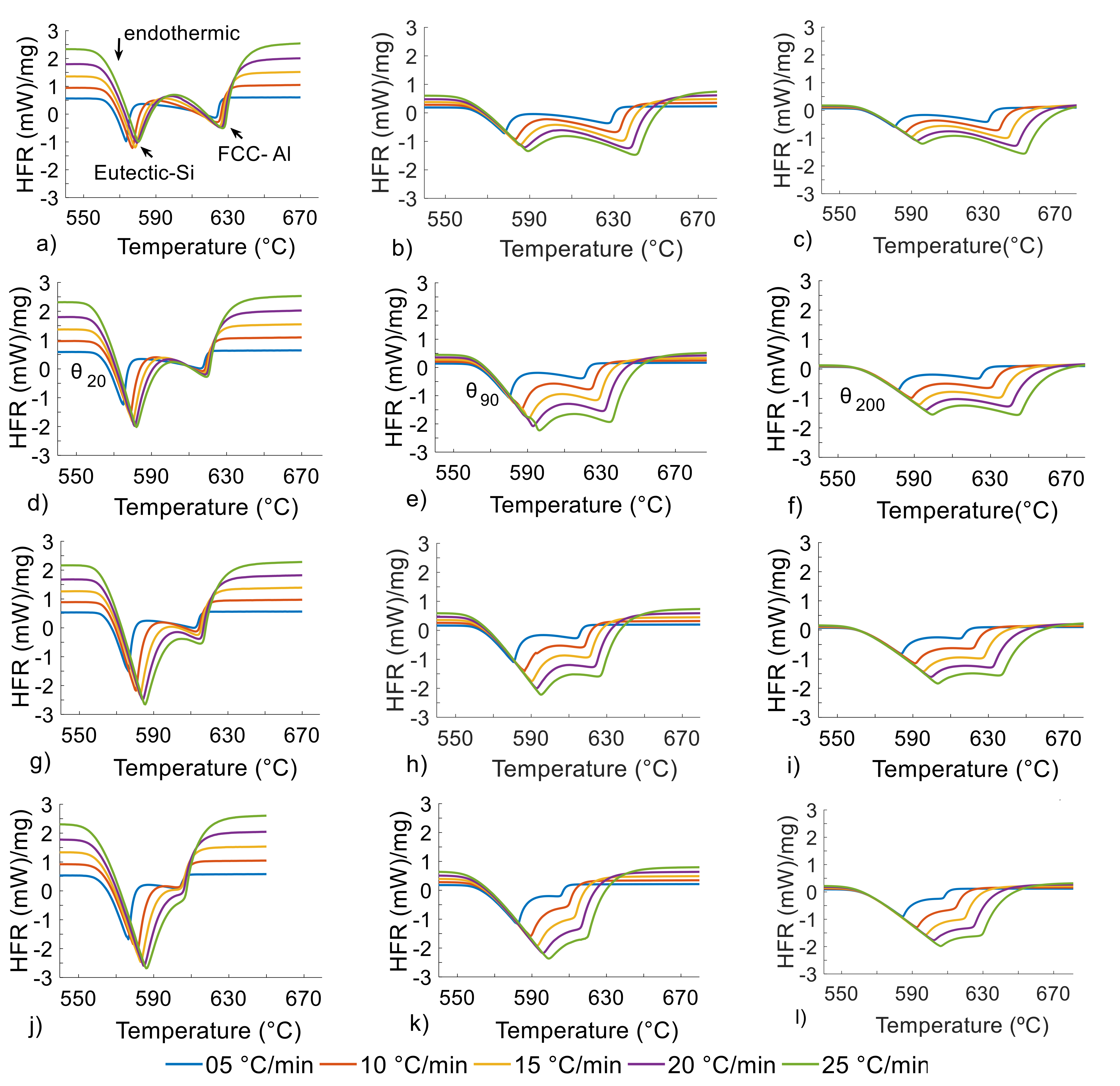
Figure 1.
DSC heat flow rate (y-axis) vs. temperature (x-axis) curves during melting of the AlXSi4Zn alloys, X = 4 (a–c), 5 (d–f), 6 (g–i) and 7 wt.%Si (j–l), at 5–25 °C/min with DSC sample masses of 20 (left), 90 (middle) and 200 mg (right).
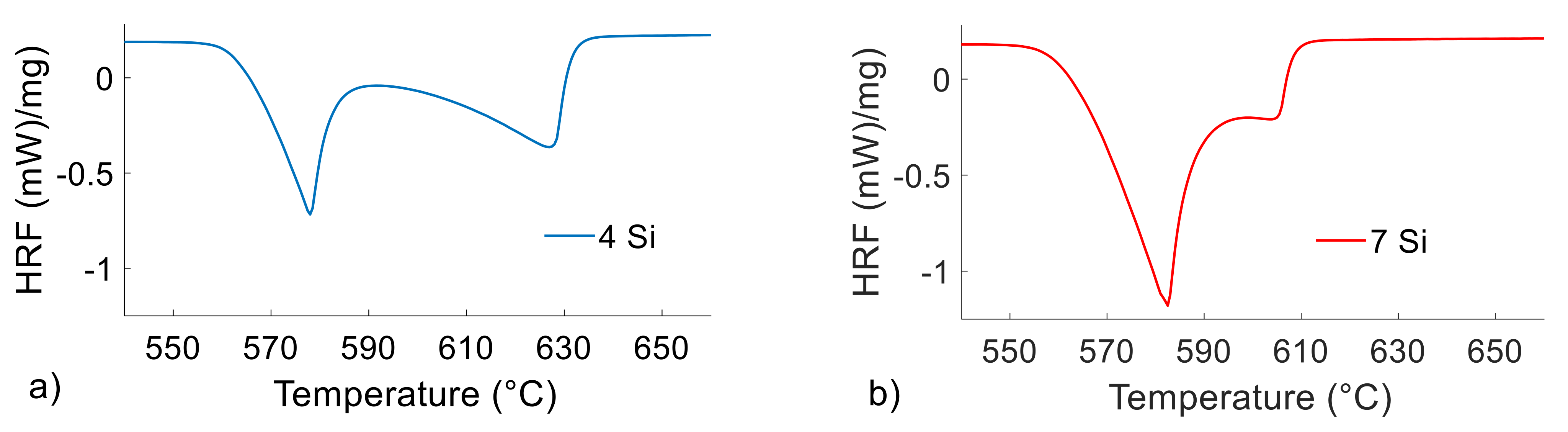
Figure 2.
