Abstract
We developed a method to determine the activation energy for hydride decomposition using a Sieverts-type apparatus and the Kissinger equation, not using thermal analysis methods. The quantity of hydrogen released from the sample and the temperature of the reactor were first measured as a function of time at different heating rates (Φ) in a Sieverts-type apparatus. The dehydriding rates were calculated according to time and the temperature Tm (at which the dehydriding rate was the highest). Φ and Tm were then applied to the Kissinger equation. The dehydriding rate of Mg-5Ni samples obeyed a first-order law, and the Kissinger equation could thus be used to determine the activation energy. On a heating rate of 3 K/min, the decomposition rate of hydride was the highest at 590.0 K. From a plot of ln (Φ/Tm2) versus 1/Tm, the obtained activation energy for hydride decomposition was 174 kJ/mole.
1. Introduction
Researchers are increasingly interested in hydrogen as a next-generation energy carrier. However, issues surrounding its storage and transportation first need to be addressed. One potential solution is to store hydrogen through metal hydride, which has the advantages of being both compact and safe.
To find the hydride decomposition temperature, the quantity of hydrogen released, and the heat involved in the hydride decomposition, thermal analysis methods—such as thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) analysis, differential thermal analysis (DTA), and thermal desorption spectroscopy (TDS) analysis—have been conducted. The data from thermal analysis methods were used to calculate the activation energy for MgH2 decomposition applying the Kissinger equation [1,2,3,4,5].
In this work, we determined the activation energy for hydride decomposition using a Sieverts-type apparatus and the Kissinger equation. This method is supposed to be useful without employing high-quality thermal analysis systems (calorimetric technique apparatuses with high- or ultra-high-vacuum performance and enabling samples to be reloaded without contact with atmosphere). We can perform experiments to obtain activation energy for hydride decomposition at the same apparatus where samples were activated.
Reacted fraction is expressed by F and time by t. The rate of reaction (dF/dt) is proportional to a function of the amount of reactant [f(F)]:
dF/dt = k f(F).
A rate constant k in Equation (1) is generally given by the Arrhenius equation:
where Z is a constant, E an activation energy, R the gas constant, and T temperature.
k = Z exp (−E/RT),
The Kissinger equation is expressed by [6]
where Φ = dT/dt is the heating rate. Tm is the temperature at which the decomposition rate of hydride (dHd/dt) has the maximum value. E is the activation energy for hydride decomposition. Here, Hd is the quantity of hydrogen released from the sample. For the derivation of the Kissinger equation, the first-order reaction was assumed [6]. The Kissinger equation can thus be used for the thermal decomposition, which obeys a first-order law: at constant temperature,
where t is time, and k is a rate constant. Integration of this equation becomes
ln (Φ/Tm2) = ln (ZR/E) – E/RTm
d(ln (Φ/Tm2)/d(1/Tm) = −E/R
dF/dt = k (1 − F)
−ln (1 − F) = k t
Mg has many advantages as a hydrogen-storage material, but it has a low dehydriding rate even at 573 K around. To prepare a sample of Mg with increased hydriding and dehydriding rates, Ni was added, considering the reports of many researchers [7,8,9,10]. In the present work, Mg-5Ni samples with a composition of 95 wt% Mg + 5 wt% Ni were prepared by milling in hydrogen atmosphere. We investigated the order of the dehydriding rate of hydrided Mg–5Ni; we showed that the dehydriding rate of the Mg-5Ni sample obeys a first-order law and that the Kissinger equation can thus be used to determine the activation energy for hydride decomposition of the hydrided Mg-5Ni. To determine the activation energy for hydride decomposition, the quantity of hydrogen released from the sample and the temperature of the reactor were first measured as a function of time at different heating rates (Φ) in a Sieverts-type apparatus. The dehydriding rates were calculated according to time and the temperature at which the dehydriding rate was the highest (Tm) was obtained. Φ and Tm were then applied to the Kissinger equation. As far as we know, the Kissinger method is not applied by using a volumetric technique for determining the activation energy for the dehydrogenation of hydride materials. The Kissinger method is usually applied by using calorimetric techniques.
