Grain Refinement of Aluminum and Aluminum Alloys by Sc and Zr
Abstract
1. Introduction
2. The Mechanism of Grain Refinement
2.1. Constitutional Supercooling
2.2. Heterogeneous Nucleation
2.2.1. Lattice Matching Principle
2.2.2. Free Growth Model
3. Al-M Master Alloy
3.1. Al-Sc
3.2. Al-Zr
3.3. Al-Er
3.4. Al-Sc-Zr
4. Prospects
- (1)
- The refinement mechanism of Al3Sc needs further analysis. At present, the understanding of the refinement mechanism is still in the crystal structure, lattice constant and lattice mismatch. With the help of E2E model, it is helpful to explore the crystallographic relationship between Al3Sc and α-Al and to improve the refinement theory. Sc is a eutectic reaction type element, and the primary Al3Sc phase in the eutectic structure during the solidification process is isolated at the grain boundary. The influence of the size, distribution and number density of the primary phase on the refining effect is still unclear.
- (2)
- The primary Al3Zr phase will have different morphologies when the melting conditions are different. The law and mechanism of the influence of morphologies on the refining effect need to be explored using experiments. The size and distribution of the primary phase are also factors to be considered.
- (3)
- The analysis of the strength of the grain refinement effect of Sc and Zr elements can provide data in support of the optimization of the refinement effect and the design of ternary alloy composition.
- (4)
- When the pairwise addition of Sc and Zr elements to prepare ternary master alloys is performed, the primary phase type of the master alloys is affected by the mass ratio of Sc and Zr, and whether the morphology and distribution will have the same law. Whether the composition ratio of Sc and Zr is also one of the factors affecting the type, morphology and distribution of primary phase is still to be discussed. In addition, if the type and morphology of primary phase change, will the refinement mechanism also change?
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McCartney, D.G. Grain refining of aluminium and its alloys using inoculants. Int. Mater. Rev. 1989, 34, 247–260. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 319–324. [Google Scholar] [CrossRef]
- Nowak, M.; Bolzoni, L.; Hari Babu, N. Grain refinement of Al–Si alloys by Nb–B inoculation. Part I: Concept development and effect on binary alloys. Mater. Des. 2015, 66, 366–375. [Google Scholar] [CrossRef]
- Langdon, T.G. Twenty-five years of ultrafine-grained materials: Achieving exceptional properties through grain refinement. Acta Mater. 2013, 61, 7035–7059. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Baharvandi, H.R.; Shirvanimoghaddam, K. Tensile and fracture behavior of nano/micro TiB2 particle reinforced casting A356 aluminum alloy composites. Mater. Des. 2015, 66, 150–161. [Google Scholar] [CrossRef]
