Al-Mn Intermetallics in High Pressure Die Cast AZ91 and Direct Chill Cast AZ80
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Laboratory Study on Al-Mn IMCs in Solidification and Solution Heat Treatment
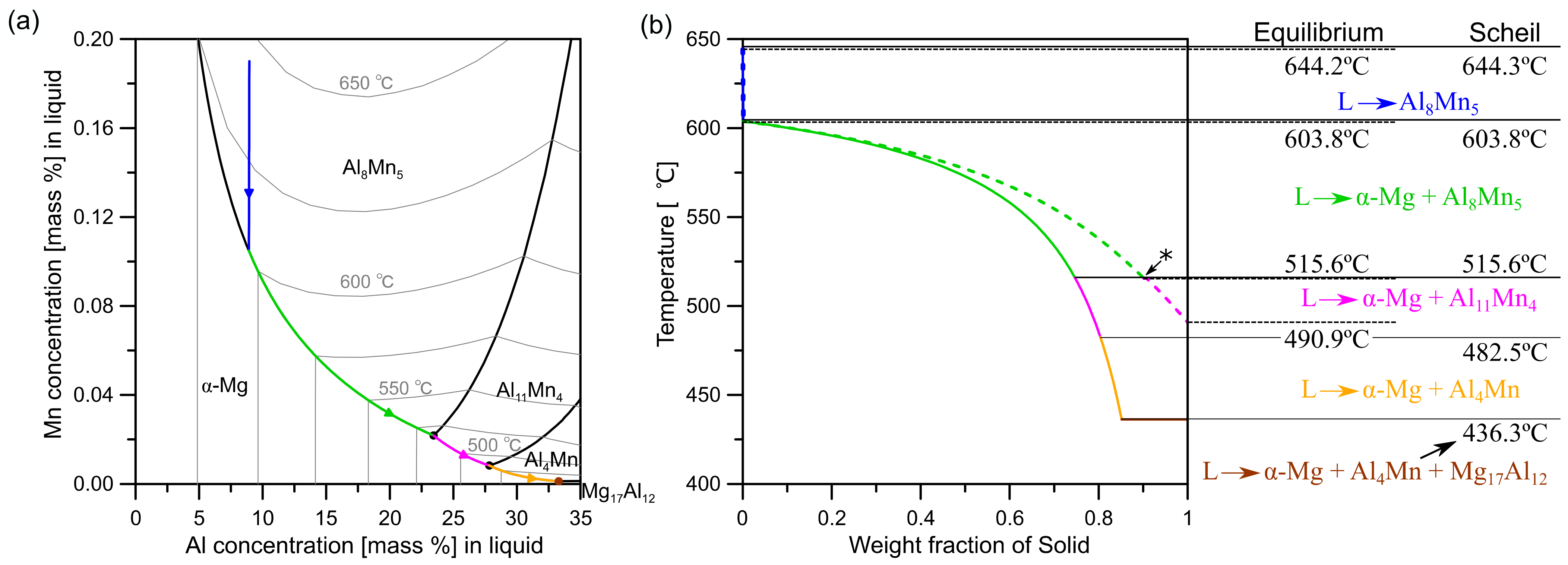
3.1.1. Thermodynamic Calculations
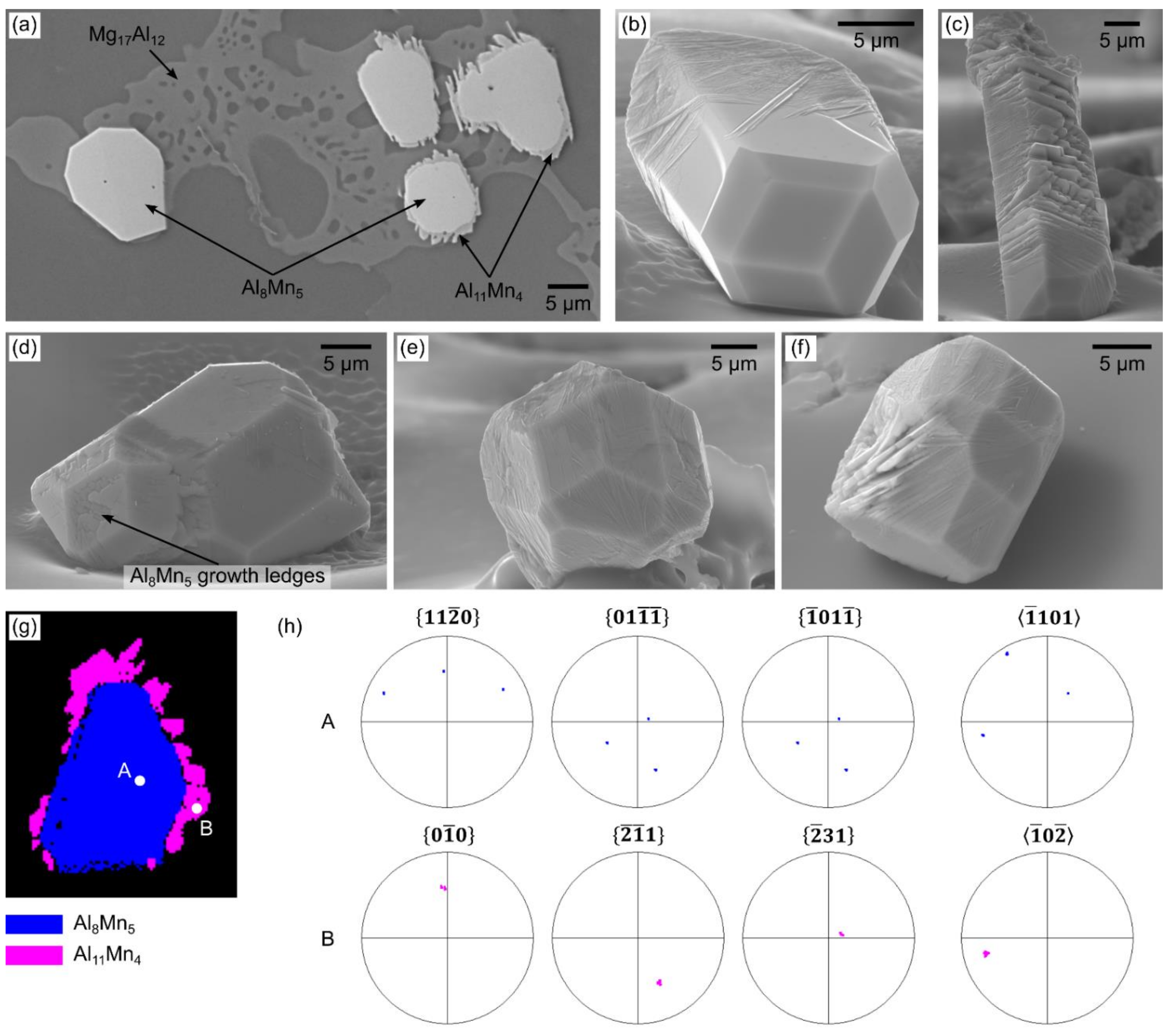
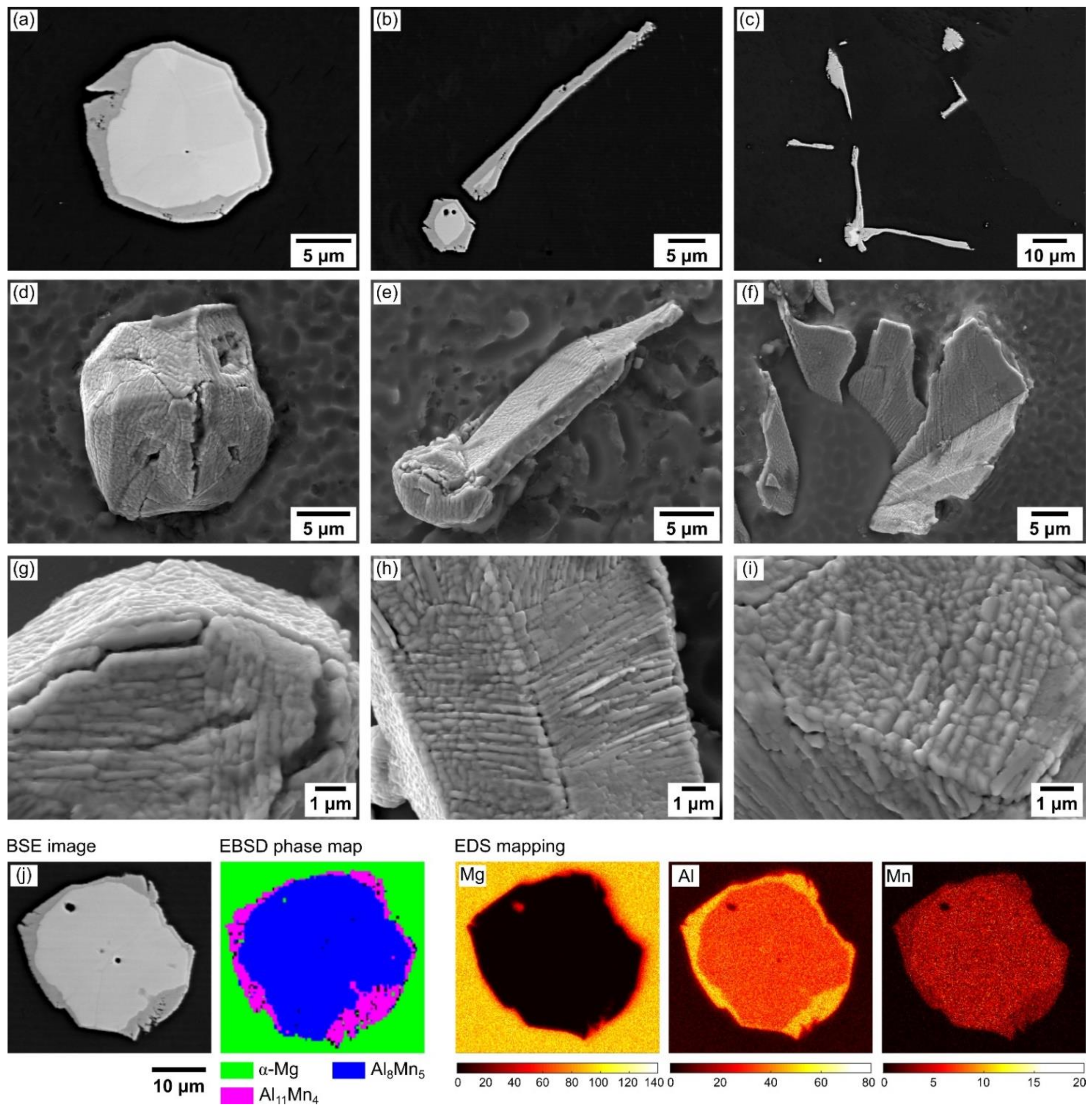
3.1.2. Growth of Al8Mn5 during Solidification
3.1.3. Al11Mn4 Nucleation and Growth on Al8Mn5 during Solidification
