Abstract
In this paper, the effect of rare earth Ce content on the morphology, composition, type and size distribution of inclusions in W350 non-oriented silicon steel was investigated by means of ICP-MS (inductively coupled plasma mass spectrometry), SEM/EDS (scanning electron microscope-energy Dispersive Spectrometer), and ASPEX (automated SEM/EDS inclusion analysis). The results showed that with the increase of Ce content in the steel, the modification sequence of inclusions was CeAlO3→Ce2O2S→CexSy. The type and size distribution of inclusions in the steel obviously changed with the difference in added Ce content. When the added Ce content in the steel was 10 ppm, 14 ppm, 20 ppm and 30 ppm respectively, the rare earth inclusions were mainly CeAlO3-Ce2O2S. Furthermore, when the added Ce content increased to 60 ppm, the rare earth inclusions were mainly Ce2O2S with a small amount of CeAlO3 contained in part inclusions. When the added Ce content increased continually to 95 ppm, the rare earth inclusions were mainly CexSy-Ce2O2S. The critical Ce content for the conversion between CeAlO3 and Ce2O2S was 41 ppm. To ensure that inclusions transform from CeAlO3 to Ce2O2S, the Ce content in the steel should be greater than 41 ppm. Under the current experimental conditions, it was found that when the Ce content was 20 ppm, the number density and proportion of inclusions in the steel were lower, and their average size was larger. When the added Ce content increased to 95 ppm, the number density of inclusions in the steel significantly increased, which deteriorated the steel cleanliness.
1. Introduction
High-grade, non-oriented silicon steel, as the functional material for high-end power equipment [1], is widely applied to the core of large generators and high-efficiency, energy-saving motors and high-efficiency, energy-saving appliances and electric vehicle manufacturing because of its magnetic characteristics of low iron loss and high magnetic induction [2,3,4]. With the implementation and promotion of frequency conversion technology in the home appliance industry, the rise of the new-energy automobile industry and the development of energy-saving, high-efficiency motors, there is an increasing demand for the magnetic properties of high-grade, non-oriented silicon steel with low iron loss and high magnetic induction [5]. The factors affecting the magnetic properties of high-grade, non-oriented silicon steel are mainly manifested in two aspects: one is the precise control of the composition and cleanliness of the molten steel that is also the basis for determining the magnetic properties of high-grade, non-oriented silicon steel [6]; and the other is a suitable plastic processing and heat treatment process, as there are many micro-sized inclusions in steel which can have adverse effect on the magnetic domain movement, favorable texture formation and recrystallization process in silicon steel [7]. Rare earth elements have attracted the attention of metallurgical workers much more, because of their unique metallurgical properties. By adding a small amount of rare earth elements into molten steel, the active oxygen and sulfur in the molten steel can be reduced to a lower level, and the microstructure of the finished product can be improved by refining the solidification structure. Meanwhile, rare earth elements can also change the inherent type and morphology of inclusions, reduce the harm of inclusions in experimental molten steel, and improve the related properties of steel products [8,9,10,11,12,13,14]. Takashima et al. [15,16] proposed the addition of composite rare earth and Al to non-oriented silicon steel. The results showed that after adding rare earth alloy and Al, the inclusion size became larger, the grain growth rate was significantly improved, the residual stress after annealing was reduced, and the product performance was significantly improved. Wu [17], Liu [18], and Yuan [19] have investigated the application of rare earth elements in non-oriented silicon steel. The research content was mainly based on the addition of pure rare earth elements or rare earth alloy-modified inclusions. Due to their strong deoxidation and desulfurization ability, it was easy to generate rare earth oxides, rare earth sulfides and rare earth oxysulfide compounds by adding appropriate amount of rare earth elements after pre-deoxidation of molten steel. By adding rare earth elements or trace amounts of alloying elements, this could increase the size of inclusions, weaken the pinning effect of original fine inclusions on grain boundaries and magnetic domain walls, make the magnetization process easier, reduce hysteresis loss, and achieve the combination of high magnetic induction and low iron loss. The dispersion precipitation of rare earth elements in molten steel can refine the solidification structure and increase the beneficial texture composition, further improving the magnetic properties of the product. However, it is still unclear whether the mechanism of high melting point phases, such as rare earth oxides (sulfides) as nucleation particles to modify inclusions, can change the type, size and distribution of inclusions in non-oriented silicon steel. Therefore, in this work, efforts have been made to investigate the modification of rare earth Ce on inclusions in W350 non-oriented silicon steel in the laboratory with thermodynamic calculation and experimental analysis, aiming to lay a theoretical foundation for rare earth Ce to modify inclusions in non-oriented silicon steel and provide guidance for production.
2. Materials and Methods
The experimental slab of W350 non-oriented silicon steel was produced by a domestic steel company. The slag was removed cleanly before the hot metal entered the desulfurization station, and the slag was removed twice after the deep desulfurization treatment to [S] ≤0.0010%, aiming to minimize the high-sulfur slag entering the converter. The scrap steel required self-produced, high-quality scrap steel, of which about 50% was silicon steel scrap. When the converter was tapping, the slide plate and slag stopper were used to stop the slag, and the thickness of the top slag of the ladle was required to be less than 60 mm. Lime was added to the top slag treatment during the tapping process, and the argon flow rate was controlled during the tapping process to prevent the top slag of the ladle from agglomeration. In the RH refining process, it was forbidden to heat up the molten steel by adding aluminum, after the molten steel had arrived at the RH refining station. After the decarburization, the oxygen content of the molten steel was less than 300 ppm before alloying. Following this, the molten steel was cast into a slab. The raw materials used in the experiment were from the continuous casting slab. The chemical composition of raw materials is listed in Table 1.

Table 1.
Chemical composition of experimental raw materials (wt.%).
The 150 kg vacuum induction furnace was used for smelting, and the furnace lining was rebuilt before the experiment. The first furnace used pure iron as the raw material to wash the furnace. During the experiment, it was necessary to control the vacuum degree in the furnace, keep the vacuum degree stable; the composition was then adjusted before tapping and rare earth cerium (purity 99.99%) was added, as shown in Table 2. These rare earth cerium alloys were purchased from Beijing Dream Material Technology Co., Ltd. (Beijing, China).

Table 2.
Chemical composition of rare earth Ce alloy (wt.%).
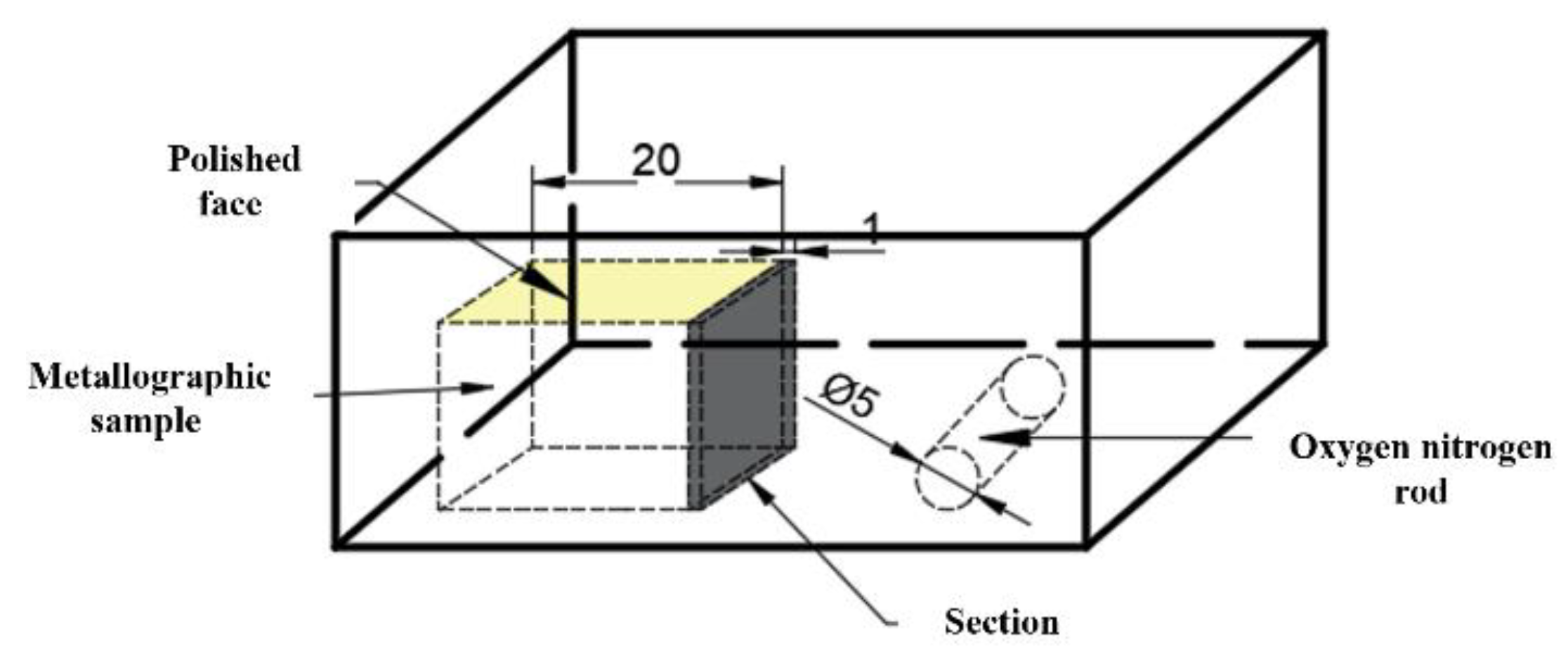
The steel composition was detected using inductively coupled plasma mass spectrometry (ICP-MS). Table 3 shows the chemical composition of the experimental steel. The rare earth Ce content in 1#~6# experimental steels was 10 ppm, 14 ppm, 20 ppm, 30 ppm, 60 ppm, and 95 ppm, respectively. Samples (20 mm × 15 mm × 15 mm) were taken from the edge of the slab after removing the oxide scale of the slab surface, as shown in Figure 1. After grinding and polishing, the morphology, size and composition of inclusions in the steel were detected and analyzed using automated SEM/EDS inclusion analysis (ASPEX), scanning electron microscope (SEM) and energy dispersive spectrum (EDS) apparatus. The content of total oxygen (T.O.) and nitrogen in the steel were detected by inert gas fusion pulse-infrared absorption spectroscopy.

Table 3.
Chemical composition of experimental steel (wt.%).
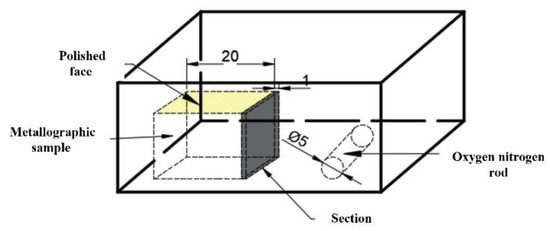
Figure 1.
Sampling diagram of the experimental steel.
