Research Progress of Low Density and High Stiffness of Be-Al Alloy Fabricated by Investment Casting
Abstract
:1. Introduction
2. The Characteristics of Investment Be-Al Alloy
3. The Effect of Adding Elements on the Casting Process
3.1. Ag, Co, and Ge Elements
3.2. Ni Element
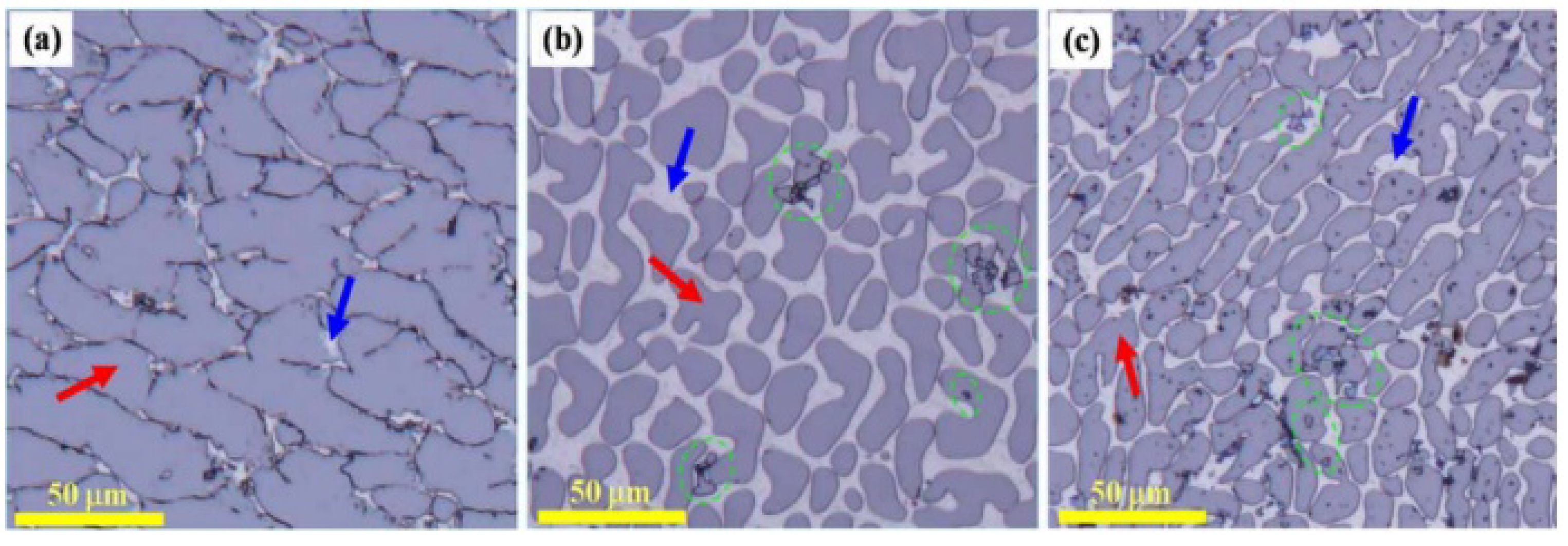
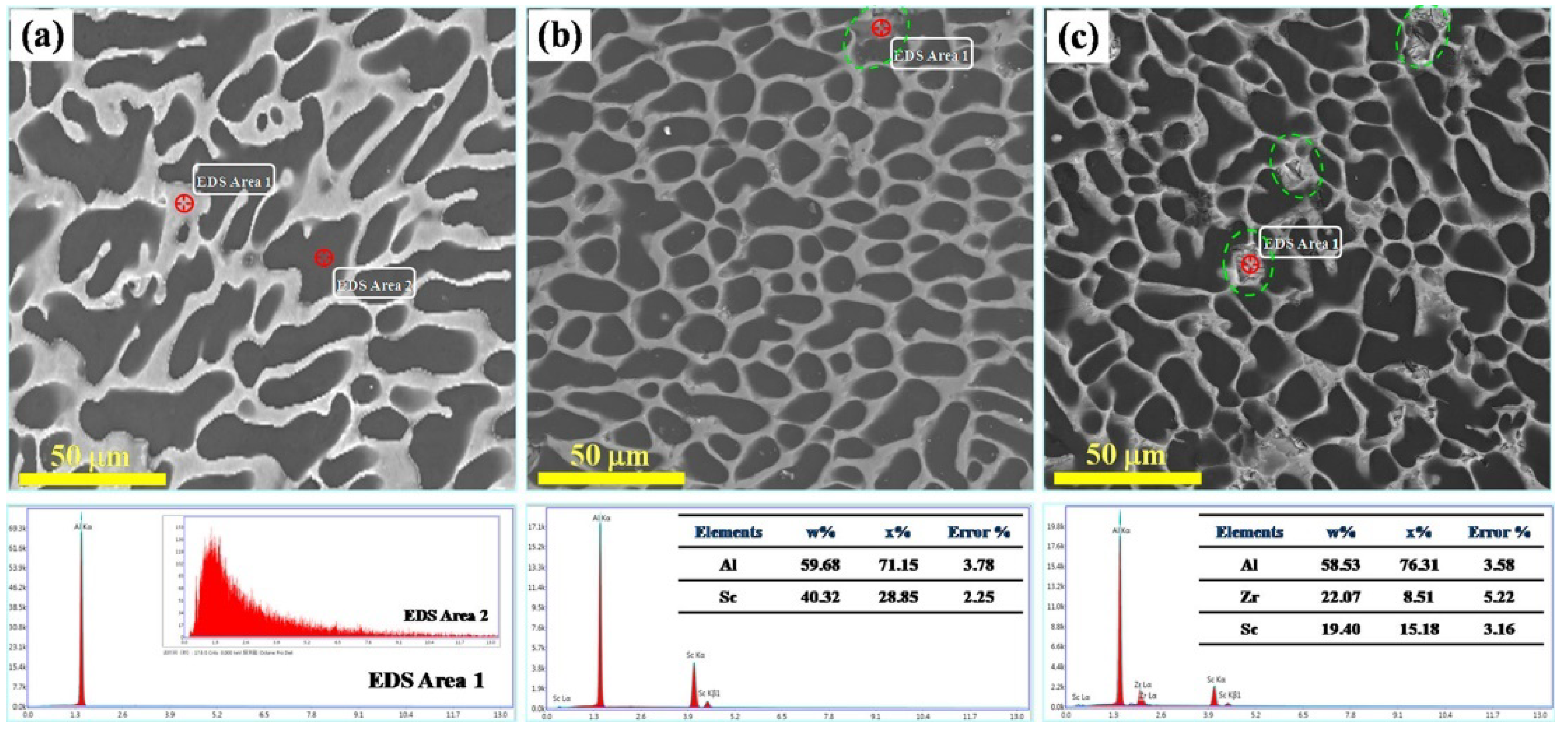
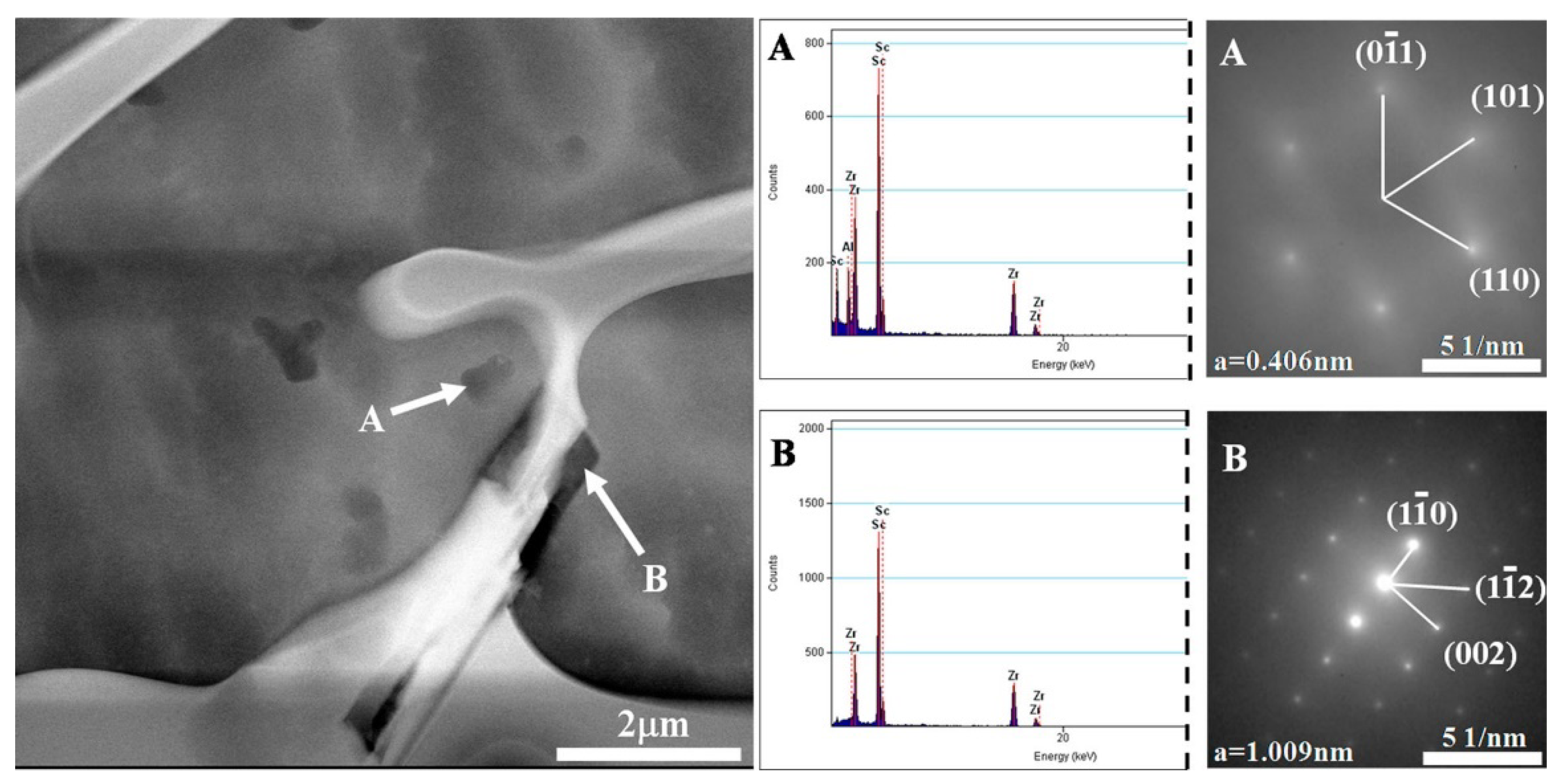
3.3. Sc Element
3.4. Si Element
3.5. Cu Element
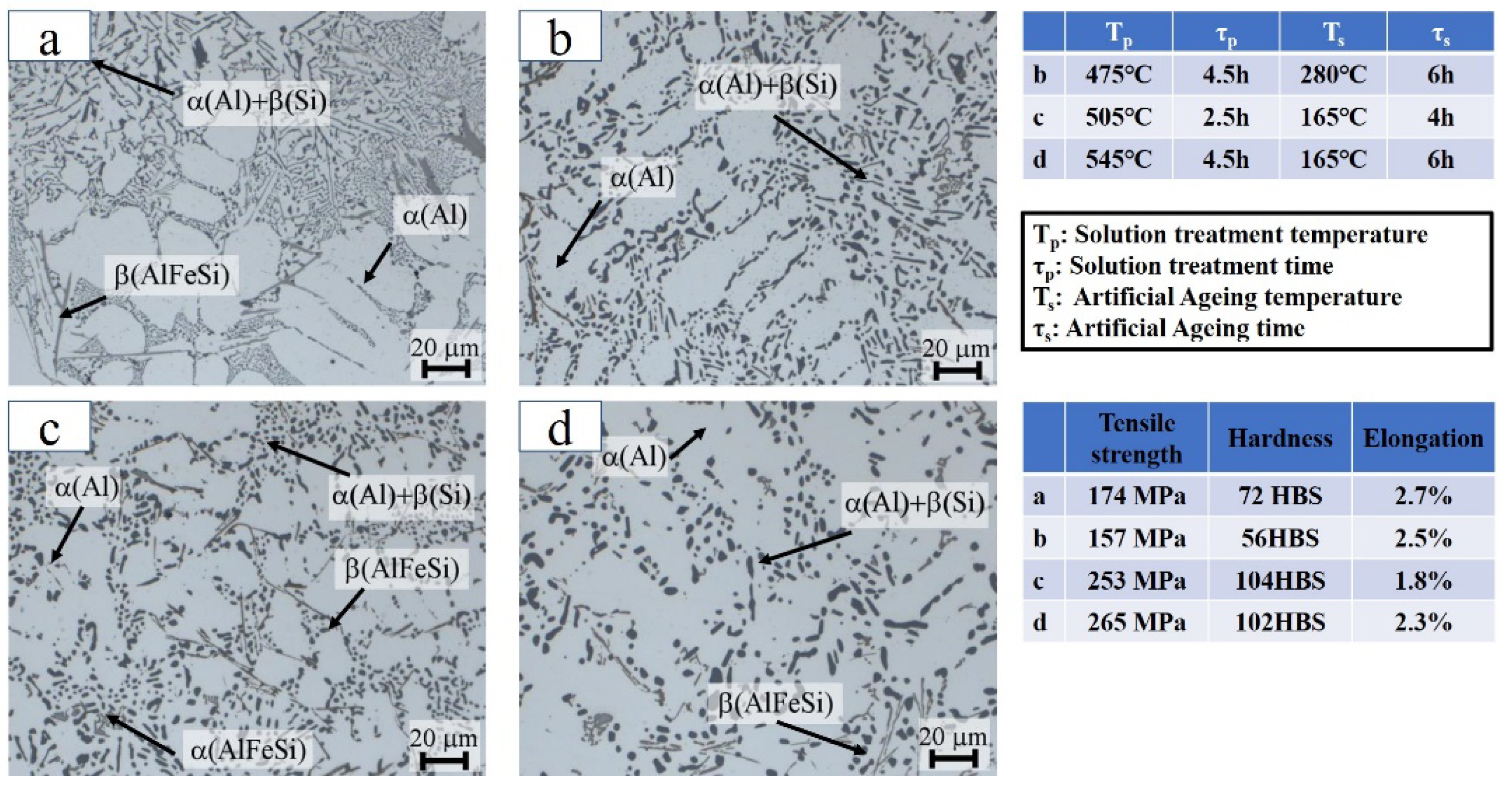
4. The Heat Treatment of Casting Be-Al Alloy
| Heat Treatment | Composition | Advantages Due to the Heat Treatment | Reference |
|---|---|---|---|
| Solution and aging treatment. | 65Be-31Al-2Si-2Ag-0.04 Sr(0.25Cu, Ni and Co) | Strength improved | [31] |
| Homogenizing and aging treatment. | 62Be-37.6Al-0.4Sc | Hardness improved Electrical conductivity improved | [59,60] |
| Composition | Conditions | Tensile Strength MPa | Yield Strength MPa | Elongation(%) |
|---|---|---|---|---|
| 60Be-40Al | as-cast | 110.5 | 91.7 | 1.0 |
| 65Be-31Al-2Si-2Ag-0.04Sr | as-cast heat treated | 190.3 217.9 | 138.6 158.6 | 2.3 2.5 |
5. The Defects Repair of Be-Al Alloy Casting
6. The Development of Be-Al Alloy Casting in China
- It should further accelerate the improvement of Be-Al alloy casting technology and equipment and improve casting product quality and yield in order to reduce the price of Be-Al alloy products. At the same time, standards and systems for Be-Al alloy products based on the casting Al alloy model should be established as soon as possible. More importantly, the construction of the Be-Al alloy product system must be accelerated according to the different application requirements of customers. For example, the development of a different Be content in alloy and the different processing technology of alloy series; it is also necessary to strengthen the collaboration between enterprises and universities, promote the basic theoretical and applied research of Be-Al alloy, establish the genetic database of Be-Al alloy, and provide technical support for the casting simulation and new product development of Be-Al alloy.
- The construction of the standard of Be-Al alloy casting needs to be accelerated, especially the establishment of casting defects evaluation and usability evaluation system. The Be-Al alloy products and the standard manufacturing system in China require the form combined with the characteristics of the user. As a result, the process of “production, study, research and application” of Be-Al alloy will be accelerated.