- Wang, M.; Chen, D.; Chen, Z.; Wu, Y.; Wang, F.; Ma, N.; Wang, H. Mechanical properties of in-situ TiB2/A356 composites. Mater. Sci. Eng. A 2014, 590, 246–254. [Google Scholar] [CrossRef]
- Zhao, K.; Gao, T.; Yang, H.; Hu, K.; Liu, G.; Sun, Q.; Nie, J.; Liu, X. Enhanced grain refinement and mechanical properties of a high–strength Al–Zn–Mg–Cu–Zr alloy induced by TiC nano-particles. Mater. Sci. Eng. A 2021, 806, 140852. [Google Scholar] [CrossRef]
- Ali, Y.; Qiu, D.; Jiang, B.; Pan, F.; Zhang, M.X. Current research progress in grain refinement of cast magnesium alloys: A review article. J. Alloys Compd. 2015, 619, 639–651. [Google Scholar] [CrossRef]
- Wannasin, J.; Canyook, R.; Wisutmethangoon, S.; Flemings, M.C. Grain refinement behavior of an aluminum alloy by inoculation and dynamic nucleation. Acta Mater. 2013, 61, 3897–3903. [Google Scholar] [CrossRef]
- Tamirisakandala, S.; Bhat, R.B.; Tiley, J.S.; Miracle, D.B. Grain refinement of cast titanium alloys via trace boron addition. Scr. Mater. 2005, 53, 1421–1426. [Google Scholar] [CrossRef]
- Murty, B.S.; Kori, S.A.; Chakraborty, M. Grain refinement of aluminium and its alloys by heterogeneous nucleation and alloying. Int. Mater. Rev. 2002, 47, 3–29. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al–Ti–B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.; Fu, H.; Xu, J.; Fu, Y.; Li, T. Grain refining potency of Al–B master alloy on pure aluminum. Scr. Mater. 2011, 64, 1121–1124. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M. Accurate orientation relationships between ferrite and cementite in pearlite. Scr. Mater. 1997, 37, 2009–2015. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Easton, M.A.; Taylor, J.A. Crystallographic study of grain refinement in aluminum alloys using the edge-to-edge matching model. Acta Mater. 2005, 53, 1427–1438. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, S.; Easton, M.A.; Zhang, M.; Qiu, D.; Wu, G.; Liu, W.; Ding, W. Heat treatment, microstructure and mechanical properties of a Mg–Gd–Y alloy grain-refined by Al additions. Mater. Sci. Eng. A 2013, 576, 298–305. [Google Scholar] [CrossRef]
- Qiu, D.; Zhang, M.X.; Taylor, J.A.; Kelly, P.M. A new approach to designing a grain refiner for Mg casting alloys and its use in Mg–Y-based alloys. Acta Mater. 2009, 57, 3052–3059. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, L.; Kang, M.; Peng, G.; Li, W.; Miao, S.; Huang, Y.; Zhao, Y.; Xu, Y. Effect of Warm Rolling on the Grain-Refining Performance of Al-5Ti-1B Grain Refiner in Al. JOM 2022, 74, 1210–1217. [Google Scholar] [CrossRef]
- Li, Z.; Hu, B.; Li, D.; Zhang, W.; Zeng, X.; Lin, Z.; Jin, C.; Zhao, S. Microstructure-dependent thermal conductivity and mechanical properties in cast Mg-4Sm-xAl alloys. Mater. Sci. Eng. A 2022, 861, 144336. [Google Scholar] [CrossRef]