3. Results
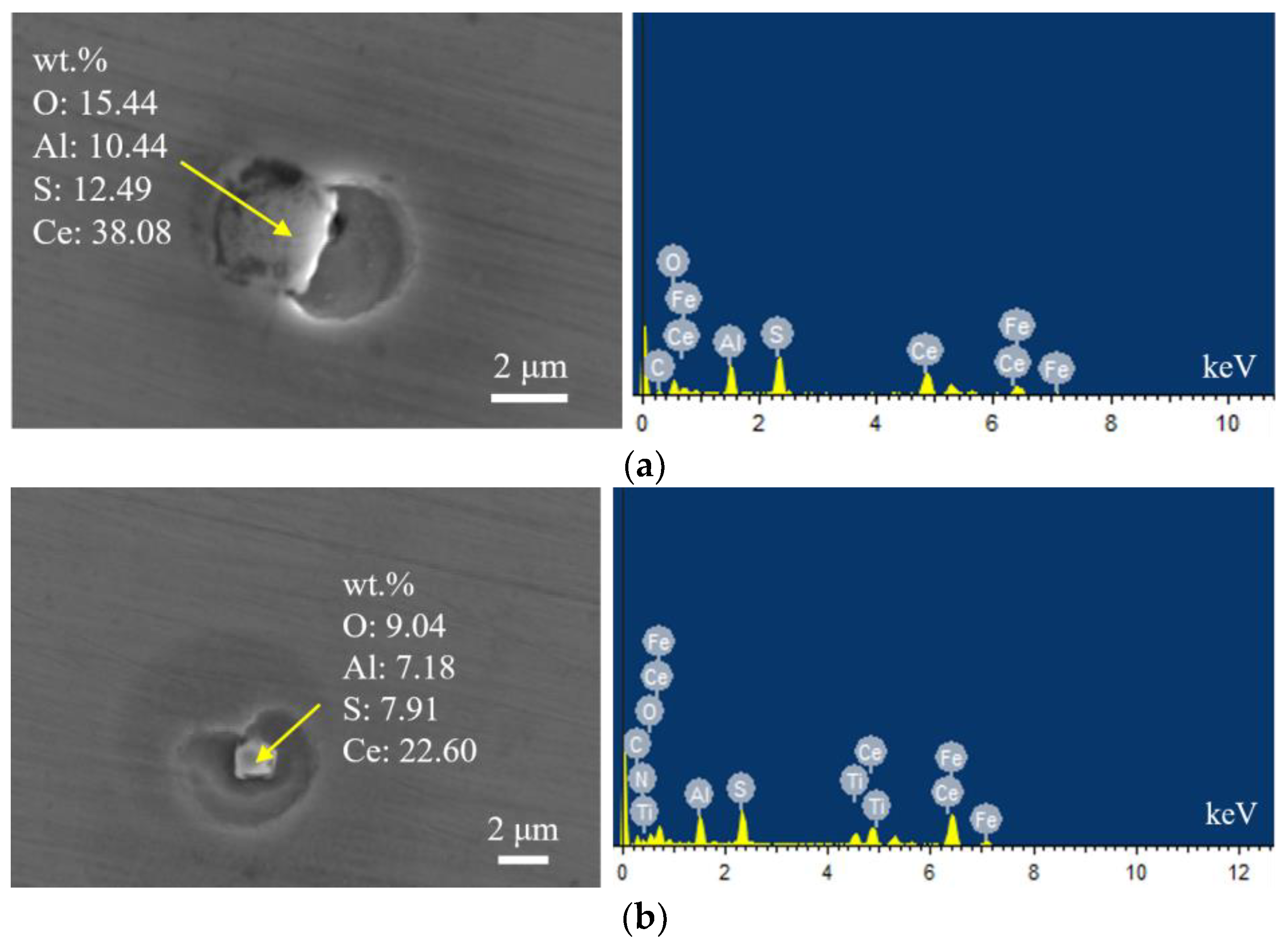
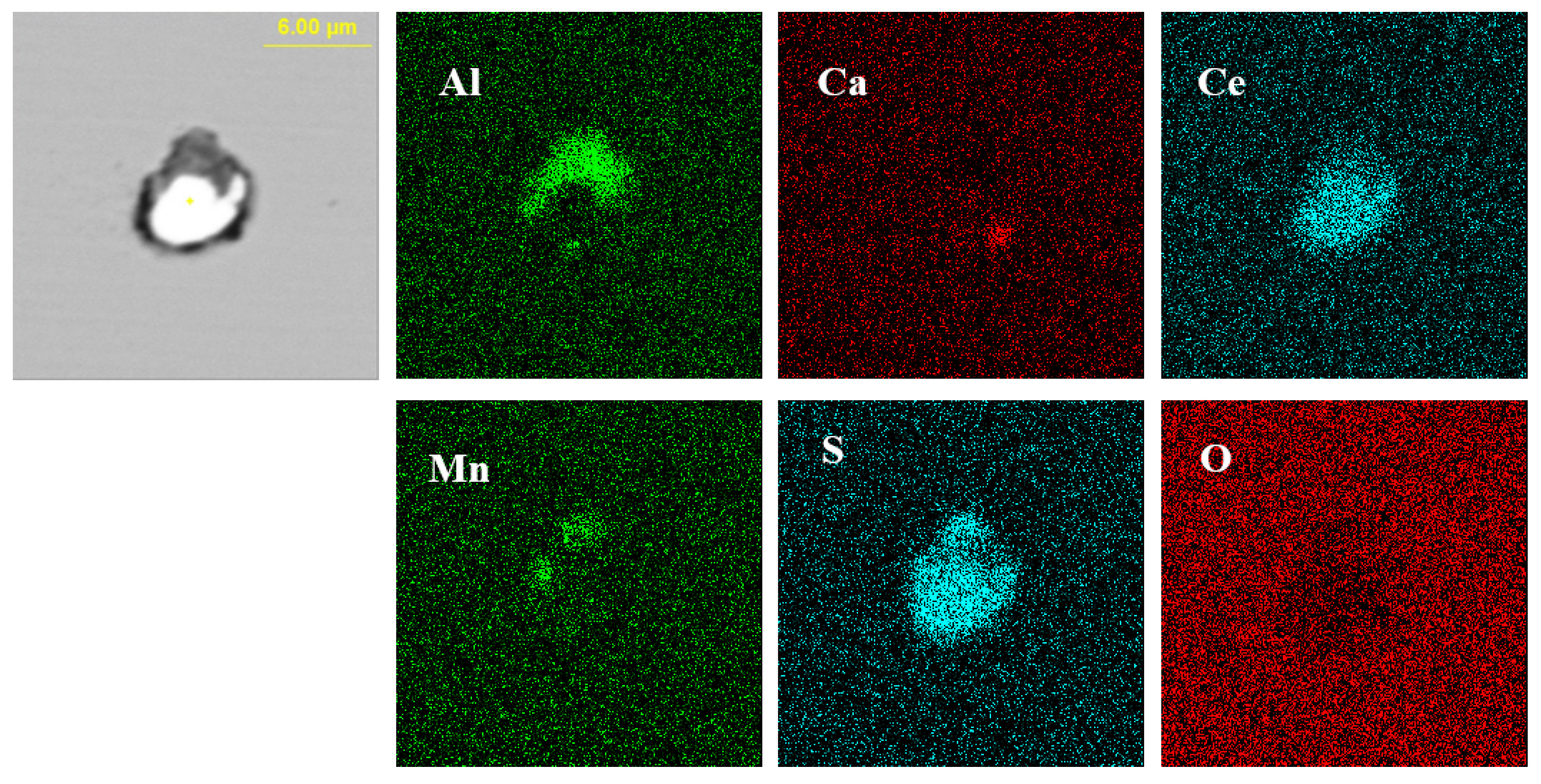
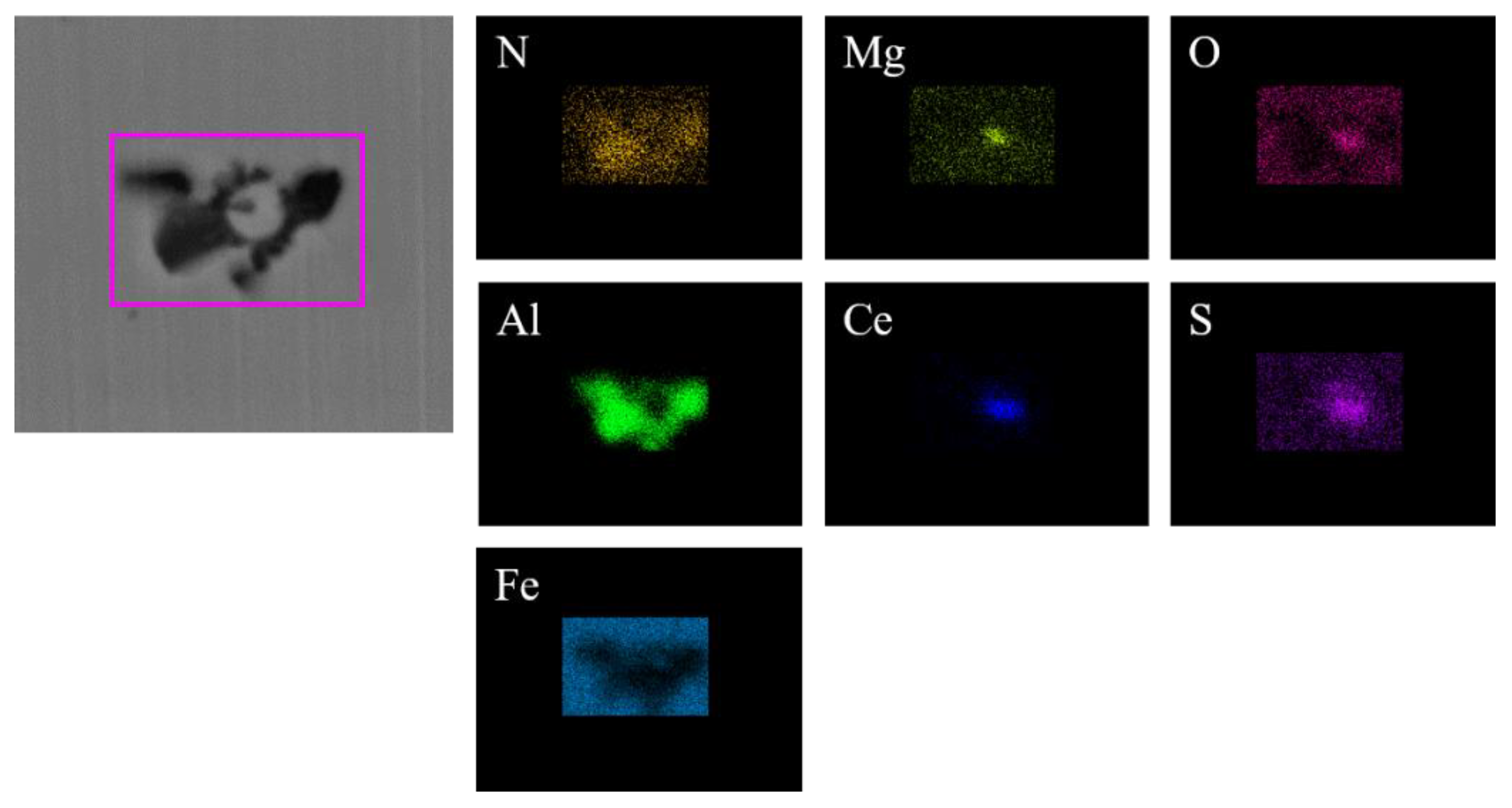
Figure 2 shows the typical morphology and energy spectrum of inclusions in the steel with 10 ppm Ce content. It can be seen that the type of inclusions in the steel was mainly CeAlO3-Ce2O2S-(AlN), where CeAlO3-Ce2O2S are light gray, and AlN in some inclusions was coated with CeAlO3-Ce2O2S. The main morphology of CeAlO3-Ce2O2S-(AlN) inclusions was spherical or ellipsoidal. The elemental mapping of a typical inclusion is shown in Figure 3.

