Kinetics of Reordering in Quenched Ni2Mn0.8Cu0.2Ga Ferromagnetic Shape Memory Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
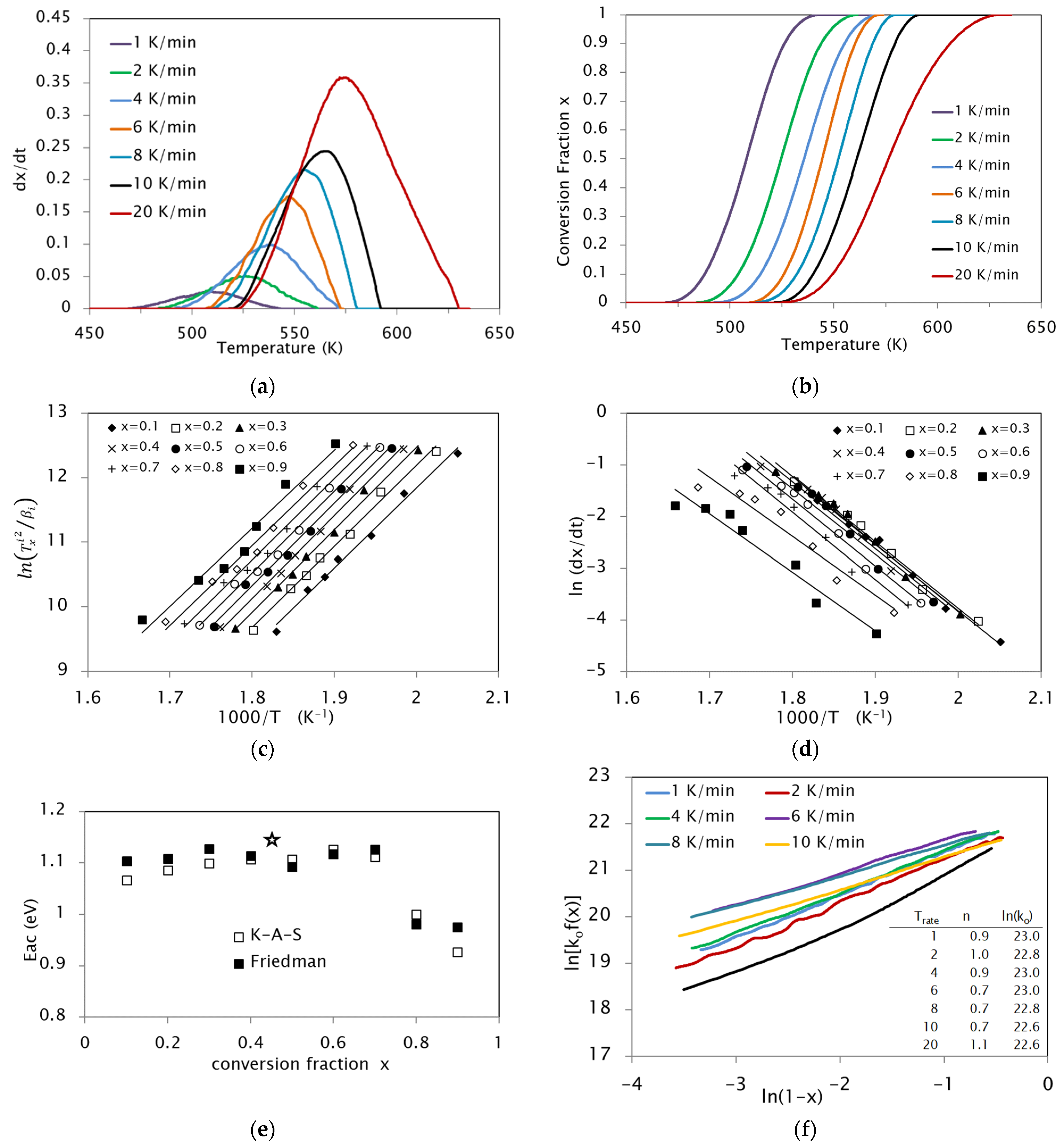
3.1. Activation Energies
3.2. Pre-Exponentional Factor
3.3. Reaction Model
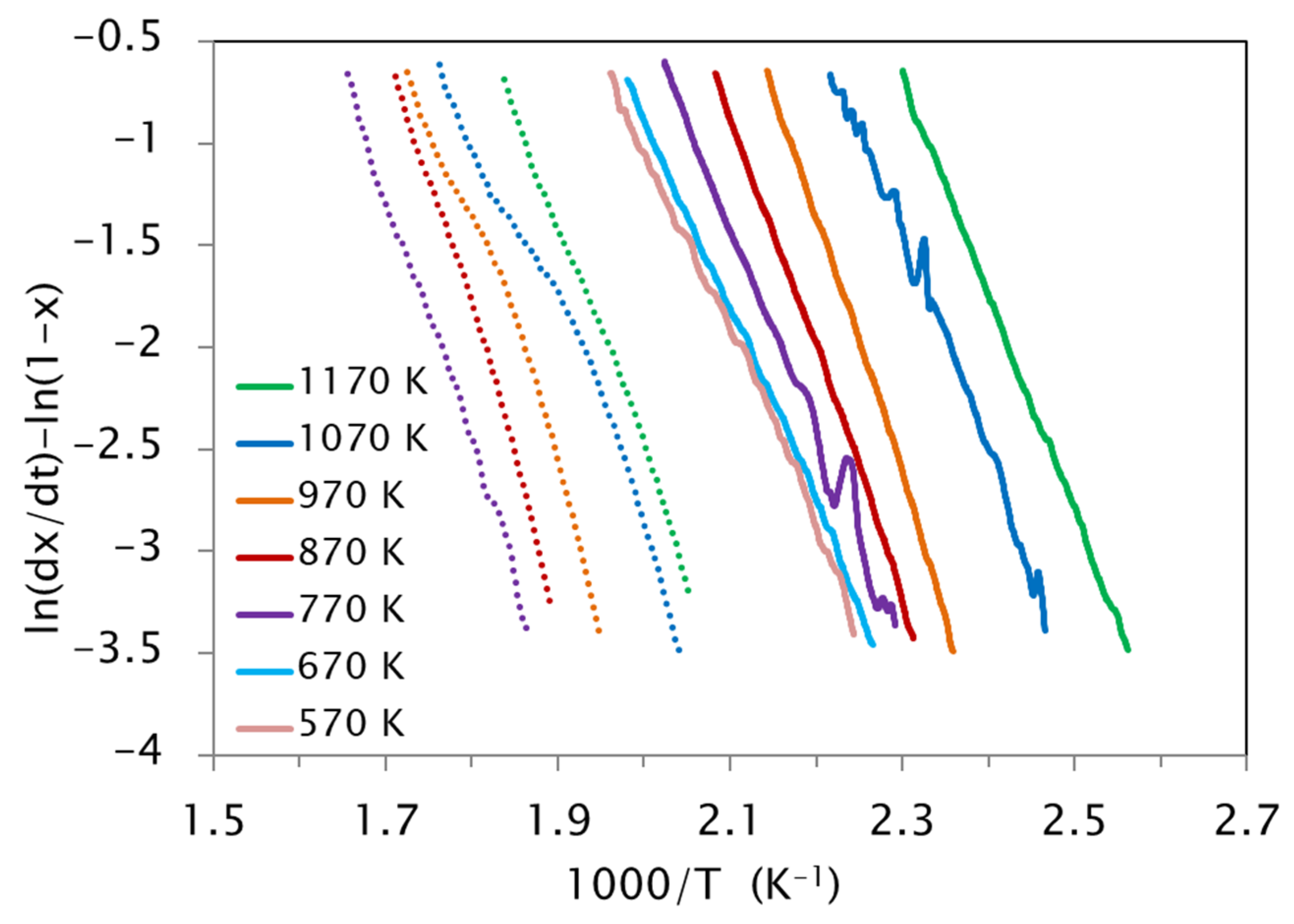
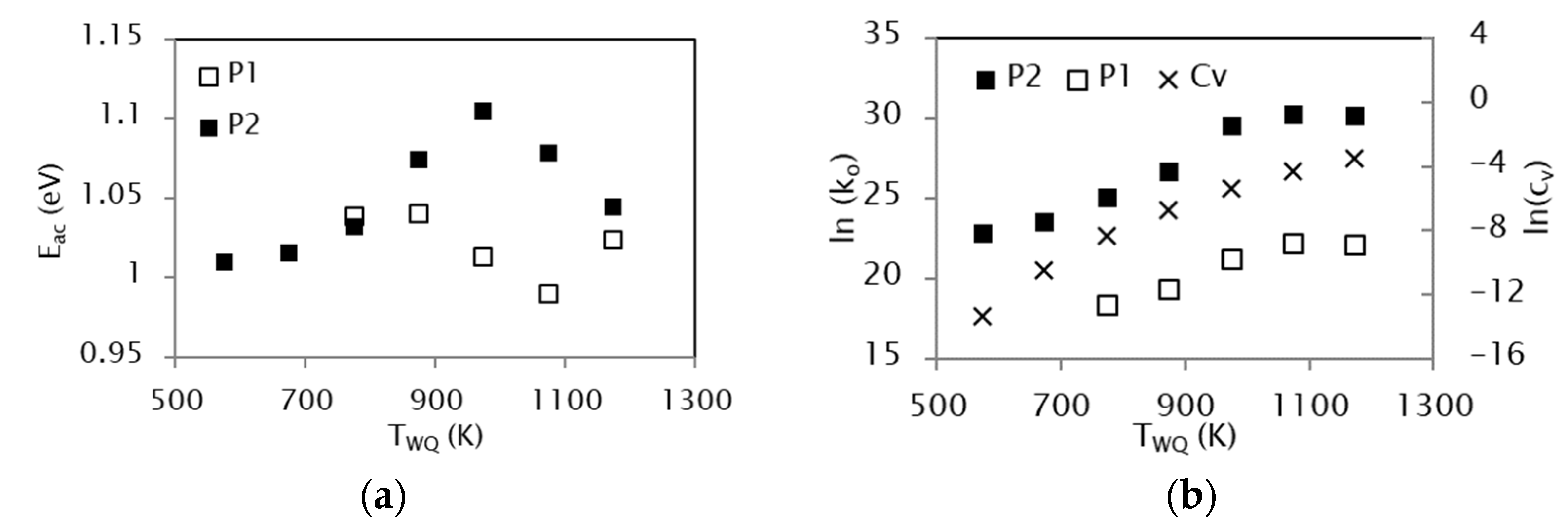
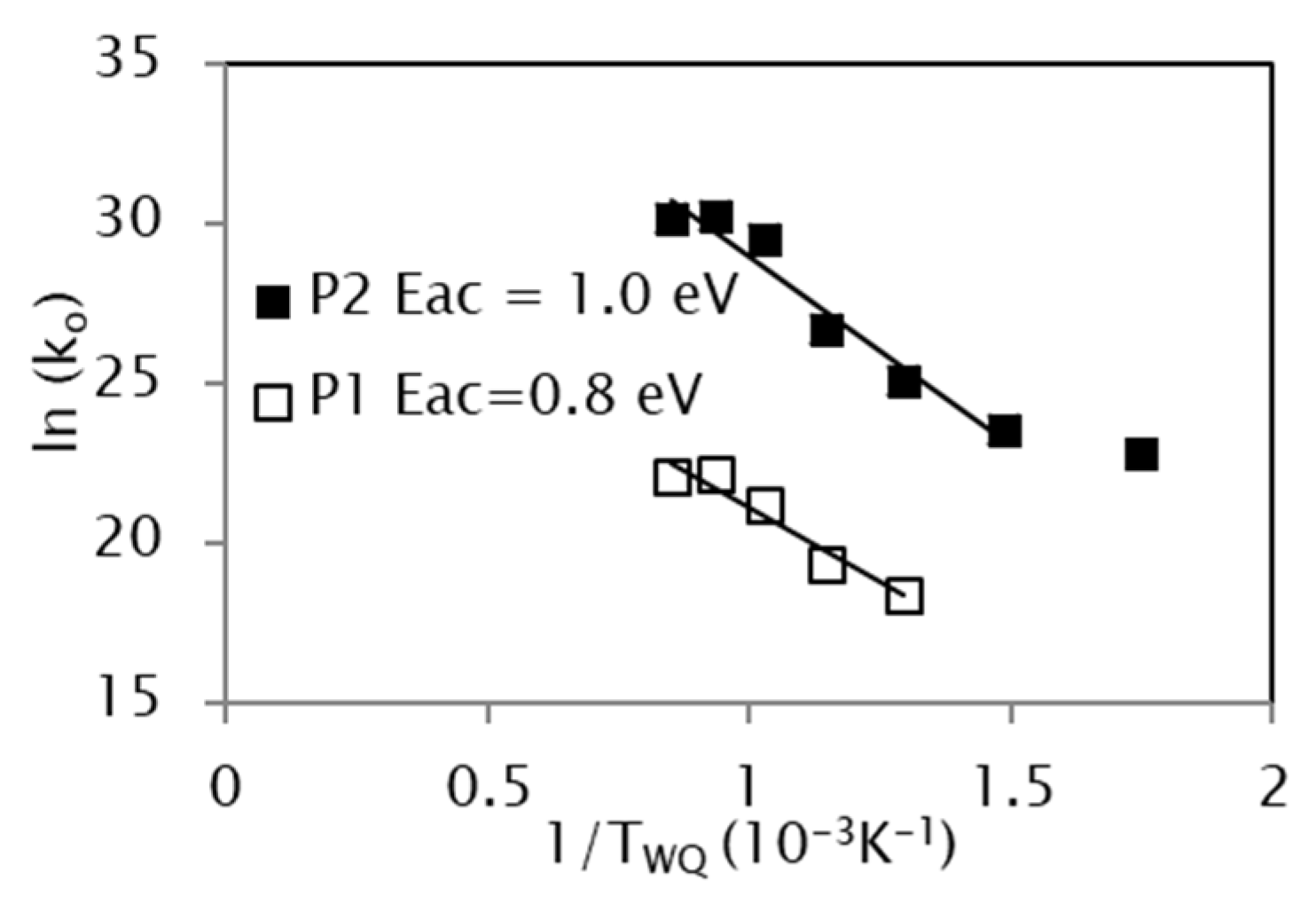
3.4. Effect of the Quenching Temperature on the Kinetic Parameters
4. Summary and Conclusions
- The activation energies associated with the two reordering processes are very close to each other (1.1−1.2 eV) and lower than those corresponding to Mn and Cu self-diffusion. The presence of a high concentration of non-equilibrium vacancies after quench, favouring the reordering processes, is likely lowering the activation energies.
- The pre-exponential factors obtained for the reordering phenomena are basically affected by the quenched excess vacancy concentrations. During post-quench heating, part of the retained excess vacancies is eliminated, which partly explains why the pre-exponential factor for the two processes are different. Therefore, the pre-exponential factors here obtained emphasize the role of the vacancies in the atomic diffusion related to ordering processes.
- An effective exponent of n = 1 can be taken for the reaction model of both the analysed processes. Therefore, a first-order kinetic model is ruling these reactions. Such a model is consistent with the site-saturation nucleation and homogeneous diffusion-controlled advance of the processes.
- The kinetic analysis extended to samples quenched from different temperatures TWQ shows a dependence of the activation energy on TWQ, attributable to the different degree of frozen atomic disorder when quenching from different temperatures. In turn, the evolution of pre-exponential factors runs parallel to the equilibrium vacancy concentration at TWQ, making evident the crucial role of vacancies in assisting the reordering processes.