- Li, H.-Y.; Li, D.-W.; Zhu, Z.-X.; Chen, B.-A.; Chen, X.; Yang, C.-L.; Zhang, H.-Y.; Kang, W. Grain refinement mechanism of as-cast aluminum by hafnium. Trans. Nonferrous Met. Soc. China 2016, 26, 3059–3069. [Google Scholar] [CrossRef]
- Chuvil’deev, V.N.; Shadrina, I.S.; Nokhrin, A.V.; Kopylov, V.I.; Bobrov, A.A.; Gryaznov, M.Y.; Shotin, S.V.; Tabachkova, N.Y.; Chegurov, M.K.; Melekhin, N.V. An investigation of thermal stability of structure and mechanical properties of Al-0.5Mg–Sc ultrafine-grained aluminum alloys. J. Alloys Compd. 2020, 831, 154805. [Google Scholar] [CrossRef]
- Min, B.K.; Kim, H.W.; Kang, S.B. Effect of Al3Sc precipitate on the microstructural evolution during accumulative roll bonding in Al–0.2wt.% Sc alloy. J. Mater. Process. Technol. 2005, 162, 355–361. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C. Creep resistance of cast and aged Al–0.1Zr and Al–0.1Zr–0.1Ti (at.%) alloys at 300–400 °C. Scr. Mater. 2008, 59, 387–390. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Nucleation and Precipitation Strengthening in Dilute Al-Ti and Al-Zr Alloys. Metall. Mater. Trans. A 2007, 38, 2552–2563. [Google Scholar] [CrossRef]
- Su, N.; Guan, R.; Wang, X.; Wang, Y.; Jiang, W.; Liu, H. Grain refinement in an AlEr alloy during accumulative continuous extrusion forming. J. Alloys Compd. 2016, 680, 283–290. [Google Scholar] [CrossRef]
- Wen, S.P.; Gao, K.Y.; Li, Y.; Huang, H.; Nie, Z.R. Synergetic effect of Er and Zr on the precipitation hardening of Al–Er–Zr alloy. Scr. Mater. 2011, 65, 592–595. [Google Scholar] [CrossRef]
- Wen, S.P.; Xing, Z.B.; Huang, H.; Huang, H.; Nie, Z.R. The effect of erbium on the microstructure and mechanical properties of Al–Mg–Mn–Zr alloy. Mater. Sci. Eng. A 2009, 516, 42–49. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, D.; Liu, Z.-L.; Taylor, J.A.; Easton, M.A.; Zhang, M.-X. The grain refinement mechanism of cast aluminium by zirconium. Acta Mater. 2013, 61, 5636–5645. [Google Scholar] [CrossRef]
- Gao, Z.; Li, H.; Lai, Y.; Ou, Y.; Li, D. Effects of minor Zr and Er on microstructure and mechanical properties of pure aluminum. Mater. Sci. Eng. A 2013, 580, 92–98. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Lin, G.; Wang, X.; Sun, Y.; Wang, X.; Ye, J.; Sun, Y.; Yu, Y.; Jiang, F.; et al. Microstructure and properties of novel Al-Ce-Sc, Al-Ce-Y, Al-Ce-Zr and Al-Ce-Sc-Y alloy conductors processed by die casting, hot extrusion and cold drawing. J. Mater. Sci. Technol. 2020, 58, 155–170. [Google Scholar] [CrossRef]
- Tian, S.; Li, J.; Zhang, J.; Wulabieke, Z.; Lv, D. Effect of Zr and Sc on microstructure and properties of 7136 aluminum alloy. J. Mater. Res. Technol. 2019, 8, 4130–4140. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, F. Effects of Sc or/and Ge addition on microstructure and mechanical properties of as-cast 6016 Al alloy. J. Alloys Compd. 2019, 809, 151829. [Google Scholar] [CrossRef]