2. Materials and Methods
Mg (−20 + 100 mesh, 99.8% (metals basis), Alfa Aesar) and Ni (Nickel powder APS, 2.2–3.0 µm, purity 99.9 % metal basis, C typically < 0.1%, Alfa Aesar) were used as starting materials.
For the synthesis of Mg-5Ni, milling was carried out in a planetary ball mill (Planetary Mono Mill; Pulverisette 6, Fritsch, Idar-Oberstein, Germany). A mixture of Mg and Ni at the weight ratio of 95:5 (total weight = 8 g) was mixed in a hermetically sealed stainless steel container (with 105 hardened steel balls, total weight = 360 g). The sample to ball weight ratio was 1:45. All sample handling was performed in a glove box under an Ar atmosphere in order to minimize oxidation. The mill container (volume of 250 mL) was then filled with high purity hydrogen gas (about 12 bar), which was refilled every 2 h. Milling was performed at the disc revolution speed of 400 rpm for 6 h.
Hydrogen absorption and release of Mg-5Ni were investigated using a Sieverts-type volumetric apparatus, which was described previously [11]. Before hydrogen release measurements, samples were hydrided at 593 K under 12 bar H2 for 60 min. The quantity of the hydrogen released under 1.0 bar H2 and the temperature of the reactor were measured as a function of time as the sample was heated at the heating rates (Φ) of 3, 6, 9, 12, and 15 K/min to increase the temperature from room temperature up to 673 K.
3. Results and Discussion
Figure 1 shows micrographs captured by SEM at various magnifications of Mg-5Ni dehydrogenated at the eighth cycle. Shapes of particles are irregular. Particle size is not homogeneous. Particles are agglomerated. Quite small particles are on the surfaces of agglomerates.
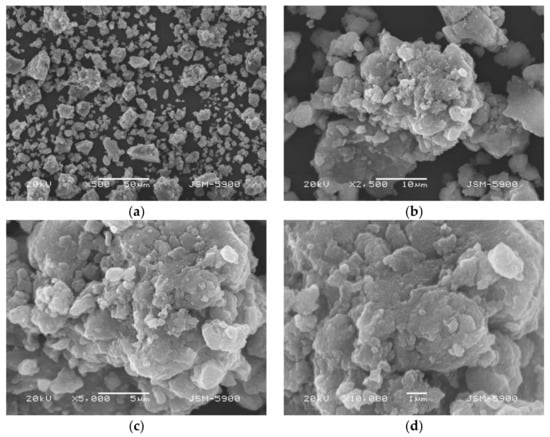
Figure 1.
Micrographs captured by SEM at various magnifications ((a) ×500, (b) ×2,500, (c) ×5000, and (d) ×10,000) of Mg-5Ni dehydrogenated at the eighth cycle.
An X-ray diffractogram of Mg-5Ni dehydrogenated at the eighth cycle is shown in Figure 2. The sample contains Mg and small amounts of β-MgH2, Mg2Ni, and MgO. Mg2Ni is believed to have been formed during heating at the first cycle [12]. Mg and Mg2Ni absorb hydrogen and then release hydrogen during hydriding-dehydriding cycling at temperatures of the present work.
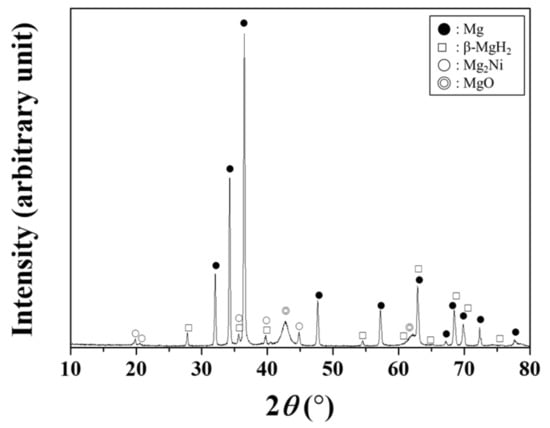
Figure 2.