DSC heat flow rate (y-axis) vs. temperature (x-axis) curves during melting of the Al4Si4Zn (a) and Al7Si4Zn (b) alloys at 5 °C/min with a DSC sample mass of 90 mg.
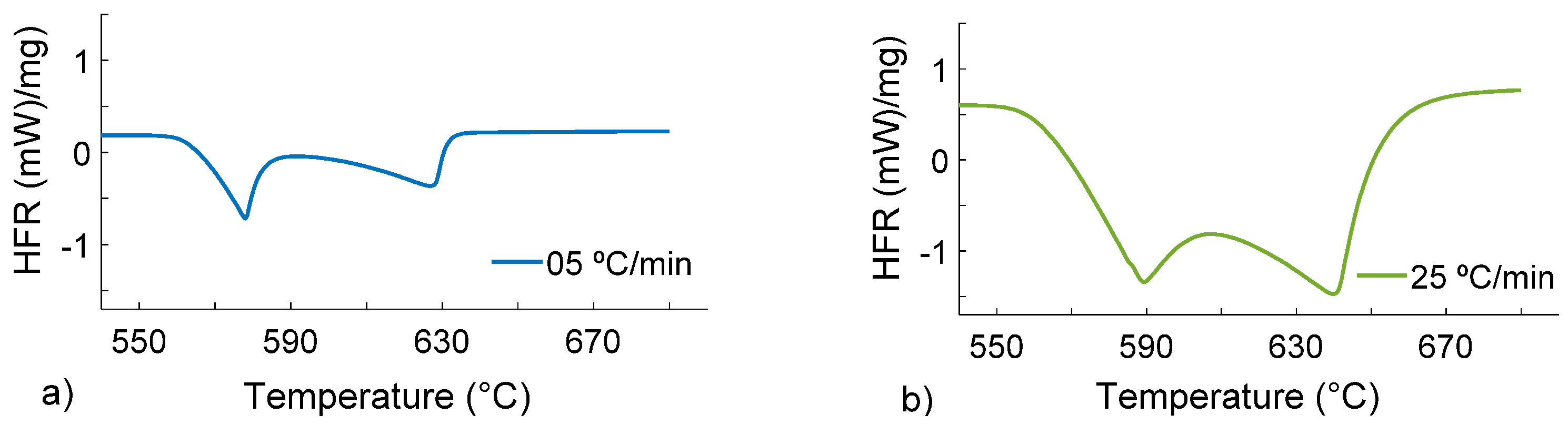
Figure 3.
DSC heat flow rate (y-axis) vs. temperature (x-axis) curves during melting of the Al4Si4Zn alloy at 5 (a) and 25 (b) °C/min with a DSC sample mass of 90 mg.
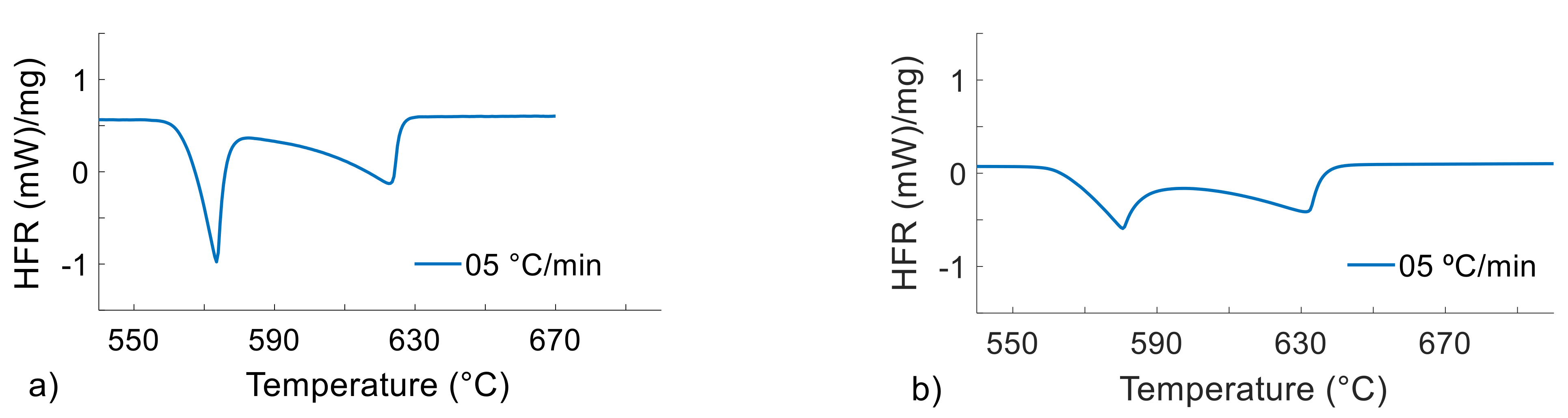
Figure 4.
DSC heat flow rate (y-axis) vs. temperature (x-axis) curves during melting of the Al4Si4Zn alloy at 5 °C/min with DSC sample masses of 20 (a) and 200 (b) mg.
Figure 5.
