Effect of Slag Basicity on Non-Metallic Inclusions and Cleanliness of 15-5PH Stainless Steel
Abstract
1. Introduction
2. Experimental Method
3. Results and Discussion
3.1. Characterization of Inclusions
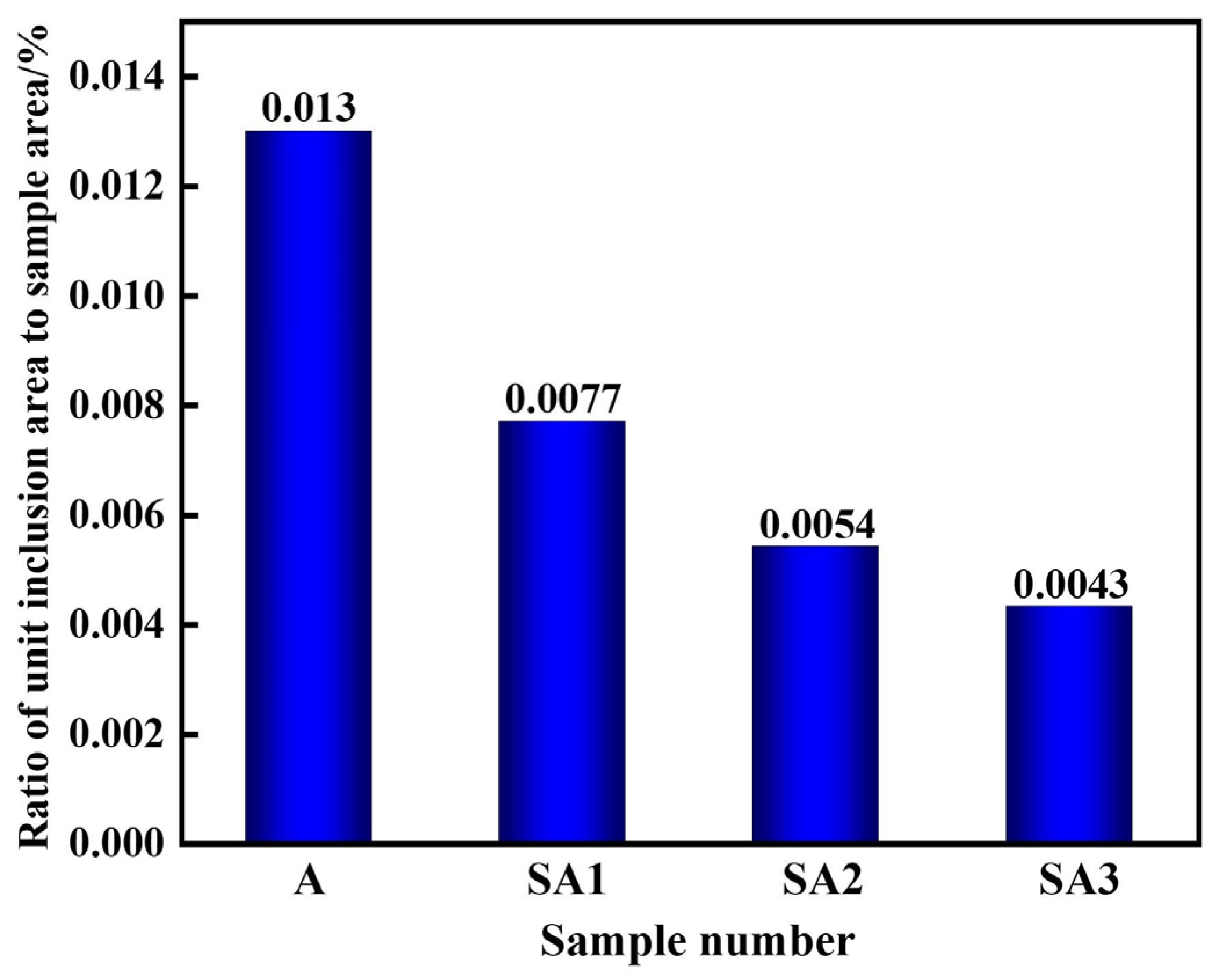
3.2. Sizes of the Inclusions and Their Densities
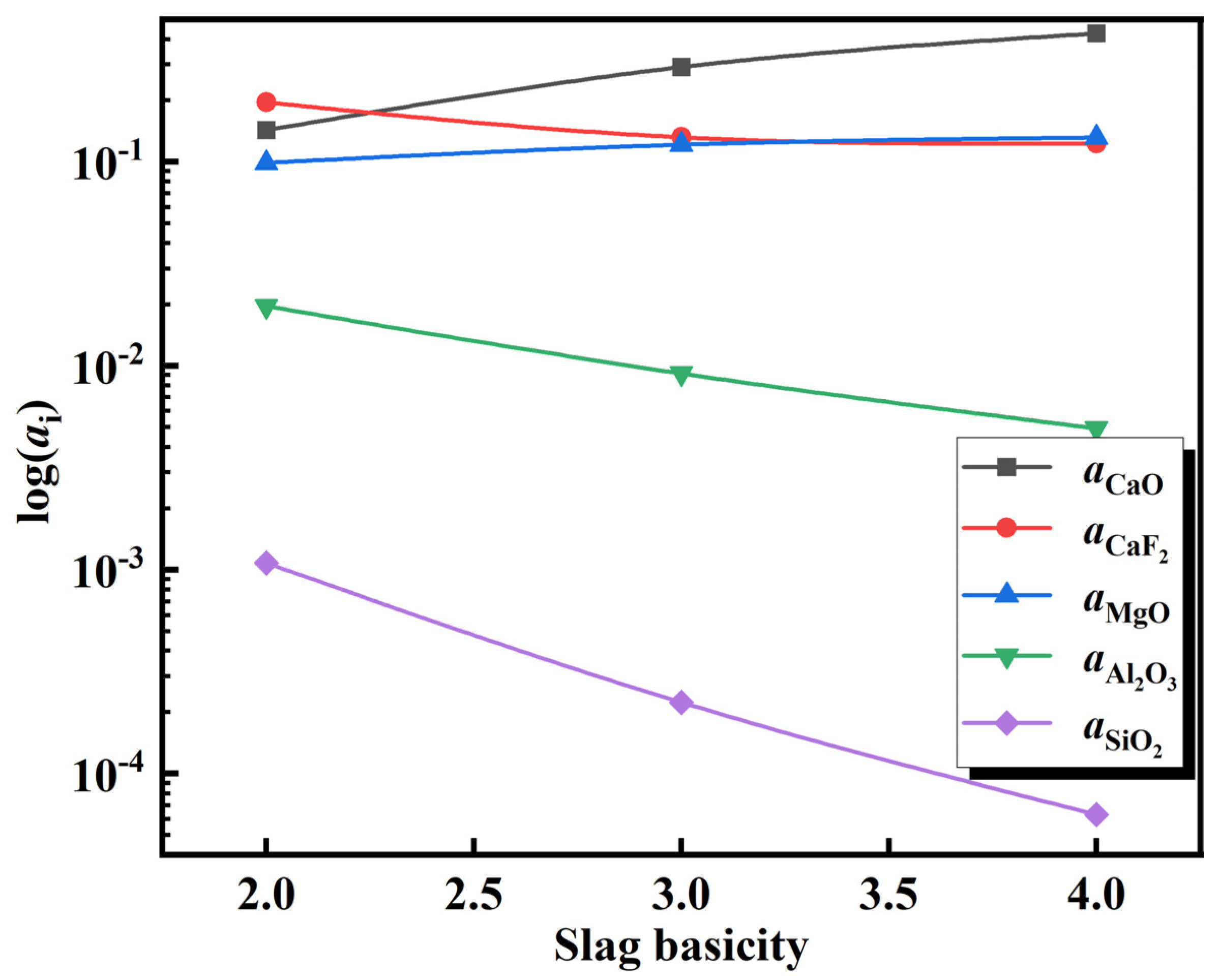
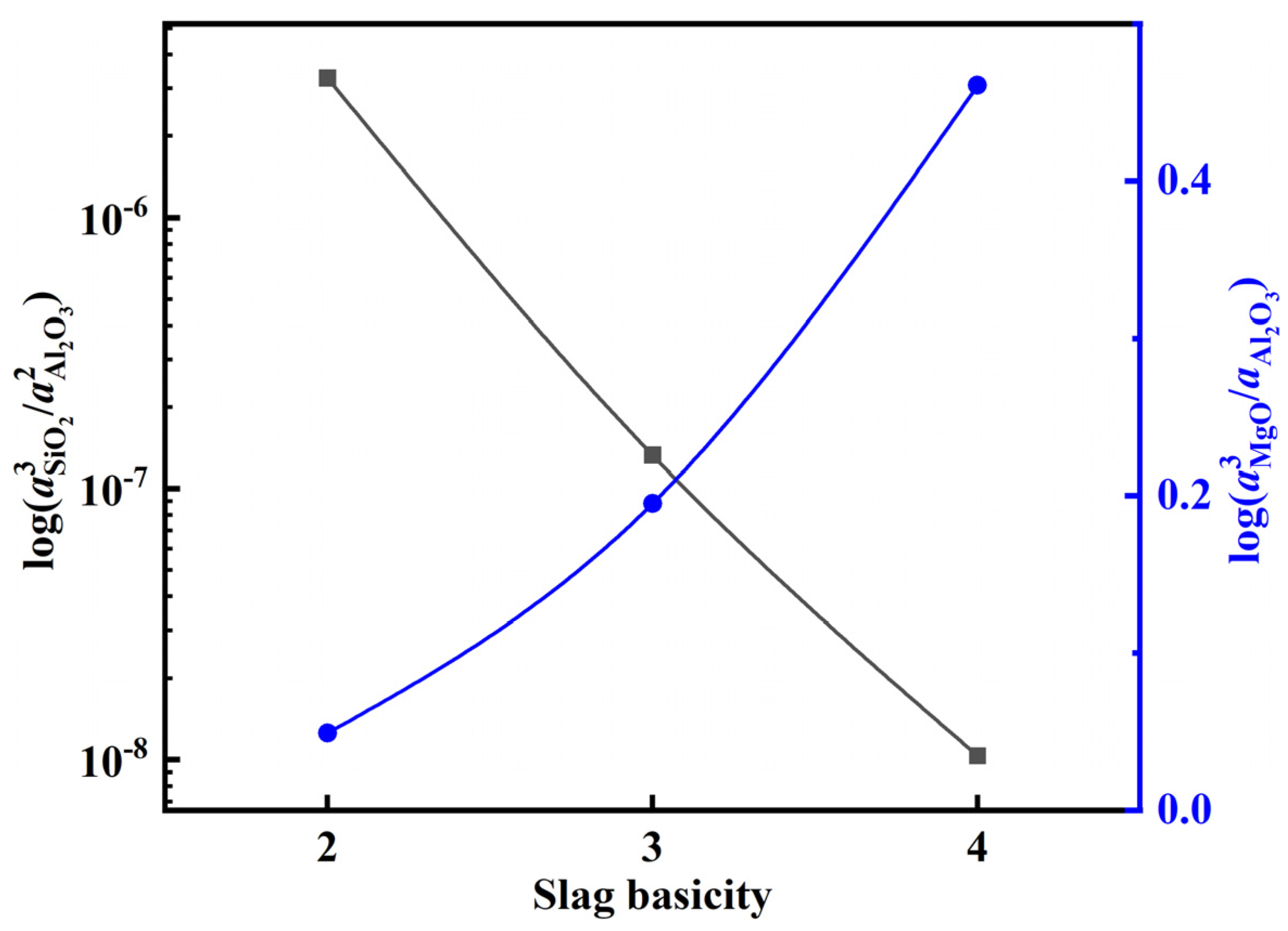
3.3. Impact of Slag Composition on Molten Steel Inclusions
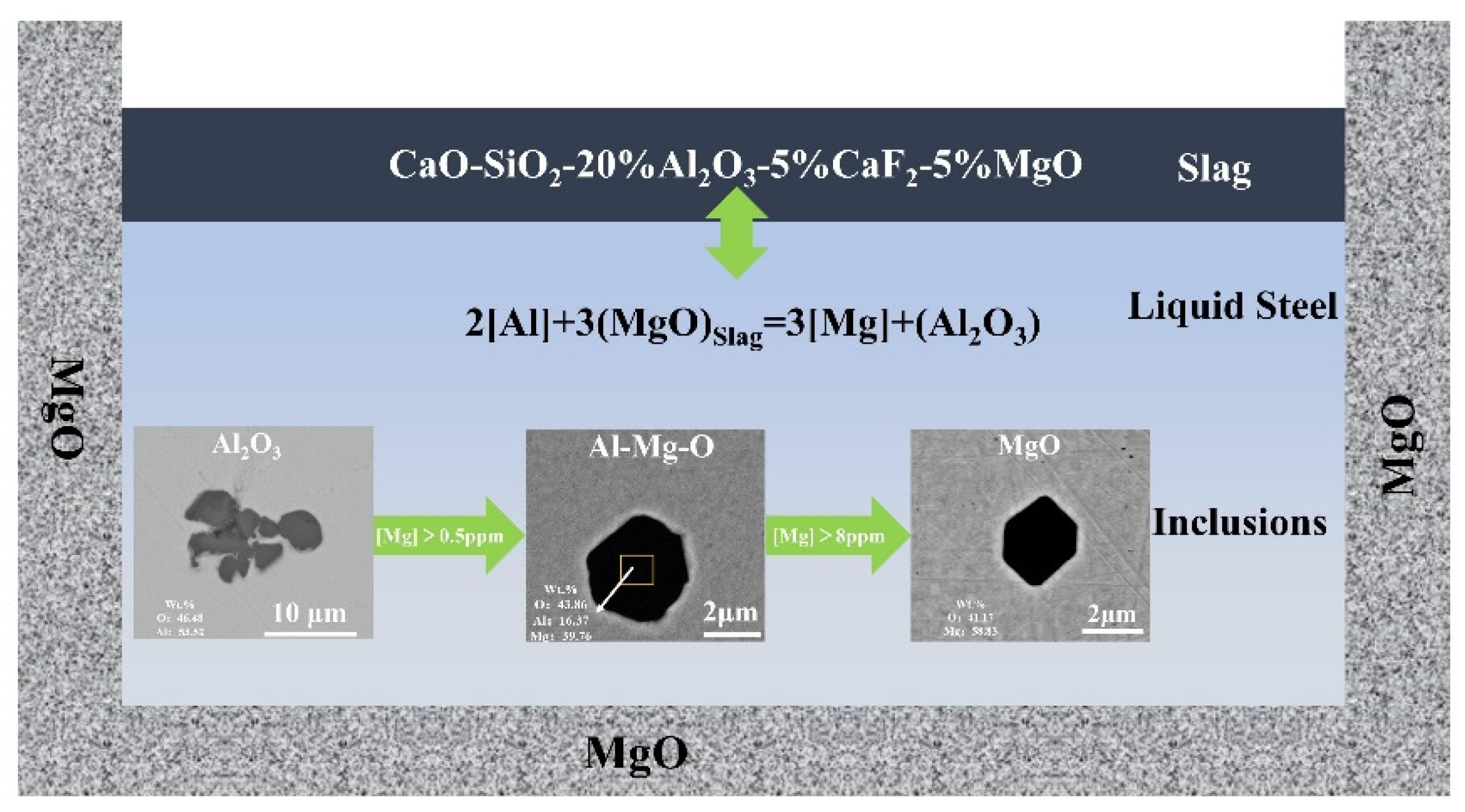
3.4. Evolution Mechanism of the Inclusions in Steel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Peng, Y.; Zhang, S.; Liu, C.; Cheng, R.; Ni, H. Effects of refining slag on transformation and removal of inclusions in type 430 stainless steel. Met. Mater. Trans. B 2022, 53, 702–715. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, C.; Yang, L.; Hou, S.; Cheng, R.; Zhang, H.; Ni, H. Improving cleanliness of 30Cr2Ni4MoV low-pressure rotor steel by CaO–SiO2–MgO–Al2O3 slag refining. J. Iron Steel Res. 2022, 29, 1434–1445. [Google Scholar] [CrossRef]
- Sasai, K. Kinetics on formation, growth and removal of alumina inclusions in molten steel. Tetsu-to-Hagane 2021, 107, 785–795. [Google Scholar] [CrossRef]