- Ocenasek, V.; Slamova, M. Resistance to recrystallization due to Sc and Zr addition to Al–Mg alloys. Mater. Charact. 2001, 47, 157–162. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.L.; Qiu, D.; Taylor, J.A.; Easton, M.A.; Zhang, M.X. The Influence of the Effect of Solute on the Thermodynamic Driving Force on Grain Refinement of Al Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 505–515. [Google Scholar] [CrossRef]
- Chen, Z.w.; He, Z.; Jie, W.q. Growth restriction effects during solidification of aluminium alloys. Trans. Nonferrous Met. Soc. China 2009, 19, 410–413. [Google Scholar] [CrossRef]
- Easton, M.A.; StJohn, D.H. A model of grain refinement incorporating alloy constitution and potency of heterogeneous nucleant particles. Acta Mater. 2001, 49, 1867–1878. [Google Scholar] [CrossRef]
- Greer, A.L.; Bunn, A.M.; Tronche, A.; Evans, P.V.; Bristow, D.J. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al–Ti–B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Minouei, H.; Rizi, M.S.; Akbari, G.; Hong, S.I. Effect of residual nanocrystals on thermal stability and mechanical properties of metalloid-containing amorphous alloys. Mater. Charact. 2021, 173, 110914. [Google Scholar] [CrossRef]
- Bruce, L.B. The effect of carbide and nitride additions on the eterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar]
- Turnbull, D.; Vonnegut, B. Nucleation Catalysis. Ind. Eng. Chem. 1952, 44, 1292–1298. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Ma, Q.; Taylor, J.A. Crystallography of grain refinement in Mg–Al based alloys. Acta Mater. 2005, 53, 3261–3270. [Google Scholar] [CrossRef]
- Xiao, F.; Wu, M.; Wang, Y.; Wang, S.; Shu, D.; Wang, D.; Zhu, G.; Mi, J.; Sun, B. Design of newly effective grain refiner for aluminum based on medium-entropy metal diboride. Vacuum 2022, 205, 111462. [Google Scholar] [CrossRef]
- Li, G.-J.; Liao, H.-C. E2EM’s prediction of LaB6 as nucleation substrate for primary Mn-rich phase in Al-Si-Cu-Mn heat-resistant alloy and its refining effect. Trans. Nonferrous Met. Soc. China 2022, 32, 1795–1804. [Google Scholar] [CrossRef]
- Sunitha, K.; Gurusami, K. Study of Al-Si alloys grain refinement by inoculation. Mater. Today Proc. 2021, 43, 1825–1829. [Google Scholar] [CrossRef]
- Greer, A.L.; Cooper, P.S.; Meredith, M.W.; Schneider, W.; Schumacher, P.; Spittle, J.A.; Tronche, A. Grain refinement of aluminium alloys by inoculation. Adv. Eng. Mater. 2003, 5, 81–91. [Google Scholar] [CrossRef]
- Johnsson, M. Grain refinement of aluminium studied by use of a thermal analytical technique. Thermochim. Acta 1995, 256, 107–121. [Google Scholar] [CrossRef]
- Easton, M.; StJohn, D. An analysis of the relationship between grain size, solute content, and the potency and number density of nucleant particles. Metall. Mater. Trans. A 2005, 36, 1911–1920. [Google Scholar] [CrossRef]
