Study of Thermodynamic for Low-Reactive CaO-BaO-Al2O3-SiO2-CaF2-Li2O Mold Flux Based on the Model of Ion and Molecular Coexistence Theory
Abstract
:1. Introduction
2. Methodology
2.1. Structural Units and Mass Action Concentration
2.2. Calculation of Standard Gibbs Free Energy for Complex Molecules
2.3. Mass Action Concentration for Structural Units and Ion Couples
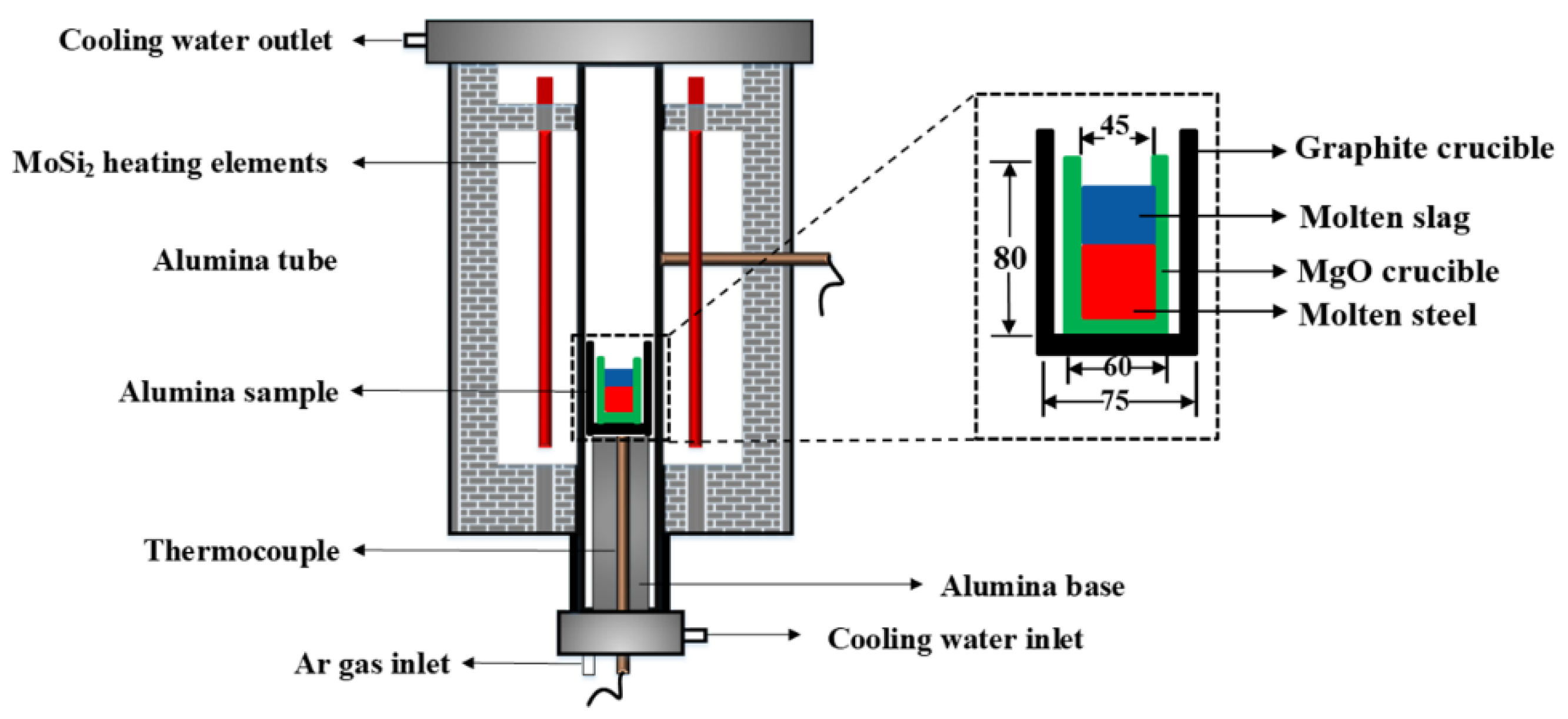
2.4. Steel–Slag Contact Experiment
3. Model Validation
CaO-Al2O3-SiO2-BaO and CaO-Al2O3-SiO2-CaF2
4. Contact Experiment of Steel-Slag Reaction
5. Effect of Different Factors
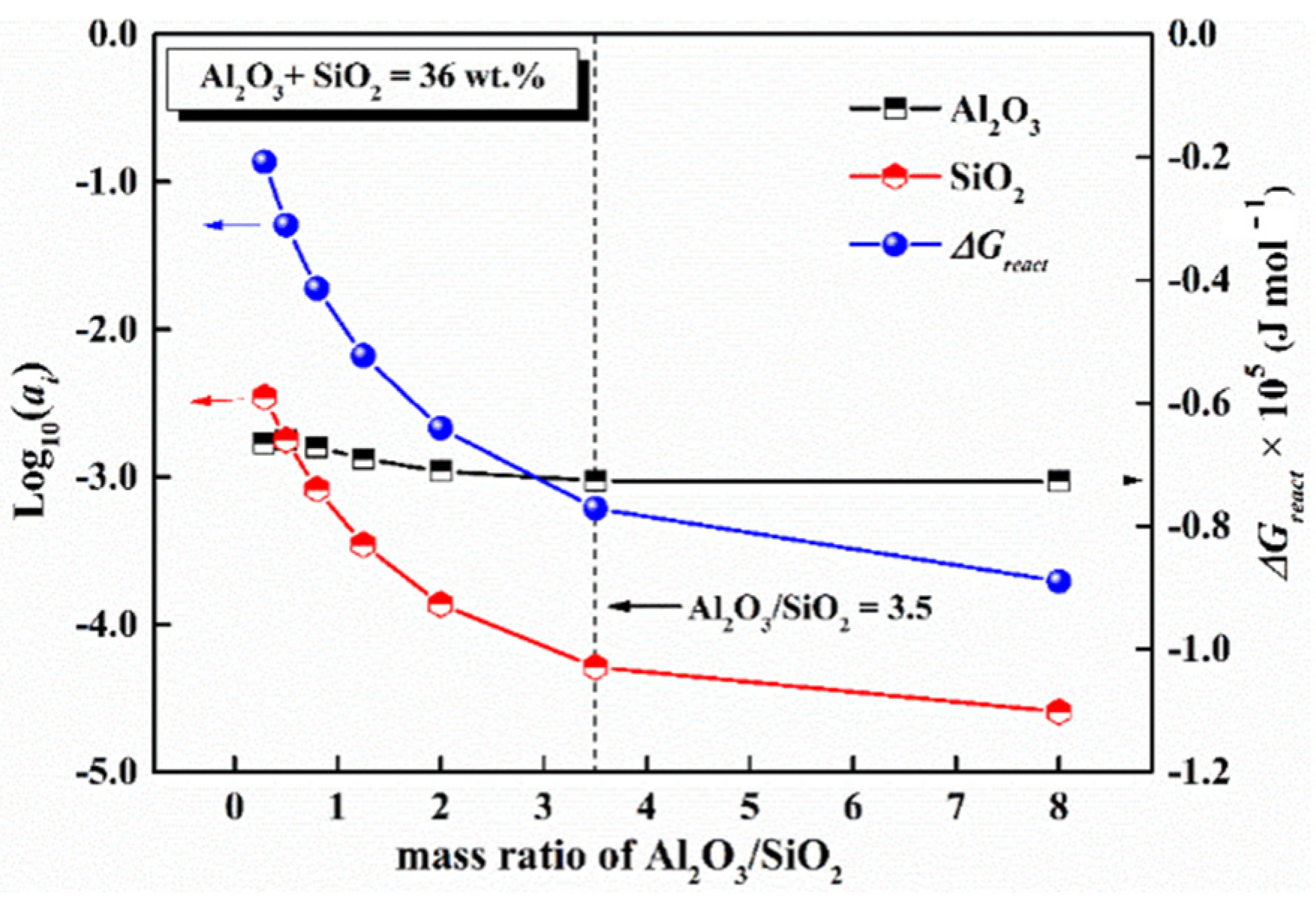
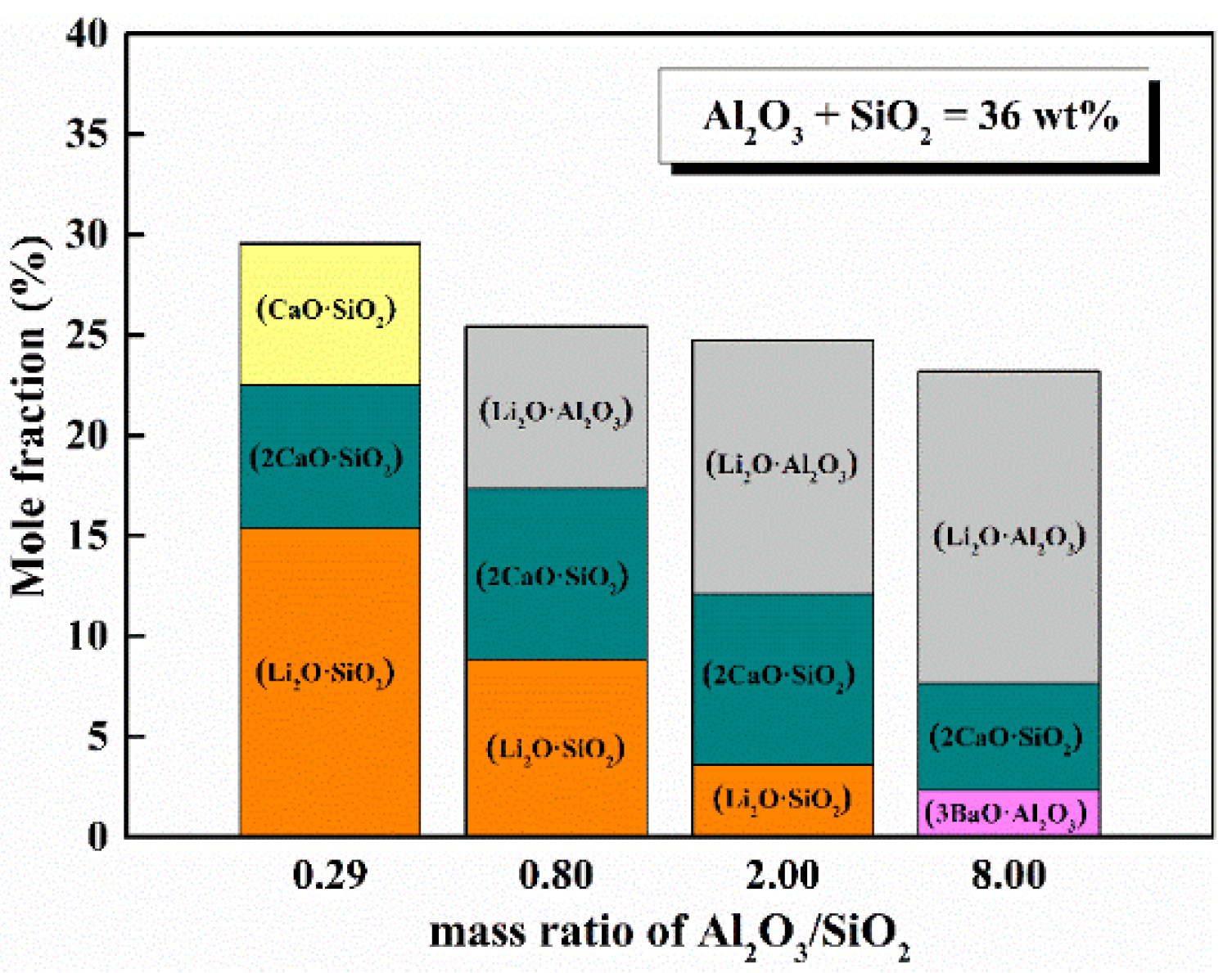
5.1. Mass Ratio of Al2O3/SiO2
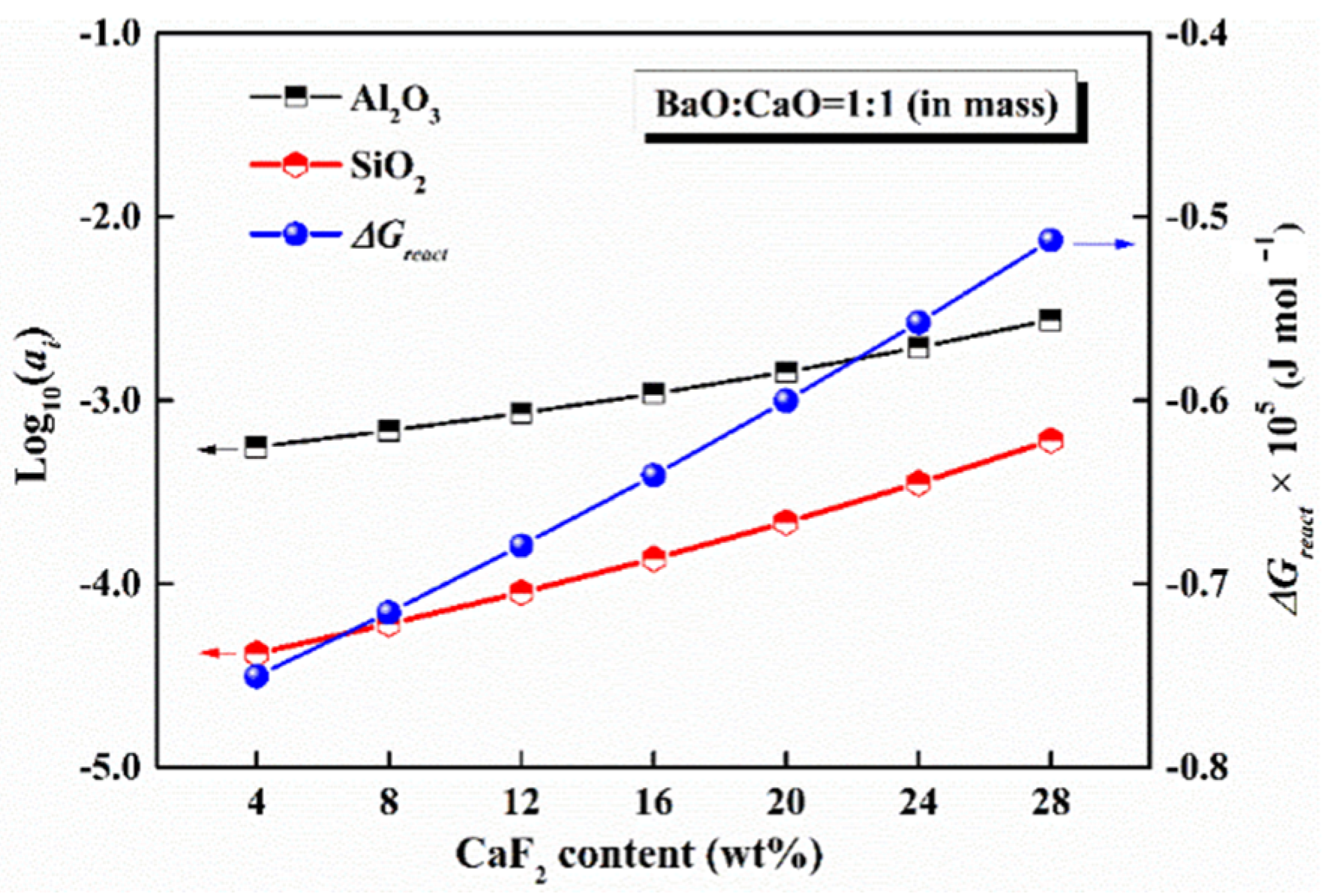
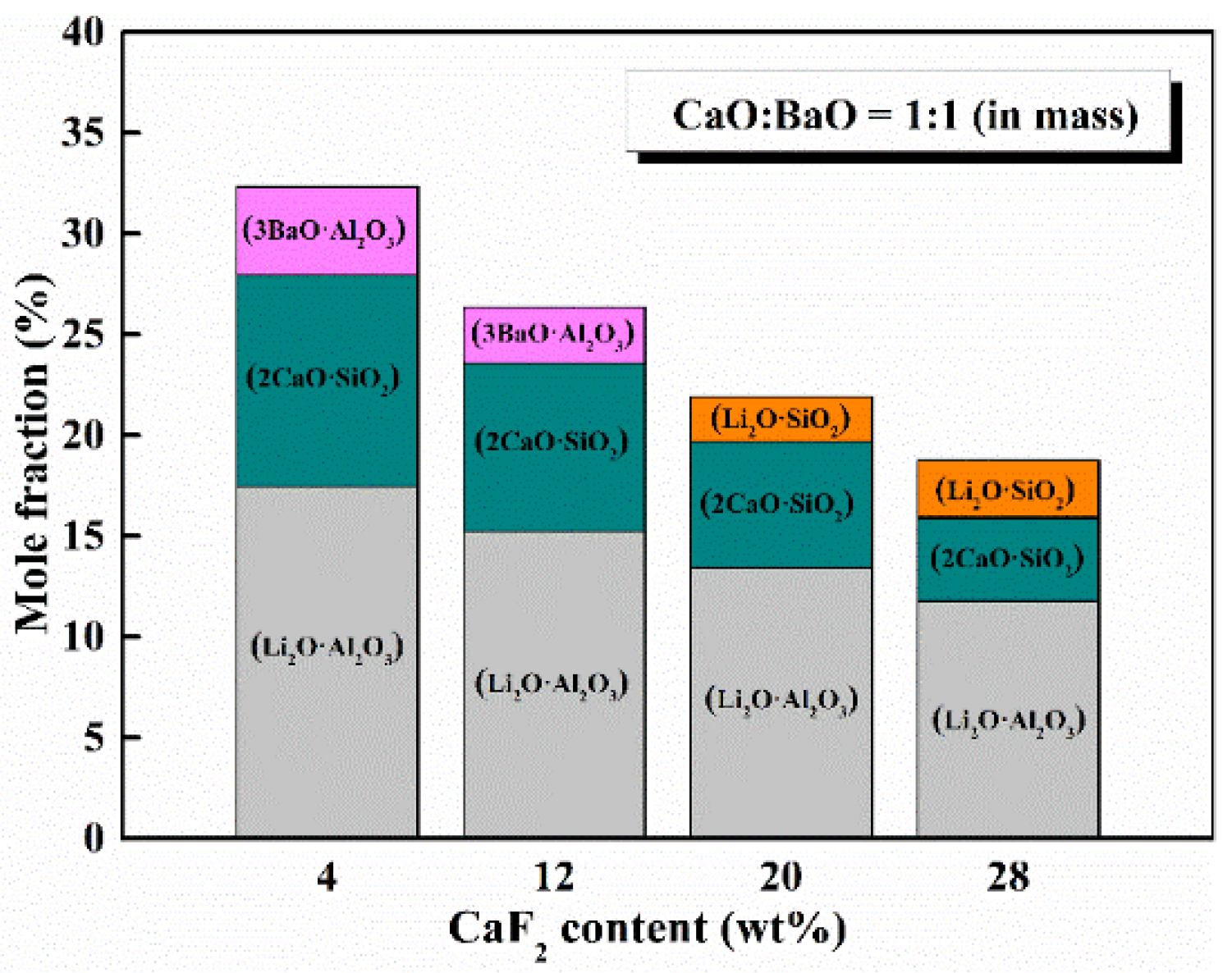
5.2. Content of CaF2
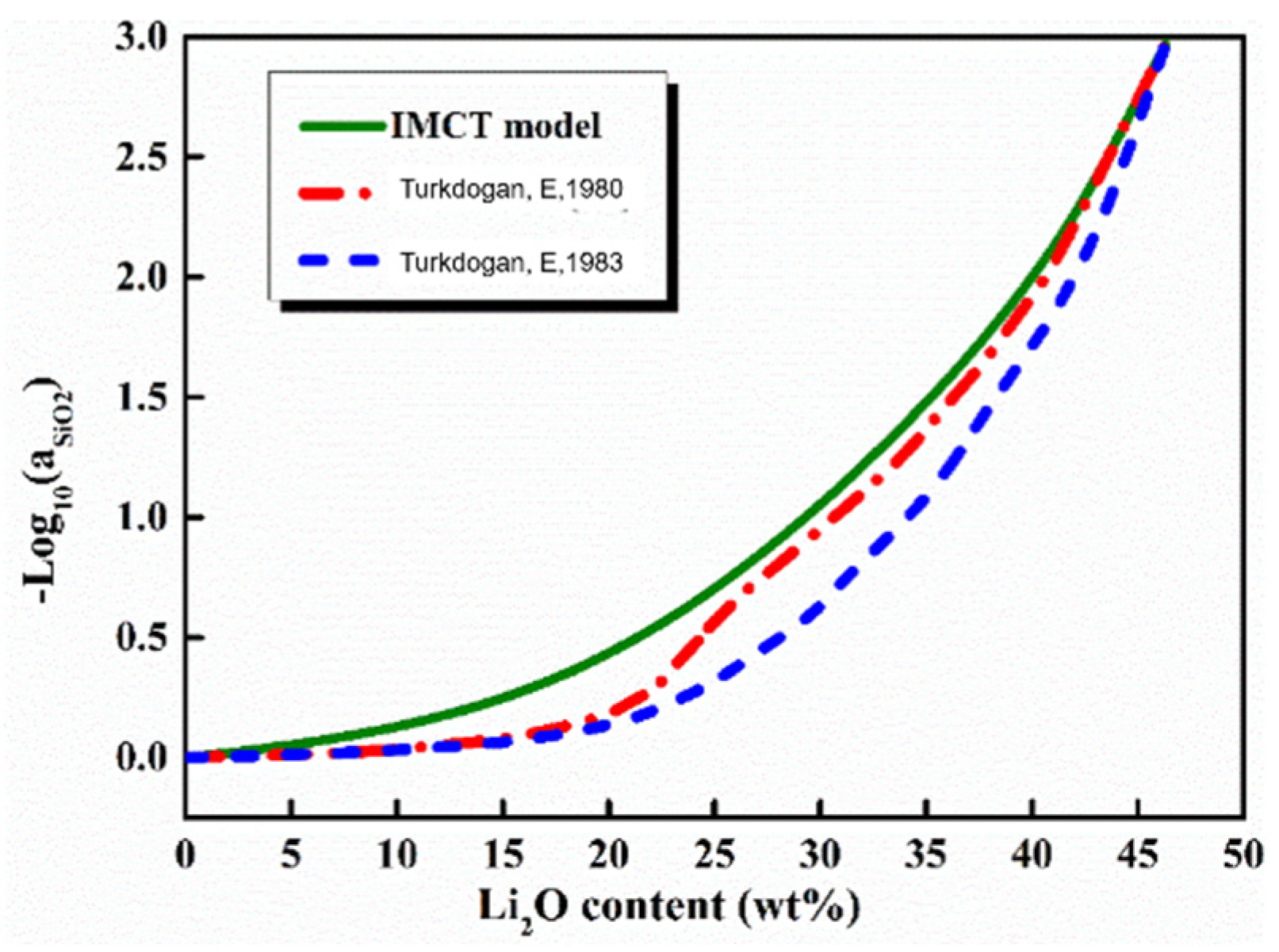
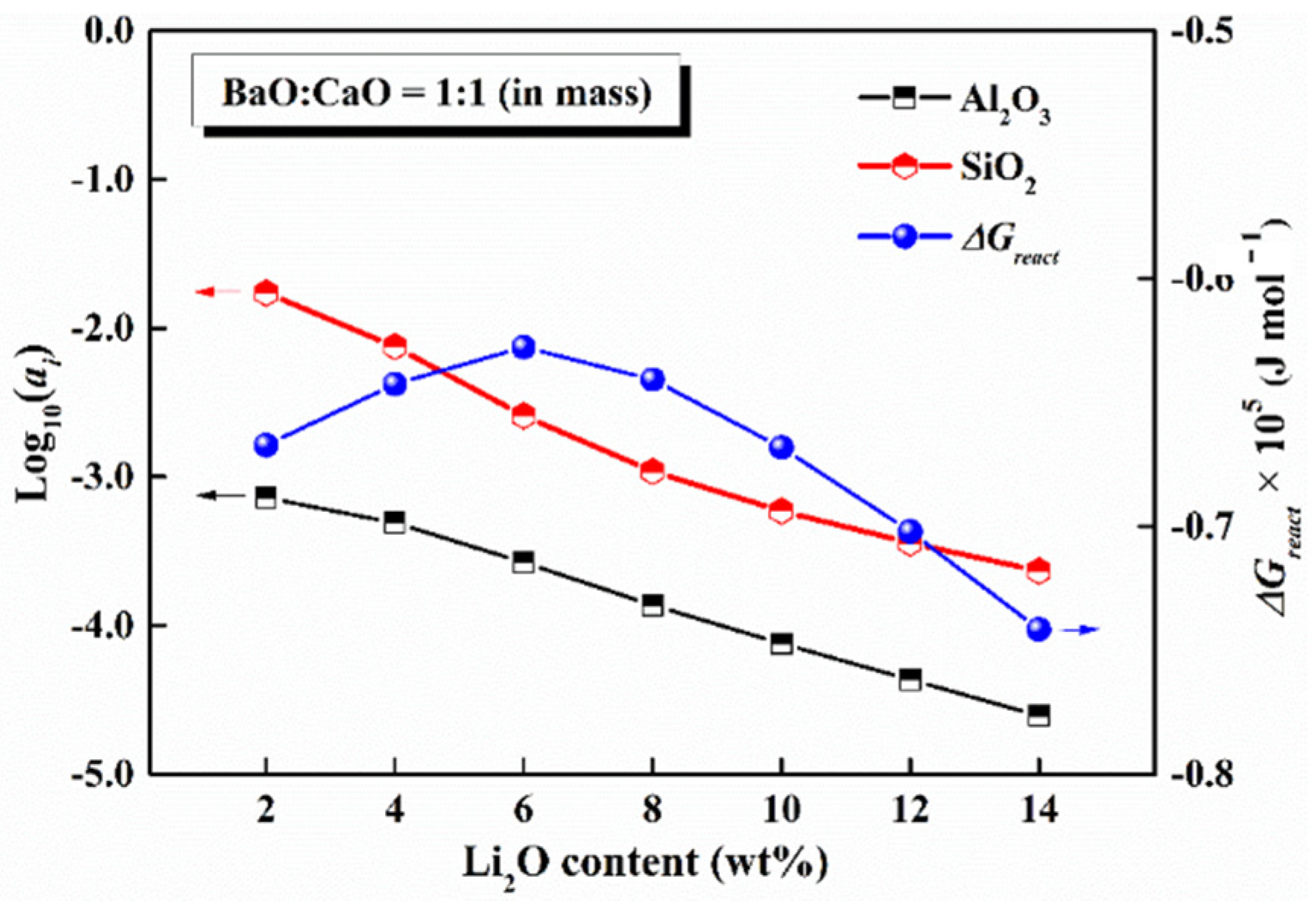
5.3. Content of Li2O
6. Conclusions
- The results calculated by IMCT model are good accordance to the experiment results and Factsage calculation. The thermodynamic model based on IMCT could predict the activity of each component in the low-reactive CaO-BaO-Al2O3-SiO2-CaF2-Li2O mold flux accurately and has good reliability.
- With the increase in mass ratio of Al2O3/SiO2, the decreases in the activity of SiO2 and the reactivity of mold flux had a turning point when the ratio of Al2O3/SiO2 was 3.5, where the content of SiO2 was 8 wt%.
- The activities of SiO2 and Al2O3 and the reactivity of mold flux increased continuously with an increase in the content of CaF2, which is unfavorable for developing low-reactivity mold flux. However, to avoid compromising other physical properties, the CaF2 should be kept to a minimum.
