Reduction Kinetics of Compact Hematite with Hydrogen from 600 to 1050 °C
Abstract
1. Introduction
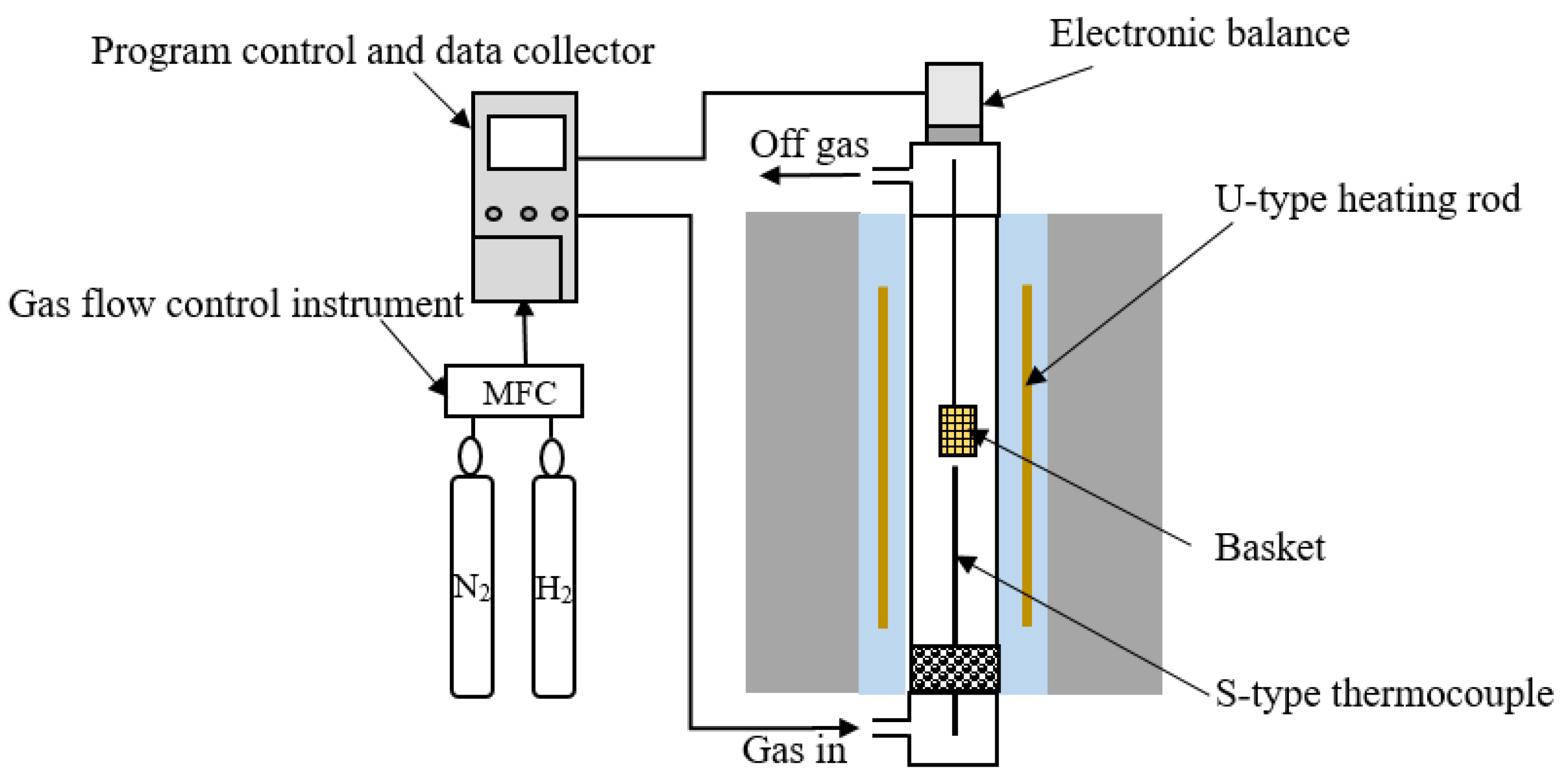
2. Materials and Methods
3. Results and Discussion
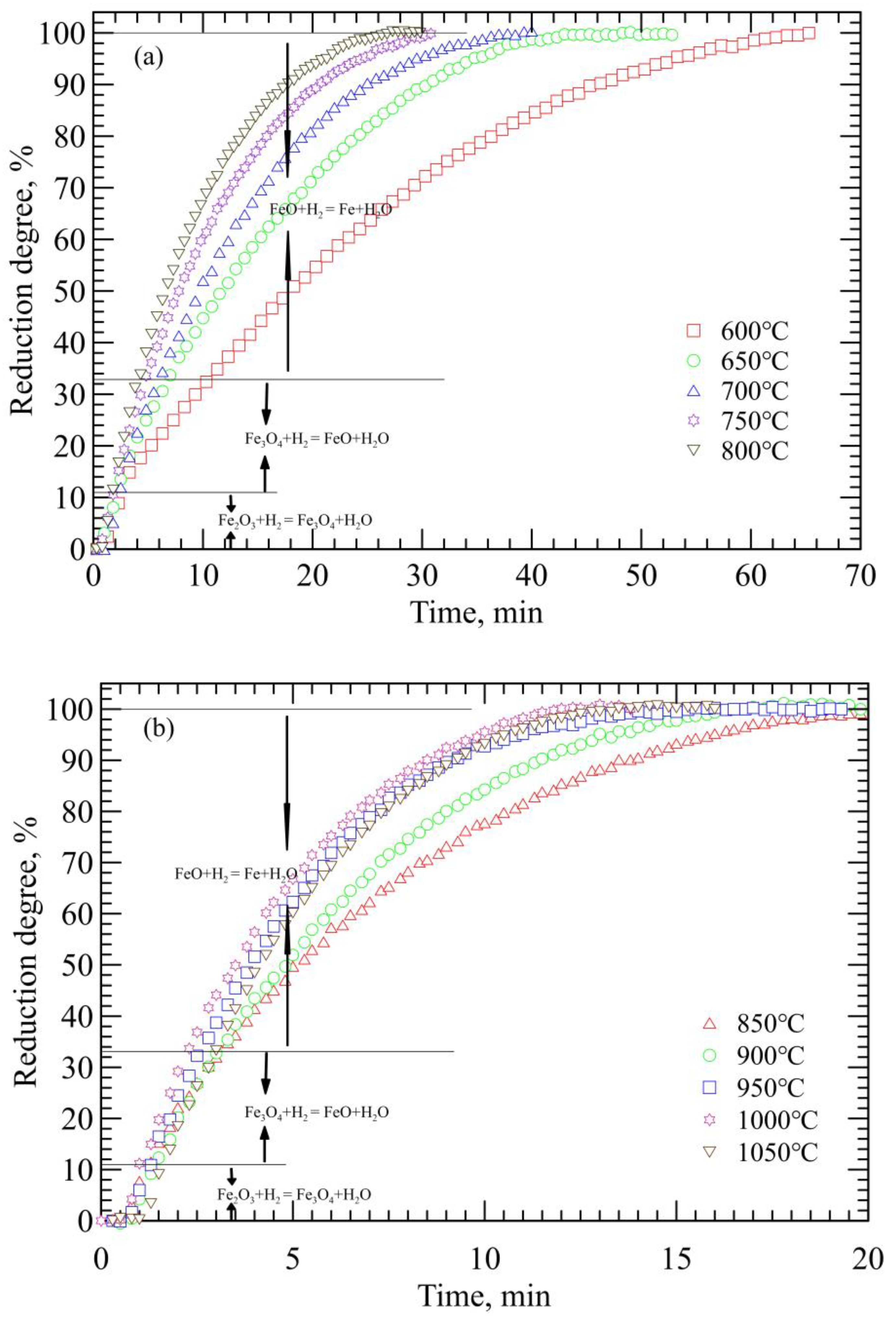
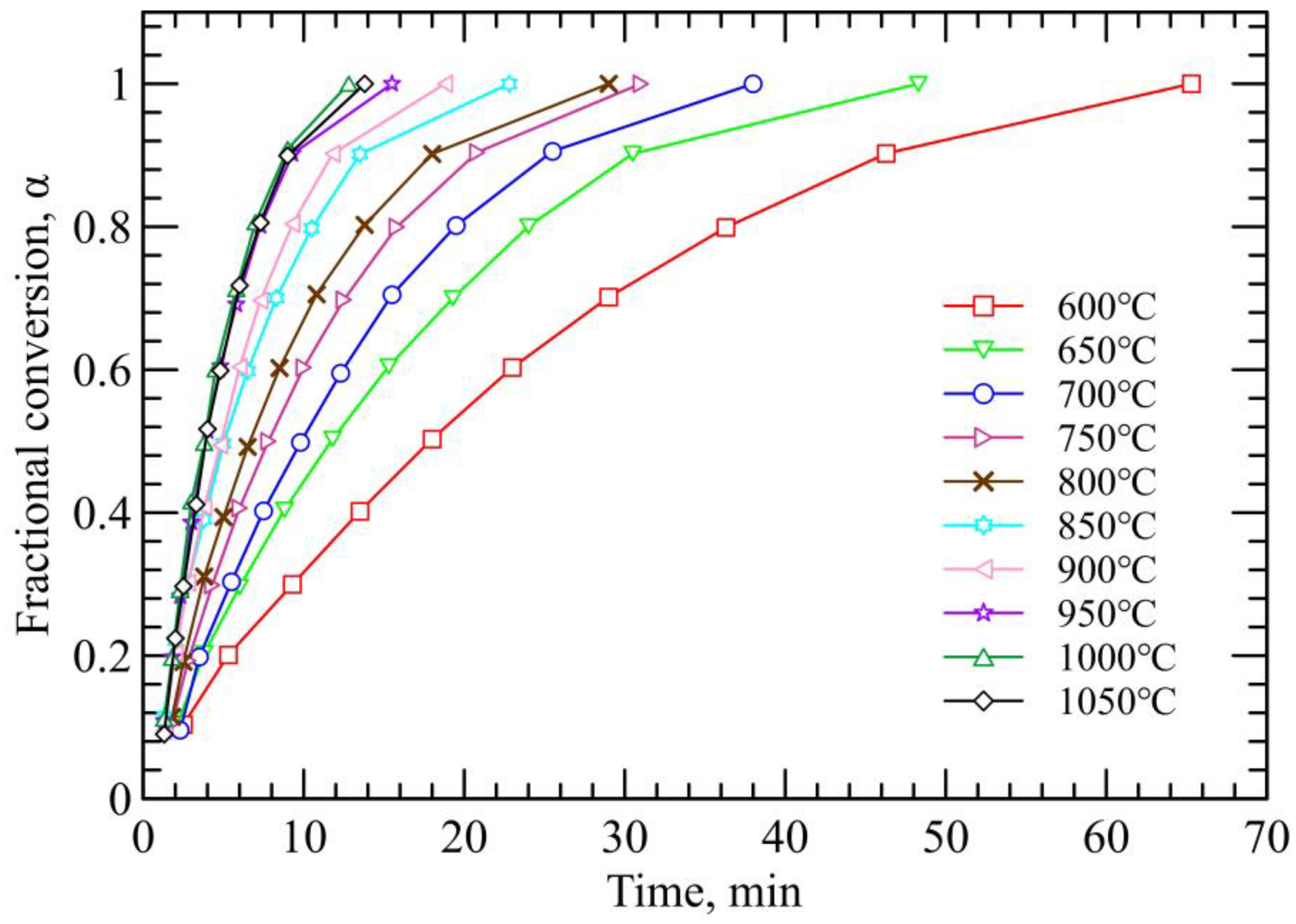
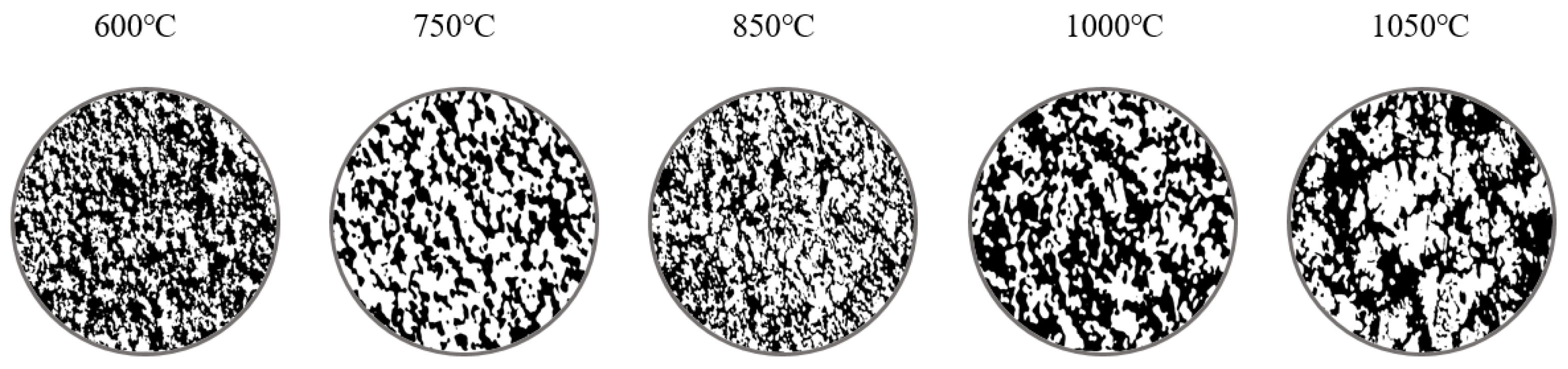
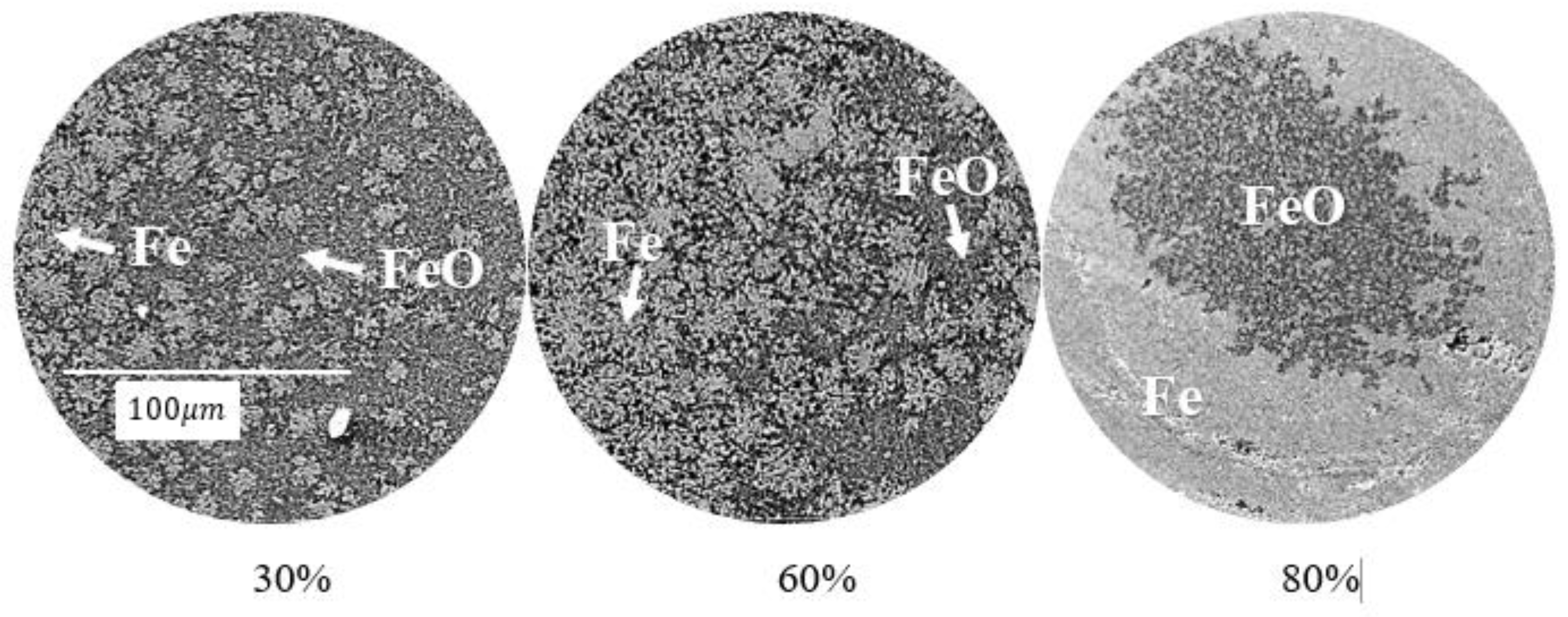
3.1. Reduction Kinetics
3.2. Morphology Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holappa, L. A general vision for reduction of energy consumption and CO2 emissions from the steel industry. Metals 2020, 10, 1117. [Google Scholar] [CrossRef]
- Fan, Z.; Friedmann, S.J. Low-carbon production of iron and steel: Technology options, economic assessment, and policy. Joule 2021, 5, 829–862. [Google Scholar] [CrossRef]
- Kim, J.; Sovacool, B.K.; Bazilian, M.; Griffiths, S.; Lee, J.; Yang, M.; Lee, J. Decarbonizing the iron and steel industry: A systematic review of sociotechnical systems, technological innovations, and policy options. Energy Res. Soc. Sci. 2022, 89, 102565. [Google Scholar] [CrossRef]
- Pan, C.; Pang, J.M. Development trace and application prospect of hydrogen metallurgy technology. China Metall. 2021, 31, 73–77. [Google Scholar]
- Zhang, B.; Zhao, Z.; Guo, H.; Quan, Q.; Xue, Q. Development of direct- reduction ironmaking technology in gas-based shaft furnace. Res. Iron Steel 2016, 44, 59–62. [Google Scholar]