X-ray diffractogram of Mg-5Ni dehydrogenated at the eighth cycle.
The quantity of hydrogen released from the sample was expressed by Hd in the unit of wt% H, which was defined as the percentage of the released hydrogen on the basis of the sample weight. Variation in the Hd versus t curve with the number of cycles, n, at 593 K under 1.0 bar H2 for hydrided Mg-5Ni is shown in Figure 3. At n = 1, the initial dehydriding rate is quite high, the dehydriding rate is the highest after about 5 min, and the dehydriding rate becomes low after 20 min. At n = 2, the initial dehydriding rate and the dehydriding rate after 5 min are higher than at n = 1. The Hd versus t curves at n = 2 and n = 3 are very similar, showing that the activation of the samples was completed after the first cycle.
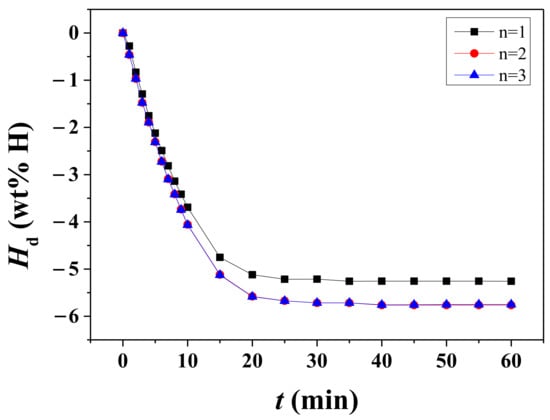
Figure 3.
Variation in the Hd versus t curve with the number of cycles, n, at 593 K under 1.0 bar H2 for hydrided Mg-5Ni.
Dehydrided fraction was expressed by F, which was calculated by dividing Hd by the quantity of hydrogen released completely from the fully hydrided Mg-5Ni sample (8.07 wt% H). To check whether the dehydriding reaction of the Mg-5Ni sample obeys a first-order law or not, −ln (1 − F) was plotted against t. Figure 4 shows variation of −ln (1 − F) with t at heating rates of 3, 6, and 9 K/min at 593 K under 1.0 bar H2 for hydrided Mg-5Ni and variation of −ln (1 − F) with t in the range where most of the hydrogen absorbed during hydriding is released. Figure 4a shows that in the beginning −ln (1 − F) is small, then increases very rapidly, and finally increases very slowly. Figure 4b shows that −ln (1 − F) versus t plots exhibit good linearity. The good linearity of these plots supports that the dehydriding rate of the Mg–5Ni sample obeys a first-order law and that the Kissinger equation can thus be used to determine the activation energy for hydride decomposition of the hydrided Mg–5Ni.
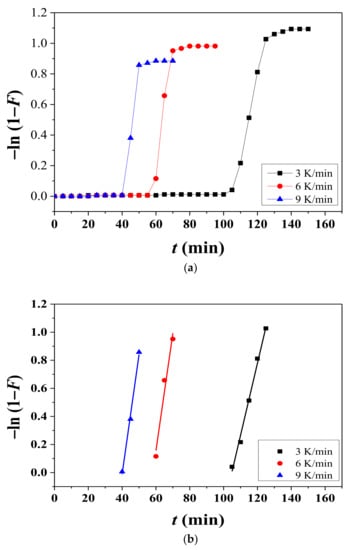
Figure 4.
(a) Variations in −ln (1 − F) with t at heating rates of 3, 6, and 9 K/min at 593 K under 1.0 bar H2 for hydrided Mg–5Ni and (b) variation in −ln (1 − F) with t in the range where most of the hydrogen absorbed during hydriding is released.
To determine the onset temperature and the temperature at which the dehydriding rate was the highest (Tm) for hydride decomposition of hydrided Mg-5Ni and to calculate the activation energy for hydride decomposition of hydrided Mg-5Ni applying the Kissinger equation, variations in the released hydrogen quantity, Hd, with time, t, were obtained after activation at n = 4–8.