- The activities of SiO2 and Al2O3 decreased with an increase in Li2O content, whereas the reactivity of mold flux had a maximum with 6 wt% Li2O content, indicating that the compositional regions involving around 6 wt% Li2O content should be avoided to design the low-reactive flux system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grässel, O.; Krüger, L.; Frommeyer, G.; Meyer, L.W. High strength Fe-Mn-(Al-Si) Trip/Twip steels development-properties-application. Int. J. Plast. 2000, 16, 1391–1409. [Google Scholar] [CrossRef]
- Gutiérrez-Urrutia, I.; Raabe, D. Multistage strain hardening through dislocation substructure and twinning in a high strength and ductile weight-reduced Fe–Mn–Al–C steel. Acta Mater. 2012, 60, 5791–5802. [Google Scholar] [CrossRef]
- Bouaziz, O.; Zurob, H.; Huang, M.X. Driving Force and Logic of Development of Advanced High Strength Steels for Automotive Applications. Steel Res. Int. 2013, 84, 937–947. [Google Scholar] [CrossRef]
- Fu, X.; Wen, G.H.; Liu, Q.; Tang, P.; Li, J.; Li, W. Development and evaluation of CaO–SiO2 based mould fluxes for casting high aluminum TRIP steel. Steel Res. Int. 2015, 86, 110–120. [Google Scholar] [CrossRef]
- Fu, X.; Wen, G.H.; Tang, P.; Ma, F.J.; Wang, H. Characteristics of heat flux through slag film of mold slag used for high Al-TRIP steel casting. J. Iron Steel Res. Int. 2010, 17, 11–16. [Google Scholar]
- Wu, T.; He, S.P.; Zhu, L.L.; Wang, Q. Study on Reaction Performances and Applications of Mold Flux for High-Aluminum Steel. Mater. Trans. 2016, 57, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Ji, C.X.; Yang, C.; Zhi, Z.; Tian, Z.H.; Zhao, C.L.; Zhu, G.S. Continuous casting of high-Al steel in shougang jingtang steel works. J. Iron Steel Res. Int. 2015, 22, 53–56. [Google Scholar] [CrossRef]
- He, S.P.; Li, Z.R.; Chen, Z.; Wu, T.; Wang, Q. Review of mold fluxes for continuous casting of high-alloy (Al, Mn, Ti) steels. Steel Res. Int. 2019, 90, 1800424. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.L.; Lu, B.X.; Xiao, D. A Review of Mold Flux Development for the Casting of High-Al Steels. Metall. Mater. Trans. B 2016, 47, 384–389. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P. The widespread applicability of the mass action law to metallurgical melts and organic solutions. Calphad 2001, 25, 343–354. [Google Scholar] [CrossRef]