- Tang, J.; Chu, M.S.; Li, F.; Feng, C.; Liu, Z.G.; Zhou, Y.S. Development and progress on hydrogen metallurgy. Int. J. Miner. Metall. Mater. 2020, 27, 713–723. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Heidari, A.; Niknahad, N.; Iljana, M.; Fabritius, T. A review on the kinetics of iron ore reduction by hydrogen. Materials 2021, 14, 7540. [Google Scholar] [CrossRef]
- Ma, Y.; Souza Filho, I.R.; Bai, Y.; Schenk, J.; Patisson, F.; Beck, A.; van Bokhoven, J.A.; Willinger, M.G.; Li, K.; Xie, D.; et al. Hierarchical nature of hydrogen-based direct reduction of iron oxides. Scr. Mater. 2022, 213, 114571. [Google Scholar] [CrossRef]
- Turkdogan, E.T.; Vinters, J.V. Gaseous reduction of iron oxides Part I. Reduction of hematite in hydrogen. Metall. Trans. 1971, 2, 3175–3188. [Google Scholar] [CrossRef]
- Garg, P.; Hu, X.; Li, Y.; Li, K.; Nag, S.; Zhang, J. Kinetics of Iron Oxide Reduction in H2/ H2O Gas Mixture: Global and Stepwise Reduction. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2022, 53, 1759–1774. [Google Scholar] [CrossRef]
- II-Joon, M.; Chang-Hee, R.; Dong-Joon, M. Reduction of hematite compact by H2-CO gas mixtures. Steel Res. 1998, 69, 302–306. [Google Scholar]