- Jiang, M.; Li, K.; Wang, R.; Yang, E.; Wang, X. Cleanliness and control of inclusions in Al-deoxidized bearing steel refined by basic slags during LF-VD-Ar Bubbling. ISIJ Int. 2021, 62, 124–132. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Chen, B.; Wang, W. Formation of MgO-Al2O3 inclusions in high strength alloyed structural steel refined by CaO-SiO2-Al2O3-MgO Slag. ISIJ Int. 2008, 48, 885–890. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, Y. Inclusions in stainless steels—A review. Steel Res. Int. 2017, 88, 1700130. [Google Scholar] [CrossRef]
- Park, J.S.; Park, J.H. Effect of physicochemical properties of slag and flux on the removal rate of oxide inclusion from molten steel. Met. Mater. Trans. B 2016, 47, 3225–3230. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W. Influence of refining slag composition on cleanness and fatigue life of 60Si2MnA spring steel. Ironmak. Steelmak. 2016, 43, 340–350. [Google Scholar] [CrossRef]
- Li, M. Study on Cleanliness of Shougang Cord Steel; University of Science and Technology Beijing: Beijing, China, 2006. [Google Scholar]
- Yoon, B.; Heo, K.; Kim, J. Improvement of steel cleanliness by controlling slag composition. Ironmak. Steelmak. 2002, 29, 215–218. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, I.H.; Lee, H.G. Dissolution behavior of Al2O3 and MgO inclusions in the CaO–Al2O3–SiO2 slags: Formation of ring-like structure of MgAl2O4 and Ca2SiO4 around MgO inclusions. ISIJ Int. 2006, 46, 1626–1634. [Google Scholar] [CrossRef]