Figure 2.
Typical morphology and energy spectrum of inclusions in the steel with 10 ppm Ce content. (a) CeAlO3-Ce2O2S, (b) CeAlO3-Ce2O2S.

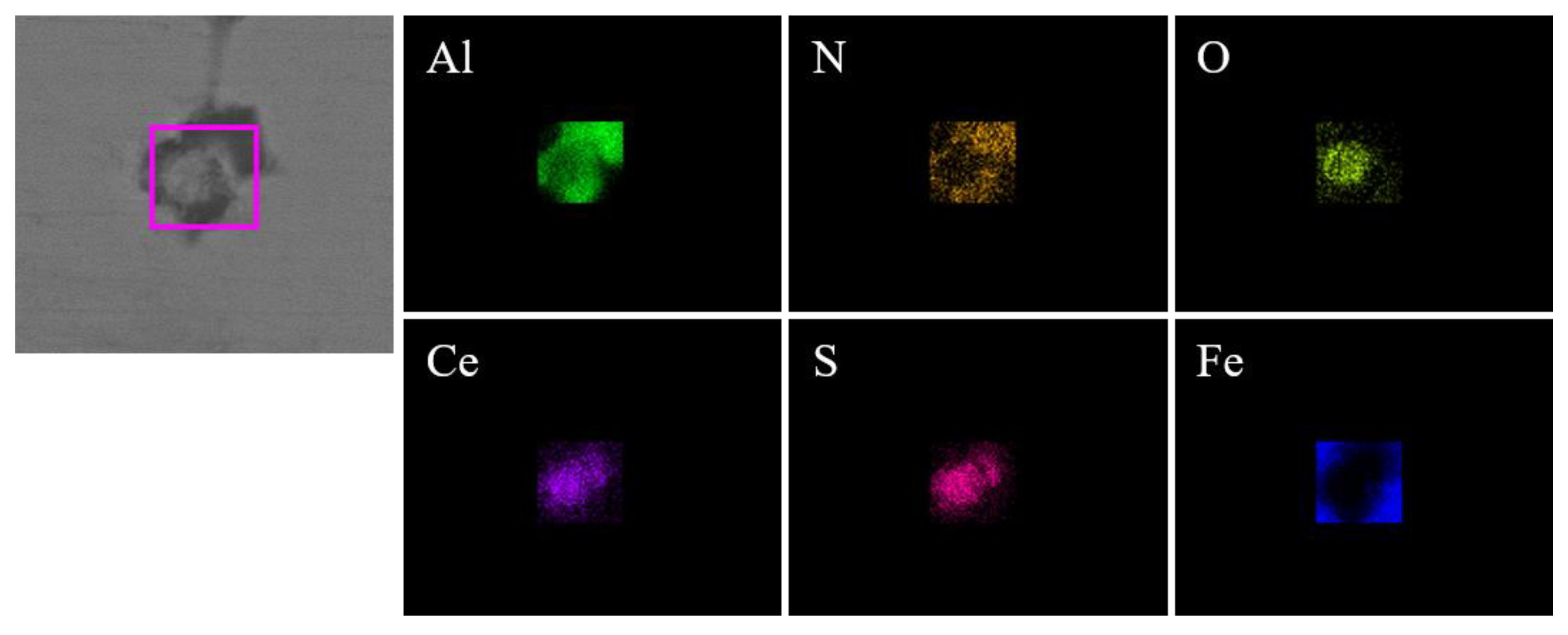
Figure 3.
Elemental mapping of a typical inclusion in the steel with 10 ppm Ce content.
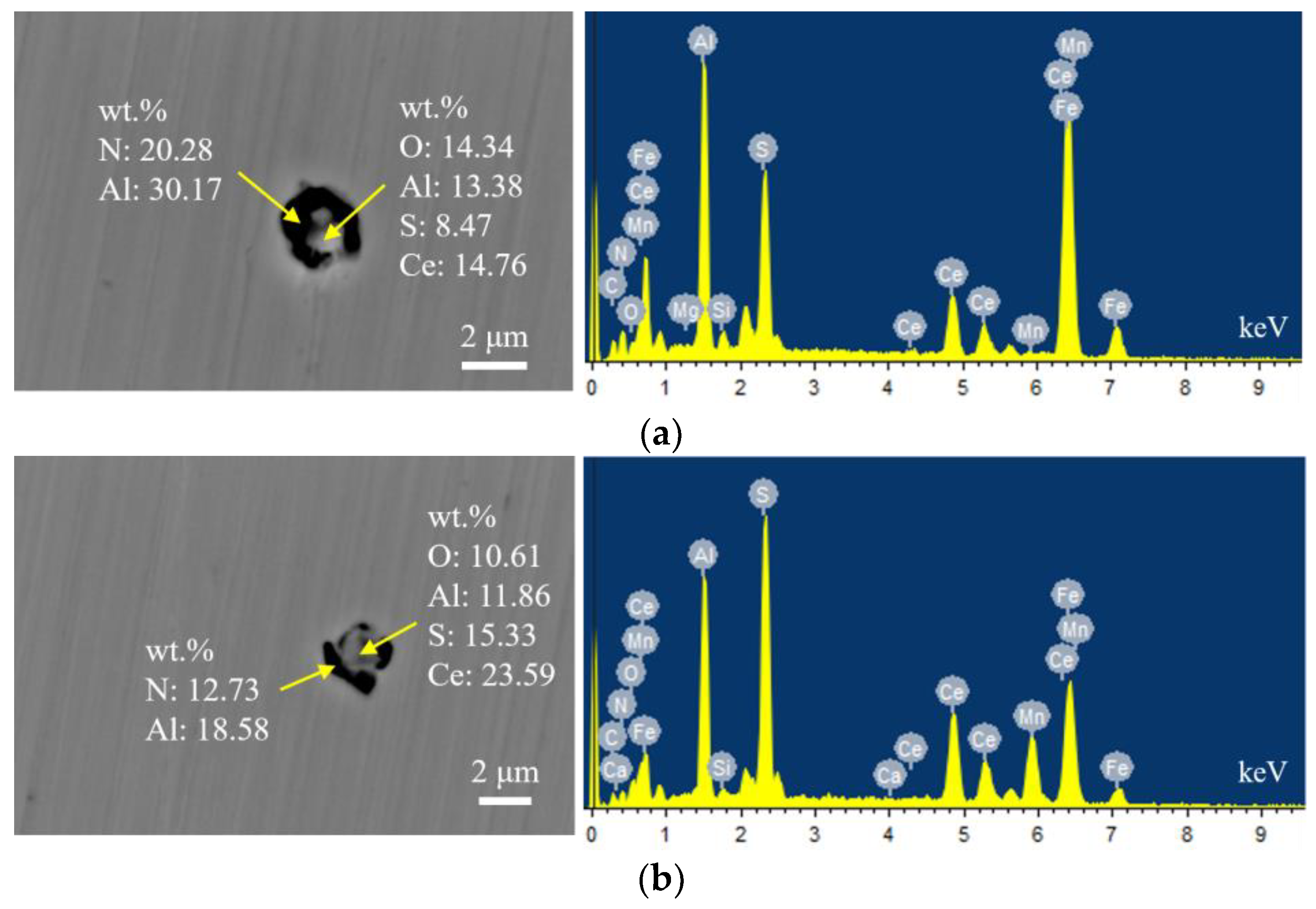
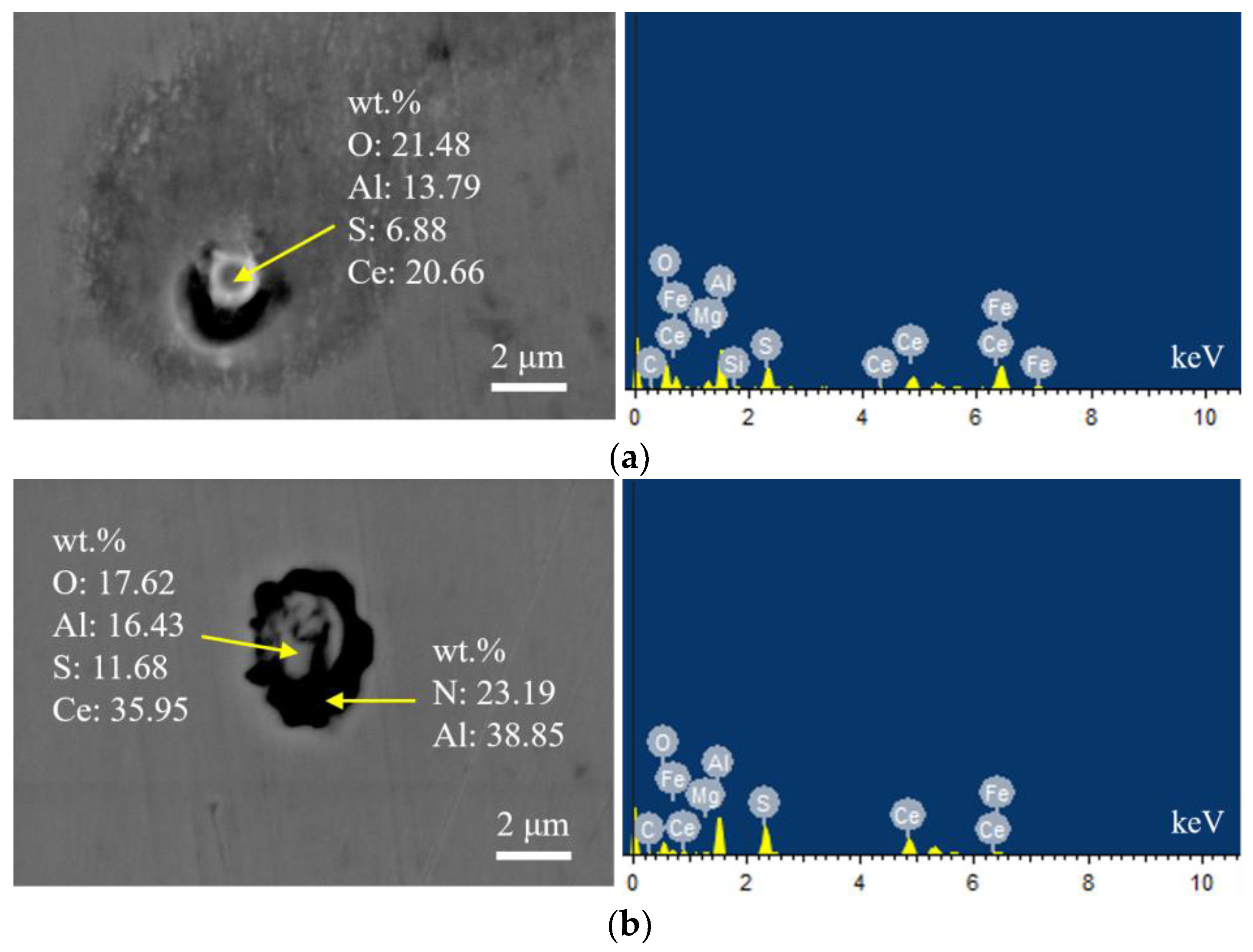
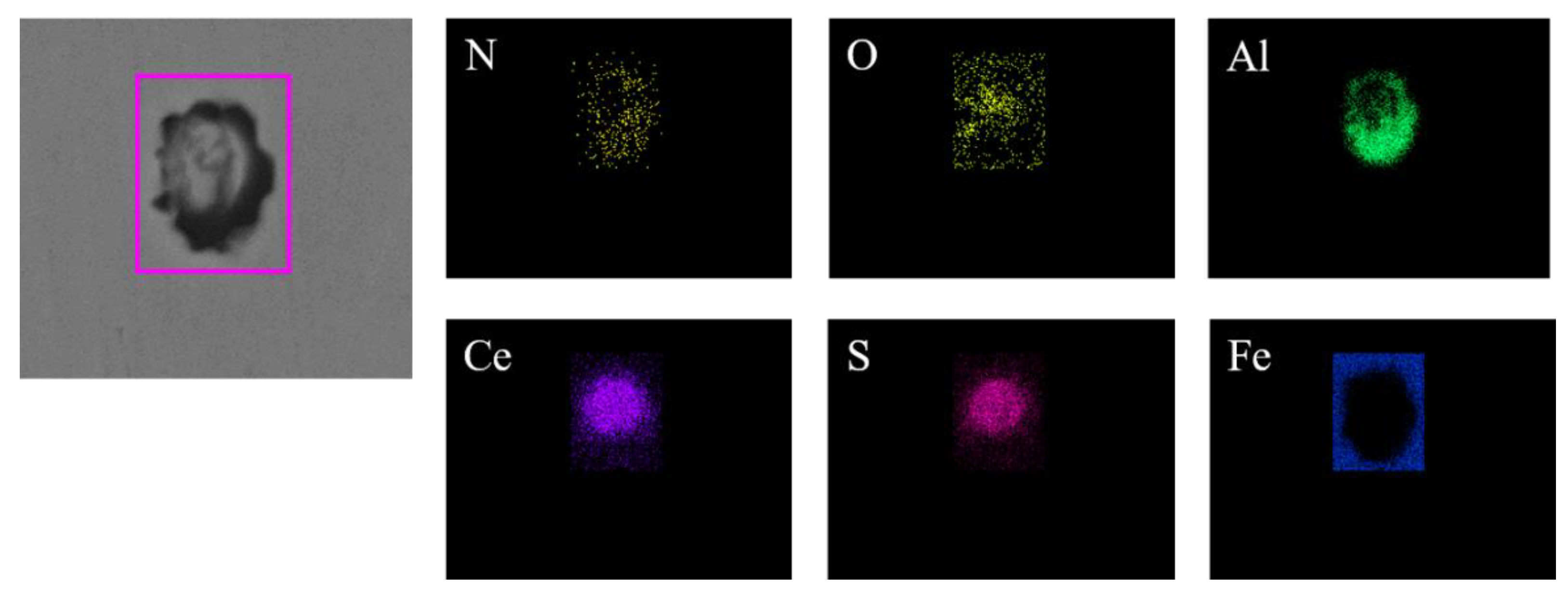
Figure 4 presents the typical morphology and energy spectrum results of inclusions in the steel with 14 ppm Ce content. The inclusions were composite phases, mainly with CeAlO3-Ce2O2S as the core, and the outer layer was wrapped with AlN. The core of some inclusions was CexSy-CeAlO3 and its morphology was spherical. The outer layer was wrapped with sharp-angled Al2O3-SiO2. The elemental mapping of a typical inclusion is shown in Figure 5.

Figure 4.
Typical morphology and energy spectrum of inclusions in the steel with 14 ppm Ce content. (a) Inner layer: CeAlO3-Ce2O2S, Outer layer: AlN, (b) Inner layer: CeAlO3-Ce2O2S, Outer layer: AlN.
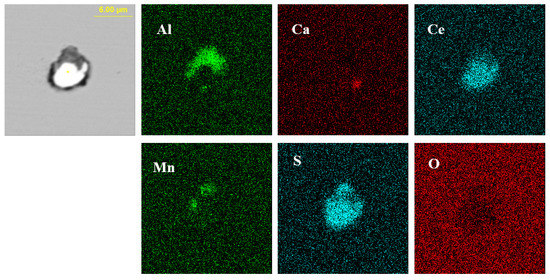
Figure 5.
Elemental mapping of a typical inclusion in the steel with 14 ppm Ce content.