- The defects repairing technology of Be-Al alloy casting should be studied as soon as possible. Due to the characteristics of Be-Al alloy, it is feasible to repair casting defects with laser and electron beams. In the future research plan, it is important to study the thermodynamics and kinetics of laser and electron beam welding of Be-Al alloy and further develop the specific process. On the other hand, it is necessary to develop the application of additive manufacturing technology, semi-solid casting and low-pressure casting technology in the preparation of Be-Al alloy.
- Technical communication with customers should be strengthened in order to improve customers’ objective understanding of Be-Al alloy and promote the rapid development of Be-Al alloy. Most importantly, the research on industrial preparation technology of the alloy should be paid attention to, and the application scale of the alloy in the civil field should be continuously enhanced.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nikitin, S.N.; Tarasov, B.A.; Shornikov, D.P. Interaction between U-Mo alloys and alloys Al-Be. Phys. Procedia 2015, 72, 370–373. [Google Scholar] [CrossRef]
- Xu, Q.D.; Yang, L.; He, S.X.; Liu, X.D.; Shi, T.; Zhang, P.C. Three-dimensional microstructure and solidification behavior in laser remelting of beryllium-aluminum alloy. Mater. Lett. 2020, 274, 127963. [Google Scholar] [CrossRef]
- William, S. Application of aluminum-beryllium composite for structural aerospace component. Eng. Fail. Anal. 2004, 11, 895. [Google Scholar]
- Parsonage, T. Beryllium metal matrix composites for aerospace and commercial applications. Mater. Sci. Technol. 2010, 16, 732–738. [Google Scholar] [CrossRef]
- Anon, S. Be-Al alloy show promise for spacecraft component. J. Fail. Anal. Prev. 2004, 4, 31. [Google Scholar]
- US Air Force Research Laboratory, Wright-Patterson Air Force Base. Beryllium-aluminum alloys reduce weight in spacecraft. Adv. Mater. Processes. 2004, 162, 12. [Google Scholar]
- Infante, P.F.; Newman, L.S. Beryllium exposure and chronic beryllium disease. Lancet 2004, 363, 415. [Google Scholar] [CrossRef]