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Handley, R.C.; Murray, S.J.; Marioni, M.; Nembach, H.; Allen, S.M. Phenomenology of giant magnetic-field-induced strain in ferromagnetic shape-memory materials (invited). J. Appl. Phys. 2000, 87, 4712–4717. [Google Scholar] [CrossRef]
- Ullakko, K.; Huang, J.K.; Kantner, C.; O’Handley, R.C.; Kokorin, V.V. Large magnetic-field-induced strains in Ni2MnGa single crystals. Appl. Phys. Lett. 1996, 69, 1966–1968. [Google Scholar] [CrossRef]
- Webster, P.J.; Ziebeck, K.R.A.; Town, S.L.; Peak, M.S. Magnetic order and phase transformation in Ni2MnGa. Philos. Mag. Part B 1984, 49, 295–310. [Google Scholar] [CrossRef]
- Planes, A.; Mañosa, L.; Acet, M. Magnetocaloric effect and its relation to shape-memory properties in ferromagnetic Heusler alloys. J. Phys. Condens. Matter 2009, 21, 233201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.H.; Liu, H.; Zhang, X.X.; Xiao, J.Q.; Zhu, Z.Y.; Dai, X.F.; Liu, G.D.; Chen, J.L.; Wu, G.H. Large negative magnetoresistance in quaternary Heusler alloy Ni50Mn8Fe17Ga25 melt-spun ribbons. Appl. Phys. Lett. 2005, 86, 182507. [Google Scholar] [CrossRef] [Green Version]
- Segui, C.; Cesari, E. Composition and atomic order effects on the structural and magnetic transformations in ferromagnetic Ni–Co–Mn–Ga shape memory alloys. J. Appl. Phys. 2012, 111, 043914. [Google Scholar] [CrossRef]
- Seguí, C. Effects of the interplay between atomic and magnetic order on the properties of metamagnetic Ni-Co-Mn-Ga shape memory alloys. J. Appl. Phys. 2014, 115, 113903. [Google Scholar] [CrossRef]
- Kataoka, M.; Endo, K.; Kudo, N.; Kanomata, T.; Nishihara, H.; Shishido, T.; Umetsu, R.Y.; Nagasako, M.; Kainuma, R. Martensitic transition, ferromagnetic transition, and their interplay in the shape memory alloys Ni2Mn1−xCuxGa. Phys. Rev. B 2010, 82, 214423. [Google Scholar] [CrossRef]