- Lin, H.-Y.; Chen, Y.-W.; Li, C. The mechanism of reduction of iron oxide by hydrogen. Thermochim. Acta 2003, 400, 61–67. [Google Scholar] [CrossRef]
- Bahgat, M.; Khedr, M.H. Reduction kinetics, magnetic behavior and morphological changes during reduction of magnetite single crystal. Mater. Sci. Eng. B 2007, 138, 251–258. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Nasr, M.I. Influence of the original structure on the kinetics of hydrogen reduction of hematite compacts. Trans. Iron Steel Inst. Jpn. 1988, 28, 650–658. [Google Scholar] [CrossRef]
- Rao, Y.K.; Moinpour, M. Kinetics of reduction of hematite with hydrogen gas at modest temperatures. Metall. Trans. B 1983, 14, 711–723. [Google Scholar] [CrossRef]
- Yi, L. Fundamental Research on Gas-based DirectReduction of Iron Ore Pellets with Carbon Monoxide and Hydrogen Mixtures. Doctoral Dissertation, Central South University, Changsha, China, 2013. [Google Scholar]
- Turkdogan, E.T.; Olsson, R.G.; Vinters, J.V. Gaseous reduction of iron oxides: Part II. Pore characteristics of iron reduced from hematite in hydrogen. Metall. Mater. Trans. B 1971, 2, 3189–3196. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2. Part II. Low temperature reduction of magnetite. Thermochim. Acta 2007, 456, 75–88. [Google Scholar] [CrossRef]