- Wu, C.C.; Cheng, G.G.; Long, H. Calculating model of action concentration of slag systems containing Ce2O3. Zhongguo Youse Jinshu Xuebao/Chin. J. Nonferrous Met. 2013, 23, 2999–3005. [Google Scholar]
- Zhang, B.; Jiang, M.F.; Qi, J.; Liu, C.J. Activity Calculation Model for a Slag System Consisting of CaO-SiO2-Al2O3-FeO-CaF2-La2O3-Nb2O5-TiO2. J. Northeast. Univ. Nat. Sci. 2011, 32, 524–528. [Google Scholar]
- Duan, S.C.; Li, C.; Guo, X.L.; Guo, H.J.; Guo, J.; Yang, W.S. A thermodynamic model for calculating manganese distribution ratio between CaO-SiO2-MgO-FeO-MnO-Al2O3-TiO2-CaF2 ironmaking slags and carbon saturated hot metal based on the IMCT. Ironmak. Steelmak. 2018, 45, 655–664. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Q.; He, S.P.; Xu, J.F.; Long, X.; Lu, Y.J. Study on properties of alumina-based mould fluxes for high-Al steel slab casting. Steel Res. Int. 2012, 83, 1194–1202. [Google Scholar] [CrossRef]
- Levin, E.; McMurdie, H. Phase Diagrams for Ceramists; The American Ceramic Society: Westerville, OH, USA, 1975. [Google Scholar]
- Shukla, A.; Jung, I.-H.; Decterov, S.A.; Pelton, A.D. Thermodynamic evaluation and optimization of the BaO-SiO2 and BaO-CaO-SiO2 systems. Calphad 2018, 61, 140–147. [Google Scholar] [CrossRef]
- Allibert, M. Slag Atlas; Verein Deutscher Eisenhüttenleute: Dusseldorf, Germany, 1995; p. 616. [Google Scholar]
- Yang, X.M.; Jiao, J.S.; Ding, R.C.; Shi, C.B.; Guo, H.J. A thermodynamic model for calculating sulphur distribution ratio between CaO-SiO2-MgO-Al2O3 ironmaking slags and carbon saturated hot metal based on the ion and molecule coexistence theory. ISIJ Int. 2009, 49, 1828–1837. [Google Scholar] [CrossRef] [Green Version]