HFR vs. T (black), dHFR/dT vs. T (red), fs vs. T (dark blue) and dfs/dT vs. T (light blue) curves for Al7Si4Zn alloy with a 5 °C/min heating rate and 20 mg sample mass; the temperatures/solid fractions at the beginning (SSML) and end (SSMH) of the thixoforming processing working window are shown as solid dots. (a) Firt step-obtaining of the dFHR/dT/dT from FHR, (b) Second step-Partial integration of the HFR curve to obtain the fs × T curve, (c) derivative curve of the solid fraction as equivalent of the sensitivity, (d) solid fraction and its corresponding sensitivity as a function of temperature.
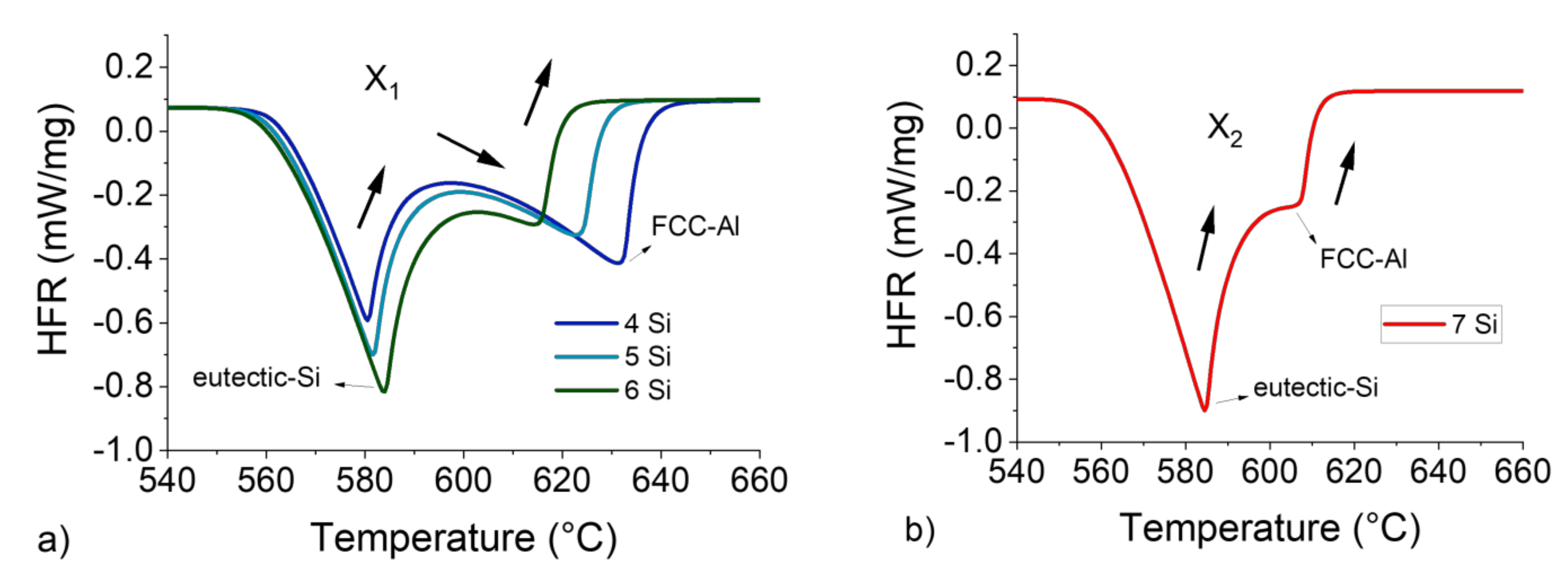
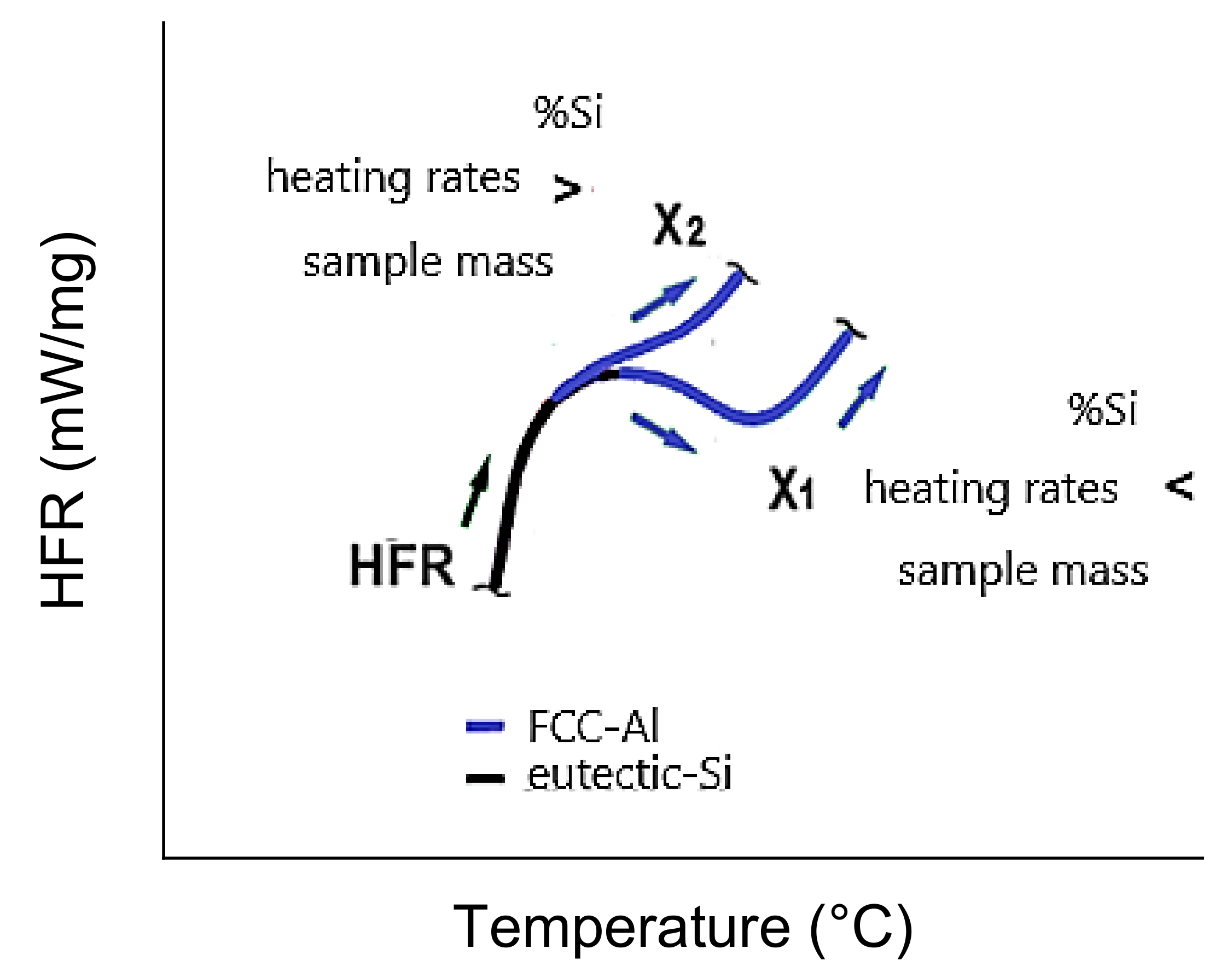
Figure 6.