- Kanomata, T.; Endo, K.; Kudo, N.; Umetsu, R.Y.; Nishihara, H.; Kataoka, M.; Nagasako, M.; Kainuma, R.; Ziebeck, K.R. Magnetic Moment of Cu-Modified Ni2MnGa Magnetic Shape Memory Alloys. Metals 2013, 3, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Seguí, C.; Torrens-Serra, J.; Cesari, E.; Lázpita, P. Optimizing the Caloric Properties of Cu-Doped Ni–Mn–Ga Alloys. Materials 2020, 13, 419. [Google Scholar] [CrossRef] [Green Version]
- Kanomata, T.; Nozawa, T.; Kikuchi, D.; Nishihara, H.; Koyama, K.; Watanabe, K. Magnetic properties of ferromagnetic shape memory alloys Ni2−xCuxMnGa. Int. J. Appl. Electromagn. Mech. 2005, 21, 151–157. [Google Scholar] [CrossRef]
- Overholser, R.; Wuttig, M.; Neumann, D. Chemical ordering in Ni-Mn-Ga Heusler alloys. Scr. Mater. 1999, 40, 1095–1102. [Google Scholar] [CrossRef]
- Khovailo, V.V.; Takagi, T.; Vasilev, A.N.; Miki, H.; Matsumoto, M.; Kainuma, R. On Order–Disorder (L21→B2′) Phase Transition in Ni2+xMn1−xGa Heusler Alloys. Phys. Status Solidi 2001, 183, R1–R3. [Google Scholar] [CrossRef]
- Sánchez-Alarcos, V.; Recarte, V.; Pérez-Landazábal, J.; Cuello, G. Correlation between atomic order and the characteristics of the structural and magnetic transformations in Ni–Mn–Ga shape memory alloys. Acta Mater. 2007, 55, 3883–3889. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Ohtoyo, D.; Umemoto, M.H.; Ohtsuka, H. Effect of Isothermal Aging on Martensitic Transformations in Ni-Mn-Ga Alloy. Trans. Mater. Res. Soc. Jpn. 2000, 25, 521–524. [Google Scholar]
- Kreissl, M.; Neumann, K.-U.; Stephens, T.; Ziebeck, K.R.A. The influence of atomic order on the magnetic and structural properties of the ferromagnetic shape memory compound Ni2MnGa. J. Phys. Condens. Matter 2003, 15, 3831–3839. [Google Scholar] [CrossRef]
- Goryczka, T.; Gigla, M.; Morawiec, H. Effect of quenching on martensitic transformation course in non-stoichiometric NiMnGa alloy. Int. J. Appl. Electromagn. Mech. 2006, 23, 81–88. [Google Scholar] [CrossRef]