- Wei, S. Thermodynamics of Metallurgical Processes; Series Book of Modern Metallurgy; Shanghai Scientific & Technical Publishers: Shanghai, China, 1980; 52p. [Google Scholar]
- Ye, D.L.; Hu, J. Practical Inorganic Thermodynamic Data Handbook; Metallurgical Industry Press: Beijing, China, 2002; 561p. [Google Scholar]
- Yang, X.M.; Duan, J.P.; Shi, C.B.; Zhang, M.; Zhang, Y.L.; Wang, J.C. A thermodynamic model of phosphorus distribution ratio between CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 slags and molten steel during a top–bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2011, 42, 738–770. [Google Scholar] [CrossRef]
- Shukla, A. Development of a Critically Evaluated Thermodynamic Database for the Systems Containing Alkaline-Earth Oxides; École Polytechnique de Montréal: Montreal, QC, Canada, 2012. [Google Scholar]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Duan, J.P.; Zhang, J. A thermodynamic model of phosphate capacity for CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 slags equilibrated with molten steel during a top–bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Metall. Mater. Trans. B 2011, 42, 951–977. [Google Scholar] [CrossRef]
- Duan, S.C.; Guo, X.L.; Guo, H.J. A manganese distribution prediction model for CaO-SiO2-FeO-MgO-MnO-Al2O3 slags based on IMCT. Ironmak. Steelmak. 2017, 44, 168–184. [Google Scholar] [CrossRef]
- Gzieo, A.; Jowsa, J. Activity of SiO2 in slags CaO–SiO2–Al2O3–CaF2. Arch. Hut. 1984, 29, 319–331. [Google Scholar]
- Gao, J.X.; Wen, G.H.; Huang, T.; Bai, B.W.; Tang, P.; Liu, Q. Effect of slag-steel reaction on the structure and viscosity of CaO-SiO2-based mold flux during high-Al steel casting. J. Non-Cryst. Solids 2016, 452, 119–124. [Google Scholar] [CrossRef]
- Rey, M. Discuss the thermodynamic activity of silica and of oxides in silicate melts. Discuss. Faraday Soc. 1948, 4, 257–265. [Google Scholar] [CrossRef]