HFR vs. T curves for 4 to 6 wt.%Si with a 5 °C/min heating rate and 200 mg sample mass, complete curvature inversion at the Al-α/Si-eutectic interface X1 (a), and HFR vs. T curve for 7 wt.%Si with a 5 °C/min heating rate and 200 mg sample mass, with incomplete curvature X2 (b).
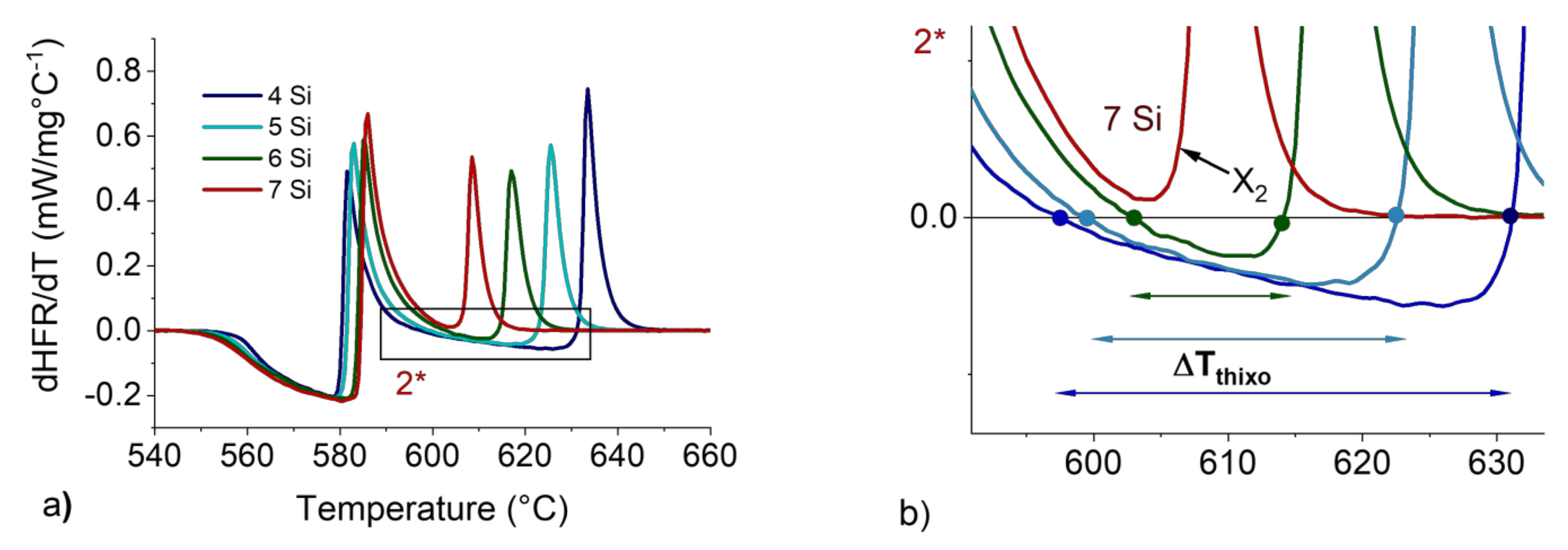
Figure 7.
dHFR/dT vs. T curves for 4 to 7 wt.%Si with a 5 °C/min heating rate and 200 mg sample mass (a). Magnification at the ΔTTHIXO zone (b).