Variations in Hd and temperature T with t under 1.0 bar H2 for hydrided Mg–5Ni are shown in Figure 5. The sample was heated with a heating rate of 3 K/min. As the time elapses, the temperature increases and thus the rate of Hd change, dHd/dt, will increase. The sample begins to release hydrogen at 572.1 K (6060 s), releases hydrogen most rapidly at 590.0 K (6780 s), and releases hydrogen very slowly above 640 K (7500 s). Correspondingly, the rate of Hd change, dHd/dt, is the highest at 590.0 K. In a part of the T versus t curve, a plateau appears. This plateau is believed to indicate the temperature Tm at which the dHd/dt is the highest under 1.0 bar H2 for hydrided Mg–5Ni.
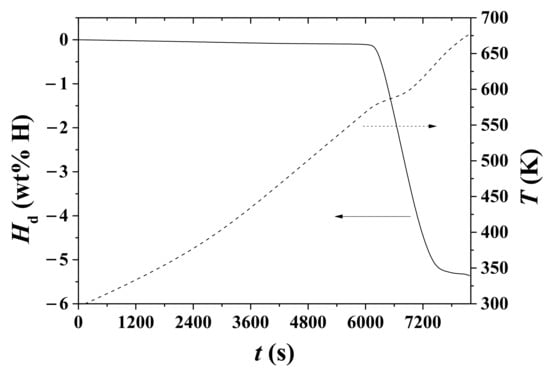
Figure 5.
Variations in Hd and temperature T with t under 1.0 bar H2 for hydrided Mg–5Ni. The sample was heated with a heating rate of 3 K/min.
Variations in Hd and temperature T with t under 1.0 bar H2 for hydrided Mg–5Ni are shown in Figure 6. The sample was heated with a heating rate of 9 K/min. The sample begins to release hydrogen at 586.6 K (2441 s), releases hydrogen most rapidly at 606.3 K (2570 s), and releases hydrogen very slowly above 650 K (2921 s). Correspondingly, the rate of Hd change, dHd/dt, is the highest at 606.3 K.
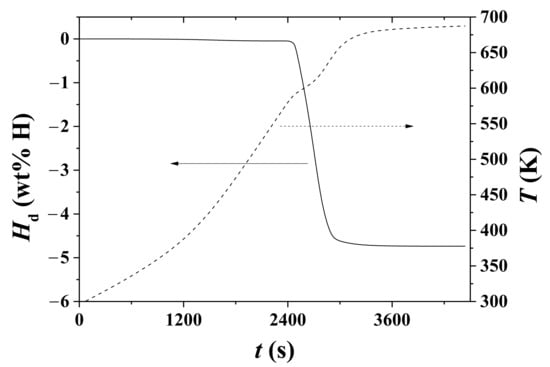
Figure 6.
Variations in Hd and T with t under 1.0 bar H2 for hydrided Mg–5Ni. The sample was heated with a heating rate of 9 K/min.
Figure 7 shows variations with time, t, of the temperature, T, and the rate of Hd change, dHd/dt, under 1.0 bar H2 for hydrided Mg–5Ni. The sample was heated with a heating rate of 3 K/min. The temperature increases as time elapses and a plateau appears after about 6780 s. The rate of Hd change begins to increase after 6060 s (572.1 K) and becomes very low after 7500 s. The rate of Hd change, dHd/dt, is the highest at 6780 s (590.0 K).
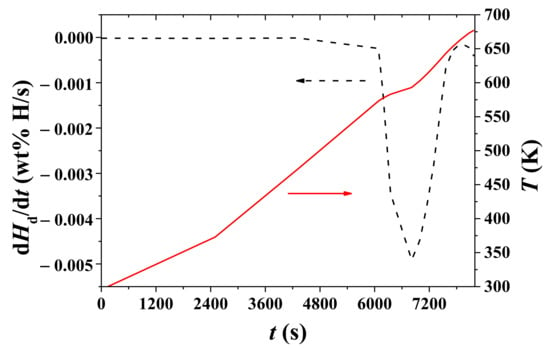
Figure 7.