- Valdez, M.; Shannon, G.S.; Sridhar, S. The ability of slags to absorb solid oxide inclusions. ISIJ Int. 2006, 46, 450–457. [Google Scholar] [CrossRef]
- Lee, S.; Tse, C.; Yi, K.W.; Misra, P.; Chevrier, V.; Orrling, C.; Sridhar, S.; Cramb, A.W. Separation and dissolution of Al2O3 inclusions at slag/metal interfaces. J. Non-Cryst. Solids 2001, 282, 41–48. [Google Scholar] [CrossRef]
- Ni, P.; Goto, H.; Nakamoto, M.; Tanaka, T. Neural network modelling on contact angles of liquid metals and oxide ceramics. ISIJ Int. 2020, 60, 1586–1595. [Google Scholar] [CrossRef]
- Mills, K.; Karagadde, S.; Lee, P.; Yuan, L.; Shahbazian, F. Calculation of physical properties for use in models of continuous casting process-Part 1: Mould slags. ISIJ Int. 2016, 56, 264–273. [Google Scholar] [CrossRef]
- Abdeyazdan, H.; Dogan, N.; Rhamdhani, M.; Chapman, M.; Monaghan, B. Dynamic wetting of CaO-Al2O3-SiO2-MgO liquid oxide on MgAl2O4 spinel. Met. Mater. Trans. B 2014, 46, 208–219. [Google Scholar] [CrossRef]
- Furukawa, T.; Saito, N.; Nakashima, K. Evaluation of interfacial energy between molten Fe and Fe-18%Cr-9%Ni alloy and non-metallic inclusion-type oxides. ISIJ Int. 2021, 61, 2381–2390. [Google Scholar] [CrossRef]
- Reis, B.H.; Bielefeldt, W.V.; Vilela, A.C.F. Absorption of non-metallic inclusions by steelmaking slags—A review. J. Mater. Res. Technol. 2014, 3, 179–185. [Google Scholar] [CrossRef]