- Richard, M.L.; Feuchtwanger, J.; Allen, S.M.; O’Handley, R.C.; Lázpita, P.; Barandiaran, J.M.; Gutierrez, J.; Ouladdiaf, B.; Mondelli, C.; Lograsso, T.; et al. Chemical order in off-stoichiometric Ni–Mn–Ga ferromagnetic shape-memory alloys studied with neutron diffraction. Philos. Mag. 2007, 87, 3437–3447. [Google Scholar] [CrossRef]
- Sánchez-Alarcos, V.; Pérez-Landazábal, J.; Gómez-Polo, C.; Recarte, V. Influence of the atomic order on the magnetic characteristics of a Ni–Mn–Ga ferromagnetic shape memory alloy. J. Magn. Magn. Mater. 2008, 320, e160–e163. [Google Scholar] [CrossRef]
- Sánchez-Alarcos, V.; Recarte, V.; Pérez-Landazábal, J.; Gómez-Polo, C.; Rodríguez-Velamazán, J. Role of magnetism on the martensitic transformation in Ni–Mn-based magnetic shape memory alloys. Acta Mater. 2012, 60, 459–468. [Google Scholar] [CrossRef]
- Recarte, V.; Pérez-Landazábal, J.; Sánchez-Alarcos, V.; Rodríguez-Velamazán, J. Dependence of the martensitic transformation and magnetic transition on the atomic order in Ni–Mn–In metamagnetic shape memory alloys. Acta Mater. 2012, 60, 1937–1945. [Google Scholar] [CrossRef]
- Seguí, C.; Cesari, E. Ordering mechanism and kinetics in Ni2Mn1−Cu Ga ferromagnetic shape memory alloys. J. Alloys Compd. 2021, 887, 161302. [Google Scholar] [CrossRef]
- Soto-Parra, D.; Moya, X.; Mañosa, L.; Planes, A.; Flores-Zúñiga, H.; Alvarado-Hernández, F.; Ochoa-Gamboa, R.; Matutes-Aquino, J.; Ríos-Jara, D. Fe and Co selective substitution in Ni2MnGa: Effect of magnetism on relative phase stability. Philos. Mag. 2010, 90, 2771–2792. [Google Scholar] [CrossRef] [Green Version]
- Kopecký, V.; Rameš, M.; Veřtát, P.; Colman, R.; Heczko, O. Full Variation of Site Substitution in Ni-Mn-Ga by Ferromagnetic Transition Metals. Metals 2021, 11, 850. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-State Kinetic Models: Basics and Mathematical Fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef]
