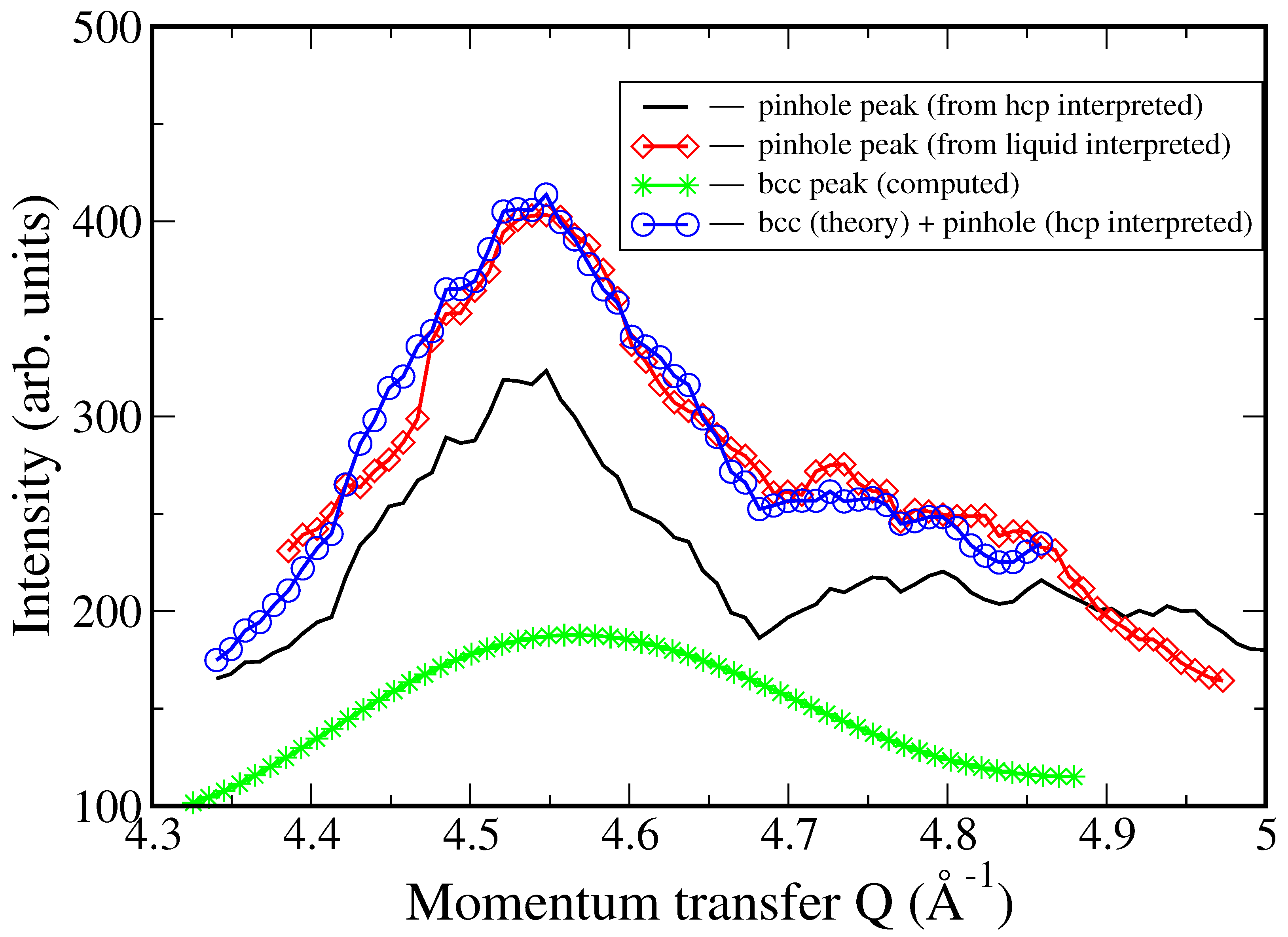
3. Results
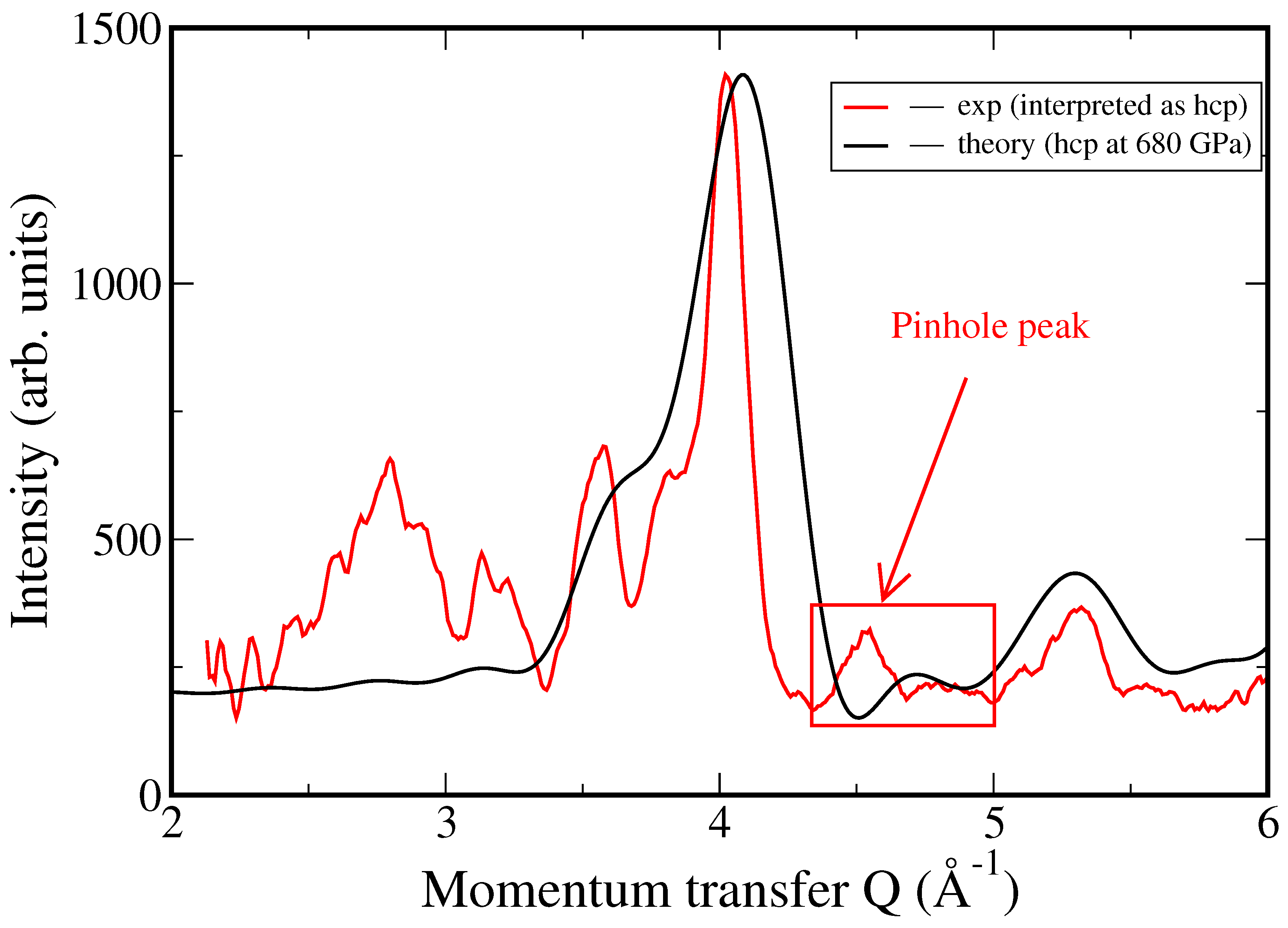
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, I.P. Publications du Bureau Central séIsmologique Internationale. Série A: Travaux Scientifiques; International Council of Scientific Unions: Paris, France, 1936; Volume 14, pp. 87–115. [Google Scholar]
- Birch, F. Elasticity and constitution of the Earth’s interior. In Elastic Properties and Equations of State; American Geophysical Union: Washington, DC, USA, 1988; pp. 31–90. [Google Scholar]
- Al’tshuler, L.V.; Krupnikov, K.K.; Ledenev, B.N.; Zhuchikhin, V.I.; Brazhnik, M.I. Dynamic Compressibility and Equation of State of Iron under High Pressure. J. Exp. Theor. Phys. 1958, 7, 606–614. [Google Scholar]
- Dziewonski, A.M.; Anderson, D.L. Preliminary reference Earth model. Phys. Earth Planet. Inter. 1981, 25, 297–356. [Google Scholar] [CrossRef]
- Hemley, R.J.; Mao, H.K. In Situ Studies of Iron under Pressure: New Windows on the Earth’s Core. Int. Geol. Rev. 2001, 43, 1–30. [Google Scholar]
- Belonoshko, A.B.; Ahuja, R.; Johansson, B. Stability of the body-centred-cubic phase of iron in the Earth’s inner core. Nature 2003, 424, 1032–1034. [Google Scholar] [CrossRef]
- Belonoshko, A.B.; Skorodumova, N.V.; Davis, S.; Osiptsov, A.N.; Rosengren, A.; Johansson, B. Origin of the low rigidity of the Earth’s inner core. Science 2007, 316, 1603–1605. [Google Scholar] [CrossRef]
- Belonoshko, A.B.; Skorodumova, N.V.; Rosengren, A.; Johansson, B. Elastic anisotropy of Earth’s inner core. Science 2008, 319, 797–800. [Google Scholar] [CrossRef]
- Belonoshko, A.B.; Lukinov, T.; Fu, J.; Zhao, J.; Davis, S.; Simak, S.I. Stabilization of body-centred cubic iron under inner-core conditions. Nat. Geosci. 2017, 10, 312–316. [Google Scholar] [CrossRef]
- Belonoshko, A.B.; Fu, J.; Bryk, T.; Simak, S.I.; Mattesini, M. Low viscosity of the Earth’s inner core. Nat. Commun. 2019, 10, 2483. [Google Scholar] [CrossRef]