- Charles, R. Activities in Li2O-, Na2O-, and K2O-SiO2 Solutions. J. Am. Ceram. Soc. 1967, 50, 631–641. [Google Scholar] [CrossRef]
- Li, Z.R.; You, X.C.; Li, M.; Wang, Q.Q.; He, S.P.; Wang, Q. Effect of substituting CaO with BaO and CaO/Al2O3 ratio on the viscosity of CaO–BaO–Al2O3–CaF2–Li2O mold flux system. Metals 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.C.; Däcker, C.Å. The Casting Powders Book; Springer: Berlin, Germany, 2017. [Google Scholar]
- Li, G.J.; Du, D.X.; Chi, J.H. Role of Li2O in the mold powder. Iron Steel Vanadium Titan. 1996, 17, 15–18. [Google Scholar]


| Items | Structural Units | Mole Number | Mass Action Concentration |
|---|---|---|---|
| Ion couples | (Ca2+ + O2−) | ||
| (Ba2+ + O2−) | |||
| (Ca2+ + 2F−) | |||
| (2Li+ + O2−) | |||
| Simple molecules | Al2O3 | ||
| SiO2 | |||
| Complex molecules | CaO·SiO2 | ||
| 2CaO·SiO2 | |||
| 3CaO·SiO2 | |||
| 3CaO·2SiO2 | |||
| CaO·Al2O3 | |||
| CaO·2Al2O3 | |||
| CaO·6Al2O3 | |||
| 3CaO·Al2O3 | |||
| 12CaO·7Al2O3 | |||
| BaO·SiO2 | |||
| BaO·2SiO2 | |||
| 2BaO·SiO2 | |||
| 2BaO·3SiO2 | |||
| BaO·Al2O3 | |||
| BaO·6Al2O3 | |||
| 3BaO·Al2O3 | |||
| 3Al2O3·2SiO2 | |||
| Li2O·SiO2 | |||
| Li2O·2SiO2 | |||
| 2Li2O·SiO2 | |||
| Li2O·Al2O3 | |||
| BaO·3CaO·2SiO2 | |||
| 2BaO·4CaO·3SiO2 | |||
| BaO·2CaO·4Al2O3 | |||
| 3BaO·CaO·Al2O3 | |||
| CaO·Al2O3·2SiO2 | |||
| 2CaO·Al2O3·SiO2 | |||
| BaO·Al2O3·2SiO2 | |||
| 3CaO·2SiO2·CaF2 | |||
| 3CaO·3Al2O3·CaF2 | |||
| 11CaO·7Al2O3·CaF2 | |||
| Li2O·Al2O3·2SiO2 | |||
| Li2O·Al2O3·4SiO2 |
| Reaction | Ki | Ni | Ref. | |
|---|---|---|---|---|
| (Ca2+ + O2−) + (SiO2)→(CaO·SiO2) | −92,500 + 1.25T | [10] | ||
| 2(Ca2+ + O2−) + (SiO2)→(2CaO·SiO2) | −102,090 − 24.267T | [21] | ||
| 3(Ca2+ + O2−) + (SiO2)→(3CaO·SiO2) | −118,826 − 6.694T | [21] | ||
| 3(Ca2+ + O2−) + 2(SiO2)→(3CaO·2SiO2) | −236,814 + 9.623T | [21] | ||
| (Ca2+ + O2−) + (Al2O3)→(CaO·Al2O3) | 59,413 − 59.413T | [21] | ||