Variations with time, t, of temperature, T, and the rate of Hd change, dHd/dt, under 1.0 bar H2 for hydrided Mg–5Ni. The sample was heated with a heating rate of 3 K/min.
Figure 8 shows variations with time, t, of T and the dHd/dt under 1.0 bar H2 for hydrided Mg–5Ni. The sample was heated with a heating rate of 9 K/min. As time elapses, the temperature increases, and a plateau appears after about 2570 s. After 3400 s, the temperature increases very slowly. The rate of Hd change begins to increase after 2441 s (586.6 K) and becomes very low after 3400 s. The rate of Hd change, dHd/dt, is the highest at 2570 s (606.3 K).
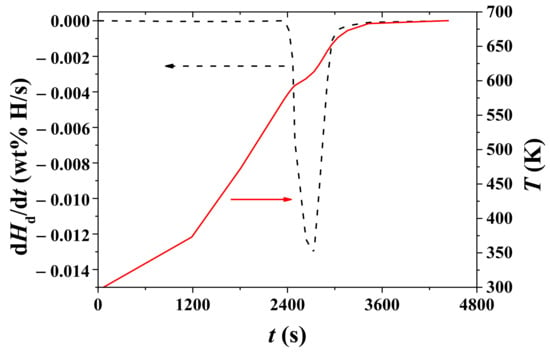
Figure 8.
Variations with t of T and dHd/dt under 1.0 bar H2 for hydrided Mg–5Ni. The sample was heated with a heating rate of 9 K/min.
Table 1 presents variations, with heating rate, in the onset time, onset temperature, and time tm and temperature Tm at which the dHd/dt has the maximum value under 1.0 bar H2 for hydrided Mg–5Ni.

Table 1.
Variations, with heating rate Φ, in the onset time, onset temperature, and time tm and temperature Tm at which the dHd/dt has the maximum value under 1.0 bar H2 for hydrided Mg–5Ni.
Table 2 presents variations in Tm, ln (Φ/Tm2), and 1/Tm, with heating rate Φ under 1.0 bar H2 for hydrided Mg–5Ni.

Table 2.
Variations in Tm, ln (Φ/Tm2), and 1/Tm, with heating rate Φ under 1.0 bar H2 for hydrided Mg–5Ni.
Figure 9 shows a plot of ln (Φ/Tm2) versus 1/Tm. The linearity of the plot is good (correlation coefficient: 0.977). From this plot, the activation energy for hydride decomposition, E, was calculated as 174 ± 5 kJ/mole.
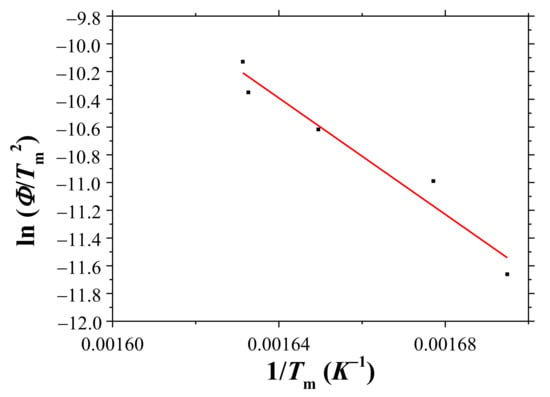
Figure 9.
Plot of ln (Φ/Tm2) versus 1/Tm.
Comparing the equation obtained from Figure 9 with Equation (3), E was 174 ± 5 kJ/mole, and Z was 5.49 × 1014. And thus
k = (5.49 × 1014) exp [−174 ± 5 (kJ/mole)/RT].
Consequently, the rate equation is given by
dF/dt = (5.49 × 1014) (1 − F) exp [−174 ± 5 (kJ/mole)/RT].