- Belonoshko, A.B.; Fu, J.; Smirnov, G. Free energies of iron phases at high pressure and temperature: Molecular dynamics study. Phys. Rev. B 2021, 104, 104103. [Google Scholar] [CrossRef]
- Belonoshko, A.B.; Simak, S.I.; Olovsson, W.; Vekilova, O.Y. Elastic properties of body-centered cubic iron in Earth’s inner core. Phys. Rev. B 2022, 105, L180102. [Google Scholar] [CrossRef]
- Mattesini, M.; Belonoshko, A.B.; Buforn, E.; Ramírez, M.; Simak, S.I.; Udías, A.; Mao, H.K.; Ahuja, R. Hemispherical anisotropic patterns of the Earth’s inner core. Proc. Natl. Acad. Sci. USA 2010, 107, 9507–9512. [Google Scholar] [CrossRef] [PubMed]
- Tkalcic, H. The Earth’s Inner Core; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Stephenson, J.; Tkalčić, H.; Sambridge, M. Evidence for the innermost inner core: Robust parameter search for radially varying anisotropy using the neighborhood algorithm. J. Geophys. Res. Solid Earth 2021, 126, e2020JB020545. [Google Scholar] [CrossRef]
- Mattesini, M.; Belonoshko, A.B.; Tkalčić, H.; Buforn, E.; Udías, A.; Ahuja, R. Candy Wrapper for the Earth’s inner core. Sci. Rep. 2013, 3, 2096. [Google Scholar] [CrossRef] [PubMed]
- Modak, P.; Verma, A.K.; Rao, R.S.; Godwal, B.K.; Stixrude, L.; Jeanloz, R. Stability of the hcp phase and temperature variation of the axial ratio of iron near Earth-core conditions. J. Phys. Condens. Matter 2007, 19, 016208. [Google Scholar] [CrossRef]
- Stixrude, L. Structure of iron to 1 gbar and 40,000 K. Phys. Rev. Lett. 2012, 108, 055505. [Google Scholar] [CrossRef] [PubMed]
- Godwal, B.K.; González-Cataldo, F.; Verma, A.K.; Stixrude, L.; Jeanloz, R. Stability of iron crystal structures at 0.3–1.5 TPa. Earth Planet. Sci. Lett. 2015, 409, 299–306. [Google Scholar] [CrossRef]
- Vocadlo, L.; Alfè, D.; Gillan, M.J.; Wood, I.G.; Brodholt, J.P.; Price, G.D. Possible thermal and chemical stabilization of body-centred-cubic iron in the Earth’s core. Nature 2003, 424, 536–539. [Google Scholar] [CrossRef]