| (Ca2+ + O2−) + 2(Al2O3)→(CaO·2Al2O3) | −16,736 − 25.522T | [21] | ||
| (Ca2+ + O2−) + 6(Al2O3)→(CaO·6Al2O3) | −22,594 − 31.798T | [21] | ||
| 3(Ca2+ + O2−) + (Al2O3)→(3CaO·Al2O3) | −21,757 − 29.288T | [21] | ||
| 12(Ca2+ + O2−) + 7(Al2O3)→(12CaO·7Al2O3) | 617,977 − 612.119 T | [21] | ||
| (Ba2+ + O2−) + (SiO2)→(BaO·SiO2) | −154,238 − 2.926T | [22] | ||
| (Ba2+ + O2−) + 2(SiO2)→(BaO·2SiO2) | −169,365 + 1.496T | [22] | ||
| 2(Ba2+ + O2−) + (SiO2)→(2BaO·SiO2) | −264,183 − 3.395T | [22] | ||
| 2(Ba2+ + O2−) + 3(SiO2)→(2BaO·3SiO2) | −337,580 + 7.039T | [22] | ||
| (Ba2+ + O2−) + (Al2O3)→(BaO·Al2O3) | −99,760 − 25.413T | [22] | ||
| (Ba2+ + O2−) + 6(Al2O3)→(BaO·6Al2O3) | −126,813 − 24.293T | [22] | ||
| 3(Ba2+ + O2−) + (Al2O3)→(3BaO·Al2O3) | −187,633 − 37.528T | [23] | ||
| 3(Al2O3) + 2(SiO2)→(3Al2O3·2SiO2) | −4354 − 10.467T | [22] | ||
| (2Li+ + O2−) + (SiO2)→(Li2O·SiO2) | −143,757 + 3.796T | [22] | ||
| (2Li+ + O2−) + 2(SiO2)→(Li2O·2SiO2) | −145,174 − 1.372T | [22] | ||
| 2(2Li+ + O2−) + (SiO2)→(2Li2O·SiO2) | −230,237 + 15.442T | [22] | ||
| (2Li+ + O2−) + (Al2O3)→(Li2O·Al2O3) | −106,327 − 16.567T | [22] | ||
| (Ba2+ + O2−) + 3(Ca2+ + O2−) + 2(SiO2)→(BaO·3CaO·2SiO2) | −376,298 + 8.751T | [22] | ||
| 2(Ba2+ + O2−) + 4(Ca2+ + O2−) + 3(SiO2)→(2BaO·4CaO·3SiO2) | −533,550 + 269.292T | [22] | ||
| (Ba2+ + O2−) + 2(Ca2+ + O2−) + 4(Al2O3)→(BaO·2CaO·4Al2O3) | −157,255 − 85.113T | [22] | ||
| 3(Ba2+ + O2−) + (Ca2+ + O2−) + (Al2O3)→(3BaO·CaO·Al2O3) | −139,905 − 42.192T | [22] | ||
| (Ca2+ + O2−) + (Al2O3) + 2(SiO2)→(CaO·Al2O3·2SiO2) | −4184 − 73.638T | [23,24] | ||
| 2(Ca2+ + O2−) + (Al2O3) + (SiO2)→(2CaO·Al2O3·SiO2) | −116,315 − 38.911T | [23,24] | ||
| (Ba2+ + O2−) + (Al2O3) + 2(SiO2)→(BaO·Al2O3·2SiO2) | −198,791 − 38.497T | [22] | ||
| 3(Ca2+ + O2−) + 2(SiO2) + (Ca2+ + 2F−)→(3CaO·2SiO2·CaF2) | −255,180 − 8.20T | [23,24] | ||