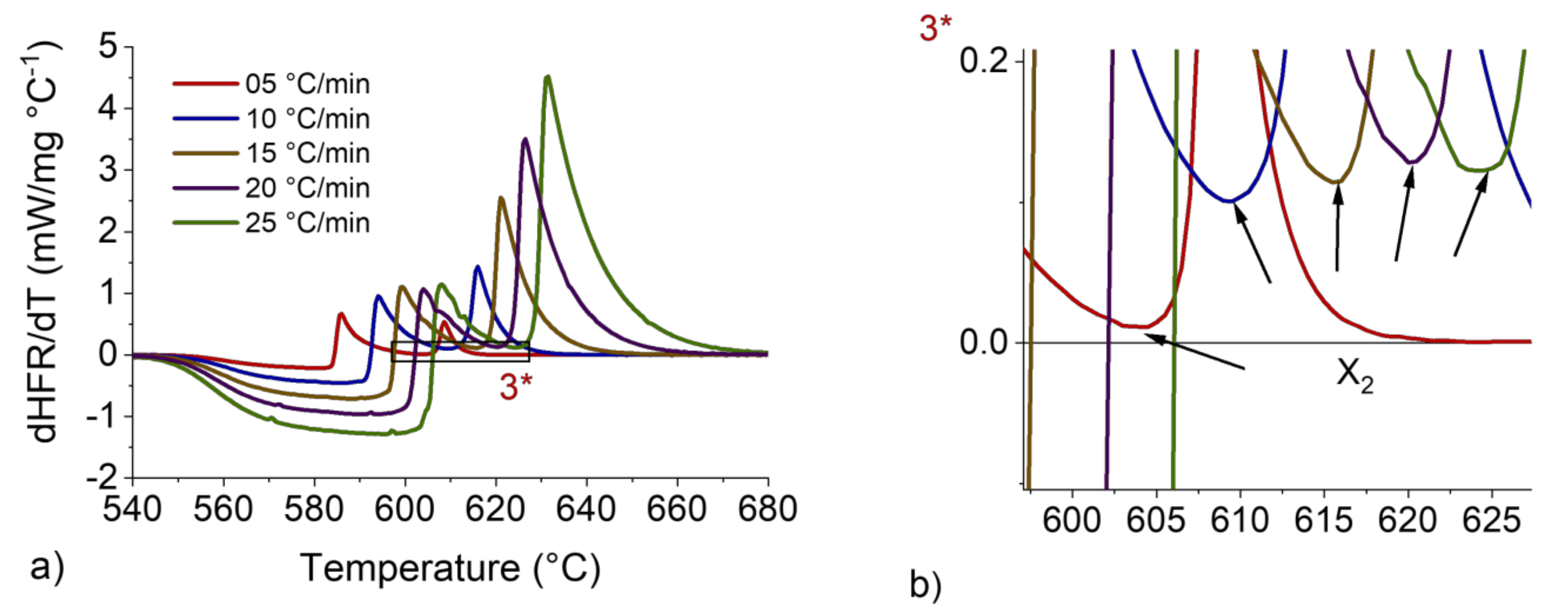
Figure 8.
dHFR/dT vs. T curve for 7 wt.%Si with heating rates from 5 to 25 °C/min and sample mass of 200 mg (a). Magnification at the ΔTTHIXO zone (b).
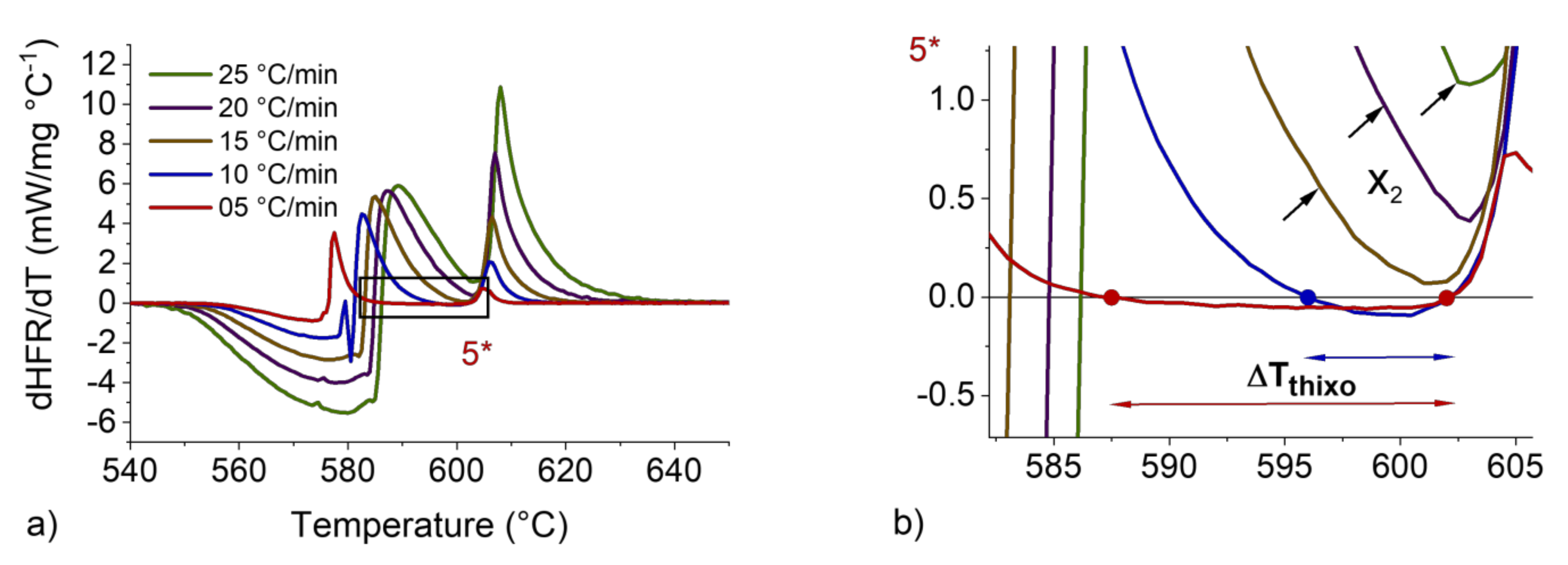
Figure 9.
dHFR/dT vs. T curve for 7 wt.%Si with heating rates from 5 to 25 °C/min and sample mass of 90 mg (a). Magnification at the ΔTTHIXO zone (b).
Figure 10.
dHFR/dT vs. T curve for 7 wt.%Si with heating rates from 5 to 25 °C/min and sample mass of 20 mg (a). Magnification at the ΔTTHIXO zone (b).
Figure 11.
Correlation between Si content, heating rate and DSC sample mass and the feasibility of identifying ΔTTHIXO for the Al-Si-Zn system.