- Caminero, M.A.; Chacón, J.M.; García-Plaza, E.; Núñez, P.J.; Reverte, J.M.; Bécar, J.P. Additive manufacturing of PLA-based composites using fused filament fabrication: Effect of graphene nanoplatelet reinforcement on mechanical properties, dimensional accuracy and texture. Polymers 2019, 11, 799. [Google Scholar] [CrossRef]
- Chacón, J.M.; Caminero, M.A.; García-Plaza, E.; Nuñez, P.J. Additive manufacturing of PLA structures using fused deposition modelling: Effect of process parameters on mechanical properties and their optimal selection. Mater. Des. 2017, 124, 143–157. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies, 3rd ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Panahizadeh, V.; Ghasemi, A.H.; Asl, Y.D.; Mohammadmahdi, D. Optimization of LB-PBF process parameters to achieve best relative density and surface roughness for Ti6Al4V samples: Using NSGA-II algorithm. Rapid Prototyp. J. 2022. [Google Scholar] [CrossRef]
- Khorasani, M.; Ghasemi, A.H.; Leary, M.; Sharabian, E.; Cordova, L.; Gibson, I.; Downing, D.; Bateman, S.; Brandt, M.; Rolfe, B. The effect of absorption ratio on meltpool features in laser-based powder bed fusion of IN718. Opt. Laser Technol. 2022, 153, 108263. [Google Scholar] [CrossRef]
- Ángel Caminero, M.; Romero Gutiérrez, A.; Miguel Chacón, J.; García-Plaza, E.; José Núñez, P. Effects of fused filament fabrication parameters on the manufacturing of 316L stainless-steel components: Geometric and mechanical properties. Rapid Prototyp. J. 2022. [Google Scholar] [CrossRef]
- Yao, J.; Ding, R.C.; Li, K.; Du, B.R.; Zhao, L.; Yuan, Y.X. Study on the impact behavior of arch micro-strut (ARCH) lattice structure by selective laser melting (SLM). Rapid Prototyp. J. 2022, 28, 1541–1557. [Google Scholar] [CrossRef]
- Louvis, E.; Fox, P.; Sutcliffe, C.J. Selective laser melting of aluminium components. J. Mater. Process. Technol. 2011, 211, 275–284. [Google Scholar] [CrossRef]
- Sweeney, M.; Acreman, M.; Vettese, T.; Myatt, R.; Thompson, M. Application and testing of additive manufacturing for mirrors and precision structures. In Material Technologies and Applications to Optics, Structures, Components, and Sub-Systems II; SPIE: Washington, DC, USA, 2015; Volume 957406. [Google Scholar]
- Wang, Z.H.; Wang, J.; Yu, L.B.; Wu, J.; Wang, M.; Su, B. Numerical simulation and process optimization of vacuum investment casting for Be–Al alloys. Int. J. Met. 2019, 13, 74–81. [Google Scholar] [CrossRef]