- Todoroki, H.; Mizuno, K. Effect of silica in slag on inclusion compositions in 304 stainless steel deoxidized with aluminum. ISIJ Int. 2004, 44, 1350–1357. [Google Scholar] [CrossRef]
- Monaghan, B.; Chen, L.; Sorbe, J. Comparative study of oxide inclusion dissolution in CaO–SiO2–Al2O3 slag. Ironmak. Steelmak. 2013, 32, 258–264. [Google Scholar] [CrossRef]
- Ma, W.; Bao, Y.; Wang, M.; Zhao, D. Influence of slag composition on bearing steel cleanness. Ironmak. Steelmak. 2013, 41, 26–30. [Google Scholar] [CrossRef]
- Rocha, V.; Pereira, J.; Yoshioka, A.; Bielefeldt, W.; Vilela, A. Evaluation of secondary steelmaking slags and their relation with steel cleanliness. Met. Mater. Trans. B 2017, 48, 1423–1432. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Chen, S.; Jiang, M.; Huang, F.; Wang, W. Inclusion composition control in tyre cord steel by top slag refining. Ironmak. Steelmak. 2014, 41, 676–684. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Yang, S.; Zhuang, C. Effect of slag composition on the cleanliness of drill rod steel. Ironmak. Steelmak. 2018, 46, 416–423. [Google Scholar] [CrossRef]
- Liu, C.; Gao, X.; Ueda, S.; Kitamura, S. Change in composition of inclusions through the reaction between Al-killed Steel and the slag of CaO and MgO saturation. ISIJ Int. 2019, 59, 268–276. [Google Scholar] [CrossRef]
- Mu, H.; Zhang, T.; Fruehan, R.; Webler, B. Reduction of CaO and MgO slag components by Al in Liquid Fe. Met. Mater. Trans. B 2018, 49, 1665–1674. [Google Scholar] [CrossRef]