Zhao-Karger et al. [1] prepared an infiltrated MgH2/ACF (activated carbon fiber) composite by direct hydrogenation of Bu2Mg inside the voids of the carbon scaffolds. They also ball-milled and catalyzed a commercial MgH2 with graphite. They performed thermal analysis using high pressure DSC and simultaneous TGA-DSC-MS (mass spectrometry). They reported that the Kissinger plots showed that the activation energies for MgH2 decomposition of the commercial MgH2, the ball-milled MgH2 with graphite, and the infiltrated MgH2/ACF composite were 195, 165, and 143 KJ/mol, respectively [1]. Sabitu et al. [2] prepared MgH2 + 4 mol% MO (MO = Nb2O5, Fe3O4, ZrO2, and CeO2) by ball milling. MgH2 + 4 mol% Nb2O5 had the highest dehydriding rate, followed in order by MgH2 + 4 mol% Fe3O4, MgH2 + 4 mol% ZrO2, MgH2 + 4 mol% CeO2, and MgH2 (without oxide). The Kissinger plots, obtained from TG/DTA data, showed that the activation energies for MgH2 decomposition of MgH2 + 4 mol% MO (MO = Nb2O5, Fe3O4, ZrO2, and CeO2) and MgH2 (without oxide) were 95, 108, 113, 140, and 174 KJ/mol, respectively [2]. The order of the activation energies for MgH2 decomposition of these samples is exactly opposite to that of the dehydriding rates of these samples. The results of Sabitu et al. [2] prove that the main role of oxide additives on increasing the dehydriding rates of MgH2 is lowering the activation energy of MgH2 decomposition. The effects of addition by mechanical milling in hydrogen are reportedly creating defects, producing cracks and clean surfaces, and decreasing particle size [10,13,14,15]. Figen et al. [3] synthesized MgH2 from modified waste magnesium chips (WMC), which was prepared by mechanically milling waste magnesium with tetrahydrofuran (THF) and NaCl. They performed thermal analysis using DSC. Doyle and Kissinger non-isothermal kinetic models were applied to calculate desorption activation energies, which were found equal to 255 kJ/mol and 256 kJ/mol, respectively [3]. Xiao et al. [4] reported that the Kissinger plots showed that the activation energies for MgH2 decomposition of an as-received MgH2, a ball-milled MgH2, and a ball-milled MgH2/LiCl mixture after treatment with tetrahydrofuran (THF) were 213, 138, and 115 KJ/mol, respectively. Xiao et al. [4] performed thermal analysis using TG-DTA [4]. Mustafa et al. [5] studied the effect of PdCl2 on the hydrogen storage properties of MgH2. They reported that, from the Kissinger analysis applied to data obtained by DSC, the apparent activation energy for hydrogen desorption was reduced from 142 kJ/mol for as-milled MgH2 to 99 kJ/mol after the addition of 20 wt % PdCl2 [5]. Campostrini et al. [16] investigated the thermal desorption of hydrogen from commercial MgH2 powders by coupled thermogravimetry and mass spectroscopy (TG–MS). They reported that, considering temperatures corresponding to iso-conversion points of transformed fraction = 0.25 and calculating the relative values of the reaction rate, an activation energy for the hydrogen release of 240 kJ/mol was obtained via Friedman method [16]. Table 3 presents the activation energies of reported Mg-based hydrogen storage materials.

Table 3.
Activation energies of reported Mg-based hydrogen storage materials.
The activation energy for hydride decomposition (174 kJ/mole) obtained in the present work is smaller than that of Zhao-Karger et al. [1] for the hydrogen release of the commercial MgH2 (195 kJ/mol), that of Campostrini et al. [16] for the hydrogen release of the commercial MgH2 powders (240 kJ/mol), and that of Figen et al. [3] for the hydrogen desorption of MgH2 synthesized from ball-milled waste magnesium with tetrahydrofuran (THF) (255 kJ/mol) or NaCl (256 kJ/mol), but larger than ball-milled MgH2 [4,5]. Our result has a value very similar to that of Sabitu et al. [2] for MgH2 decomposition (174 KJ/mol).
Because the first-order reaction was assumed for the derivation of the Kissinger equation [6], this equation can be used for some reactions on heating that obey a first-order law. Reaction order should be checked before applying the Kissinger equation. Even in a multi-step reaction, the Kissinger plot may show good linearity in cases when the activation energies are similar in multi-steps. Vyazovkin [17] reported that the Kissinger method should be used under a temperature program of linear heating, never being applied to the data obtained on cooling. This method must be applied to single-step kinetics, not to multi-step kinetics; the method tends to yield a single activation energy even though the process is controlled by more than one energy barrier.