- Donoso, E.; Diaz, G.; Varschavsky, A. Thermal behavior of short-range-order in quenched Cu-12 at% Mn assessed by DSC. J. Therm. Anal. 2005, 81, 425–433. [Google Scholar] [CrossRef]
- Merida, D.; García, J.; Sánchez-Alarcos, V.; Pérez-Landazábal, J.; Recarte, V.; Plazaola, F. Characterisation and modelling of vacancy dynamics in Ni–Mn–Ga ferromagnetic shape memory alloys. J. Alloys Compd. 2015, 639, 180–186. [Google Scholar] [CrossRef]
- Erdélyi, G.; Mehrer, H.; Imre, A.; Lograsso, T.; Schlagel, D. Self-diffusion in Ni2MnGa. Intermetallics 2007, 15, 1078–1083. [Google Scholar] [CrossRef]
- López-Ferreño, I.; Breczewski, T.; López, G.; Nó, M.; Juan, J.S. Stress-assisted atomic diffusion in metastable austenite D03 phase of Cu-Al-Be shape memory alloys. Scr. Mater. 2016, 124, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Alarcos, V.; Pérez-Landazábal, J.; Recarte, V.; A Rodriguez-Velamazán, J.; Chernenko, V. Effect of atomic order on the martensitic and magnetic transformations in Ni–Mn–Ga ferromagnetic shape memory alloys. J. Phys. Condens. Matter 2010, 22, 166001. [Google Scholar] [CrossRef] [PubMed]
- Askill, J. Tracer Diffusion Data for Metals, Alloys and Simple Oxides; Springer: Boston, MA, USA, 1970. [Google Scholar] [CrossRef]
- Abrahams, K.; Zomorodpoosh, S.; Khorasgani, A.R.; Roslyakova, I.; Steinbach, I.; Kundin, J. Automated assessment of a kinetic database for fcc Co–Cr–Fe–Mn–Ni high entropy alloys. Model. Simul. Mater. Sci. Eng. 2021, 29, 055007. [Google Scholar] [CrossRef]
- Murch, G.E. Diffusion Kinetics in Solids, in Phase Transformations in Materials; Kostorz, G., Ed.; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Liu, J.; Riddiford, L.; Floristean, C.; Goncalves-Neto, F.; Rezaeeyazdi, M.; Lewis, L.; Barmak, K. Kinetics of order-disorder transformation of L12 FeNi3 in the Fe-Ni system. J. Alloys Compd. 2016, 689, 593–598. [Google Scholar] [CrossRef]