- Sun, Y.; Mendelev, M.I.; Zhang, F.; Liu, X.; Da, B.; Wang, C.Z.; Wentzcovitch, R.M.; Ho, K.M. Ab initio melting temperatures of bcc and hcp iron under the earth’s inner core condition. Geophys. Res. Lett. 2023, 50, e2022GL102447. [Google Scholar] [CrossRef]
- Nguyen, J.H.; Holmes, N.C. Melting of iron at the physical conditions of the Earth’s core. Nature 2004, 427, 339–342. [Google Scholar] [CrossRef]
- Anzellini, S.; Errandonea, D.; Burakovsky, L.; Proctor, J.E.; Turnbull, R.; Beavers, C.M. Characterization of the high-pressure and high-temperature phase diagram and equation of state of chromium. Sci. Rep. 2022, 12, 6727. [Google Scholar] [CrossRef]
- Tateno, S.; Hirose, K.; Ohishi, Y.; Tatsumi, Y. The structure of iron in Earth’s inner core. Science 2010, 330, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Turneaure, S.J.; Sharma, S.M.; Gupta, Y.M. Crystal structure and melting of Fe shock compressed to 273 GPa: In situ X-ray diffraction. Phys. Rev. Lett. 2020, 125, 215702. [Google Scholar] [CrossRef] [PubMed]
- Merkel, S.; Hok, S.; Bolme, C.; Rittman, D.; Ramos, K.J.; Morrow, B.; Lee, H.J.; Nagler, B.; Galtier, E.; Granados, E.; et al. Femtosecond visualization of hcp-iron strength and plasticity under shock compression. Phys. Rev. Lett. 2021, 127, 205501. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Ohtani, E.; Hirao, N.; Ohishi, Y. Stability field of the hcp-structure for Fe, Fe-Ni, and Fe-Ni-Si alloys up to 3 Mbar. Geophys. Res. Lett. 2011, 38, L09302. [Google Scholar] [CrossRef]
- Ping, Y.; Coppari, F.; Hicks, D.G.; Yaakobi, B.; Fratanduono, D.E.; Hamel, S.; Eggert, J.H.; Rygg, J.R.; Smith, R.F.; Swift, D.C.; et al. Solid iron compressed up to 560 GPa. Phys. Rev. Lett. 2013, 111, 065501. [Google Scholar] [CrossRef]