| 3(Ca2+ + O2−) + 3(Al2O3) + (Ca2+ + 2F−)→(3CaO·3Al2O3·CaF2) | −44,492 − 73.15T | [23,24] | ||
| 11(Ca2+ + O2−) + 7(Al2O3) + (Ca2+ + 2F−)→(11CaO·7Al2O3·CaF2) | −228,760 − 155.8T | [23,24] | ||
| (2Li+ + O2−) + (Al2O3) + 2(SiO2)→(Li2O·Al2O3·2SiO2) | −136,270 − 37.516T | [19] | ||
| (2Li+ + O2−) + (Al2O3) + 4(SiO2)→(Li2O·Al2O3·4SiO2) | −128,739 − 48.253T | [19] |
| CaO | BaO | Al2O3 | SiO2 | F | Li2O | ||
|---|---|---|---|---|---|---|---|
| Fluxes | S-1 | 36.5 | 24 | 21 | 6 | 8 | 4.5 |
| S-2 | 36.5 | 24 | 19 | 8 | 8 | 4.5 | |
| S-3 | 36.5 | 24 | 17 | 10 | 8 | 4.5 | |
| S-4 | 36.5 | 24 | 15 | 12 | 8 | 4.5 | |
| Steel | C | Al | Mn | Si | S | Fe | |
| 0.17 | 1.49 | 22.7 | 0.22 | 0.02 | Bal. | ||
| CaO | BaO | Al2O3 | SiO2 | CaF2 | ki,cal | ki,fit | ΔX | |
|---|---|---|---|---|---|---|---|---|
| CaO-Al2O3-SiO2-BaO | 20 | 30 | 10 | 40 | 0 | −0.670 | −0.884 | 0.242 |
| 20 | 40 | 10 | 30 | 0 | −1.241 | −1.549 | 0.199 | |
| 20 | 50 | 10 | 20 | 0 | −2.378 | −2.234 | 0.064 | |
| 30 | 30 | 10 | 30 | 0 | −1.494 | −1.711 | 0.127 | |
| 40 | 20 | 10 | 30 | 0 | −1.763 | −1.943 | 0.093 | |
| 50 | 10 | 10 | 30 | 0 | −2.093 | −2.411 | 0.132 | |
| 50 | 20 | 20 | 10 | 0 | −4.632 | −4.653 | 0.005 | |
| 40 | 20 | 30 | 10 | 0 | −3.983 | −4.049 | 0.016 | |
| 30 | 20 | 40 | 10 | 0 | −2.851 | −3.383 | 0.157 | |
| CaO-Al2O3-SiO2-CaF2 | 36 | 0 | 16 | 40 | 8 | −0.969 | −1.006 | 0.037 |
| 40 | 0 | 16 | 36 | 8 | −1.297 | −1.294 | 0.002 | |
| 40 | 0 | 22 | 30 | 8 | −1.734 | −1.623 | 0.068 | |
| 40 | 0 | 34 | 18 | 8 | −2.552 | −2.627 | 0.029 | |
| 40 | 0 | 40 | 12 | 8 | −2.879 | −3.236 | 0.110 | |
| 36 | 0 | 8 | 40 | 16 | −0.876 | −0.846 | 0.035 | |
| 40 | 0 | 8 | 36 | 16 | −1.188 | −1.111 | 0.069 | |
| 40 | 0 | 14 | 30 | 16 | −1.729 | −1.529 | 0.131 | |
| 40 | 0 | 26 | 18 | 16 | −2.731 | −2.795 | 0.023 | |
| 40 | 0 | 32 | 12 | 16 | −3.106 | −3.302 | 0.059 |
| Mold Flux | Before/After | CaO | BaO * | Al2O3 | SiO2 | F | Li2O | MgO |
|---|---|---|---|---|---|---|---|---|