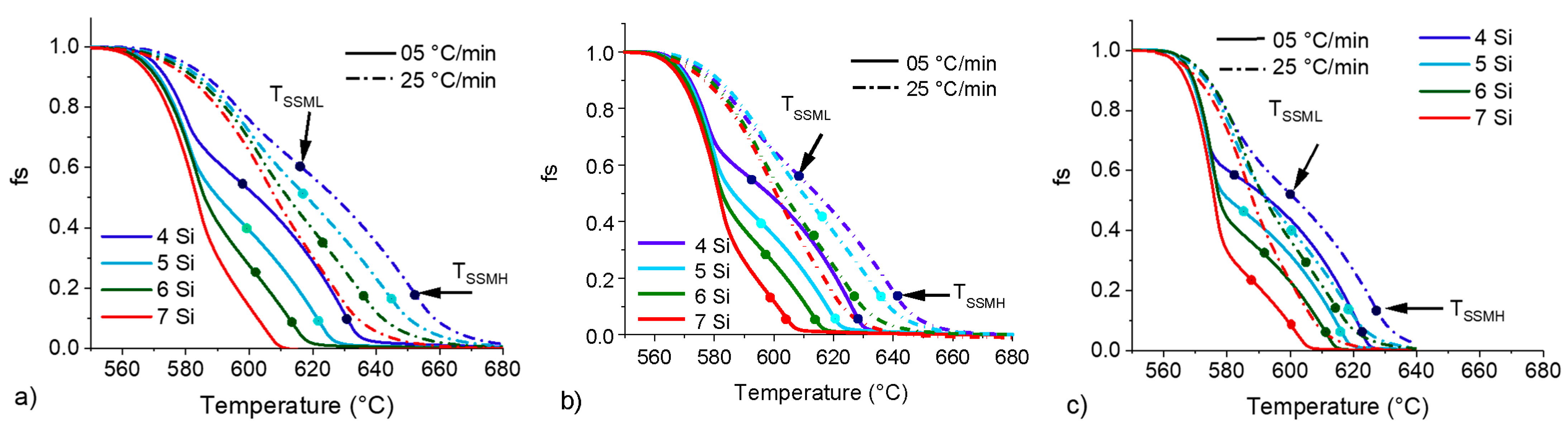
Figure 12.
fS vs. T curve for 4, 5, 6 and 7 wt.%Si with heating rates of 5 and 25 °C/min and sample masses of 200 (a) 90 (b) and 20 mg (c).
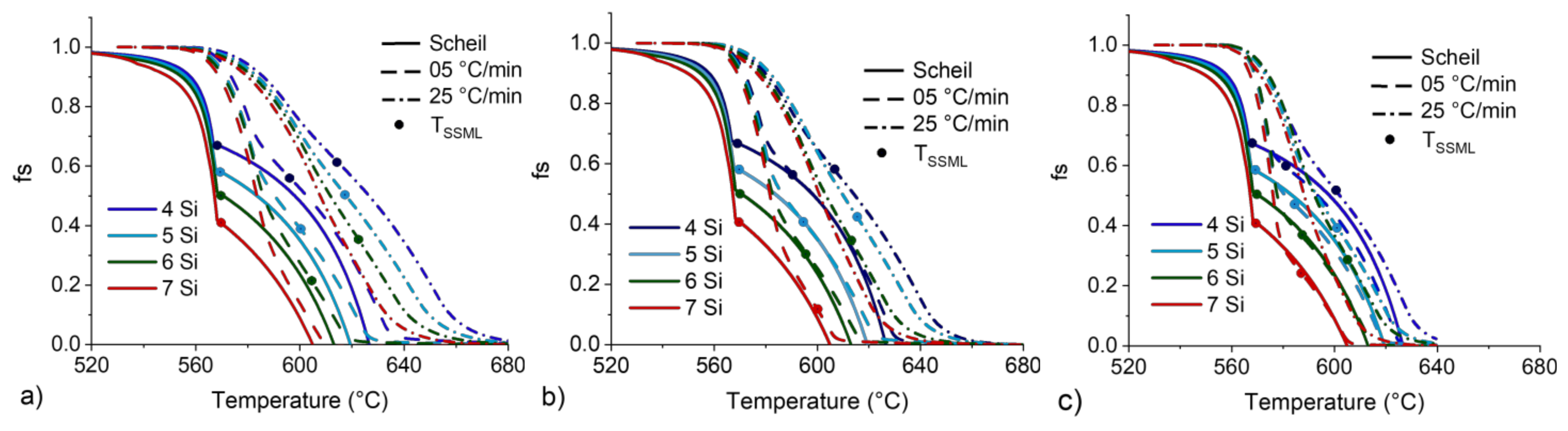
Figure 13.
fS vs. T curves for 4 to 7 wt.%Si from CALPHAD simulation at the Scheil condition and DSC curves for heating rates of 5 and 25 °C/min and 200 (a), 90 (b) and 20 mg (c) DSC samples.