- AlBeCast® 910 Composite. Available online: https://materion.com/products/metal-matrix-composites/albemet-albecast (accessed on 10 August 2022).
- Elmer, J.W.; Aziz, M.J.; Tanner, L.E.; Smith, P.M.; Wall, M.A. Formation of bands of ultrafine beryllium particles during rapid solidification of Al-Be alloys: Modeling and direct observations. Acta Metall. Mater. 1994, 42, 1065. [Google Scholar] [CrossRef]
- Zhang, X.D.; Grensing, F.C.; Meisenkothen, F.; Meyrick, G.; Fraser, H.; Wiezorek, J. Microstructural characterization of novel in-situ Al-Be composites. Metall. Mater. Trans. A 2000, 31, 2963–2971. [Google Scholar] [CrossRef]
- Nardone, V.C.; Garosshen, T.J. Evaluation of the tensile and fatigue behaviour of ingot metallurgy beryllium/aluminium alloys. J. Mater. Sci. 1997, 32, 3975–3985. [Google Scholar] [CrossRef]
- Liu, X.D.; Zhang, P.C.; He, S.X.; Xu, Q.D.; Dou, Z.Y.; Wang, H.J. Effect of beryllium content and heat treatment on microstructure and yield strength in Be/6061Al composites. J. Alloys Compd. 2018, 743, 746–755. [Google Scholar] [CrossRef]
- Niessen, A.K.; De Boer, F.R.; Boom, R.; de Châtel, P.F.; Mattens, W.C.M.; Miedema, A.R. Model predictions for the enthalpy of formation of transition metal alloys II. Calphad 1983, 7, 51–70. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s handbook of chemistry. J. Am. Soc. Naval Eng. 2010, 53, 904. [Google Scholar] [CrossRef]
- Molchanova, L.V.; Ilyushinb, V.N. Alloying of aluminum-beryllium alloys. Russ. Metall. 2013, 1, 84–86. [Google Scholar] [CrossRef]
- William, T.N.; Nancy, F.L.; Kevin, R.R. Ductile, Light Weight, High Strength Beryllium-Aluminum Cast Composite Alloy. U.S. Patent 5,421,916, 23 May 1995. [Google Scholar]
- Krock, R.H.; Mass, P.; Larsen, E.I.; Indianapolis, I.; Jones, C.R.; Arlington, M. Beryllium-Aluminum-Silver Composite. U.S. Patent 3,322,512, 30 May 1967. [Google Scholar]
- Noribuml, T.; Nobuyoshi, I.; Shigemi, T.; Ogawa, T. Method of Fabricating a Light Shielding Blade Composed of Beryllium Aluminum Alloy. U.S. Patent 650,229,6B2, 7 January 2003. [Google Scholar]
- Smith, B.J. High-cycle fatigue of an investment cast Be-Al metal matrix composite. Metall. Mater. Trans. A 2002, 33, 1862. [Google Scholar] [CrossRef]