- Jing, L.-J.; Pan, Y.; Lu, T.; Pi, J.-H.; Gu, T.-F. Nucleation potency prediction of LaB6 with E2EM model and its influence on microstructure and tensile properties of Al-7Si-0.3Mg alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 1687–1694. [Google Scholar] [CrossRef]
- Guan, R.G.; Misra, R.D.K.; Shang, Y.; An, Y.; Wang, Y.; Zhang, Y.; Tie, D. Mechanism of microstructural refinement of deformed aluminum under synergistic effect of TiAl3 and TiB2 particles and impact on mechanical properties. Mater. Sci. Eng. A 2018, 716, 129–139. [Google Scholar] [CrossRef]
- Han, Y.; Shu, D.; Wang, J.; Sun, B. Microstructure and grain refining performance of Al-5Ti-1B master alloy prepared under high-intensity ultrasound. Mater. Sci. Eng. A 2006, 430, 326–331. [Google Scholar] [CrossRef]
- Xu, J.; Li, R.X.; Qian, L. Effect of Agglomeration on Nucleation Potency of Inoculant Particles in the Al-Nb-B Master Alloy: Modeling and Experiments. Metall. Mater. Trans. A 2021, 52, 1077–1094. [Google Scholar] [CrossRef]
- Quested, T.E.; Greer, A.L. The effect of the size distribution of inoculant particles on as-cast grain size in aluminium alloys. Acta Mater. 2004, 52, 3859–3868. [Google Scholar] [CrossRef]
- Greer, A.L. Grain refinement of alloys by inoculation of melts. Philos. Trans. R. Soc. Lond. A 2003, 361, 479–495. [Google Scholar] [CrossRef]
- Yu, H.; Wang, N.; Guan, R.; Tie, D.; Li, Z.; An, Y.; Zhang, Y. Evolution of secondary phase particles during deformation of Al-5Ti-1B master alloy and their effect on α-Al grain refinement. J. Mater. Sci. Technol. 2018, 34, 2297–2306. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Criteria for developing castable, creep-resistant aluminum-based alloys—A review. Int. J. Mater. Res. 2006, 97, 246–265. [Google Scholar] [CrossRef]
- Marcantonio, J.; Mondolfo, L. Nucleation of aluminium by several intermetallic compounds. J. Inst. Met. 1970, 98, 23–27. [Google Scholar]
- Norman, A.F.; Prangnell, P.B.; McEwen, R.S. The solidification behaviour of dilute aluminium–scandium alloys. Acta Mater. 1998, 46, 5715–5732. [Google Scholar] [CrossRef]
- Hu, X.W.; Jiang, F.G.; Ai, F.R. Effects of rare earth Er additions on microstructure development and mechanical properties of die-cast ADC12 aluminum alloy. J. Alloys Compd. 2012, 538, 21–27. [Google Scholar] [CrossRef]
- Muhammad, A.; Cong, X.; Wang, X.J. High strength aluminum cast alloy: A Sc modification of a standard Al–Si–Mg cast alloy. Mater. Sci. Eng. A 2014, 16, 122–126. [Google Scholar] [CrossRef]
- Yang, J.J.; Nie, Z.R.; Jin, T.N.; Ruan, H.Q.; Zuo, T.Y. Form and refinement mechanism of element Er in Al-Zn-Mg alloy. Chin. J. Nonferrous Met. 2004, 4, 620–626. (In Chinese) [Google Scholar] [CrossRef]
- Jones, M.J.; Humphreys, F.J. Interaction of recrystallization and precipitation: The effect of Al3Sc on the recrystallization behaviour of deformed aluminium. Acta Mater. 2003, 51, 2149–2159. [Google Scholar] [CrossRef]