Figure 6 shows the typical morphology and energy spectrum results of inclusions in the steel with 20 ppm Ce content. The inclusions in the steel were mainly composite phases, with CeAlO3-Ce2O2S as the core. The morphology of inclusions was spherical or ellipsoidal, and the outer layer was wrapped with AlN. The elemental mapping of a typical inclusion is shown in Figure 7.
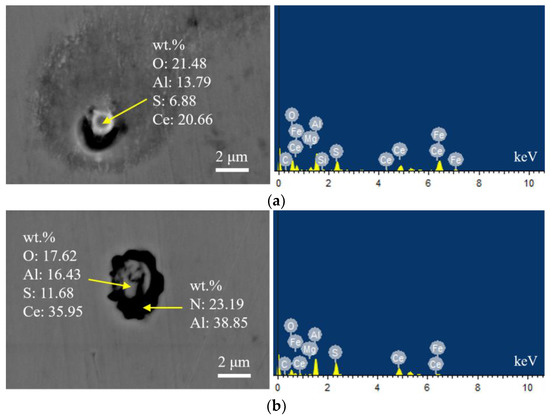
Figure 6.
Typical morphology and energy spectrum of inclusions in the steel with 20 ppm Ce content. (a) CeAlO3-Ce2O2S, (b) Inner layer: CeAlO3-Ce2O2S, Outer layer: AlN.
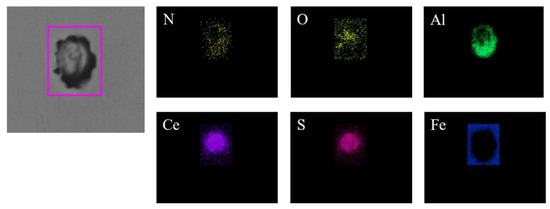
Figure 7.
Elemental mapping of a typical inclusion in the steel with 20 ppm Ce content.
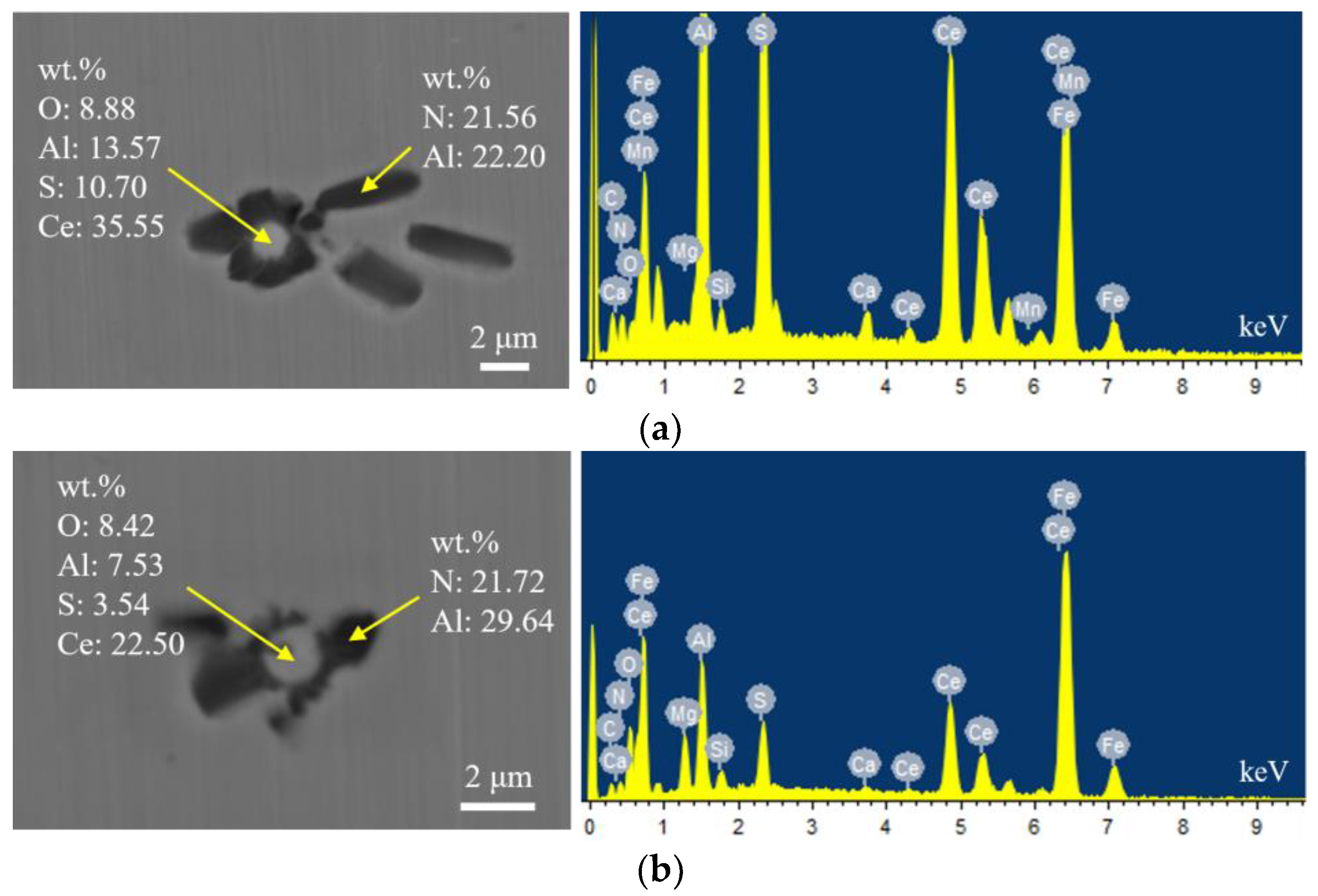
Figure 8 shows the typical morphology and energy spectrum results of inclusions in the steel with 30 ppm Ce content. It can be seen that the main types of inclusions in the steel were CeAlO3-Ce2O2S-AlN. The inner layer was wrapped with CeAlO3-Ce2O2S and its morphology was spherical. The outer layer was wrapped with irregularly shaped AlN. The elemental mapping of a typical inclusion is shown in Figure 9.