- Yu, L.B.; Wang, W.Y.; Wang, J.; Su, B.; Dong, X.F.; Wang, Z.H. The Effects of Sc Addition on the Microstructure and Mechanical Properties of Be-Al Alloy Fabricated by Induction Melting. J. Mater. Eng. Perform. 2019, 28, 2378–2387. [Google Scholar] [CrossRef]
- William, T.N.; Nancy, F.L.; Kevin, R.R. Light Weight, High Strength Beryllium-Aluminum Alloy. U.S. Patent 5,603,780, 18 February 1997. [Google Scholar]
- Ma, X.B.; Wang, D.X.; Hu, Q.D.; Li, J.Y.; Zhong, J.M.; Li, Q. Research Status of Additive Elements to Strengthen Beryllium Aluminum Alloy. Chin. J. Rare Met. 2021, 45, 1010–1017. [Google Scholar]
- Xu, H.B.; Wang, J.C.; Yang, G.C. First-principles studies on alloying effect of Ag and Ni in Be-A1 alloys. Foundry Technol. 2009, 30, 478–481. [Google Scholar]
- Marder, J.M.; Haws, W.J. Beryllium-Containing Alloys of Aluminum and Semi-Solid Processing of Such Alloys. U.S. Patent 5,551,997, 3 September 1996. [Google Scholar]
- Levoy, N.F.; Smith, B.J. Beryllium-aluminum alloy development for investment casting. Adv. Mater. Processes 1997, 151, 363–374. [Google Scholar]
- Fritz, C.G.; James, M.M.; Jere, H.B. Aluminum Alloys Containing Beryllium and Investment Casting of Such Alloys. U.S. Patent 5,667,600, 16 September, 1997. [Google Scholar]
- Wang, Z.H.; Wang, L.; Mai, X.F.; Zhao, Y.L. Study on structural material manufacturing of beryllium-aluminum alloy. Min. Technol. 2006, 6, 16. [Google Scholar]
- Jostein, R.; Nils, R. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar]
- Riva, S.; Yusenko, K.V.; Lavery, N.P.; Brown, S.G.R. The scandium effect in multicomponent alloys. Int. Mater. Rev. 2015, 26, 203–228. [Google Scholar] [CrossRef]
- Okamoto, H. Be-Sc (beryllium-scandium). J. Phase Equilib. Diffus. 2008, 29, 465. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Be-Sc (aluminum-beryllium-scandium). J. Phase Equilib. Diffus. 2009, 30, 178. [Google Scholar] [CrossRef]
- Murray, J.L. The Al-Sc (aluminum-scandium) system. J. Phase Equilib. 1998, 19, 5. [Google Scholar] [CrossRef]
- Yu, L.B.; Wang, J.; Qu, F.S.; Wang, M.; Wang, W.Y.; Bao, Y.X.; Lai, X.C. Effects of scandium addition on microstructure, mechanical and thermal properties of cast Be-Al alloy. J. Alloys. Compd. 2018, 737, 655–664. [Google Scholar] [CrossRef]
- Yu, L.B.; Wang, J.; Meng, X.D.; Wang, Q.G.; Qu, F.S. Microstructure and mechanical properties of Be-Al and Be-Al-Sc alloys with various solidification rates. Mater. Res. Express 2018, 5, 066519. [Google Scholar] [CrossRef]