- Yang, X. A Study of The Reduction Kinetics of Iron Oxide with H2-rich Gas. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2016. [Google Scholar]
- Yang, X.B.; Hu, X.J.; Chen, Z.Y.; Dang, J.; Fang, F.; Chou, K.C. Structure evolution in the reduction process of FeO powder by hydrogen. Gongcheng Kexue Xuebao/Chin. J. Eng. 2015, 37, 163–167. [Google Scholar]
- John, D.H.S.; Hayes, P.C. Microstructural features produced by the reduction of wustite in H2/H2O gas mixtures. Metall. Trans. B 1982, 13, 117–124. [Google Scholar] [CrossRef]
- Moukassi, M.; Gougeon, M.; Steinmetz, P.; Dupre, B.; Gleitzer, C. Hydrogen reduction of wustite single crystals doped with Mg, Mn, Ca, Al, and Si. Metall. Trans. B 1984, 15, 383–391. [Google Scholar] [CrossRef]
- Jung, S.S.; Lee, J.S. In-situ kinetic study of hydrogen reduction of Fe2O3 for the production of Fe nanopowder. Mater. Trans. 2009, 50, 2270–2276. [Google Scholar] [CrossRef]
- Rodriguez, R.; Bedolla, E.; Conejo, A. Kinetics of Reduction of Fe2O3 Particles with H2-CO Mixtures at Low Temperalures. Iron Steelmak. 2003, 1, 25–33. [Google Scholar]
- Fruehan, R.J.; Li, Y.; Brabie, L.; Kim, E.J. Final stage of reduction of iron ores by hydrogen. Scand. J. Metall. 2005, 34, 205–212. [Google Scholar] [CrossRef]