Figure 8.
Typical morphology and energy spectrum of inclusions in the steel with 30 ppm Ce content. (a) Inner layer: CeAlO3-Ce2O2S, Outer layer: AlN, (b) Inner layer: CeAlO3-Ce2O2S, Outer layer: AlN.
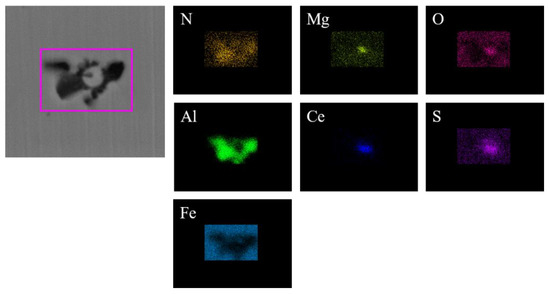
Figure 9.
Elemental mapping of a typical inclusion in the steel with 30 ppm Ce content.
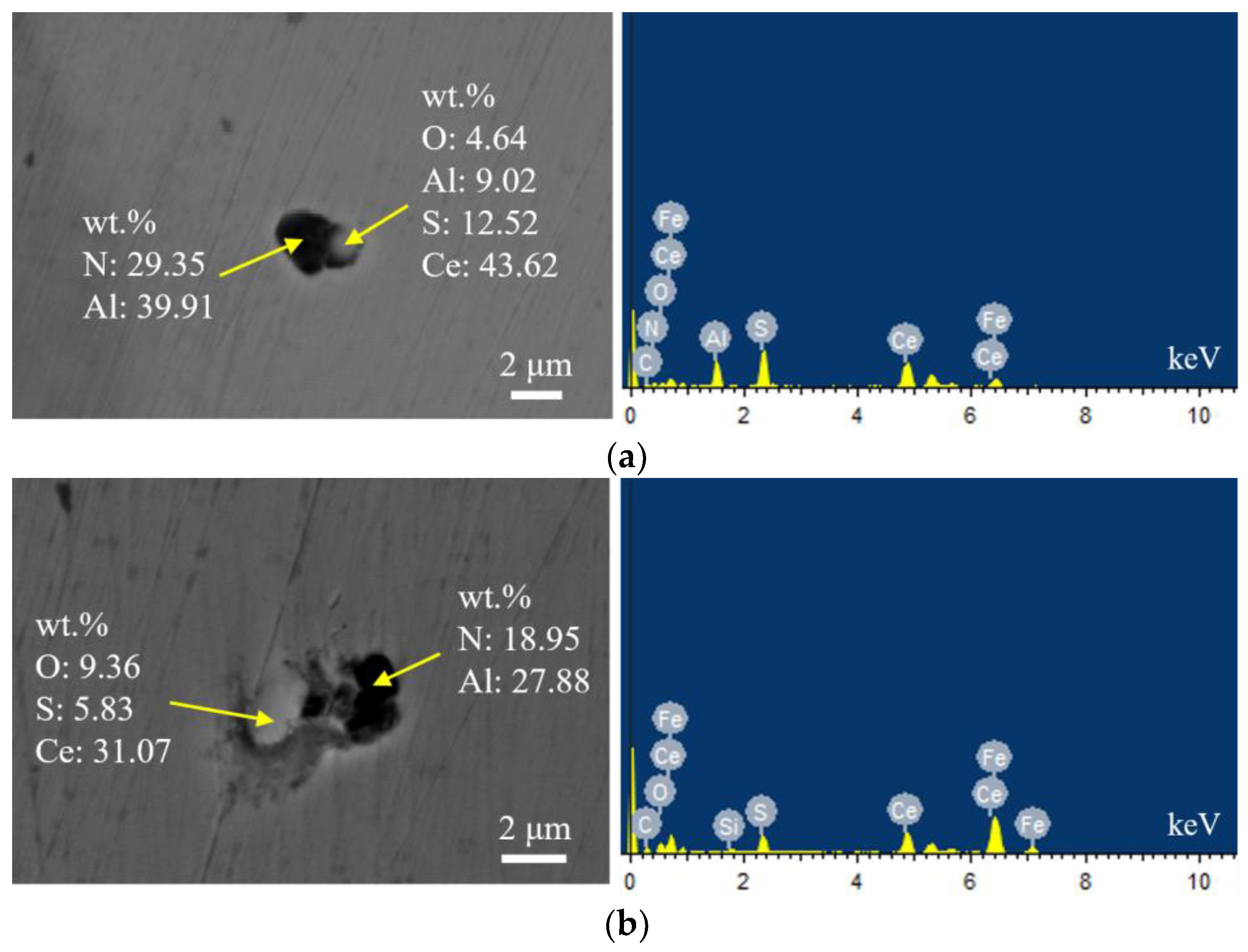
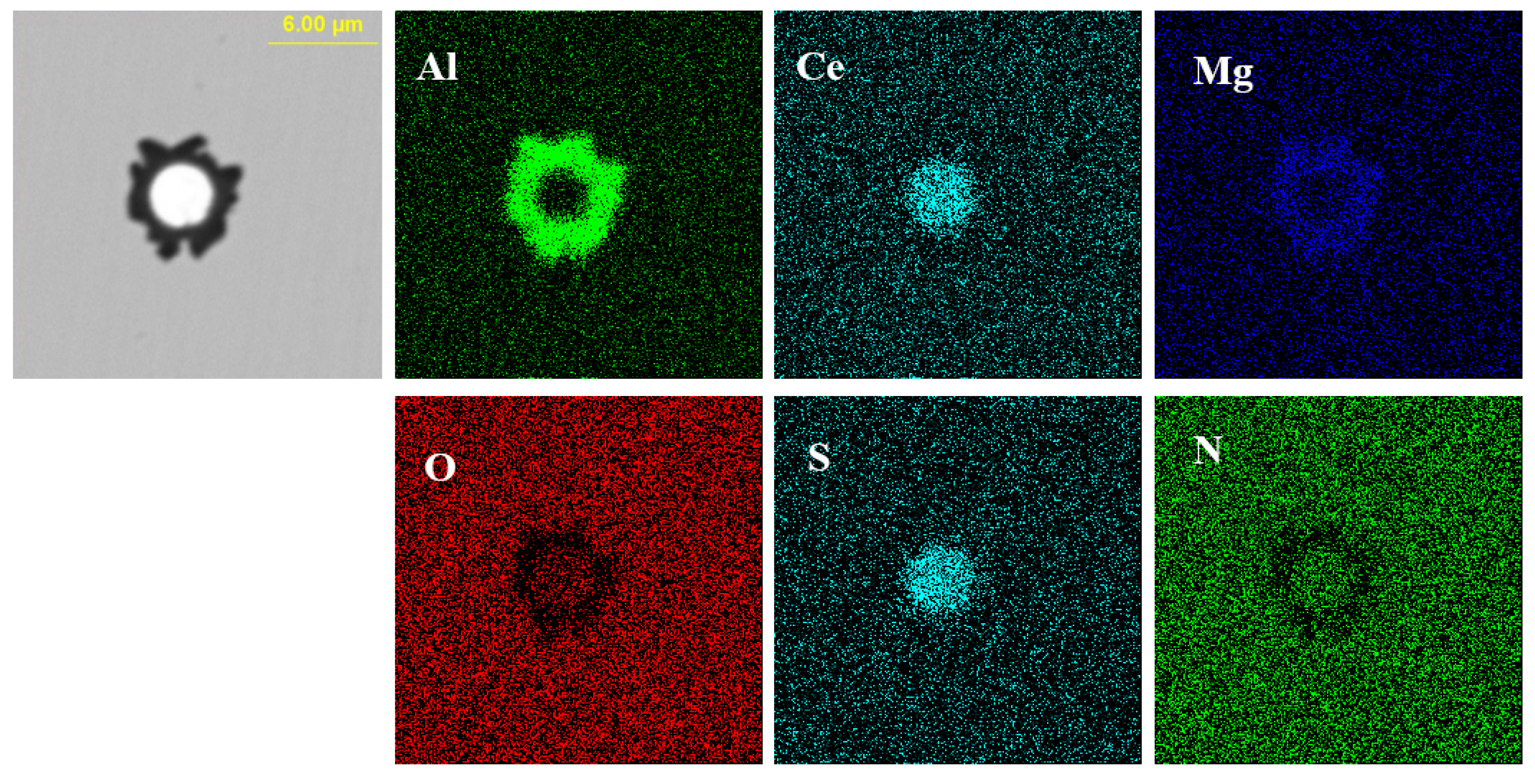
Figure 10 shows the typical morphology and energy spectrum results of inclusions in the steel with 60 ppm Ce content. It was found that when the Ce content increased to 60 ppm, the types of inclusions in the steel were mainly CeAlO3-Ce2O2S-AlN and Ce2O2S-AlN. The morphology of Ce2O2S inclusions was approximately spherical. As shown in Figure 11, the core of the inclusion was CexSy-Ce2O2S and the outer layer was wrapped with MgO·Al2O3 phase.
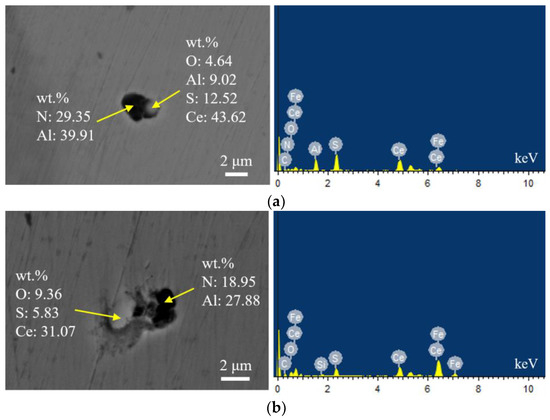
Figure 10.
Typical morphology and energy spectrum of inclusions in the steel with 60 ppm Ce content. (a) CeAlO3-Ce2O2S-AlN, (b) Ce2O2S-AlN.
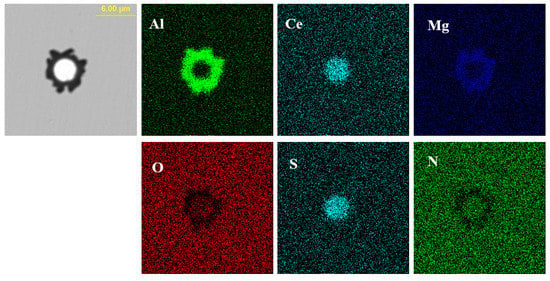
Figure 11.
Elemental mapping of a typical inclusion in the steel with 60 ppm Ce content.