Parameters that would influence the results of activation energy are measuring exact temperature variation of the samples during heating, measuring the time interval to analyze the decomposition rates, and deciding the points of the highest decomposition rates in time-dependent reaction rate curves and time-dependent temperature curves.
It is thought that the application of the Kissinger to the volumetric measurements leads to the less accurate activation energy than that of calorimetric measurements because an additional step (obtaining the dH/dt versus t curves) is involved, but they are very close. Kissinger [18] reported that the temperatures of maximum reaction rate were very close to the peak temperatures observed in DTA.
Han et al. [19,20] reported that the activation energy for hydride decomposition can be obtained from ln (b/T2) versus 1/T plot, where b is the heating rate and T is the temperature. Their equation for this plot was given by
where c is a constant, E the activation energy for hydride decomposition, and R the gas constant, and the reacted fraction was assumed to be constant. This plot is very similar to that to obtain the activation energy using the Kissinger equation. They derived the equation based on the continuous moving boundary model, assuming that the particles were spherical with a uniform diameter and that the rate-controlling step for hydride decomposition was an interfacial chemical reaction. They obtained an activation energy for magnesium hydride decomposition of 142 kJ/mol.
ln (b/T2) = ln (c/E) − E/RT
4. Conclusions
The quantity of hydrogen released from the sample and the temperature of the reactor were first measured as a function of time at different heating rates (Φ) in a Sieverts-type apparatus. The dehydriding rates were calculated according to time and the temperature at which the dehydriding rate was the highest (Tm) was obtained. Φ and Tm were then applied to the Kissinger equation to determine the activation energy for hydride decomposition. After activation, the Mg–5Ni sample with a composition of 95 wt% Mg + 5 wt% Ni contained Mg and Mg2Ni that absorbed hydrogen and then released hydrogen during hydriding–dehydriding cycling at 593 K. Because the content of Mg2Ni was small, most of the hydrogen released from the sample is believed to be from the decomposition of Mg hydride. The dehydriding rate of the hydrided Mg–5Ni sample obeyed a first-order law. Therefore, the Kissinger equation could be used to determine the activation energy for hydride decomposition of hydrided Mg–5Ni. On heating rates of 3, 6, 9, 12, and 15 K/min, Tm´s were 590.0, 596.3, 606.3, 612.5, and 613.0 K, respectively. From a plot of ln (Φ/Tm2) versus 1/Tm, the obtained activation energy for hydride decomposition was 174 kJ/mole.
Author Contributions
Conceptualization, M.-Y.S. and Y.-J.K.; methodology M.-Y.S. and Y.-J.K.; formal analysis, M.-Y.S. and Y.-J.K.; investigation, M.-Y.S. and Y.-J.K.; data curation, M.-Y.S. and Y.-J.K.; writing—original draft preparation, M.-Y.S.; writing—review and editing, M.-Y.S. and Y.-J.K.; visualization, Y.-J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1C1C2009103).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this article are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao-Karger, Z.; Hu, J.; Roth, A.; Wang, D.; Kubel, C.; Lohstroh, W.; Fichtner, M. Altered thermodynamic and kinetic properties of MgH2 infiltrated in microporous scaffold. Chem. Commun. 2010, 46, 8353–8355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabitu, S.T.; Goudy, A.J. Dehydrogenation kinetics and modeling studies of MgH2 enhanced by transition metal oxide catalysts using constant pressure thermodynamic driving forces. Metals 2012, 2, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Figen, A.K.; Coskuner, B.; Piskin, S. Hydrogen desorption kinetics of MgH2 synthesized from modified waste magnesium. Mater. Sci. 2014, 32, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Liu, Z.; Yarahmadi, S.S.; Gregorya, D.H. Facile preparation of β-/γ-MgH2 nanocomposites under mild conditions and pathways to rapid dehydrogenation. Phys. Chem. Chem. Phys. 2016, 18, 10492–10498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, N.S.; Juahir, N.; Halim Yap, F.A.; Ismail, M. Enhanced hydrogen storage properties of MgH2 by the addition of PdCl2 Catalyst. Int. J. Electroact. Mater. 2016, 4, 1–7. Available online: https://www.researchgate.net/publication/293820495_Enhanced_Hydrogen_Storage_Properties_of_MgH_2_by_The_Addition_of_PdCl_2_Catalyst (accessed on 31 December 2021).