- Kim, S.H.; Zhang, X.; Ma, Y.; Souza Filho, I.R.; Schweinar, K.; Angenendt, K.; Vogel, D.; Stephenson, L.T.; El-Zoka, A.A.; Mianroodi, J.R.; et al. Influence of microstructure and atomic-scale chemistry on the direct reduction of iron ore with hydrogen at 700 °C. Acta Mater. 2021, 212, 116933. [Google Scholar] [CrossRef]
- Abazarpoor, A.; Halali, M.; Hejazi, R.; Saghaeian, M. HPGR effect on the particle size and shape of iron ore pellet feed using response surface methodology. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2018, 127, 40–48. [Google Scholar] [CrossRef]
- Morris, M.; McMurdie, H.; Evans, E.; Peretzkin, B.; Parker, H.; Panagiotopoulos, N. Standard X-ray diffractin powder patterns. Section 18. Natl. Bur. Stand. 1981, 18, 37. [Google Scholar]
- Kimura, S.; Takagi, Y.; Tone, S.; Otake, T. A rate equation for gas-solid reactions accounting for the effect of solid structure and its application. J. Chem. Eng. Jpn. 1981, 14, 456–461. [Google Scholar] [CrossRef]
- Li, L.; Niu, L.; Guo, H.J.; Duan, S.-C.; Li, Y.-Q.; Meng, X.-L. Experimental research and kinetics analysis on hydrogen reduction of limonite. Gongcheng Kexue Xuebao/Chin. J. Eng. 2015, 37, 13–19. [Google Scholar]
- Liu, J.; Zhang, J.; Zhou, T. Assessment of Apparent Activation Energies for Reduction Reactions of Iron Oxides by Hydrogen. J. Iron Steel Res. Int. 1999, 11, 9–13. [Google Scholar]