- Riddle, Y.W.; Sanders, T.H. A study of coarsening, recrystallization, and morphology of microstructure in Al-Sc-(Zr)-(Mg) alloys. Metall. Mater. Trans. A 2004, 35, 341–350. [Google Scholar] [CrossRef]
- Yin, Z.; Pan, Q.; Zhang, Y.; Jiang, F. Effect of minor Sc and Zr on the microstructure and mechanical properties of Al–Mg based alloys. Mater. Sci. Eng. A 2000, 280, 151–155. [Google Scholar] [CrossRef]
- Meng, Y.; ZHAO, Z.H.; Cui, J.Z. Effect of minor Zr and Sc on microstructures and mechanical properties of Al–Mg–Si–Cu–Cr–V alloys. Trans. Nonferrous Met. Soc. China 2013, 23, 1882–1889. [Google Scholar] [CrossRef]
- Yan, K.; Chen, Z.W.; Zhao, Y.N.; Ren, C.C.; Lu, W.J.; Aldeen, A.W. Morphological characteristics of Al3Sc particles and crystallographic orientation relationships of Al3Sc/Al interface in cast Al-Sc alloy. J. Alloys Compd. 2021, 861, 158491. [Google Scholar] [CrossRef]
- Kelly, P.; Zhang, M.-X. Edge-to-edge matching—The fundamentals. Metall. Mater. Trans. A 2006, 37, 833–839. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M. Edge-to-edge matching and its applications: Part II. Application to Mg–Al, Mg–Y and Mg–Mn alloys. Acta Mater. 2005, 53, 1085–1096. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M. Edge-to-edge matching and its applications: Part I. Application to the simple HCP/BCC system. Acta Mater. 2005, 53, 1073–1084. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, Q.; Luo, Y.; Liu, Y.; Sun, Y.; Long, L.; Li, M.; Wang, X.; Liu, S. Study on the primary Al3Sc phase and the structure heredity of Al-Zn-Mg-Cu-Sc-Zr alloy. Mater. Charact. 2020, 169, 110601. [Google Scholar] [CrossRef]
- Hyde, K.B.; Norman, A.F.; Prangnell, P.B. The effect of cooling rate on the morphology of primary Al3Sc intermetallic particles in Al–Sc alloys. Acta Mater. 2001, 49, 1327–1337. [Google Scholar] [CrossRef]
- Qian, M.; Stjohn, D.H. Grain nucleation and formation in Mg–Zr alloys. Int. J. Cast Met. Res. 2009, 22, 256–259. [Google Scholar] [CrossRef]
- Qian, M.; Das, A. Grain refinement of magnesium alloys by zirconium: Formation of equiaxed grains. Scr. Mater. 2006, 54, 881–886. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Zr (aluminum-zirconium). J. Phase Equilibria 2002, 23, 455–456. [Google Scholar] [CrossRef]
- Villars, P.; Calvert, L.D. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases; ASM: Materials Park, OH, USA, 1991. [Google Scholar]
- Han, Y.; Ke, L.; Wang, J.; Da, S.; Sun, B. Influence of high-intensity ultrasound on grain refining performance of Al–5Ti–1B master alloy on aluminium. Mater. Sci. Eng. A 2005, 405, 306–312. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Chen, G.; Cheng, X. Effects of molten temperature on the morphologies of in situ Al3Zr and ZrB2 particles and wear properties of (Al3Zr+ZrB2)/Al composites. Mater. Sci. Eng. A 2007, 457, 156–161. [Google Scholar] [CrossRef]