- Blainea, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Bobet, J.-L.; Akiba, E.; Darriet, B. Study of Mg-M (M=Co, Ni and Fe) mixture elaborated by reactive mechanical alloying: Hydrogen sorption properties. Int. J. Hydrog. Energy 2001, 26, 493–501. [Google Scholar] [CrossRef]
- Santos, S.F.; Ishikawa, T.T.; Botta, W.J.; Huot, J. MgH2 + FeNb nanocomposites for hydrogen storage. Mater. Chem. Phys. 2014, 147, 557–562. [Google Scholar] [CrossRef]
- Asselli, A.A.C.; Santos, S.F.; Huot, J. Hydrogen storage in filed magnesium. J. Alloys Compd. 2016, 687, 586–594. [Google Scholar] [CrossRef]
- Bobet, J.-L.; Akiba, E.; Nakamura, Y.; Darriet, B. Study of Mg-M (M=Co, Ni and Fe) mixture elaborated by reactive mechanical alloying-hydrogen sorption properties. Int. J. Hydrog. Energy 2000, 25, 987–996. [Google Scholar] [CrossRef]
- Song, M.Y.; Kwak, Y.J.; Lee, S.H.; Park, H.R. Enhancement of hydrogen storage characteristics of Mg by addition of nickel and niobium (V) fluoride via mechanical alloying. Korean J. Met. Mater. 2016, 54, 210–216. [Google Scholar] [CrossRef]
- Song, M.Y.; Ivanov, E.I.; Darriet, B.; Pezat, M.; Hagenmuller, P. Hydriding properties of a mechanically alloyed mixture with a composition Mg2Ni. Int. J. Hydrog. Energy 1985, 10, 169–178. [Google Scholar] [CrossRef]
- Song, M.Y.; Choi, E.; Kwak, Y.J. Preparation of a Mg-based alloy with a high hydrogen-storage capacity by adding a polymer CMC via milling in a hydrogen atmosphere. Int. J. Hydrog. Energy 2019, 44, 3779–3789. [Google Scholar] [CrossRef]
- Song, M.Y.; Choi, E.; Kwak, Y.J. Increase in the dehydrogenation rate of Mg-CMC (Carboxymethylcellulose, Sodium Salt) by adding Ni via hydride-forming milling. Met. Mater. Int. 2019, 25, 516–527. [Google Scholar] [CrossRef]
- Song, M.Y.; Choi, E.; Kwak, Y.J. Nickel, graphene, and yttria-stabilized zirconia (YSZ)-added Mg by grinding in hydrogen atmosphere for hydrogen storage. Metals 2019, 9, 1347. [Google Scholar] [CrossRef] [Green Version]
- Campostrini, R.; Abdellatief, M.; Leoni, M.; Scardi, P. Activation energy in the thermal decomposition of MgH2 powders by coupled TG–MS measurements. J. Therm. Anal. Calorim. 2014, 116, 225–240. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger method in kinetics of materials: Things to beware and be aware of. Molecules 2014, 25, 2813. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 4, 217–221. [Google Scholar] [CrossRef]
- Han, J.S.; Pezat, M.; Lee, J.-Y. Thermal desorption of hydrogen from magnesium hydride. Scr. Metall. 1986, 20, 951–956. [Google Scholar] [CrossRef]
- Han, J.S.; Pezat, M.; Lee, J.-Y. A study of the decomposition of magnesium hydride by thermal analysis. J. Less-Common Met. 1987, 130, 195–402. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).