- El-Geassy, A.A. Gaseous reduction of Fe2O3 compacts at 600 to 1050 °C. J. Mater. Sci. 1986, 21, 3889–3900. [Google Scholar] [CrossRef]
- Yu, J.; Han, Y.; Li, Y.; Gao, P.; Li, W. Mechanism and kinetics of the reduction of hematite to magnetite with CO–CO2 in a micro-fluidized bed. Minerals 2017, 7, 209. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Shehata, K.A.; Ezz, S.Y. Mechanism of Iron Oxide Reduction with Hydrogen/Carbon Monoxide Mixtures. Trans. ISIJ 1977, 17, 629–635. [Google Scholar] [CrossRef]
- Wimmers, O.J.; Arnoldy, P.; Moulijn, J.A. Determination of the reduction mechanism by temperature-programmed reduction: Application to small iron oxide (Fe2O3) particles. J. Phys. Chem. 1986, 90, 1331–1337. [Google Scholar] [CrossRef]
- Shimokawabe, M.; Furuichi, R.; Ishii, T. Influence of the preparation history of α-Fe2O3 on its reactivity for hydrogen reduction. Thermochim. Acta 1979, 28, 287–305. [Google Scholar] [CrossRef]
- Moinpour, M.; Rao, Y.K. Kinetics of reduction of hematite with He-H2 gas mixtures at moderate temperatures. Trans. ISIJ 1988, 28, 714–720. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Moukassi, M.; Steinmetz, P.; Dupre, B.; Gleitzer, C. A study of the mechanism of reduction with hydrogen of pure wustite single crystals. Metall. Trans. B 1983, 14, 125–132. [Google Scholar] [CrossRef]