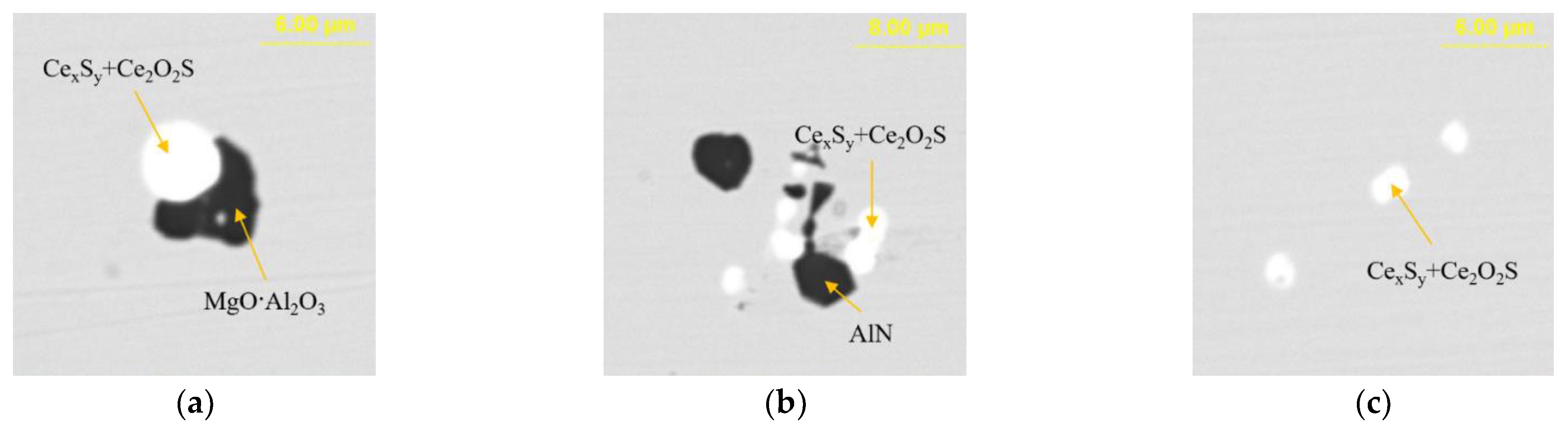
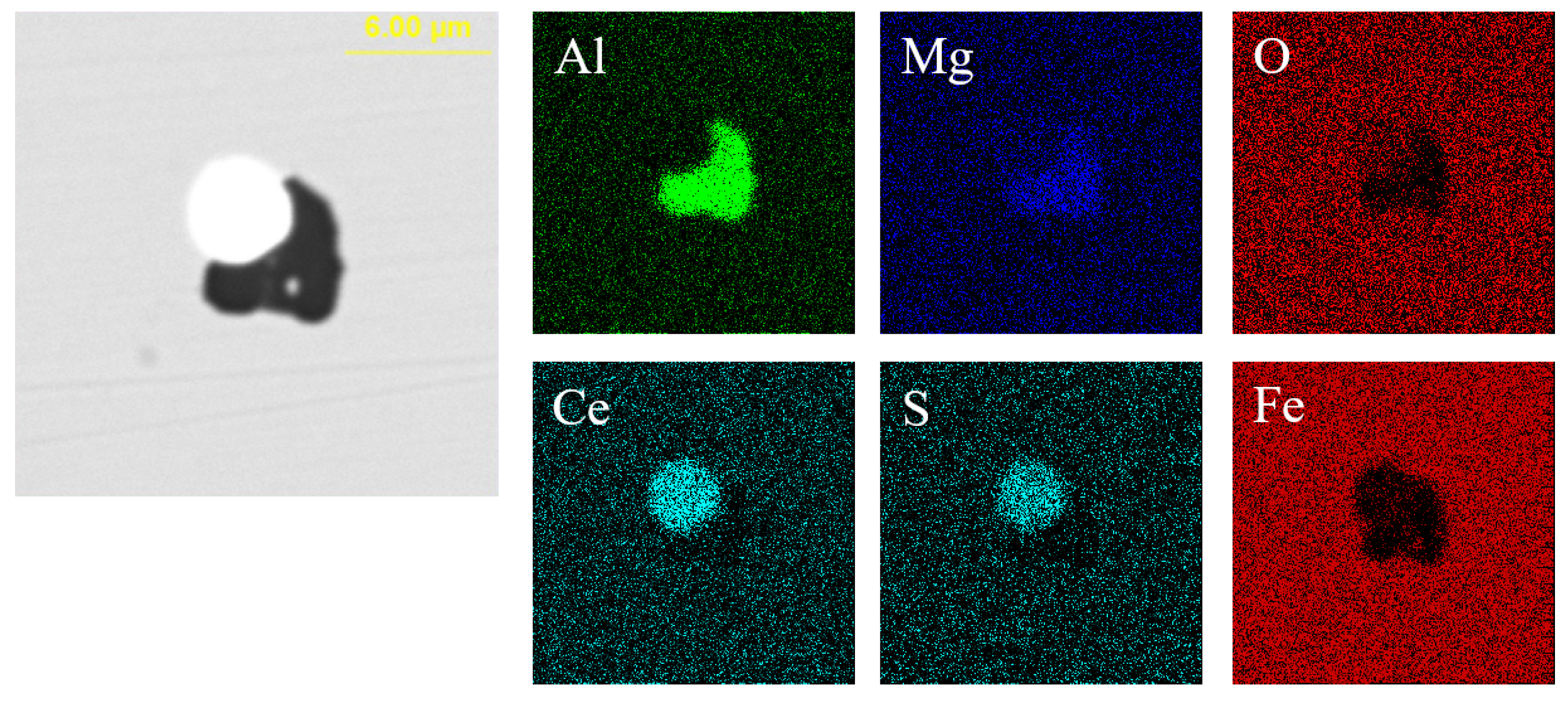
Figure 12 presents the typical morphology and composition of inclusions in the steel with 95 ppm Ce content. When the Ce content increased to 95 ppm, the core of inclusions in the steel was CexSy-Ce2O2S and its morphology was spherical or ellipsoidal. The outer layer of some inclusions was wrapped with AlN or MgO·Al2O3 phase. The elemental mapping of a typical inclusion is shown in Figure 13.

Figure 12.
Typical morphology and energy spectrum of inclusions in the steel with 95 ppm Ce content. (a) CexSy-Ce2O2S-MgO·Al2O3, (b) CexSy-Ce2O2S-AlN, (c) CexSy-Ce2O2S.
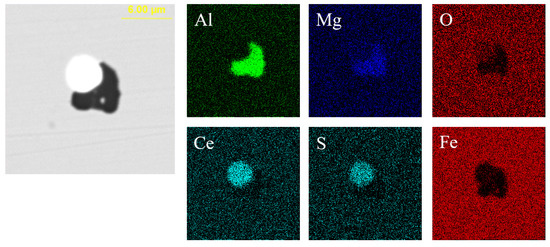
Figure 13.
Elemental mapping of a typical inclusion in the steel with 95 ppm Ce content.
4. Discussion
4.1. Effects of Rare Earth Ce Content on Inclusion Type
The type of inclusions in W350 non-oriented silicon steel was mainly Al2O3 without rare earth Ce. With the addition of Ce, reactions with O and S in molten steel would occur to form a variety of oxides, sulfides and oxysulfides. The possible reactions involved are as follows [20,21,22,23,24]:
When the rare earth Ce is fed into molten steel, it not only reacts easily with Al2O3 to form CeAlO3, but also converts the generated Ce-containing inclusions. The possible reactions involved are as follows:
The general expression of Gibbs free energy is:
where J is the ratio of the activity product of the resultant and the activity product of the reactant.
Assuming that the molten steel is an ideal dilute solution, and the solute follows Henry’s law, one can satisfy the following formula for calculating activity and activity coefficient [25]:
where ai is the activity of component i, fi is the activity coefficient of component i, and w[i] is the mass percentage of the element i. Thus:
where is the interaction coefficient between j element and component i in molten steel. The first-order interaction coefficient of each element in molten steel is listed in Table 4 [26,27,28].

Table 4.
The first-order interaction coefficient of each element in molten steel.
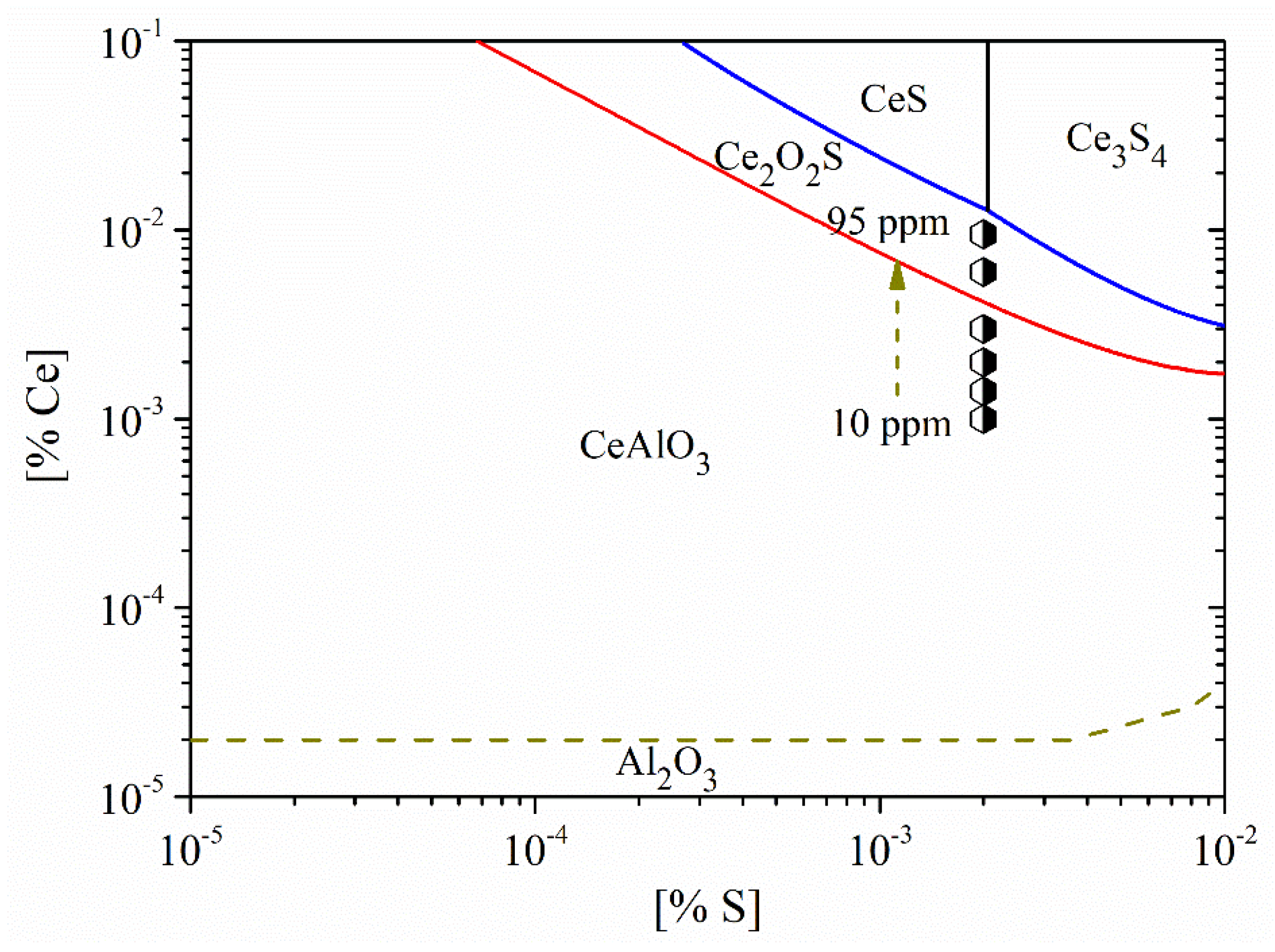
Figure 14 shows the stability diagram of Al2O3-CeAlO3-Ce2O2S-CexSy in non-oriented electrical steel. Obviously, with the increase in Ce content in the steel, the evolution sequence of inclusions was CeAlO3→Ce2O2S→CexSy. Based on the experimental results, the types of inclusions in the steel were mainly CeAlO3-Ce2O2S-(AlN) when the Ce content was 10 ppm. When the Ce content was 14 ppm, the inclusions were composed of composite phases. The core was mainly CeAlO3-Ce2O2S and the outer layer was wrapped with AlN. Meanwhile, the core of some inclusions was CexSy-CeAlO3. When the Ce content was 20 ppm, the composition of inclusions was also composite phases with CeAlO3-Ce2O2S as the core. When the Ce content was increased to 30 ppm, the main types of inclusions in the steel were CeAlO3-Ce2O2S-AlN. The above results showed that the rare earth inclusions in the steel were mainly CeAlO3 + Ce2O2S when the added Ce content was 10~30 ppm.