- Yang, G.; Wang, X.; Huang, F.; Wang, W.; Yin, Y. Transient inclusion evolution during RH Degassing. Steel Res. Int. 2014, 85, 26–34. [Google Scholar] [CrossRef]
- Nakajima, K. Estimation of surface tension for multicomponent silicate Melts. Tetsu-to-Hagane 1994, 80, 599–604. [Google Scholar] [CrossRef]
- Nakajima, K. Estimation of interfacial tensions between phases in the molten iron-slag-inclusion (Alumina) system. Tetsu-to-Hagane 1994, 80, 383–388. [Google Scholar] [CrossRef]
- Gao, M.; Yu, H.; Cheng, G.; Zhang, Y. Experimental study on high temperature wetting behavior of molten metal/slag-oxide. Exp. Technol. Manag. 2022, 39, 5–11. [Google Scholar]
- Duan, S.; Lee, M.; Kim, D.; Park, J. Oxidation behavior of boron in 9CrMoCoB steel by CaF2-CaO-Al2O3-SiO2-B2O3 electroslag remelting (ESR) type slag. J. Mater. Res. Technol. 2022, 17, 574–585. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P. The widespread applicability of the mass action law to metallurgical melts and organic solutions. Calphad 2001, 25, 343–354. [Google Scholar] [CrossRef]
- Jiang, Z.; Hou, D.; Dong, Y.; Cao, Y.; Cao, H.; Gong, W. Effect of Slag on Titanium, Silicon, and Aluminum Contents in Superalloy During Electroslag Remelting. Met. Mater. Trans. B. 2016, 47, 1465–1474. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, Z.; Geng, X. Design of ESR slag for Remelting 9CrMoCoB steel through experiments and thermodynamic calculations. Calphad 2020, 70, 342–351. [Google Scholar] [CrossRef]