- Nicolle, R.; Rist, A. The mechanism of whisker growth in the reduction of wüstite. Metall. Trans. B 1979, 10, 429–438. [Google Scholar] [CrossRef]
- Cho, Y.G.; Kim, J.Y.; Cho, H.H.; Cha, P.R.; Suh, D.W.; Lee, J.K.; Han, H.N. Analysis of transformation plasticity in steel using a finite element method coupled with a phase field model. PLoS ONE 2012, 7, e35987. [Google Scholar] [CrossRef]
- Andreola, F.; Leonelli, C.; Romagnoli, M. Techniques Used to Determine Porosity. Am. Ceram. Soc. Bull. 2000, 79, 49–52. [Google Scholar]
- Edstrom, J.O. The mechanism of reduction of iron oxides. J. Iron Steel Inst. 1953, 175, 289–304. [Google Scholar]
- Pashley, R.; Israelachvili, J. Molecular layering of water at surfaces and origin of repulsive hydration forces. Nature 1983, 306, 2. [Google Scholar]

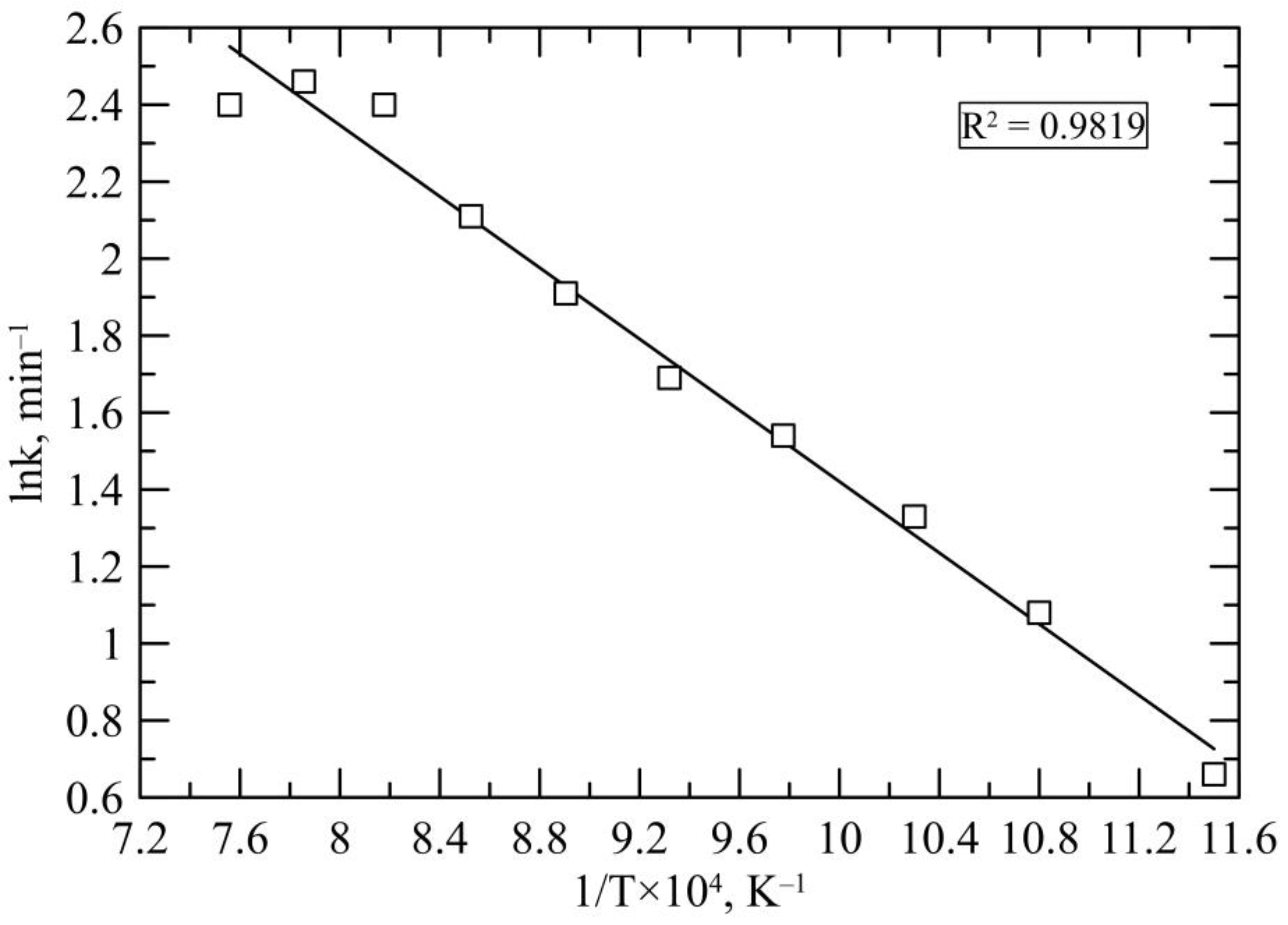




| T, °C | Rate, (%/min) | ln Rate, (min−1) | 1/T, (K−1) |
|---|---|---|---|
| 600 | 1.93 | 0.66 | 1.15 × 10−3 |
| 650 | 2.96 | 1.08 | 1.08 × 10−3 |
| 700 | 3.77 | 1.33 | 1.03 × 10−3 |
| 750 | 4.67 | 1.54 | 9.79 × 10−4 |
| 800 | 5.41 | 1.69 | 9.33 × 10−4 |
| 850 | 6.78 | 1.91 | 8.98× 10−4 |
| 900 | 8.22 | 2.11 | 8.54 × 10−4 |
| 950 | 10.97 | 2.40 | 8.19 × 10−4 |
| 1000 | 11.72 | 2.46 | 7.87 × 10−4 |
| 1050 | 10.97 | 2.40 | 7.57 × 10−4 |
| Iron Oxides | Reducing Gas | Temperature | Ea [kJ/mol] | Ref. |