- Kraus, R.G.; Hemley, R.J.; Ali, S.J.; Belof, J.L.; Benedict, L.X.; Bernier, J.; Braun, D.; Cohen, R.E.; Collins, G.W.; Coppari, F.; et al. Measuring the melting curve of iron at super-Earth core conditions. Science 2022, 375, 202–205. [Google Scholar] [CrossRef]
- Hrubiak, R.; Meng, Y.; Shen, G. Experimental evidence of a body centered cubic iron at the Earth’s core condition. arXiv 2018, arXiv:1804.05109. [Google Scholar]
- Brown, J.M.; McQueen, R.G. Phase transitions, Grüneisen parameter, and elasticity for shocked iron between 77 GPa and 400 GPa. J. Geophys. Res. 1986, 91, 7485–7494. [Google Scholar] [CrossRef]
- Brown, M.J. The equation of state of iron to 450 GPa: Another high pressure solid phase? Geophys. Res. Lett. 2001, 28, 4339–4342. [Google Scholar] [CrossRef]
- Dubrovinsky, L.; Dubrovinskaia, N.; Narygina, O.; Kantor, I.; Kuznetzov, A.; Prakapenka, V.B.; Vitos, L.; Johansson, B.; Mikhaylushkin, A.S.; Simak, S.I.; et al. Body-centered cubic iron-nickel alloy in Earth’s core. Science 2007, 316, 1880–1883. [Google Scholar] [CrossRef]
- Mikhaylushkin, A.S.; Simak, S.I.; Dubrovinsky, L.; Dubrovinskaia, N.; Johansson, B.; Abrikosov, I.A. Pure iron compressed and heated to extreme conditions. Phys. Rev. Lett. 2007, 99, 165505. [Google Scholar] [CrossRef] [PubMed]
- Belonoshko, A.B.; Derlet, P.M.; Mikhaylushkin, A.S.; Simak, S.I.; Hellman, O.; Burakovsky, L.; Swift, D.C.; Johansson, B. Quenching of bcc-Fe from high to room temperature at high-pressure conditions: A molecular dynamics simulation. New J. Phys. 2009, 11, 093039. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Mermin, N.D. Thermal properties of the inhomogeneous electron gas. Phys. Rev. 1965, 137, A1441–A1443. [Google Scholar] [CrossRef]
- Brehm, M.; Kirchner, B. TRAVIS—A free analyzer and visualizer for Monte Carlo and molecular dynamics trajectories. J. Chem. Inf. Model. 2011, 51, 2007–2023. [Google Scholar] [CrossRef]