- Duan, S.; Park, J. Comparison of Oxidation Behavior of Various Reactive Elements in Alloys during Electroslag Remelting (ESR) Process: An Overview. ISIJ Int. 2022, 62, 1561–1572. [Google Scholar] [CrossRef]
- Zouvelou, N.; Mantzouris, X.; Nikolopoulos, P. Interfacial energies in oxide/liquid metal systems with limited solubility. Int. J. Adhes. Adhes. 2007, 27, 380–386. [Google Scholar] [CrossRef]
- Li, J.; Cheng, G.; Ruan, Q.; Pan, J.; Chen, X. Formation and evolution of oxide inclusions in titanium-stabilized 18Cr stainless steel. ISIJ Int. 2018, 58, 2280–2287. [Google Scholar] [CrossRef]
- Li, J.; Cheng, G.; Ruan, Q.; Pan, J.; Chen, X. Effect of CaO-MgO-SiO2-Al2O3-TiO2 slags with different CaF2 contents on inclusions in Ti-stabilized 20Cr stainless steel. ISIJ Int. 2019, 59, 2013–2023. [Google Scholar] [CrossRef]


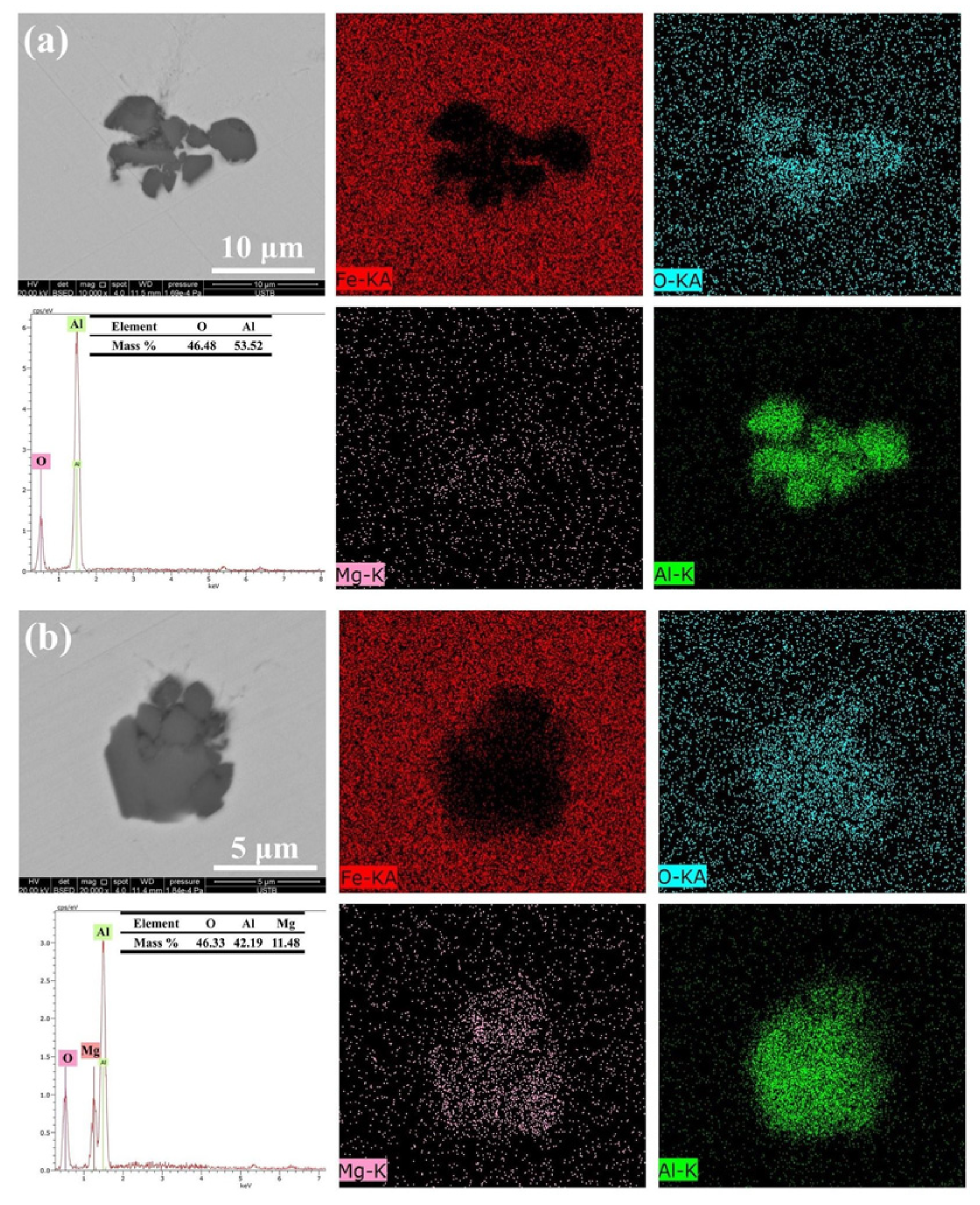
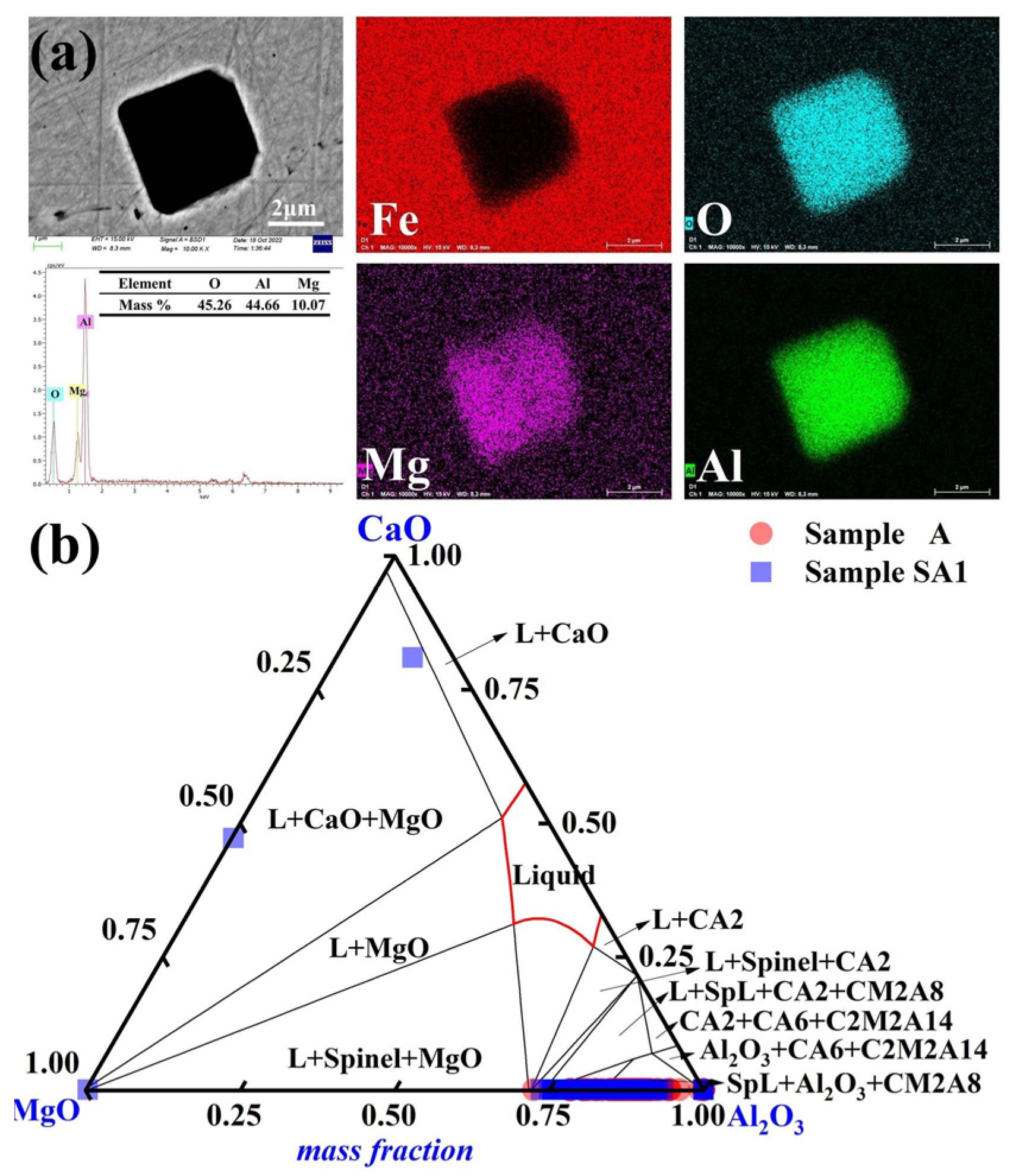

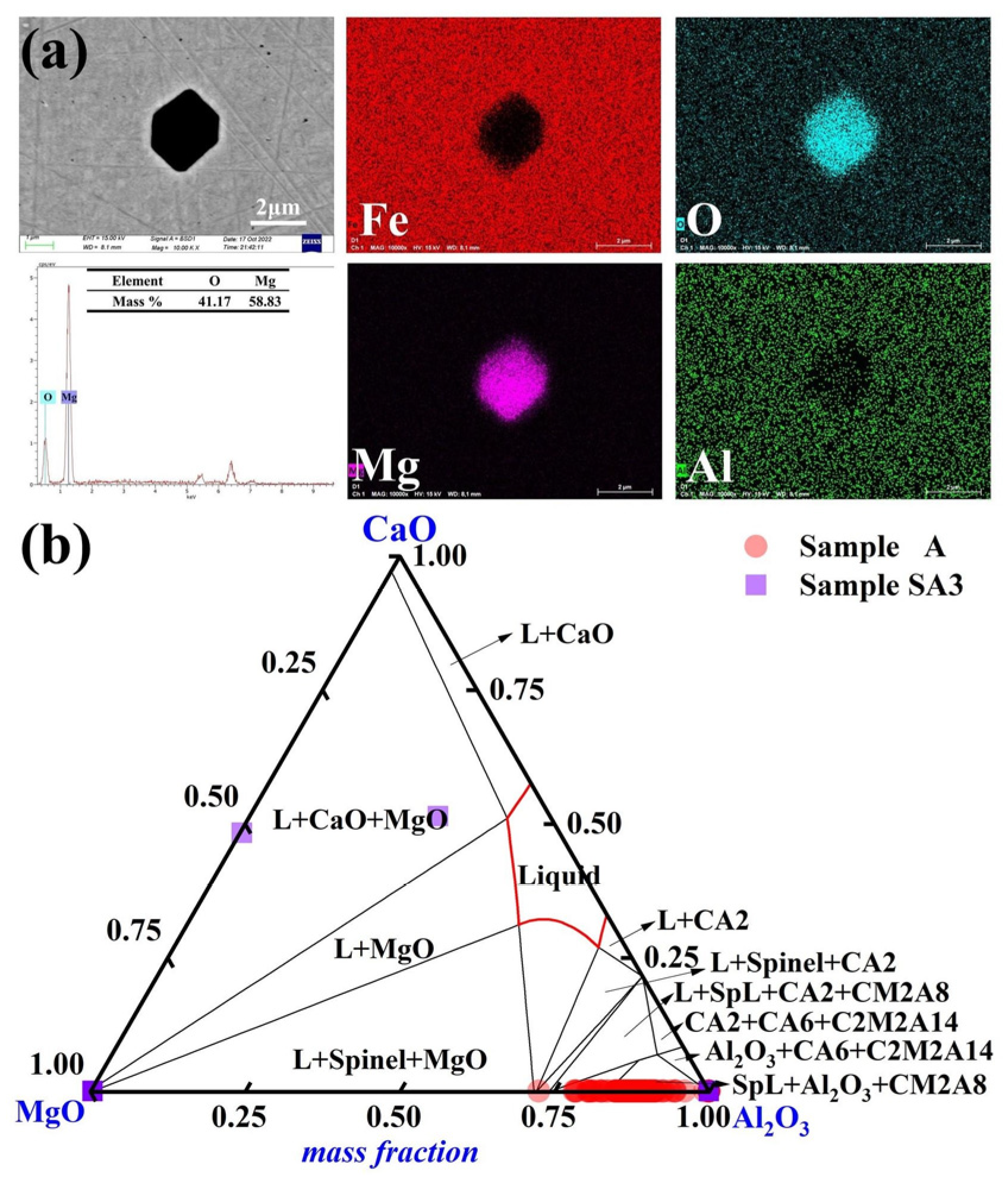
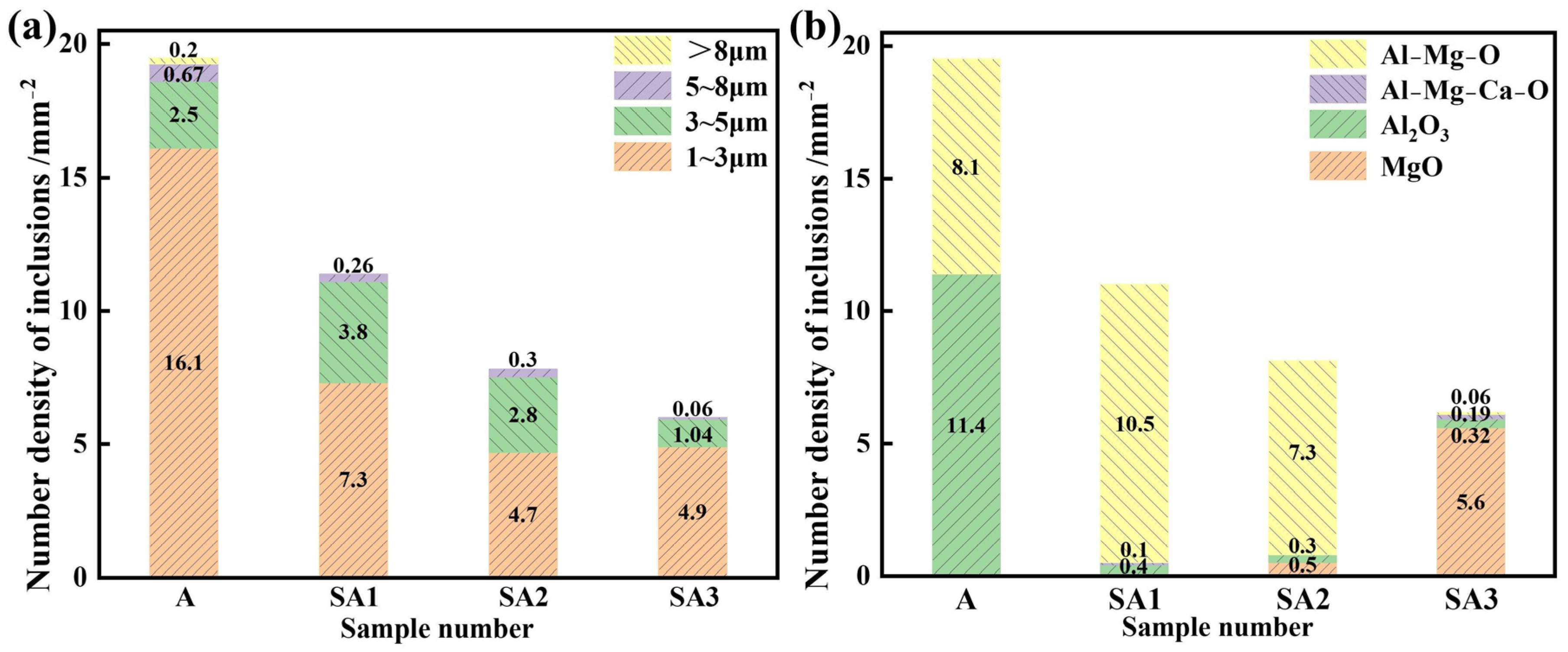



| Steel | Cr | Ni | Cu | Si | Mn | Mo | C | Nb | O | Mg | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 14.5 | 5.2 | 3.0 | 0.4 | 0.7 | 0.3 | 0.05 | 0.2 | 0.0027 | 0.0002 | 0.23 |