- Nie, Z.R.; Wen, S.P.; Huang, H.; Li, B.L.; Zuo, T.Y. Research progress of Er-containing aluminum alloy. Chin. J. Nonferrous Met. 2011, 21, 2361–2370. (In Chinese) [Google Scholar] [CrossRef]
- Nie, Z.R.; Jin, T.N.; Zou, J.X.; Fu, J.B.; Yang, J.J.; Zuo, T.Y. Development on research of advanced rare-earth aluminum alloy. Trans. Nonferrous Met. Soc. China 2003, 13, 509–514. [Google Scholar]
- Bai, S.; Liu, Z.; Li, Y.; Hou, Y.; Chen, X. Microstructures and fatigue fracture behavior of an Al–Cu–Mg–Ag alloy with addition of rare earth Er. Mater. Sci. Eng. A 2010, 527, 1806–1814. [Google Scholar] [CrossRef]
- Lu, S.; Yin, D.; Zhao, Y.C.; Liu, C.; Zhao, M.C.; Yu, Z.; Wang, H.; Atrens, A. Evolution of microstructure and texture for an Al-0.4 Er alloy during accumulative roll bonding. J. Alloys Compd. 2019, 811, 152005. [Google Scholar] [CrossRef]
- Yang, J.J.; Nie, Z.R.; Jin, T.N.; Xu, G.F.; Fu, J.B.; Ruan, H.Q.; Zuo, T.Y. Effect of trace rare earth element Er on high pure Al. Trans. Nonferrous Met. Soc. China 2003, 5, 1035–1039. [Google Scholar]
- Mondolfo, L.F. Al–Er Aluminum–Erbium system. In Aluminum Alloys; Butterworth-Heinemann: Oxford, UK, 1976; p. 280. [Google Scholar] [CrossRef]
- Zou, L.; Pan, Q.-L.; He, Y.-B.; Wang, C.-Z.; Liang, W.-J. Effect of minor Sc and Zr addition on microstructures and mechanical properties of Al-Zn-Mg-Cu alloys. Trans. Nonferrous Met. Soc. China 2007, 17, 340–345. [Google Scholar] [CrossRef]
- Esmaeili Ghayoumabadi, M.; Mochugovskiy, A.G.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. The influence of minor additions of Y, Sc, and Zr on the microstructural evolution, superplastic behavior, and mechanical properties of AA6013 alloy. J. Alloys Compd. 2022, 900, 163477. [Google Scholar] [CrossRef]
- Knipling, K.E.; Seidman, D.N.; Dunand, D.C. Ambient- and high-temperature mechanical properties of isochronally aged Al–0.06Sc, Al–0.06Zr and Al–0.06Sc–0.06Zr (at.%) alloys. Acta Mater. 2011, 59, 943–954. [Google Scholar] [CrossRef]
- Lee, S.; Utsunomiya, A.; Akamatsu, H.; Neishi, K.; Furukawa, M.; Horita, Z.; Langdon, T.G. Influence of scandium and zirconium on grain stability and superplastic ductilities in ultrafine-grained Al–Mg alloys. Acta Mater. 2002, 50, 553–564. [Google Scholar] [CrossRef]
- Yuzbekova, D.; Mogucheva, A.; Kaibyshev, R. Superplasticity of ultrafine-grained Al–Mg–Sc–Zr alloy. Mater. Sci. Eng. A 2016, 675, 228–242. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wu, R.; Sun, J.; Jiao, Y.; Hou, L.; Zhang, J.; Li, X.; Zhang, M. Ambient-temperature mechanical properties of isochronally aged 1420-Sc-Zr aluminum alloy. Mater. Sci. Eng. A 2019, 745, 411–419. [Google Scholar] [CrossRef]