- Brehm, M.; Thomas, M.; Gehrke, S.; Kirchner, B. TRAVIS-A free analyzer for trajectories from molecular simulation. J. Chem. Phys. 2020, 152, 164105. [Google Scholar] [CrossRef]
- Boehler, R. Temperatures in the Earth’s core from melting-point measurements of iron at high static pressures. Nature 1993, 363, 534–536. [Google Scholar] [CrossRef]
- Gambino, D.; Klarbring, J.; Alling, B. Phase stability of Fe from first principles: Atomistic spin dynamics coupled with ab initio molecular dynamics simulations and thermodynamic integration. Phys. Rev. B 2023, 107, 014102. [Google Scholar] [CrossRef]
- Ruban, A.V.; Belonoshko, A.B.; Skorodumova, N.V. Impact of magnetism on Fe under Earth’s core conditions. Phys. Rev. B 2013, 87, 014405. [Google Scholar] [CrossRef]
- Qiu, G.; Cai, Y.; Li, Z. Multiscale investigation of magnetic field distortion on surface of ferromagnetic materials caused by stress concentration for metal magnetic memory method. Comput. Mater. Sci. 2022, 209, 111353. [Google Scholar] [CrossRef]
- Grimvall, G. Polymorphism of Metals. III. Theory of the Temperature-Pressure Phase Diagram of Iron. Phys. Scr. 1976, 13, 59–64. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belonoshko, A.B.; Smirnov, G.S. A Comparison of Experimental and Ab Initio Structural Data on Fe under Extreme Conditions. Metals 2023, 13, 1096. https://doi.org/10.3390/met13061096
Belonoshko AB, Smirnov GS. A Comparison of Experimental and Ab Initio Structural Data on Fe under Extreme Conditions. Metals. 2023; 13(6):1096. https://doi.org/10.3390/met13061096
Chicago/Turabian StyleBelonoshko, Anatoly B., and Grigory S. Smirnov. 2023. "A Comparison of Experimental and Ab Initio Structural Data on Fe under Extreme Conditions" Metals 13, no. 6: 1096. https://doi.org/10.3390/met13061096
APA StyleBelonoshko, A. B., & Smirnov, G. S. (2023). A Comparison of Experimental and Ab Initio Structural Data on Fe under Extreme Conditions. Metals, 13(6), 1096. https://doi.org/10.3390/met13061096