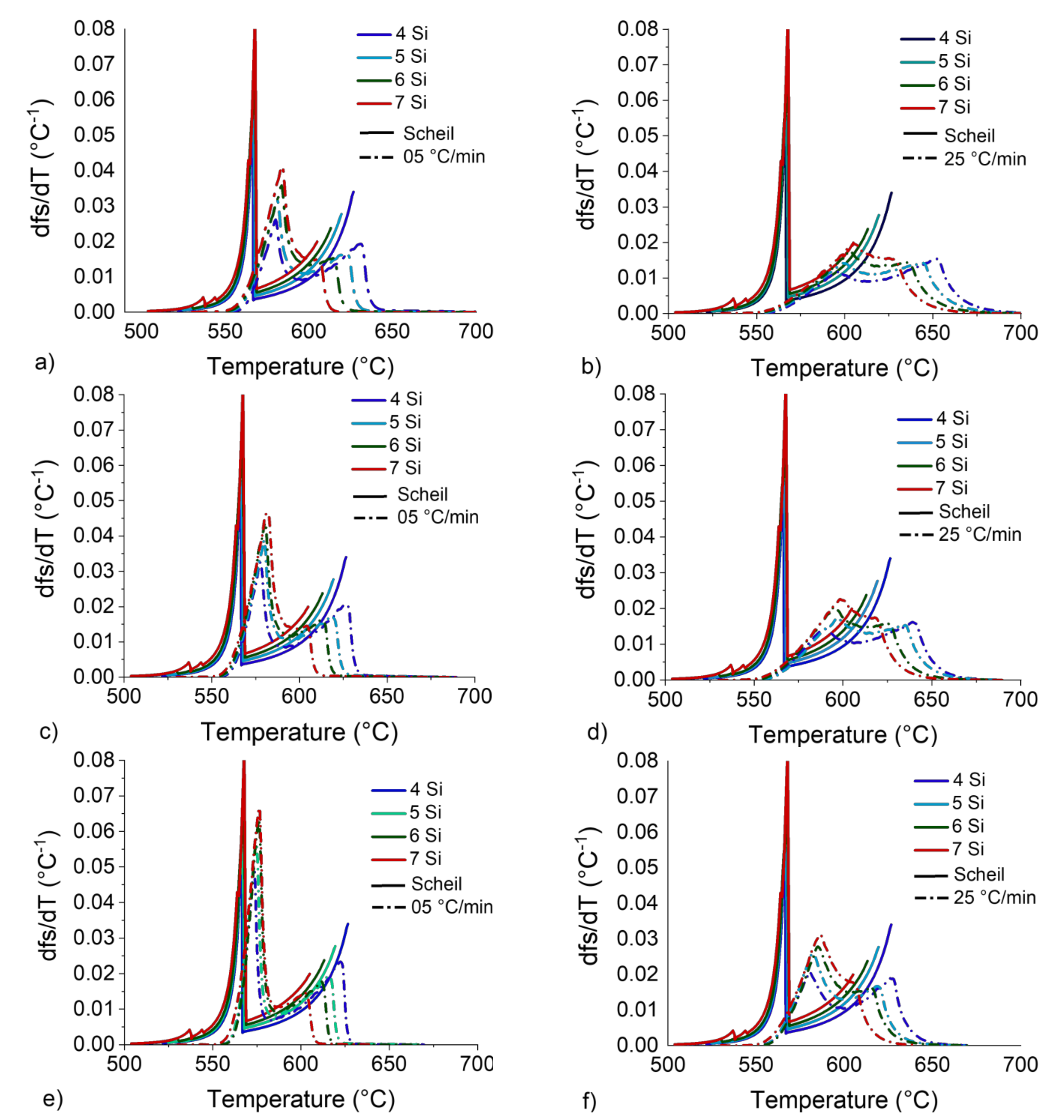
Figure 14.
dfS/dT vs. T curves for 4 to 7 wt.%Si obtained by CALPHAD simulation at the Scheil condition and DSC curves for heating rates of 5 (left) and 25 °C/min (right) and DSC sample masses of 200 (a,b), 90 (c,d) and 20 mg (e,f).
Table 1.
AlXSi4Zn alloys (composition in wt.%) for use in thixoforming processes; standard uncertainties are shown as the deviation corresponding to a 0.95 confidence interval.
| Alloy | Si | Zn | Fe | Mg 1 | Cu 1 | Mn 1 | Ti 1 | Res. 1,2 | Al |
|---|
| Al4Si4Zn | 4.15 ± 0.20 | 3.88 ± 0.13 | 0.17 ± 0.02 | 0.14 | 0.08 | 0.07 | 0.03 | 0.03 | Bal. 3 |
| Al5Si4Zn | 5.18 ± 0.16 | 3.98 ± 0.18 | 0.19 ± 0.02 | 0.17 | 0.10 | 0.09 | 0.04 | 0.04 | Bal. 3 |
| Al6Si4Zn | 6.11 ± 0.28 | 3.86 ± 0.16 | 0.19 ± 0.03 | 0.23 | 0.12 | 0.11 | 0.06 | 0.06 | Bal. 3 |
| Al7Si4Zn | 7.24 ± 0.17 | 3.72 ± 0.14 | 0.19 ± 0.02 | 0.39 | 0.14 | 0.12 | 0.07 | 0.05 | Bal. 3 |
Table 2.
Area between the DSC curves and baseline for the alloys studied here when heated at 5 and 25 °C/min with different sample masses.
| | Area between DSC Curve and Baseline (W°C/g) |
|---|
| dT/dt (°C/min) | 5 | - | - | 25 | - | - |
| Mass (mg) | 20 | 90 | 200 | 20 | 90 | 200 |
| Al4Si4Zn | 30.6 | 27.9 | 25.5 | 162.5 | 140.1 | 121.3 |
| Al5Si4Zn | 30.8 | 31.1 | 24.5 | 167.4 | 159.0 | 122.1 |
| Al6Si4Zn | 31.8 | 29.0 | 25.4 | 172.8 | 144.9 | 122.8 |
| Al7Si4Zn | 32.6 | 28.9 | 24.9 | 163.9 | 139.4 | 119.1 |
Table 3.
Enthalpy change for the melting transformation for the alloys studied here when heated at 5 and 25 °C/min with different sample masses.
| | Enthalpy Change (J/g) |
|---|
| dT/dt (°C/min) | 5 | - | - | 25 | - | - |
| Mass (mg) | 20 | 90 | 200 | 20 | 90 | 200 |
| Al4Si4Zn | 369.7 | 334.8 | 309.5 | 364.8 | 312.9 | 275.8 |
| Al5Si4Zn | 381.6 | 375.5 | 295.7 | 361.0 | 360.9 | 277.8 |
| Al6Si4Zn | 388.0 | 349.0 | 303.9 | 389.8 | 324.3 | 279.0 |
| Al7Si4Zn | 394.0 | 344.0 | 294.0 | 359.0 | 313.8 | 272.8 |
Table 4.