| S-1 | Initial | 36.70 | 24.00 | 20.82 | 6.07 | 8.00 | 4.08 | 0.00 |
| Final | 35.20 | 23.00 | 22.31 | 5.05 | 7.60 | 3.78 | 2.55 | |
| S-2 | Initial | 36.41 | 24.00 | 19.12 | 8.21 | 6.90 | 4.12 | 0.00 |
| Final | 35.80 | 23.00 | 21.01 | 6.90 | 6.80 | 3.81 | 2.46 | |
| S-3 | Initial | 36.23 | 24.00 | 17.09 | 10.11 | 7.20 | 4.05 | 0.00 |
| Final | 35.10 | 23.00 | 20.24 | 7.09 | 7.00 | 3.35 | 2.32 | |
| S-4 | Initial | 36.15 | 24.00 | 15.24 | 12.18 | 7.00 | 4.07 | 0.00 |
| Final | 33.71 | 23.00 | 20.45 | 8.58 | 6.80 | 3.74 | 2.89 |
| Variable | CaO | BaO | Al2O3 | SiO2 | CaF2 | Li2O | Sum | Interval |
|---|---|---|---|---|---|---|---|---|
| Al2O3, SiO2 | 20 | 20 | 8–32 | 28–4 | 16 | 8 | 100 | 4 |
| CaF2 | CaO:BaO = 1:1 | 24 | 12 | 4–28 | 8 | 100 | 4 | |
| Li2O | CaO:BaO = 1:1 | 24 | 12 | 16 | 2–14 | 100 | 2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Li, G.; Li, Z.; Wang, Q.; He, S. Study of Thermodynamic for Low-Reactive CaO-BaO-Al2O3-SiO2-CaF2-Li2O Mold Flux Based on the Model of Ion and Molecular Coexistence Theory. Metals 2022, 12, 1099. https://doi.org/10.3390/met12071099
Zhao M, Li G, Li Z, Wang Q, He S. Study of Thermodynamic for Low-Reactive CaO-BaO-Al2O3-SiO2-CaF2-Li2O Mold Flux Based on the Model of Ion and Molecular Coexistence Theory. Metals. 2022; 12(7):1099. https://doi.org/10.3390/met12071099
Chicago/Turabian StyleZhao, Maoguo, Gang Li, Zhirong Li, Qiangqiang Wang, and Shengping He. 2022. "Study of Thermodynamic for Low-Reactive CaO-BaO-Al2O3-SiO2-CaF2-Li2O Mold Flux Based on the Model of Ion and Molecular Coexistence Theory" Metals 12, no. 7: 1099. https://doi.org/10.3390/met12071099
APA StyleZhao, M., Li, G., Li, Z., Wang, Q., & He, S. (2022). Study of Thermodynamic for Low-Reactive CaO-BaO-Al2O3-SiO2-CaF2-Li2O Mold Flux Based on the Model of Ion and Molecular Coexistence Theory. Metals, 12(7), 1099. https://doi.org/10.3390/met12071099