|---|---|---|---|---|
| Fe2O3 → Fe3O4 | 5%H2 + 95%N2 | Non-isothermal, <325 °C | 89.1 | [13] |
| 67%H2 + Ar | Non-isothermal, 290–480 °C | 124 | [36] | |
| 5%CO + Ar | Isothermal, 25–700 °C | 33–74 | [37] | |
| Fe3O4 → Fe | 5%H2 + 95%N2 | Non-isothermal, <325 °C | 70 | [13] |
| (25–100%H2) + He | Isothermal, 310 and 377 °C | 60.7 | [38] | |
| Fe2O3 → Fe | 100% H2 | Isothermal, 700–1100 °C Porosity 7% | 53.5 | [35] |
| 100% H2 | Isothermal, 700–1100 °C Porosity 35% | 21.5 | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Li, K.; Zhang, J.; Conejo, A.N. Reduction Kinetics of Compact Hematite with Hydrogen from 600 to 1050 °C. Metals 2023, 13, 464. https://doi.org/10.3390/met13030464
He J, Li K, Zhang J, Conejo AN. Reduction Kinetics of Compact Hematite with Hydrogen from 600 to 1050 °C. Metals. 2023; 13(3):464. https://doi.org/10.3390/met13030464
Chicago/Turabian StyleHe, Junguo, Kejiang Li, Jianliang Zhang, and Alberto N. Conejo. 2023. "Reduction Kinetics of Compact Hematite with Hydrogen from 600 to 1050 °C" Metals 13, no. 3: 464. https://doi.org/10.3390/met13030464
APA StyleHe, J., Li, K., Zhang, J., & Conejo, A. N. (2023). Reduction Kinetics of Compact Hematite with Hydrogen from 600 to 1050 °C. Metals, 13(3), 464. https://doi.org/10.3390/met13030464