- Yu, L.B.; Wang, J.; Wang, Z.H.; Qu, F.S.; Zhou, F.Y.; Lai, X.C. Sc and Sc-Zr effects on microstructure and mechanical properties of Be-Al alloy. Mater. Sci. Technol. 2017, 34, 480–486. [Google Scholar] [CrossRef]
- Oz, T.; Karaköse, E.; Keskin, M. Impact of beryllium additions on thermal and mechanical properties of conventionally solidified and melt-spun Al-4.5wt.%Mn–Xwt.%Be (x = 0,1,3,5) alloys. Mater. Des. 2013, 50, 14. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Pathak, L.C.; Ray, A.K.; Das, G.; Verma, P.K.; Kumar, M.; Ghosh, R.N. Microstructure, tensile strength and wear behaviour of Al–Sc alloy. Mater. Sci Eng. A 2004, 383, 374–380. [Google Scholar] [CrossRef]
- Ang, C.Y.; Ana, S.; Helderman, E.R. Sintered Alloys of Beryllium. U.S. Patent 3,325,257, 13 June 1967. [Google Scholar]
- Larsen, E.I.; Indianapolis, Ind.; Krock, R.H.; Jones, C.R. Composites of Beryllium-Copper-Magnesium. U.S. Patent 3,378,356, 16 April 1968. [Google Scholar]
- Hashiguchi, D.H.; Grensing, F.C. Ternary aluminum-beryllium alloy. In Proceedings of the 1995 International Conference & Exposition on Powder Metallurgy & Particulate Materials, Part 3 (of 3), Seattle, WA, USA, 13–17 December 1995. [Google Scholar]
- Webster, D.; London, G.J. Beryllium Science and Technology; Plenum Publishing Corporation: New York, NY, USA, 1979. [Google Scholar]
- Milewski, J.O. Ultra-Narrow Gap Laser Welding of Be-Al Alloys Final Report; Los Alamos National Laboratory: Los Alamos, NM, USA, 1998. [Google Scholar]
- Poloczek, Ł.; Kiełbus, A. Influence of technological factors on the quality of aluminum alloys castings. Enterp. Manag. 2016, 19, 14–19. [Google Scholar]
- Szymczak, T.; Gumienny, G.; Klimek, L.; Goły, M.; Szymszal, J.; Pacyniak, T. Characteristics of Al-Si Alloys with high melting point elements for high pressure die casting. Materials 2020, 13, 4861. [Google Scholar] [CrossRef]
- Chen, J.X.; Lu, M.J.; Wu, S.S.; Lü, S.L. Study on eutectic microstructure and modification mechanism of Al-Si alloys. Mater. Sci. Forum 2016, 877, 97–103. [Google Scholar] [CrossRef]
- Wei, L.; Shuai, L.; Jie, L.; Ang, Z.; Yan, Z.; Qingsong, W.; Chunze, Y.; Yusheng, S. Effect of heat treatment on AlSi10Mg alloy fabricated by selective laser melting: Microstructure evolution, mechanical properties and fracture mechanism. Mater. Sci. Eng. A 2016, 663, 116–125. [Google Scholar]