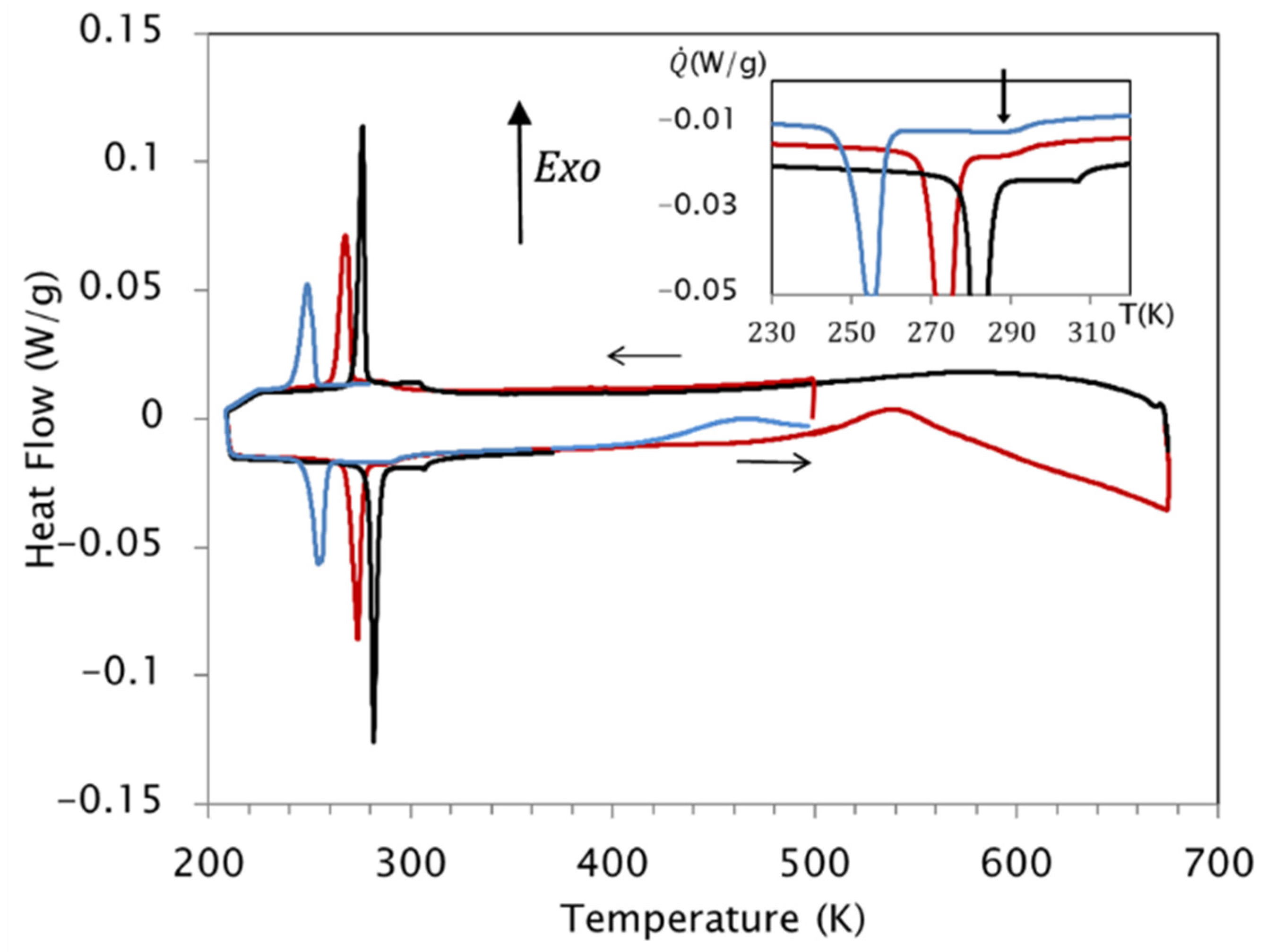
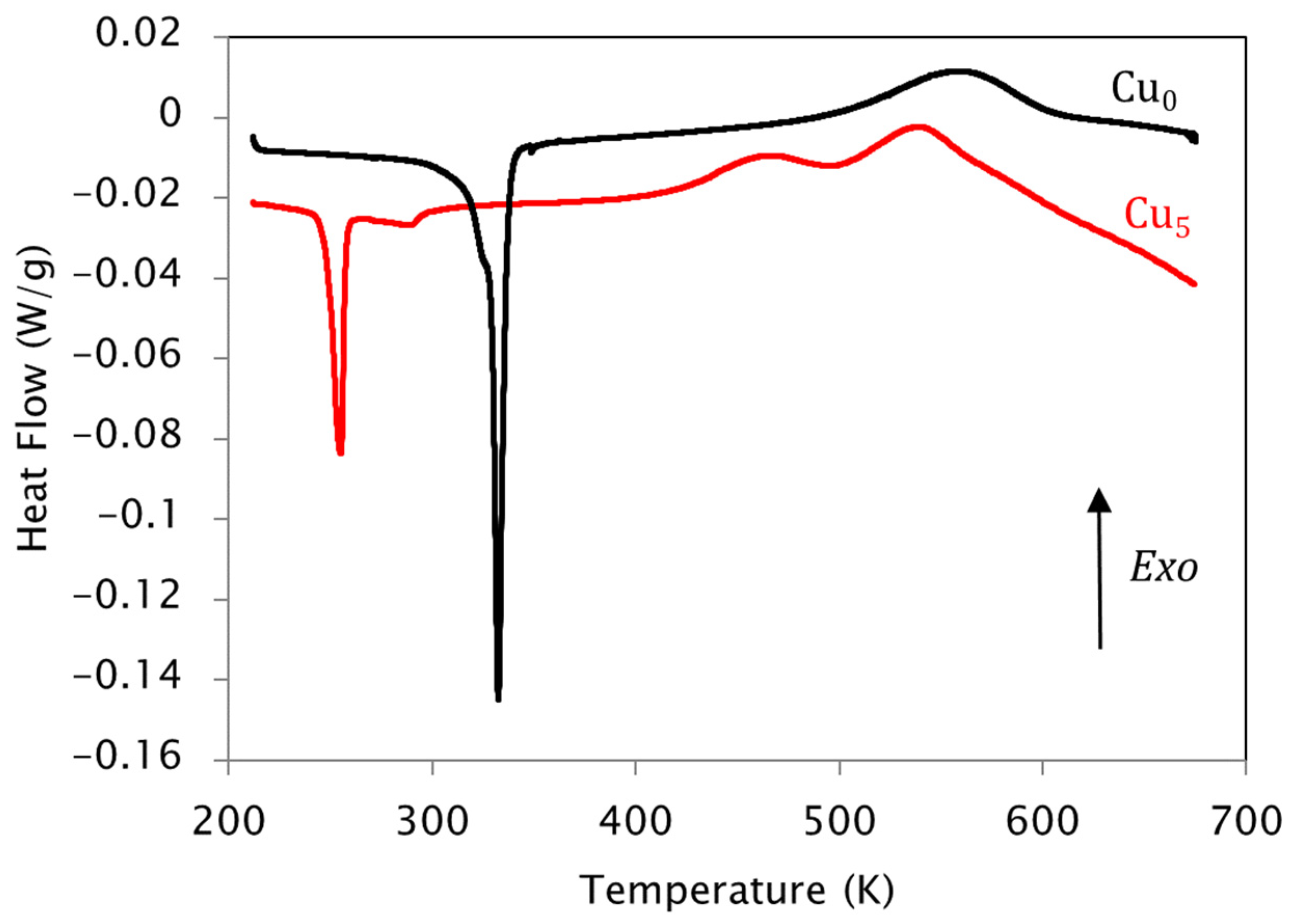
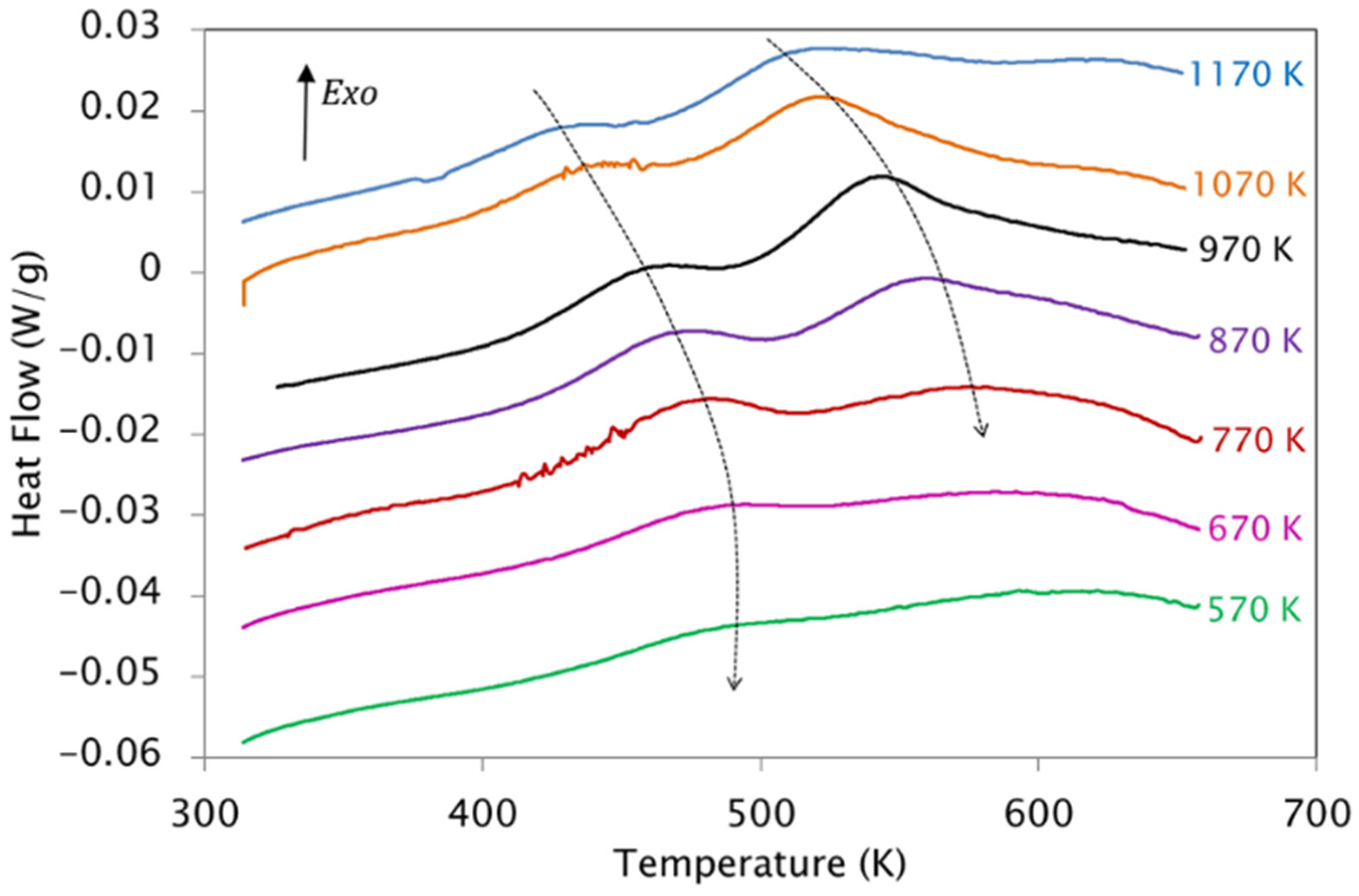
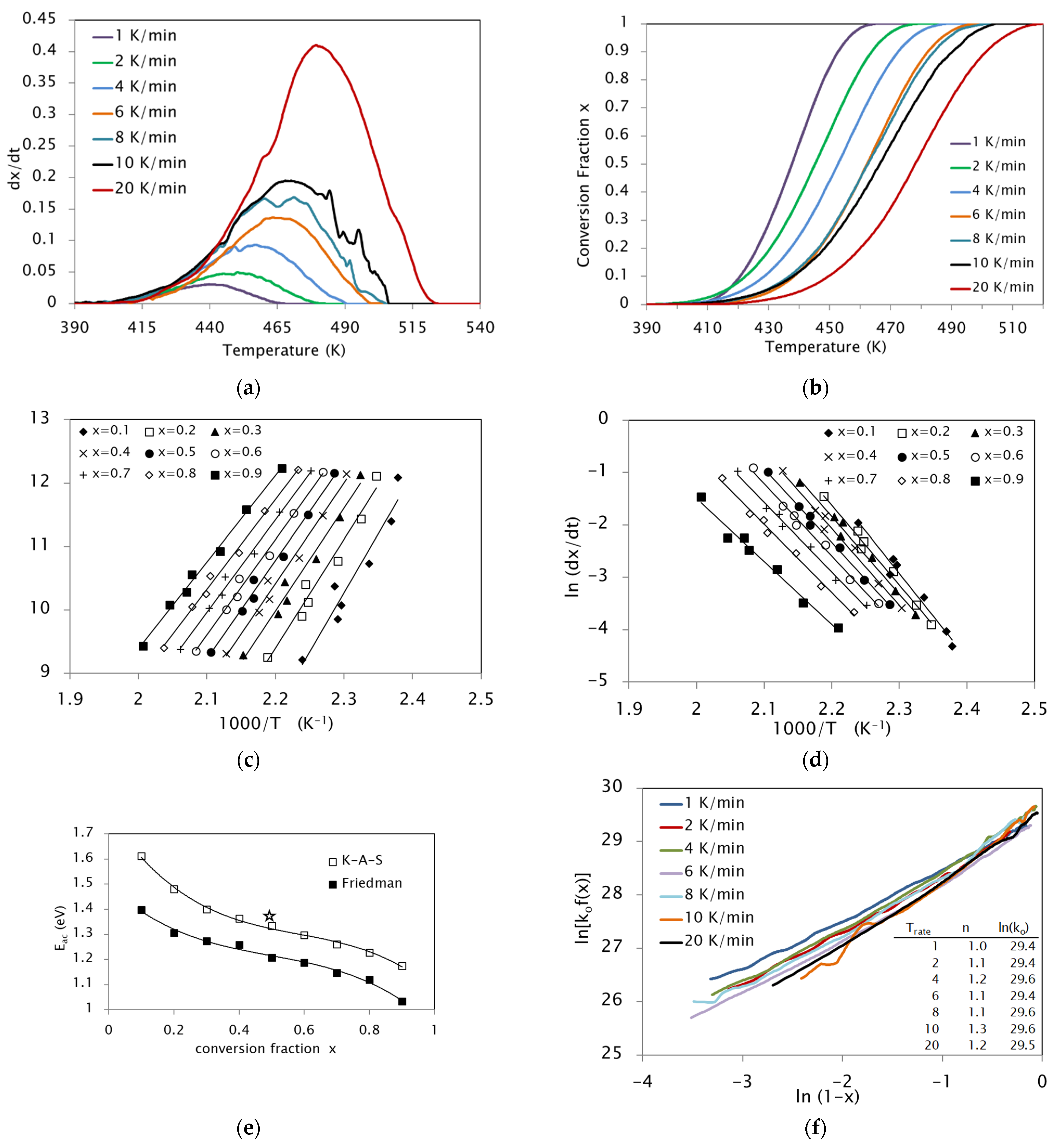

| KAS | Friedman | |||||
|---|---|---|---|---|---|---|
| Eac (eV) | Eac (kJ/mol) | Eac (eV) | Eac (kJ/mol) | n | ko (s−1) | |
| Cu5 P2 | 1.3 | 130 | 1.2 | 118 | 1.1 | 1.1 × 1011 |
| Cu5 P1 | 1.1 | 107 | 1.1 | 107 | 0.9 | 1.4 × 108 |
| Cu0 P1 | 1.3 | 126 | 1.2 | 117 | 1.2 | 1.5 × 108 |
| Diffusion | Eac (eV) | ko (s−1) | Ref. |
|---|---|---|---|
| Vacancy migration in Ni−Mn−Ga | 1.3 | 1.1 | [28] |
| Vacancy formation in Ni−Mn−Ga | 1.1 | 0.9 | [28] |
| Cu−FCC self-diffusion | 1.3 | 1.2 | [29] |
| Mn−BCC self-diffusion | 2.23 | 1.3 × 1013 | [30] |
| Mn (tracer) diffusion in L21 Ni2MnGa | 1.46 | 5.3 × 1010 | [31] |
| Improvement of L21 ordering in Ni−Mn−Ga | 1.18 (TWQ = 1173 K) 1.46 (TWQ = 873K) | [32] | |
| Cu in DO3 Cu−Al−Be | 1.38 | [33] | |
| SRO in Cu3Mn | 0.9 (TWQ = 1073K) | 6.5 × 109 | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seguí, C. Kinetics of Reordering in Quenched Ni2Mn0.8Cu0.2Ga Ferromagnetic Shape Memory Alloys. Metals 2022, 12, 738. https://doi.org/10.3390/met12050738
Seguí C. Kinetics of Reordering in Quenched Ni2Mn0.8Cu0.2Ga Ferromagnetic Shape Memory Alloys. Metals. 2022; 12(5):738. https://doi.org/10.3390/met12050738
Chicago/Turabian StyleSeguí, Concepció. 2022. "Kinetics of Reordering in Quenched Ni2Mn0.8Cu0.2Ga Ferromagnetic Shape Memory Alloys" Metals 12, no. 5: 738. https://doi.org/10.3390/met12050738
APA StyleSeguí, C. (2022). Kinetics of Reordering in Quenched Ni2Mn0.8Cu0.2Ga Ferromagnetic Shape Memory Alloys. Metals, 12(5), 738. https://doi.org/10.3390/met12050738