Solidus, liquidus and semisolid interval for the melting transformation for the alloys studied here when heated at 5 and 25 °C/min with different sample masses.
| | Solidus Temperature (°C) |
| dT/dt (°C/min) | 5 | - | - | 25 | - | - |
| Mass (mg) | 20 | 90 | 200 | 20 | 90 | 200 |
| Al4Si4Zn | 551.7 | 546.9 | 554.3 | 543.8 | 541.7 | 546.1 |
| Al5Si4Zn | 552.4 | 548.4 | 553.3 | 546.2 | 544.2 | 545.5 |
| Al6Si4Zn | 550.3 | 547.0 | 550.1 | 545.4 | 540.4 | 542.1 |
| Al7Si4Zn | 549.4 | 546.6 | 548.7 | 541.4 | 536.8 | 543.3 |
| | Liquidus Temperature (°C) |
| Al4Si4Zn | 631.8 | 641.3 | 651.7 | 664.7 | 686.9 | 714.9 |
| Al5Si4Zn | 625.5 | 633.0 | 643.0 | 654.1 | 681.3 | 701.4 |
| Al6Si4Zn | 620.0 | 625.3 | 636.1 | 651.2 | 673.0 | 694.3 |
| Al7Si4Zn | 612.9 | 615.7 | 621.4 | 642.3 | 663.9 | 682.7 |
| | Semisolid interval (°C) |
| Al4Si4Zn | 80.1 | 94.4 | 97.4 | 120.9 | 145.2 | 168.8 |
| Al5Si4Zn | 73.1 | 84.6 | 89.7 | 107.9 | 137.1 | 155.9 |
| Al6Si4Zn | 69.7 | 78.3 | 86.0 | 105.8 | 132.6 | 152.2 |
| Al7Si4Zn | 63.5 | 69.1 | 72.7 | 100.9 | 127.1 | 139.4 |
Table 5.
Comparison of the variables area between DSC curve and baseline (A in W°C/g), enthalpy change (ΔH in V/g), solidus (S in °C), liquidus (L in °C) and semisolid interval (S–L in °C) for extreme (lower and upper) values of Si content, heating rate and sample mass.
| Condition (Lower–Upper) | Ratio 4 |
|---|
| A | ΔH | S | L | S-L |
|---|
| Si (4–7 wt.%) | 1.01 | 0.24 | 0.99 | 0.96 | 0.80 |
| dT/dt (5–25 °C/min) | 5.04 | 0.93 | 0.98 | 1.07 | 1.66 |
| mass (20–200 mg) | 0.76 | 0.77 | 1.00 | 1.05 | 1.31 |
Table 6.
Average DSC sample dimensions.
| Mass (mg) | Height (mm) | Radius (mm) | As 5 (mm2) | V (mm3) | As/V (mm−1) | Ratio (-) |
|---|
| 20 | 1.0 | 3.6 | 64.06 | 41.28 | 1.55 | 1.00 |
| 90 | 2.1 | 4.2 | 108.71 | 112.54 | 0.96 | 0.62 |
| 200 | 2.6 | 5.1 | 164.49 | 212.18 | 0.77 | 0.50 |
Table 7.
The ∆TTHIXO AlXSi4Zn for 05 °C/min and 25 °C/min, with a sample mass 200 mg, 90 mg, and 20 mg.
| Mass (mg) | wt.%Si | ∆TTHIXO (°C) |
|---|
| 05 °C/min | 25 °C/min |
|---|
| 200 | 4Si | 33.8 | 38.1 |
| 5Si | 23.3 | 27.7 |
| 6Si | 10.9 | 13.5 |
| 7Si | NI | NI |
| 90 | 4Si | 35 | 32.3 |
| 5Si | 23.5 | 18.2 |
| 6Si | 16.7 | 12.7 |
| 7Si | 4.6 | NI |
| 20 | 4Si | 39.8 | 26.8 |
| 5Si | 31.4 | 17.4 |
| 6Si | 24.7 | 9.9 |
| 7Si | 14.8 | NI |
Table 8.
The TSSML for AlXSi4Zn in the Scheil condition, 05 °C/min and 25 °C/min, with sample mass 200 mg, 90 mg and 20 mg.
| Mass (mg) | wt.%Si | TSSML (°C) |
|---|
| Scheil | 05 °C/min | 25 °C/min |
|---|
| 200 | 4Si | 566 | 597.5 | 614.0 |
| 5Si | 567 | 599.0 | 616.0 |
| 6Si | 568 | 603.0 | 621.6 |
| 7Si | 568 | NI | NI |
| 90 | 4Si | 566 | 591.6 | 607.2 |
| 5Si | 567 | 595.3 | 615.4 |
| 6Si | 568 | 593.5 | 609.4 |
| 7Si | 568 | 599.2 | NI |
| 20 | 4Si | 566 | 582.4 | 600.1 |
| 5Si | 567 | 584.3 | 602.2 |
| 6Si | 568 | 586.2 | 604.8 |
| 7Si | 568 | 587.8 | NI |
Table 9.
Thixoforming working window temperatures (in °C) and solid fractions (in mass fraction) for the Al-Si-Zn alloys studied here for 20 mg samples. Values that were not identified are shown as NI.
dT/dt
(°C/min) | TSSML | TSSMH | ΔTTHIXO | fsSSML | fsSSMH | ΔfsTHIXO |
|---|
| 5 | 25 | 5 | 25 | 5 | 25 | 5 | 25 | 5 | 25 | 5 | 25 |
|---|
| 4Si | 582 | 600 | 622 | 627 | 40 | 27 | 0.58 | 0.51 | 0.07 | 0.13 | 0.51 | 0.38 |
| 5Si | 584 | 602 | 616 | 619 | 32 | 17 | 0.45 | 0.37 | 0.04 | 0.12 | 0.41 | 0.25 |
| 6Si | 586 | 605 | 612 | 615 | 26 | 10 | 0.36 | 0.28 | 0.04 | 0.13 | 0.32 | 0.15 |
| 7Si | 588 | NI | 602 | NI | 14 | NI | 0.23 | NI | 0.05 | NI | 0.18 | NI |