| Slag | CaO | SiO2 | Al2O3 | MgO | CaF2 |
|---|---|---|---|---|---|
| S1 | 46.67 | 23.33 | 19.44 | 5 | 5 |
| S2 | 52.50 | 17.50 | 19.44 | 5 | 5 |
| S3 | 56.00 | 14.00 | 19.44 | 5 | 5 |
| Steel | Si | Ni | Cr | Cu | Mo | Als | Ototal | Mg |
|---|---|---|---|---|---|---|---|---|
| A1 | 0.58 | 5.22 | 14.84 | 3 | 0.34 | 0.0066 | 0.002 | 0.0003 |
| A2 | 0.57 | 5.25 | 14.92 | 3 | 0.33 | 0.014 | 0.0012 | 0.0008 |
| A3 | 0.55 | 5.26 | 14.93 | 3 | 0.32 | 0.018 | 0.0008 | 0.0012 |
| Slag | CaO | SiO2 | Al2O3 | MgO | CaF2 |
|---|---|---|---|---|---|
| SA1 | 40.35 | 18.11 | 21.01 | 15.48 | 5.05 |
| SA2 | 48.32 | 13.59 | 21.70 | 11.09 | 5.30 |
| SA3 | 50.01 | 10.80 | 23.30 | 11.26 | 4.64 |
| i | Surface Tension /×10−3 N/m |
|---|---|
| CaO | 625 − 0.094 (T-1773) |
| SiO2 | 260 − 0.031 (T-1773) |
| MgO | 635 − 0.013 (T-1773) |
| Al2O3 | 655 − 0.177 (T-1773) |
| CaF2 | 290 − 0.070 (T-1773) |
| Slag | Substrate | Contact Angle, θ (°) |
|---|---|---|
| S1 | Alumina | 18.26 |
| S2 | 15.32 | |
| S3 | 11.37 | |
| S1 | Spinel | 11.20 |
| S2 | 10.40 | |
| S3 | 9.70 | |
| S1 | Magnesium oxide | 11.35 |
| S2 | 14.60 | |
| S3 | 20.70 |
| Structure Units as Ion Couples or Molecules | Mole Number of Structure Unit | Ion Couples or Structural Unit Mass Action Concentration |
|---|---|---|
| Ca2+ + O2− | ||
| Ca2+ + 2F− | ||
| Mg2+ + O2− | ||
| Al2O3 | ||
| SiO2 | ||
| CaO·SiO2 | ||
| 2CaO·SiO2 | ||