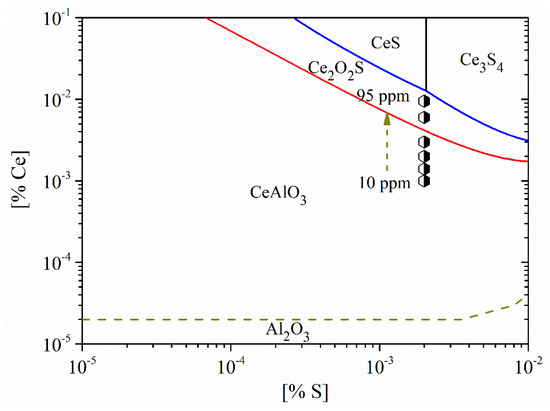
Figure 14.
Stability diagram of Al2O3-CeAlO3-Ce2O2S-CexSy in non-oriented electrical steel.
Furthermore, when the Ce content was increased to 60 ppm, the composition of inclusions in the steel was mainly Ce2O2S and some inclusions contained a small amount of CeAlO3. The inclusions were changed to CexSy-Ce2O2S when the Ce content was 95 ppm. As shown in Figure 14, when the added Ce content is 95 ppm, the composition of the inclusions is located near the boundary between CexSy and Ce2O2S, which was consistent with the experimental results.
Figure 14 also shows that the critical Ce content for the conversion between CeAlO3 and Ce2O2S was 41 ppm. To ensure the inclusions transformed from CeAlO3 to Ce2O2S, the Ce content in the steel had to be greater than 41 ppm. Due to the addition of rare earth Ce elements, the formation of rare earth aluminates, rare earth oxygen sulfides and rare earth sulfides with high melting point can occur in molten steel. These large-sized composite inclusions can be used as heterogeneous nucleation particles and it is beneficial to control the modification and precipitation of Al2O3, MnS, TiN, and AlN, thereby improving the magnetic properties of the product.
4.2. Effects of Rare Earth Ce on Size and Distribution of Inclusions
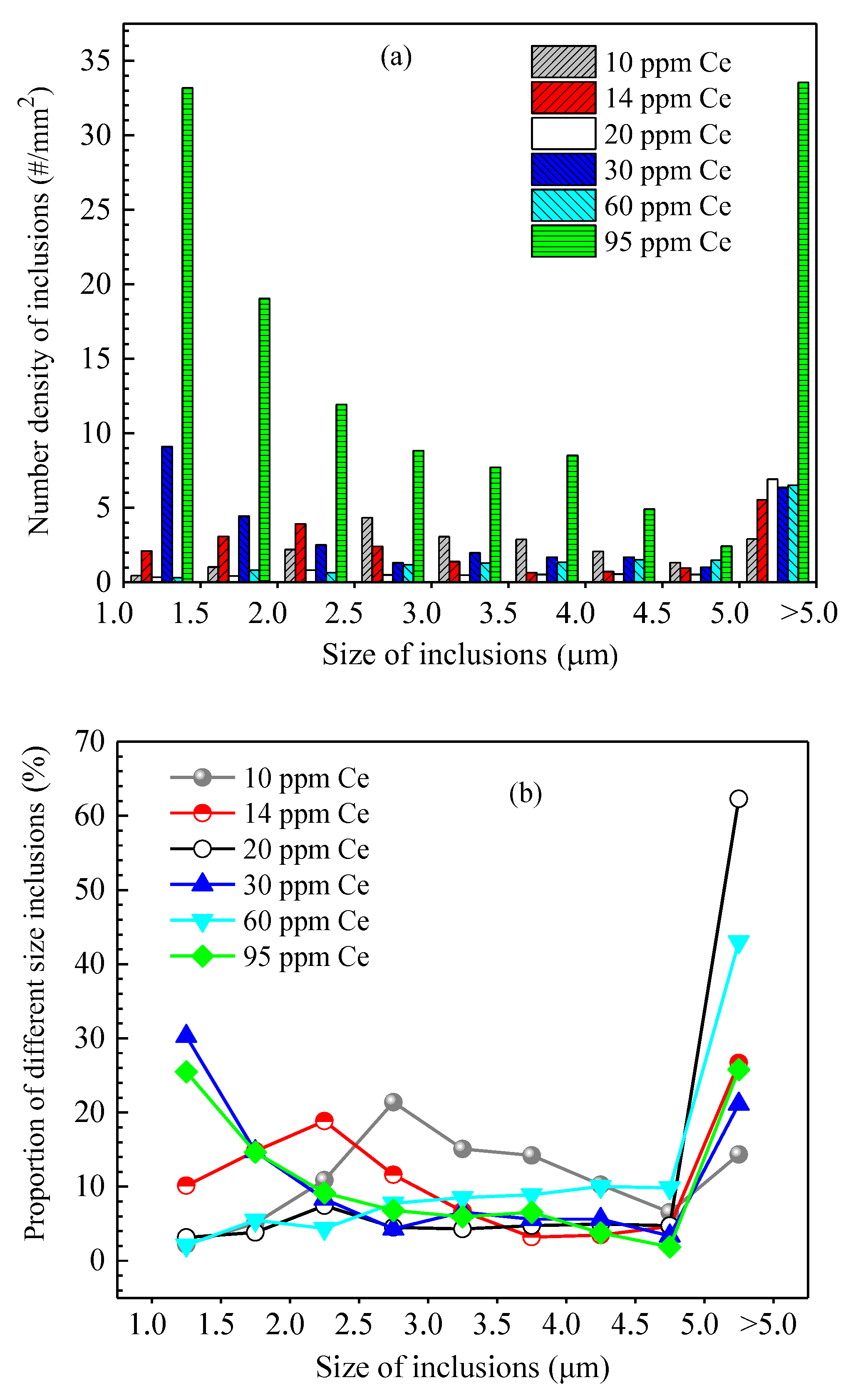
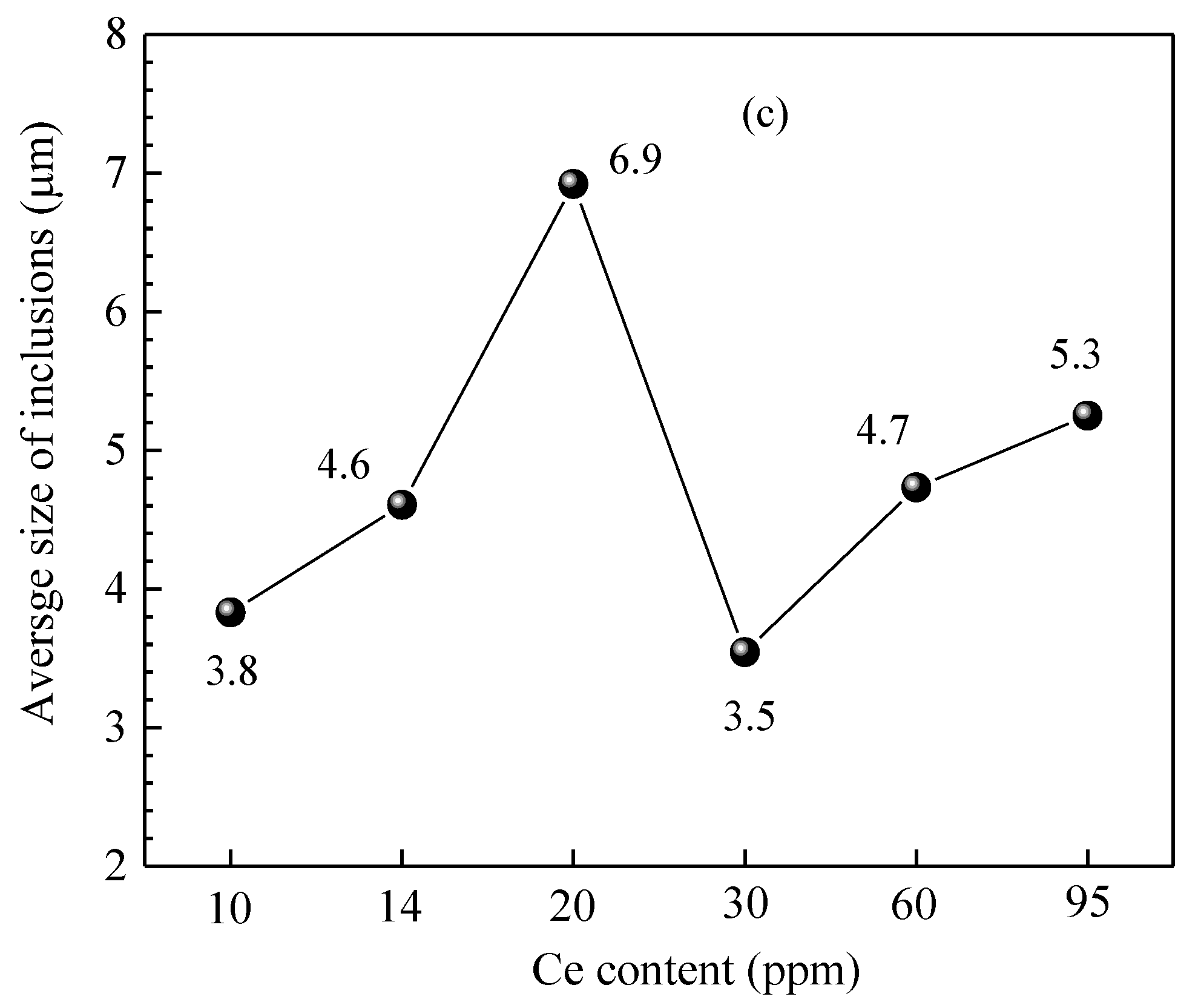
Figure 15 shows the size distribution and the average size of inclusions in steel of different heats. It shows that when the added Ce content in the steel was 10 ppm, the size of inclusions in the steel was concentrated in the range of 2.0~4.5 μm and the corresponding proportion was 10.3~21.4%. When the added Ce content was 20 ppm and 60 ppm, the number density of inclusions in the steel was relatively low (11.11 #/mm2 and 15.14 #/mm2, respectively). Addition of Ce decreased the amount of harmful inclusions (such as Al2O3, MnS) in the steel, which is beneficial for improving the steel cleanliness and the magnetic properties of the product. Moreover, the number density of >5.0 μm inclusions significantly increased (6.9 #/mm2 and 6.5 #/mm2 with Ce content of 20 ppm and 60 ppm, respectively), indicating that rare earth treatment can promote the generation of large-sized inclusions in the steel. When the Ce content was 20 ppm, the average size of inclusions in the steel was at its largest, at 6.9 μm. When the Ce content in the steel increased to 95 ppm, the number density of inclusions of all size ranges obviously increased and the total number density was 130.1 #/mm2. It was larger than that of other Ce content levels, especially for inclusions with size range of 1.0~1.5 μm and >5.0 μm. Their number densities were 33.17 #/mm2 and 33.54 #/mm2, respectively, and the corresponding proportion was about 25%, which would deteriorate the steel cleanliness and the magnetic properties of the product.