- Wang, G.Q.; Liu, Y.; Ren, G.C.; Zhao, Z.K. Analyzing Si Precipitation on Age Hardening of an Al-Si-Mg Cast Alloy. Adv. Mater. Res. 2010, 146, 1685–1689. [Google Scholar] [CrossRef]
- Jarco, A.; Pezda, J. Effect of heat treatment process and optimization of its parameters on mechanical properties and microstructure of the AlSi11 (Fe) alloy. Materials 2021, 14, 2391. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.B.; Wang, W.; Li, B.Q.; He, L.F.; Wang, Q.G.; Zhao, F.Z.; Wang, J. Determination of homogenizing conditions and aging behaviors of cast 62Be-37.6 Al-0.4 Sc alloy. J. Alloys Compd. 2019, 808, 151742. [Google Scholar] [CrossRef]
- Vo, N.Q.; Dunand, D.C.; Seidman, D.N. Improving aging and creep resistance in a dilute Al-Sc alloy by microalloying with Si, Zr and Er. Acta Mater. 2014, 63, 73–85. [Google Scholar] [CrossRef]
- Gabriel, M.N.; Alan, J.A. Precipitation of Al3Sc in binary Al-Sc alloys. Mater. Sci. Eng. A 2001, 318, 144–154. [Google Scholar]
- Parsonage, T. New Technologies for Optical Systems Utilizing Aluminum Beryllium. In Advanced Optical and Mechanical Technologies in Telescopes and Instrumentation; SPIE: Washington, DC, USA, 2008; Volume 7018, pp. 196–204. [Google Scholar]
- Xu, Q.D.; Luo, Y.; Liu, X.D.; Yang, L.; He, S.X.; Wang, X.; Zhang, P.C. Microstructural evolution and hardness of as-cast Be-Al-Sc-Zr alloy processed by laser surface remelting. Chin. J. Aeronaut. 2021, 34, 131–142. [Google Scholar] [CrossRef]
- Xu, Q.D.; Zhang, P.C.; Yang, L.; Le, G.M.; He, S.X.; He, X.H.; Liu, X.D.; Wang, W.Y. Microstructure and mechanical properties of Be–Al alloy fabricated by laser solid forming. Mater. Sci. Eng. A 2021, 799, 140335. [Google Scholar] [CrossRef]

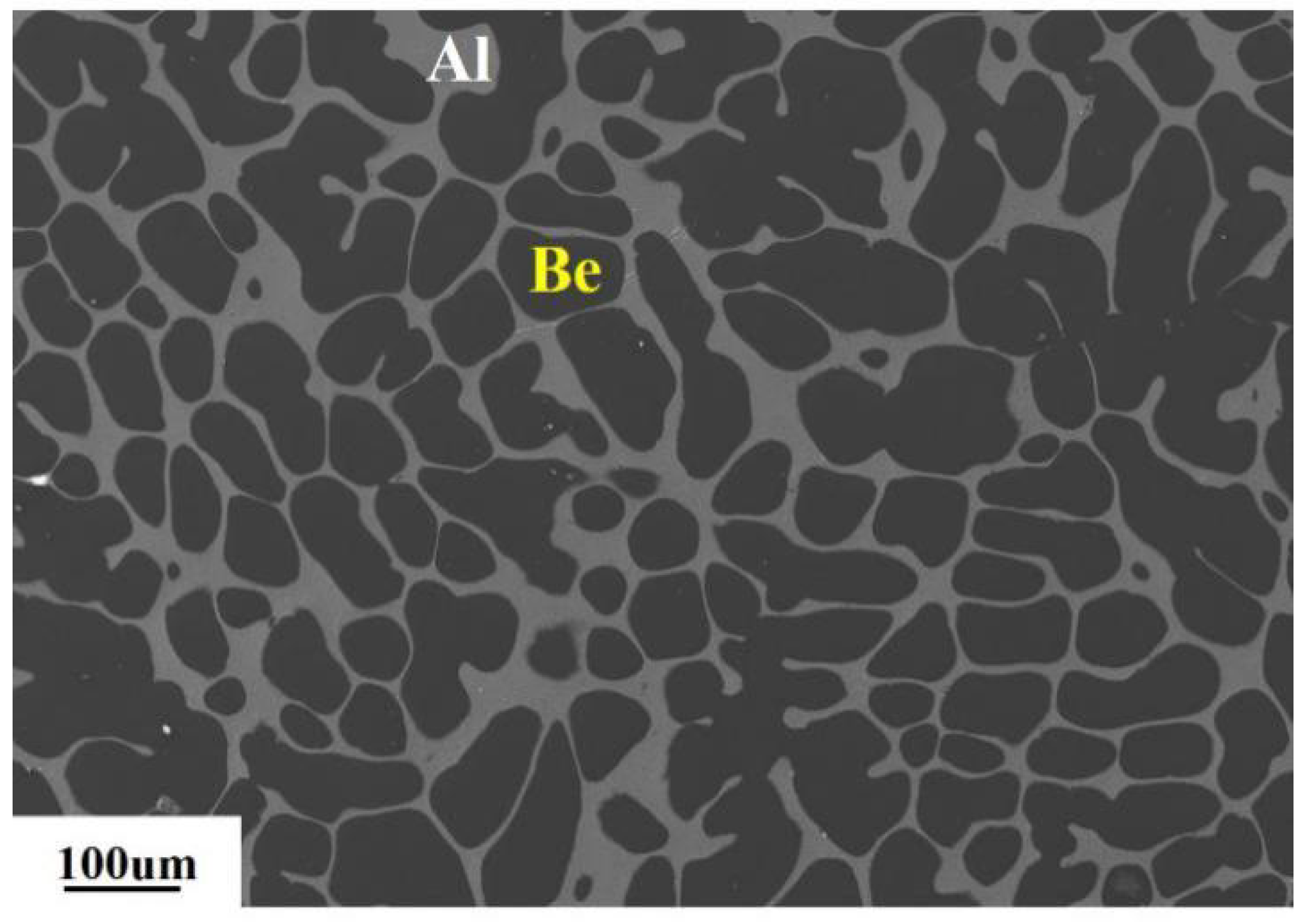
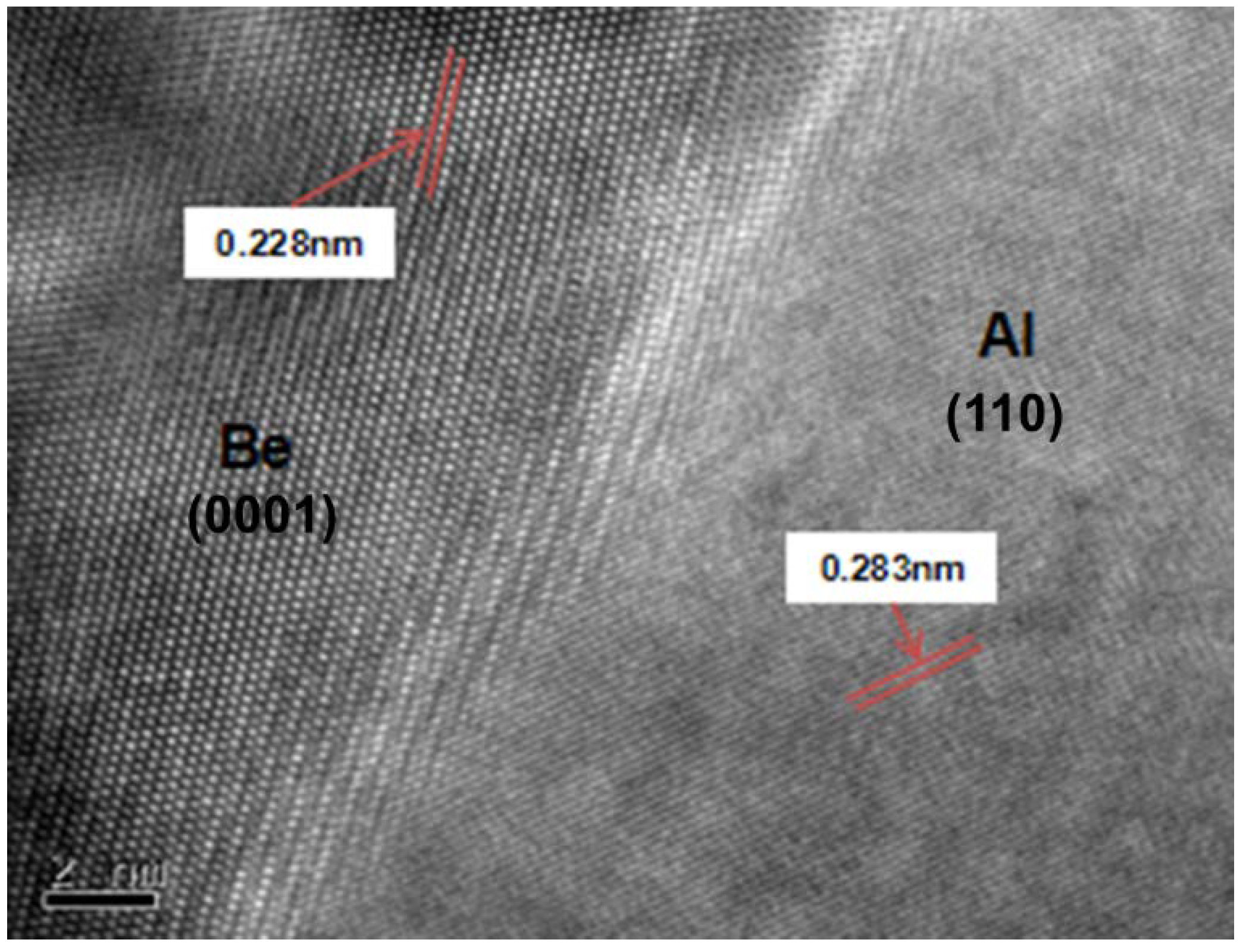

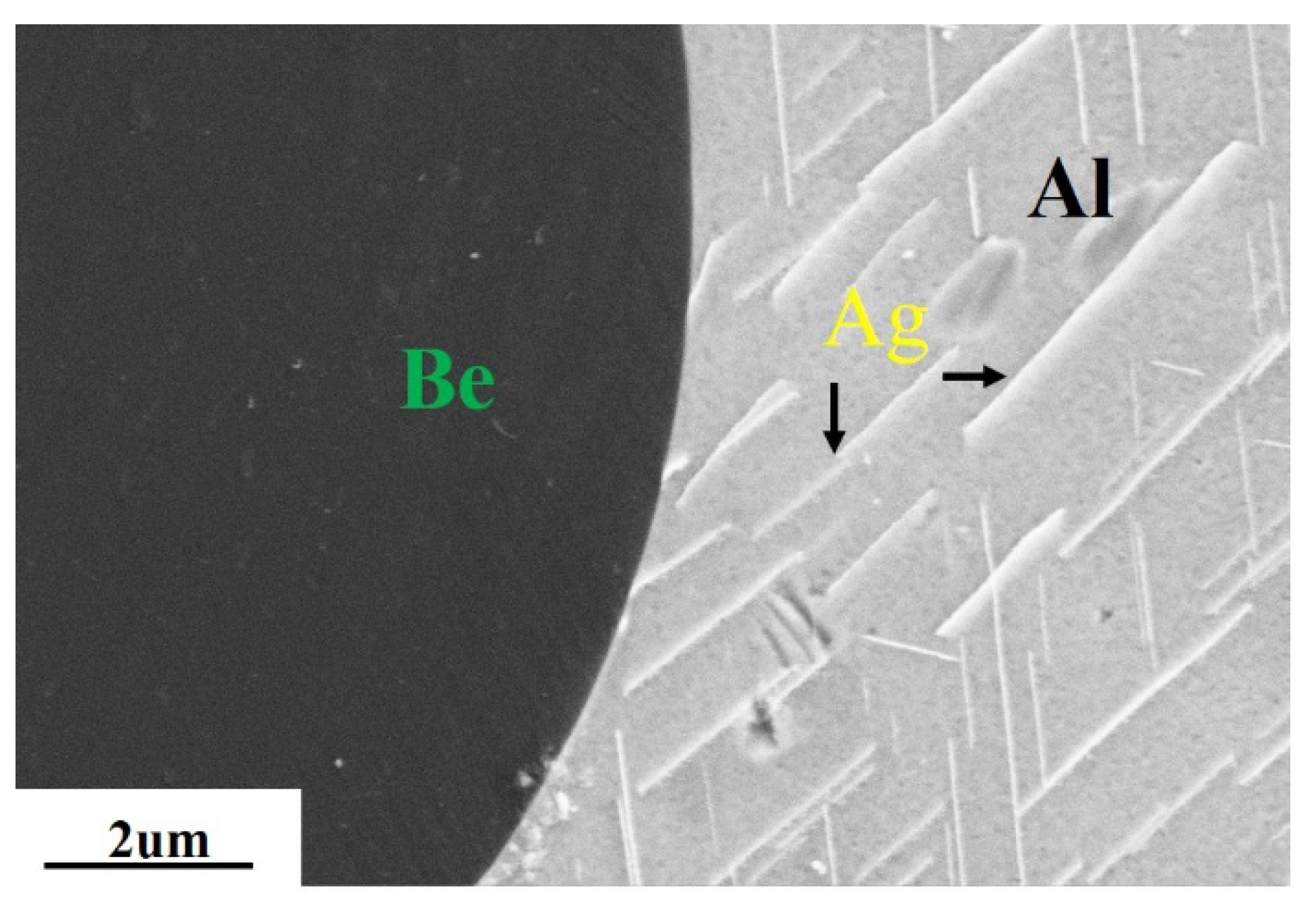
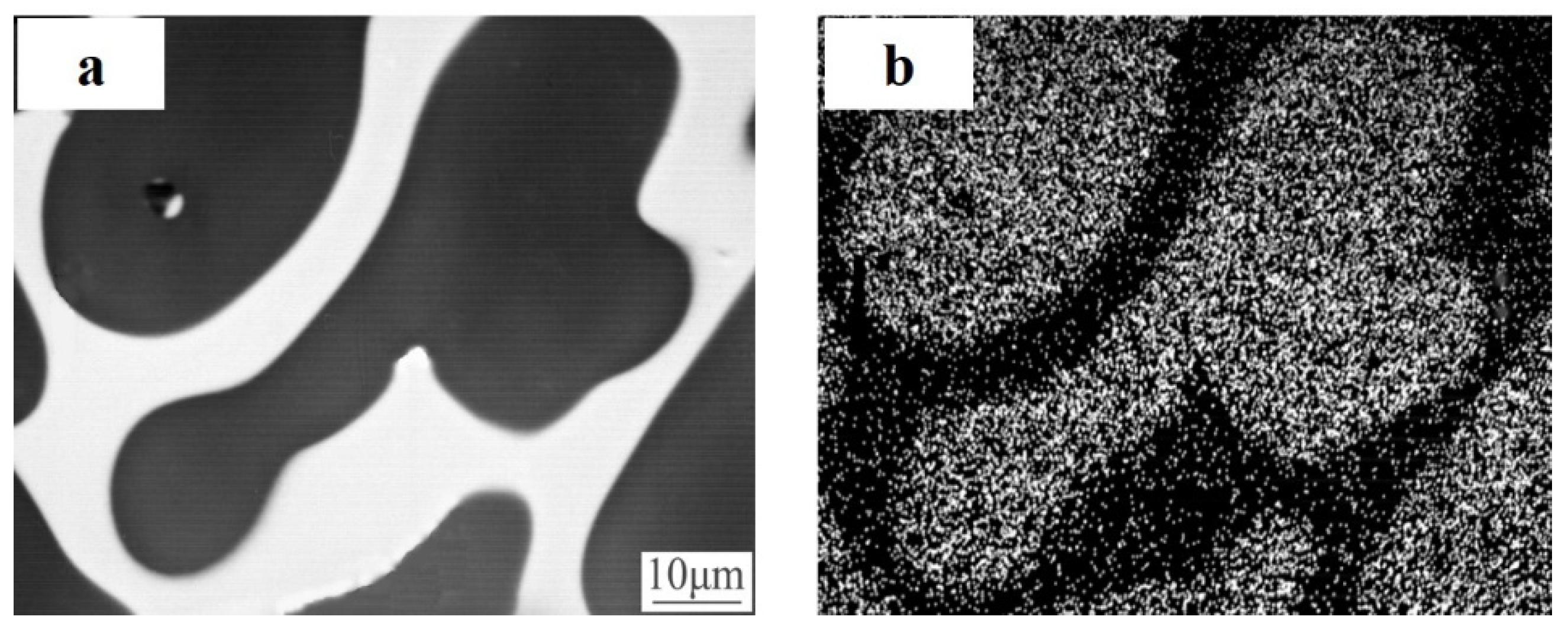

| Property | AlBeCast®910 | Aluminum A357-T6 | Titanium 6AL-4V | AlSiC F3A205-T6 |
|---|---|---|---|---|
| Density g/cm3 | 2.17 | 2.69 | 4.43 | 2.69 |
| CTE (25 °C) ppm/°C | 14.6 | 21.5 | 9.3 | 16.4 |
| Modulus GPa | 193 | 72 | 110 | 72 |
| Yield Strength, MPa | 165 | 248 | 880 | 165 |
| Ultimate Tensile Strength, MPa | 211 | 317 | 950 | 196 |
| Specific Stiffness, GPa/(g/cm3) | 88.94 | 26.77 | 24.83 | 26.77 |
| The Samples | Tensile Strength MPa | Yield Strength MPa | Elongation (%) |
|---|---|---|---|