- Kuang, Q.; Wang, R.; Peng, C.; Cai, Z. Comparison of microstructure and mechanical properties of Al-Mg-Li-Sc-Zr alloys processed by ingot metallurgy and rapid solidification. J. Alloys Compd. 2021, 883, 160937. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Wu, G.; Sun, J.; Rong, M.; Hsieh, C.-C.; Yu, Y. Influence of Sc content on the microstructure and mechanical properties of cast Al-2Li-2Cu-0.5Mg-0.2Zr alloy. Mater. Charact. 2018, 142, 223–236. [Google Scholar] [CrossRef]
- Fuller, C.B.; Seidman, D.N.; Dunand, D.C. Mechanical properties of Al(Sc,Zr) alloys at ambient and elevated temperatures. Acta Mater. 2003, 51, 4803–4814. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Xu, J.; Luo, Q.; Jiang, Y.; Xiao, Q.; Li, Q. Enhanced grain refinement of Al-Si alloys by novel Al-V-B refiners. J. Mater. Sci. Technol. 2021, 94, 104–112. [Google Scholar] [CrossRef]

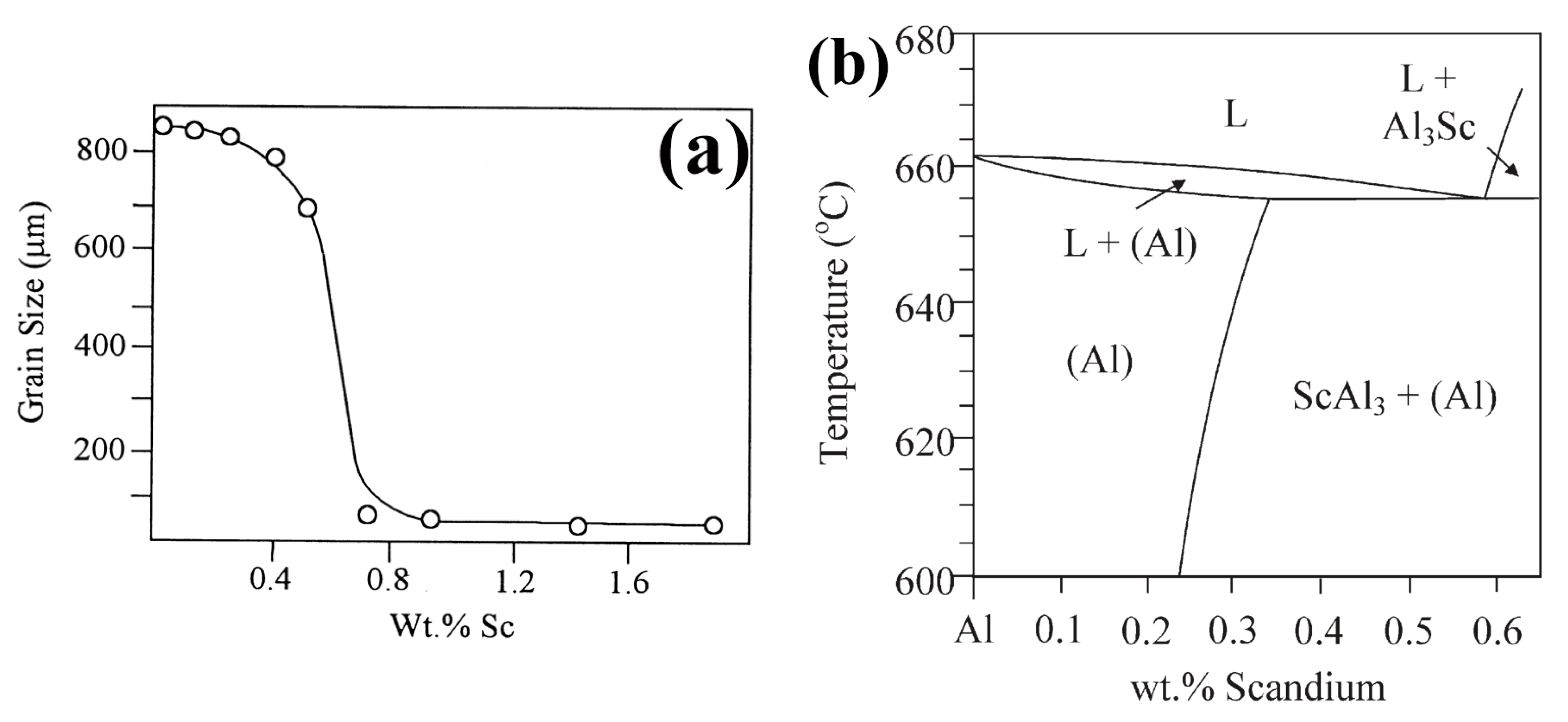
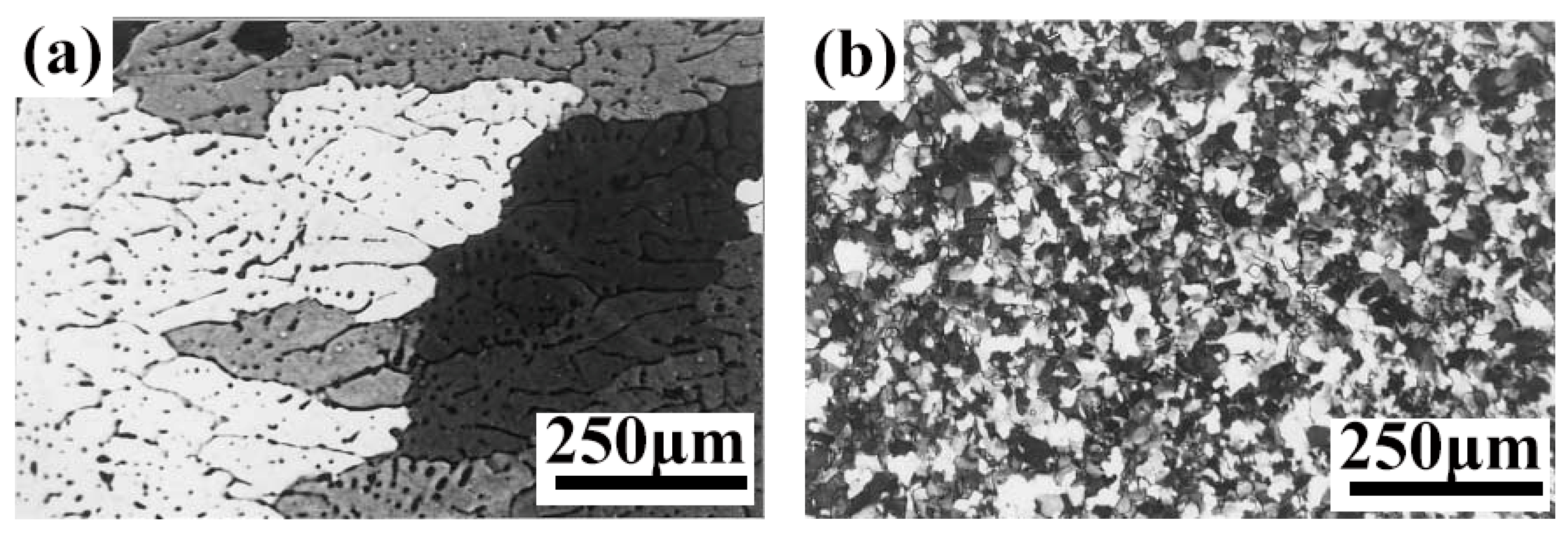
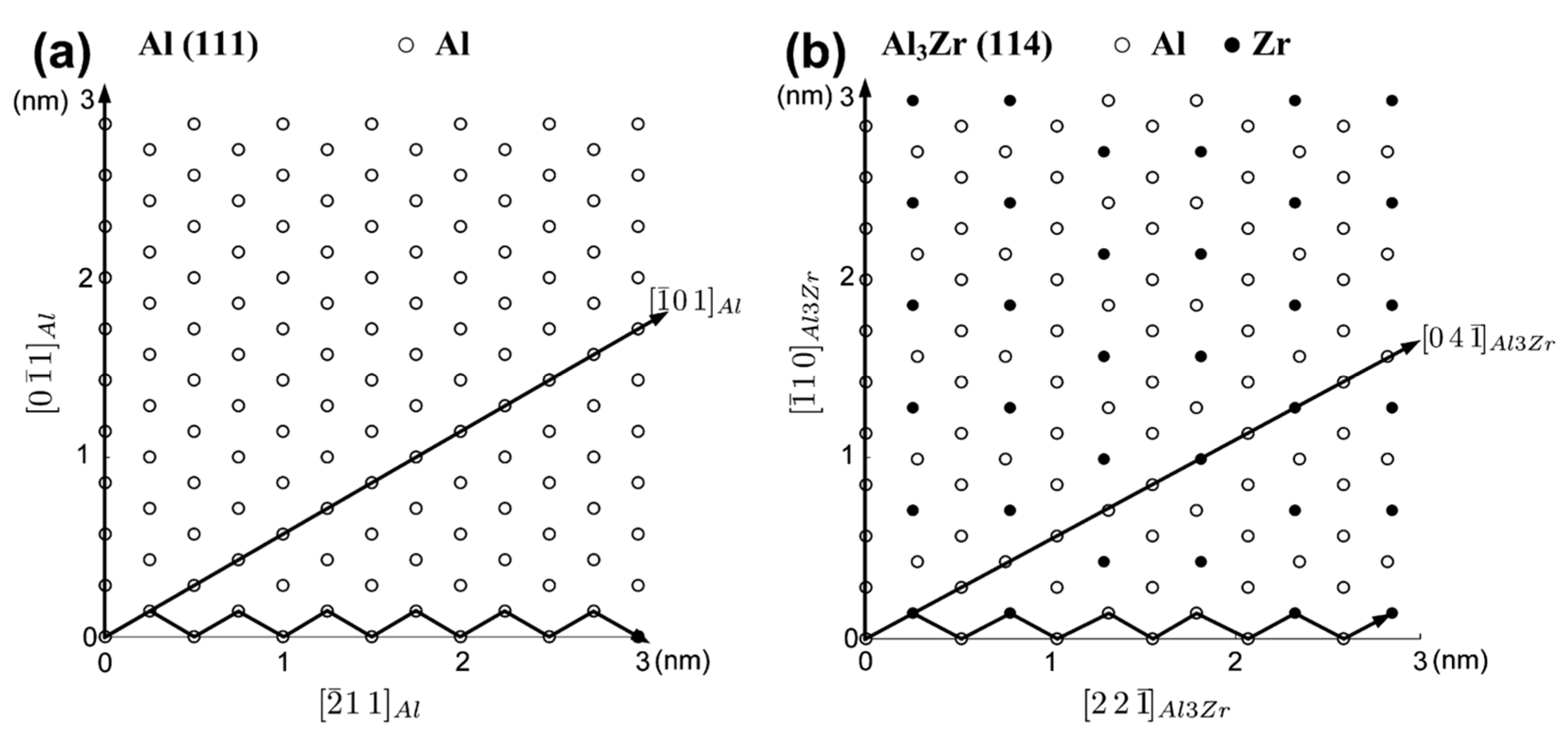
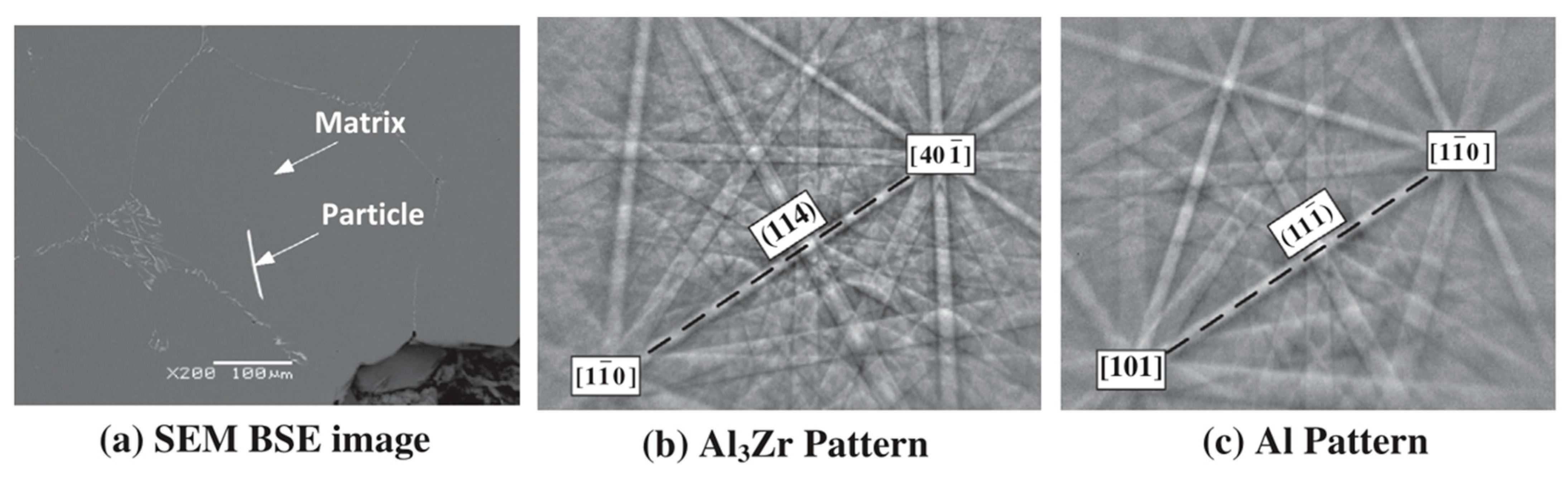
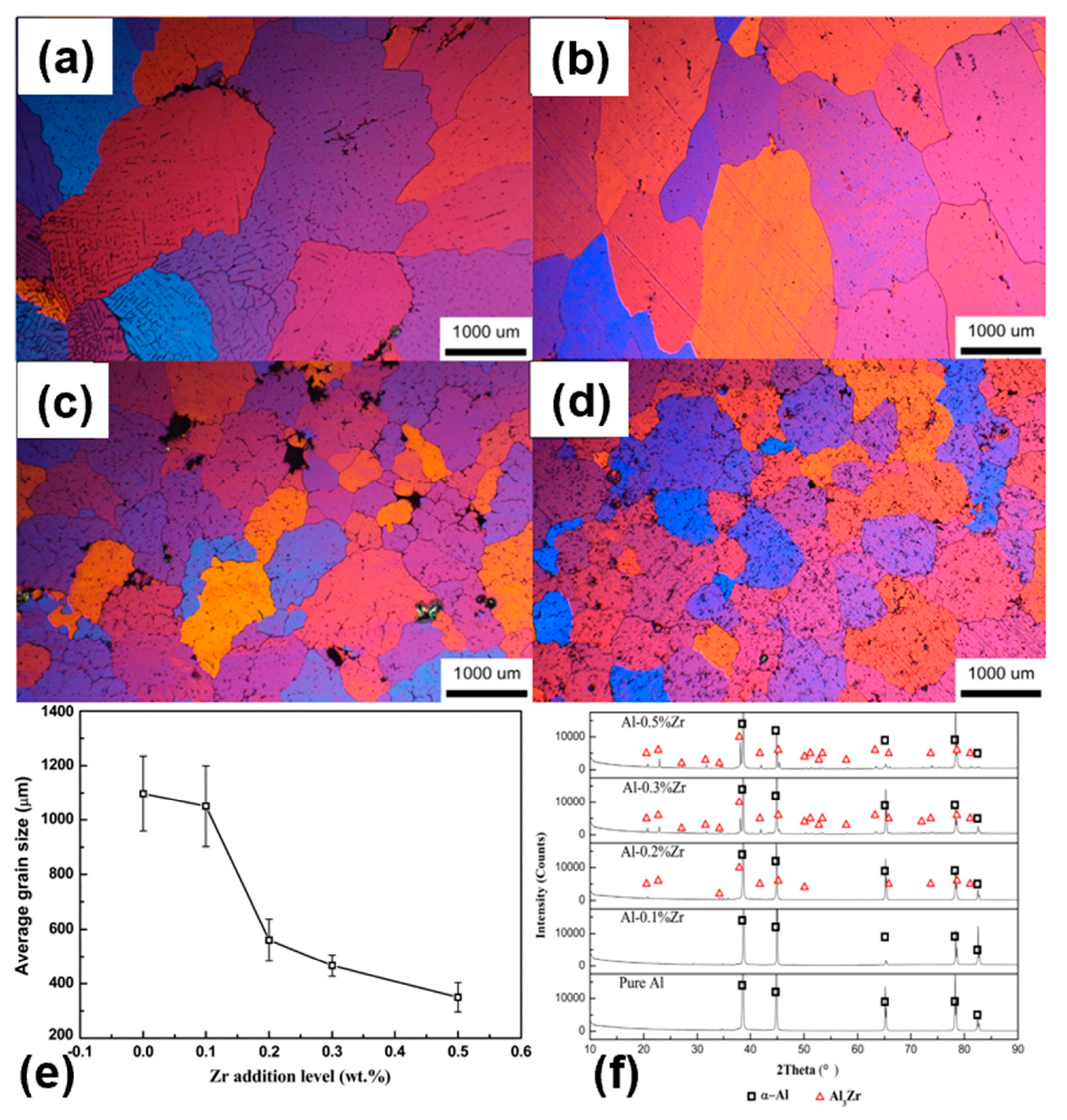
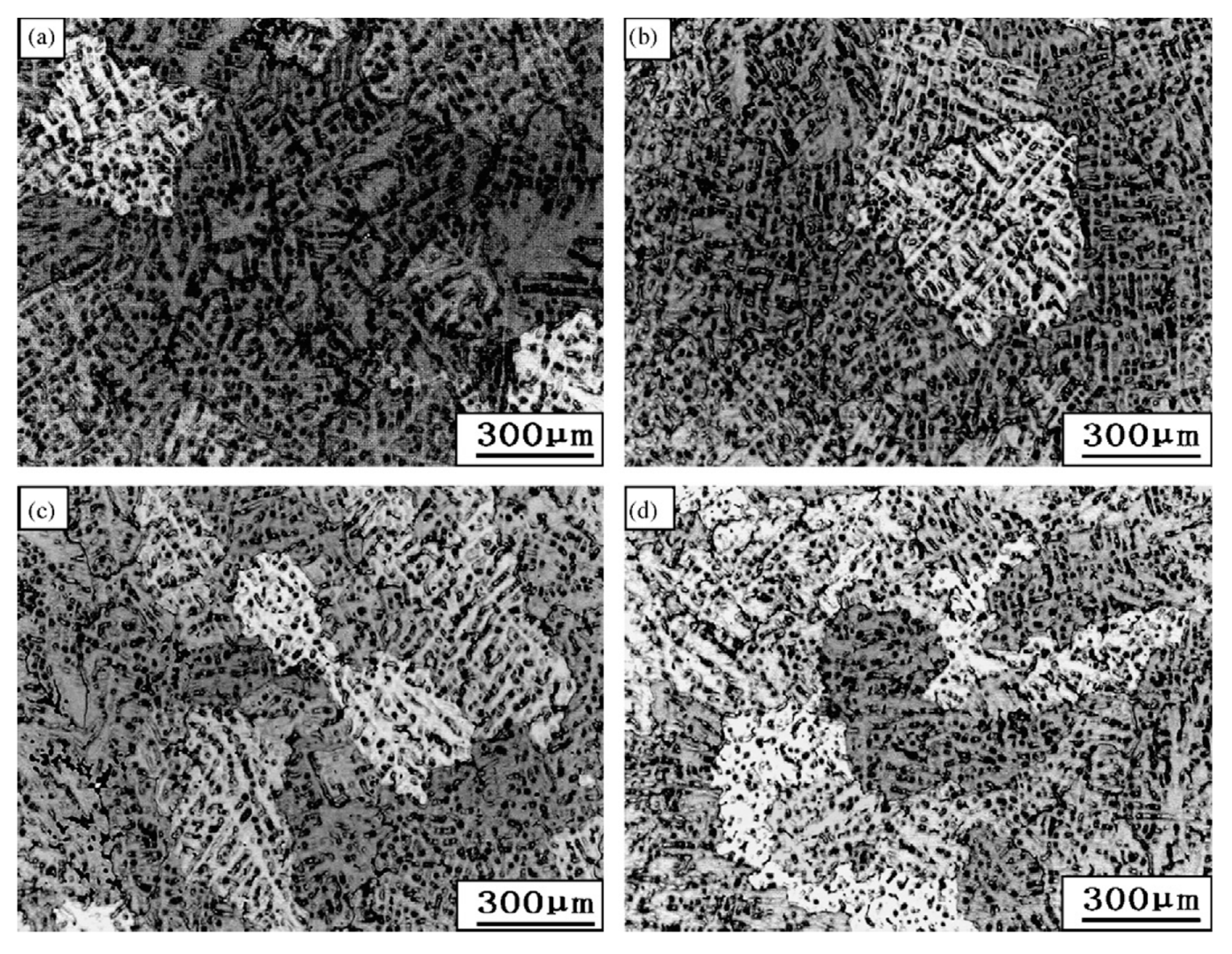
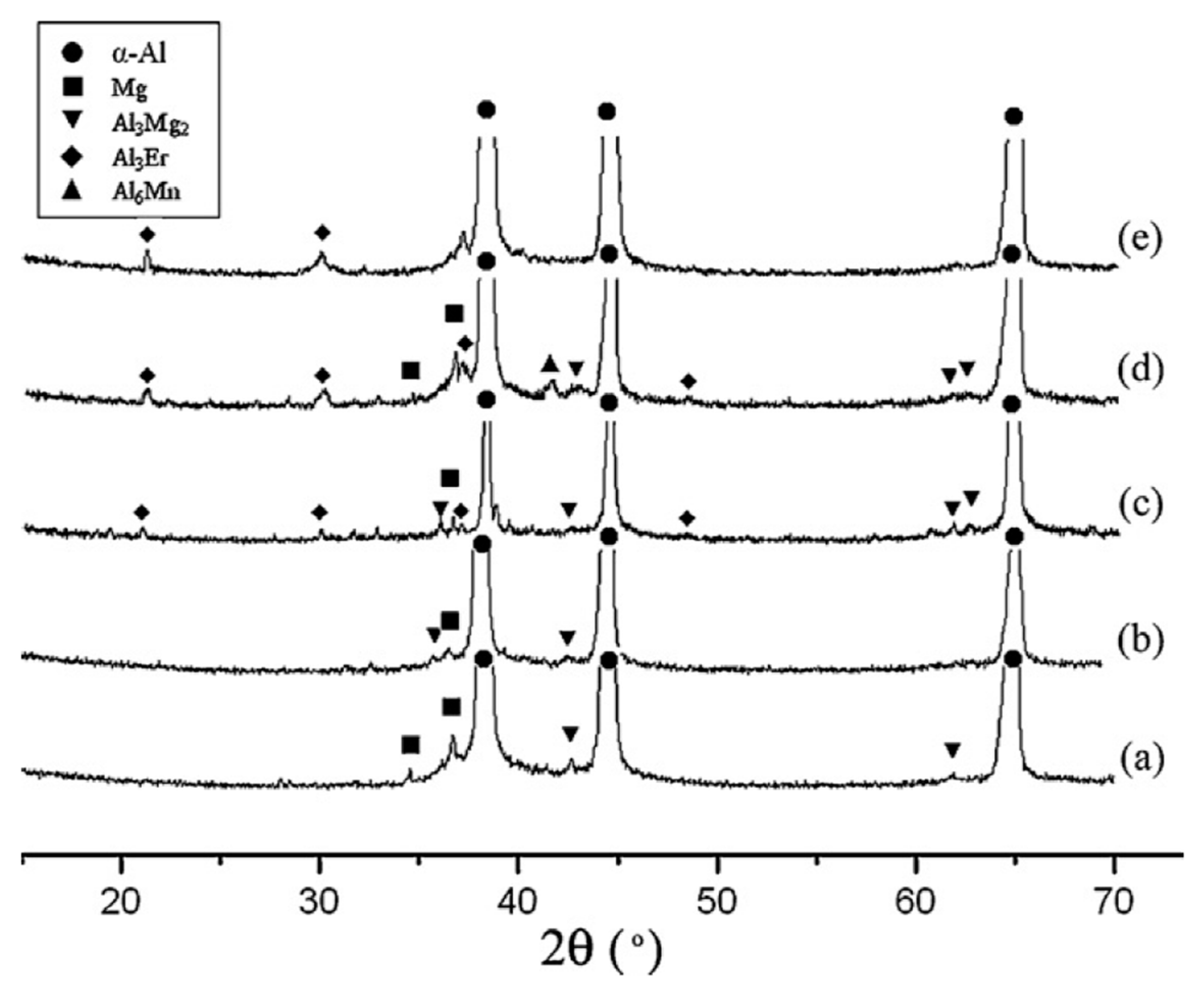
| Element | System | (K/wt.%) | K | (wt.%) | (K/wt.%) |
|---|---|---|---|---|---|
| Cu | eutectic | −3.4 | 0.17 | 33.2 | 2.8 |
| Ni | eutectic | −3.3 | 0.007 | 6 | 3.3 |
| Mg | eutectic | −6.2 | 0.51 | 3.4 | 3.0 |
| Mn | eutectic | −1.6 | 0.94 | 1.9 | 0.1 |
| Si | eutectic | −6.6 | 0.11 | 12.6 | 5.9 |
| Fe | eutectic | −3.0 | 0.02 | 1.8 | 2.9 |
| Er | eutectic | −0.91 | 0.03 | 6 | 0.88 |
| Sc | eutectic | −9.4 | 0.59 | 0.58 | 3.90 |
| Ti | peritectic | 7.8 | 33.3 | 0.15 | 220 |
| Cr | peritectic | 3.5 | 2.0 | 0.4 | 3.5 |
| Ta | peritectic | 70.0 | 7.8 | 0.10 | 105 |
| V | peritectic | 10.0 | 4.0 | 0.1 | 30.0 |
| Hf | peritectic | 8.0 | 2.4 | 0.5 | 11.2 |
| Mo | peritectic | 5.0 | 2.5 | 0.1 | 7.5 |
| Nb | peritectic | 13.3 | 1.5 | 0.15 | 6.6 |
| Zr | peritectic | 4.5 | 2.5 | 0.11 | 6.8 |
| Matching Crystal Direction | Matching Crystal Plane | ||
|---|---|---|---|
| 1% | 1% | ||
| 17% | |||
| 65% | |||
| 2% | 15% | ||
| 1% | |||
| 42% | |||
| 4% | 40% | ||
| 29% | |||
| 1% |
| Matching Crystal Direction | Matching Crystal Plane | ||
|---|---|---|---|
| 1% | 1% | ||
| 1% | |||
| 2% | 1% | ||
| 1% | |||
| 4% | 1% | ||
| 1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Z.; Wen, S.; Huang, H.; Wei, W.; Nie, Z. Grain Refinement of Aluminum and Aluminum Alloys by Sc and Zr. Metals 2023, 13, 751. https://doi.org/10.3390/met13040751
Lei Z, Wen S, Huang H, Wei W, Nie Z. Grain Refinement of Aluminum and Aluminum Alloys by Sc and Zr. Metals. 2023; 13(4):751. https://doi.org/10.3390/met13040751
Chicago/Turabian StyleLei, Zhiguo, Shengping Wen, Hui Huang, Wu Wei, and Zuoren Nie. 2023. "Grain Refinement of Aluminum and Aluminum Alloys by Sc and Zr" Metals 13, no. 4: 751. https://doi.org/10.3390/met13040751
APA StyleLei, Z., Wen, S., Huang, H., Wei, W., & Nie, Z. (2023). Grain Refinement of Aluminum and Aluminum Alloys by Sc and Zr. Metals, 13(4), 751. https://doi.org/10.3390/met13040751