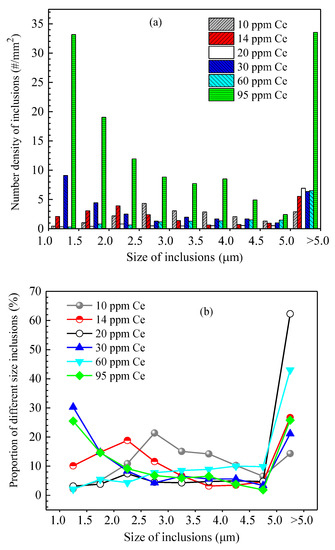
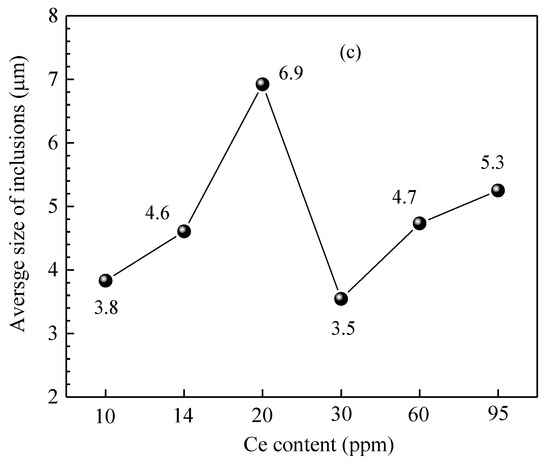
Figure 15.
Size distribution and average size of inclusions in the steel of different heats. (a) Inclusion size distribution, (b) ratio of different size inclusions, (c) average size of inclusions.
5. Conclusions
- (1)
- With the increase in the Ce content in the steel, the modification sequence of inclusions was CeAlO3→Ce2O2S→CexSy. When the added Ce content in the steel was 10 ppm, 14 ppm, 20 ppm and 30 ppm respectively, the rare earth inclusions were mainly CeAlO3-Ce2O2S. When the added Ce content increased to 60 ppm, the rare earth inclusions were mainly Ce2O2S and a small amount of CeAlO3 contained in part inclusions. When the added Ce content increased continually to 95 ppm, the rare earth inclusions were mainly CexSy + Ce2O2S.
- (2)
- When the added Ce content was 95 ppm, the composition was located near the boundary between rare earth sulfide and rare earth oxysulfide. The critical Ce content for the conversion between CeAlO3 and Ce2O2S was 41 ppm.
- (3)
- The difference in the Ce content significantly affected the number density and size distribution of inclusions in the steel. When the added Ce content was 20 ppm, the number density and proportion of inclusions in the steel were lower, and its average size was larger. When the added Ce content increased to 95 ppm, the number density of inclusions in the steel significantly increased.
Author Contributions
Conceptualization, H.W. and H.L.; methodology, H.W.; software, H.W.; validation, H.W., Y.Z. and W.Z.; formal analysis, H.W.; investigation, H.W.; resources, H.W.; data curation, H.W., J.Q. and Y.N.; writing—original draft preparation, H.W.; writing—review and editing, H.W. and S.Q.; visualization, H.W. and H.L.; supervision, S.Q.; project administration, H.W. and S.Q.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 52274312 and 51804003), the Fund of Education Department of Anhui Province (Nos. 2022AH050291, 2022AH050293), the Open Project Program of Anhui Province Key Laboratory of Metallurgical Engineering & Resources Recycling (Anhui University of Technology) (No. SKF21-04), and the Jiangxi Province Major Scientific and Technological Research and Development Special Funding Project (20213AAE01009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ji, S.C.; Shi, Y.H.; Zhang, F.; Lu, W.F. Review on vibration and noise of power transformer and its control measures. High Volt. Appar. 2019, 55, 1–17. [Google Scholar]
- Yan, M.; Xiao, H.H.; Huang, X.; Zhang, Y.D. Analysis of new energy vehicles development and electrical steel demand for driving motor. Electr. Steel 2020, 2, 1–5. [Google Scholar]
- Xu, X.X.; Qin, J.; Zhao, H.B.; Nie, J.C.; Yu, H.Y. A study on the research status and development trend of non-oriented electrical steel with high grade for new energy vehicles. Jiangxi Metall. 2020, 40, 6–11. [Google Scholar]
- Gao, L.Y.; Zhou, H.J.; Wang, C.; Pei, R.L. Development overview and trend analysis of electric drive system of new energy vehicle. Electr. Steel 2020, 2, 27–31. [Google Scholar]
- Chen, Z. Research on the development change and the demand of electrical steel in China in the new era. Electr. Steel 2019, 1, 1–6. [Google Scholar]
- Guo, F.H. Study on Control of Oxide Inclusion in Non-Oriented Electrical Steel. Master’s Thesis, Anhui University of Technology, Ma’anshan, China, 3 June 2019. [Google Scholar]
- He, Z.Z.; Zhao, Y.; Luo, H.W. Electrical Steel; Metallurgical Industry Press: Beijing, China, 2012. [Google Scholar]
- Zhang, X.F.; Tang, J.P.; Han, C.P.; Wang, A.L.; Zhi, J.G. Analysis on the role of rare earth in steel and the present situation of industrial production. Chin. Rare Earths 2020, 42, 117–130. [Google Scholar]
- Dong, H.; Lian, X.T.; Hu, C.D.; Lu, H.C.; Peng, W.; Zhao, H.S.; Xu, D.X. High performance steels: The scenario of theory and technology. Acta Metall. Sin. 2020, 56, 558–582. [Google Scholar]
- Ren, H.P.; Wu, Z.W.; Jin, Z.L. Functions and research progresses of rare earth in electrical steel. Sci. Technol. Baotou Steel 2018, 44, 1–6. [Google Scholar]
- Wang, L.M.; Tan, Q.Y.; Li, N.; Qin, Z.; Qiu, S.T.; Gan, Y. Research progress on rare earth application technology in non-oriented electrical steels. J. Chin. Soc. Rare Earths 2014, 32, 513–533. [Google Scholar]
- Zhang, F.; Li, G.Q.; Zhu, C.Y. Heredity characters of inclusions and electromagnetic properties of non-oriented silicon steel treated by rare earth alloy. J. Funct. Mater. 2013, 44, 1029–1033. [Google Scholar]
- Lin, Q.; Guo, F.; Zhu, X.Y. Behaviors of La and Ce on grain boundaries in carbon manganese cleanliness steel. J. Chin. Soc. Rare Earths 2006, 24, 427–430. [Google Scholar]
- Wang, L.M.; Du, T.; Lu, X.L.; Yue, K.X. Study of behaviors and application of micro-rare earth elements in steel. Chin. Rare Earths 2001, 22, 37–40. [Google Scholar]
- Takashima, M.; Morito, N.; Honda, A.; Maeda, C. Non-oriented electrical steel sheet with low iron loss for high-efficiency motor cores. IEEE Trans. Magn. 1999, 35, 557–561. [Google Scholar] [CrossRef]
- Takashima, M.; Ono, T.; Nishimura, K. Low iron loss non-oriented electrical steel for high efficiency motors “RMA Series”. Kawasaki Steel Tech. Rep. 1998, 39, 48–49. [Google Scholar]
- Wu, C.J.; Tang, X.L. Grain boundary segregation of Ce, Mo and P and temper embrittlement of steel. Iron Steel 1991, 26, 31–35. [Google Scholar]
- Liu, H.M.; Gao, L. The effect of rare earth metals in non-oriented silicon steel. Iron Steel 1987, 22, 41–45. [Google Scholar]
- Yuan, Z.X.; Li, J.H.; Feng, S.J.; Wu, C.J. Grain boundary segregation of Ce and temper embrittlement of steel. Chin. J. Eng. 1982, S1, 42–50. [Google Scholar]
- Chen, J.X. Manual of Chart Data for Steelmaking; Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
- Itoh, H.; Hino, M.; Ban-Ya, S. Thermodynamics on the formation of spinel nonmetallic inclusion in liquid steel. Metall. Mater. Trans. B 1997, 28, 953–956. [Google Scholar] [CrossRef]
- Vahed, A.; Kay, D.A.R. Thermodynamics of rare earths in steelmaking. Metall. Mater. Trans. B 1976, 7, 375–383. [Google Scholar] [CrossRef]
- Li, W.C. Thermodynamics of the formation of rare-earth inclusions in steel. Iron Steel 1986, 21, 7–12. [Google Scholar]
- Wang, L.M.; Du, T.; Lu, X.L.; Li, Z.B.; Gai, Y.C. Thermodynamics and application of rare earth elements in steel. J. Chin. Soc. Rare Earths 2003, 21, 251–254. [Google Scholar]
- Huang, X.H. Principles of Iron and Steel Metallurgy; Metallurgical Industry Press: Beijing, China, 2013. [Google Scholar]
- Zhang, L.F.; Ren, Y.; Duan, H.J.; Yang, W.; Sun, L.Y. Stability diagram of Mg-Al-O system inclusions in molten steel. Metall. Mater. Trans. B 2015, 46, 1809–1825. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.R.; Zeng, Z.Y.; Xi, Z.B. Crystallization of alumina modified by rare earth elements in SWRS82B steel. Iron Steel 2020, 55, 69–74. [Google Scholar]
- Pan, N.; Song, B.; Zhai, Q.J.; Wen, B. Effect of lattice disregistry on the heterogeneous nucleation catalysis of liquid steel. J. Beijing Univ. Sci. Technol. 2010, 32, 179–182. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).