| 62Be-38Al alloy | 110.5 | 91.7 | -- |
| a 65Be-30.25Al-3Ag-1Co-0.75Ge | 284.0 | 214.0 | 4.8 |
| b 62Be-33.25Al-3Ag-1Co-0.75Ge | 243.0 | 185.0 | 2.5 |
| 62Be-37.6Al -0.4Sc | 179.7 | 110.7 | 2.9 |
| 65Be-31Al -2Si-2Ag | 196.0 | 137.0 | 1.7 |
| 62Be-34Al -4Ni | 211.0 | 165.0 | 4.0 |
| Element | Microstructural Evolution | Advantages Due to the Added Element | Reference |
|---|---|---|---|
| Ag/Co/Ge | 1. Ag, Ge are solidly soluble in Al 2. Ag2Al or other Ag-Al binary alloy formed | 1. Ag improve the casting performance, strength, and plasticity of the alloy 2. Ge is mainly to improve the fluidity, strength, and toughness of the alloy 3. Co increases the strength and hardness of the Be phase, then improves the strength and hardness of the alloy | Reference: [26,27,29,31,32,33,34,35] |
| Ni | αBe (Ni is solidly soluble in Be) | reduces the thermal expansion coefficient, increases tensile properties and improves castability | [18,33,36,37] |
| Sc/Zr | second-phase particle Al3Sc, Be13Sc and Be13Zr formed | 1. Be grain refinement 2. the secondary dendritic arm spacing reduced | [30,38,39,40,41,42,43,44,45,46,47] |
| Si | 1. irregularly shaped particle within the Al matrix 2. precipitate in the alloy | 1. enhances the strengthening effect of Be-Al alloy | [31,48] |
| Cu | 1. αBe (Cu is solidly soluble in Be) 2. αAl (Cu is solidly soluble in Al) | precipitate and harden the Al phase | [49,50,51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xie, Y.; Yang, Y.; Liu, Z.; Wang, D.; Yin, Y. Research Progress of Low Density and High Stiffness of Be-Al Alloy Fabricated by Investment Casting. Metals 2022, 12, 1379. https://doi.org/10.3390/met12081379
Li J, Xie Y, Yang Y, Liu Z, Wang D, Yin Y. Research Progress of Low Density and High Stiffness of Be-Al Alloy Fabricated by Investment Casting. Metals. 2022; 12(8):1379. https://doi.org/10.3390/met12081379
Chicago/Turabian StyleLi, Junyi, Yao Xie, Yiqun Yang, Zhaogang Liu, Dongxin Wang, and Yajun Yin. 2022. "Research Progress of Low Density and High Stiffness of Be-Al Alloy Fabricated by Investment Casting" Metals 12, no. 8: 1379. https://doi.org/10.3390/met12081379
APA StyleLi, J., Xie, Y., Yang, Y., Liu, Z., Wang, D., & Yin, Y. (2022). Research Progress of Low Density and High Stiffness of Be-Al Alloy Fabricated by Investment Casting. Metals, 12(8), 1379. https://doi.org/10.3390/met12081379