| 3CaO·SiO2 | ||
| CaO·Al2O3 | ||
| CaO·2Al2O3 | ||
| CaO·6Al2O3 | ||
| 3CaO·Al2O3 | ||
| 12CaO·7Al2O3 | ||
| MgO·SiO2 | ||
| 2MgO·SiO2 | ||
| MgO·Al2O3 | ||
| 3CaO·3Al2O3·CaF2 | ||
| 11CaO·7Al2O3·CaF2 | ||
| 3CaO·2SiO2·CaF2 | ||
| CaO·MgO·SiO2 | ||
| CaO·MgO·2SiO2 | ||
| 2CaO·MgO·2SiO2 | ||
| 3CaO·MgO·2SiO2 | ||
| 2CaO·Al2O3·SiO2 | ||
| CaO·Al2O3·2SiO2 | ||
| 3Al2O3·2SiO2 |
| Reactions | (J/mol) | Ni |
|---|---|---|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Z.; Zhang, Y.; Shi, R.; Qiao, T.; Wang, G.; Cheng, G. Effect of Slag Basicity on Non-Metallic Inclusions and Cleanliness of 15-5PH Stainless Steel. Metals 2023, 13, 750. https://doi.org/10.3390/met13040750
Zhan Z, Zhang Y, Shi R, Qiao T, Wang G, Cheng G. Effect of Slag Basicity on Non-Metallic Inclusions and Cleanliness of 15-5PH Stainless Steel. Metals. 2023; 13(4):750. https://doi.org/10.3390/met13040750
Chicago/Turabian StyleZhan, Zhonghua, Yanling Zhang, Ruxing Shi, Tong Qiao, Guanbo Wang, and Guoguang Cheng. 2023. "Effect of Slag Basicity on Non-Metallic Inclusions and Cleanliness of 15-5PH Stainless Steel" Metals 13, no. 4: 750. https://doi.org/10.3390/met13040750
APA StyleZhan, Z., Zhang, Y., Shi, R., Qiao, T., Wang, G., & Cheng, G. (2023). Effect of Slag Basicity on Non-Metallic Inclusions and Cleanliness of 15-5PH Stainless Steel. Metals, 13(4), 750. https://doi.org/10.3390/met13040750