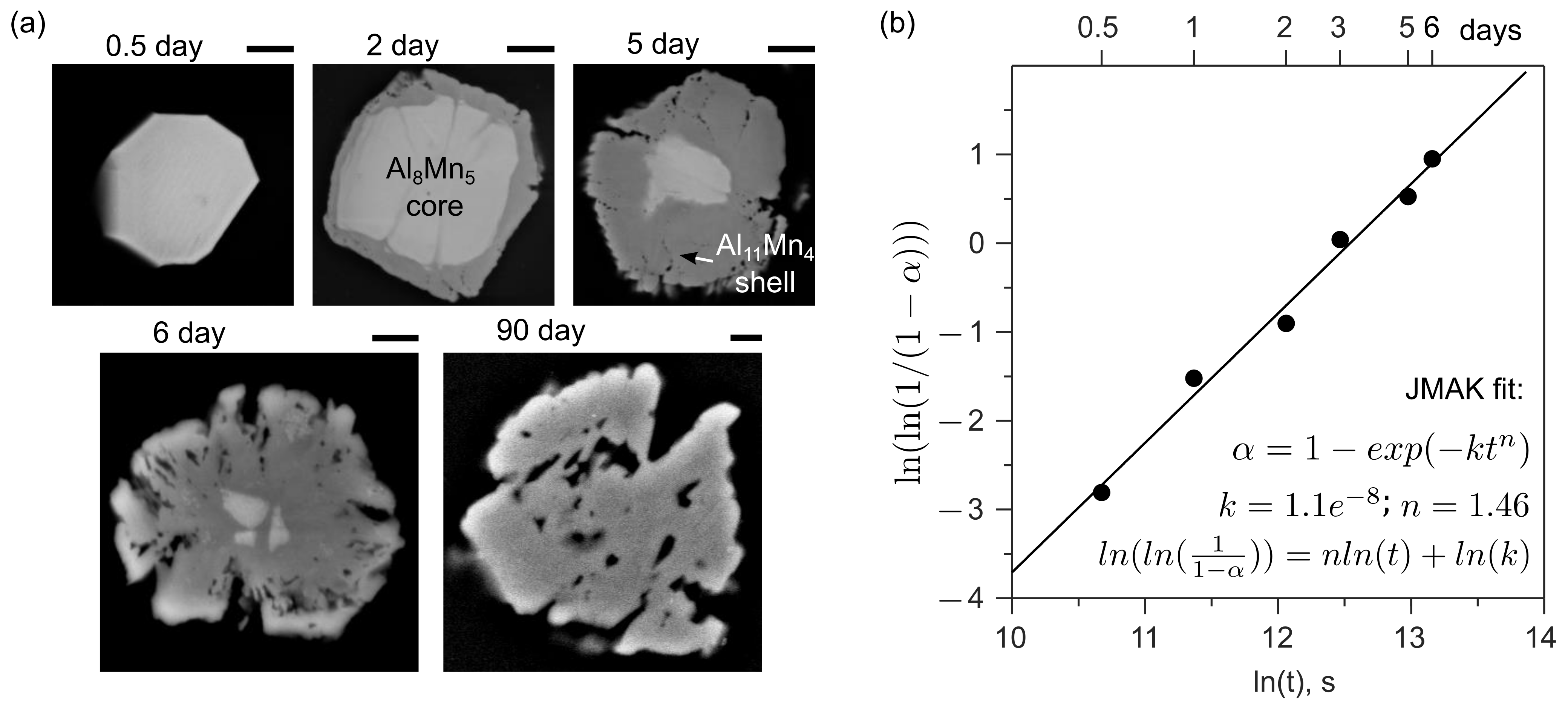
3.1.4. Transformation of Al8Mn5 into Al11Mn4 during Solution Heat Treatment
3.2. Al-Mn IMCs in HPDC AZ91
3.2.1. Sludge in Crucible
3.2.2. Microstructure Overview
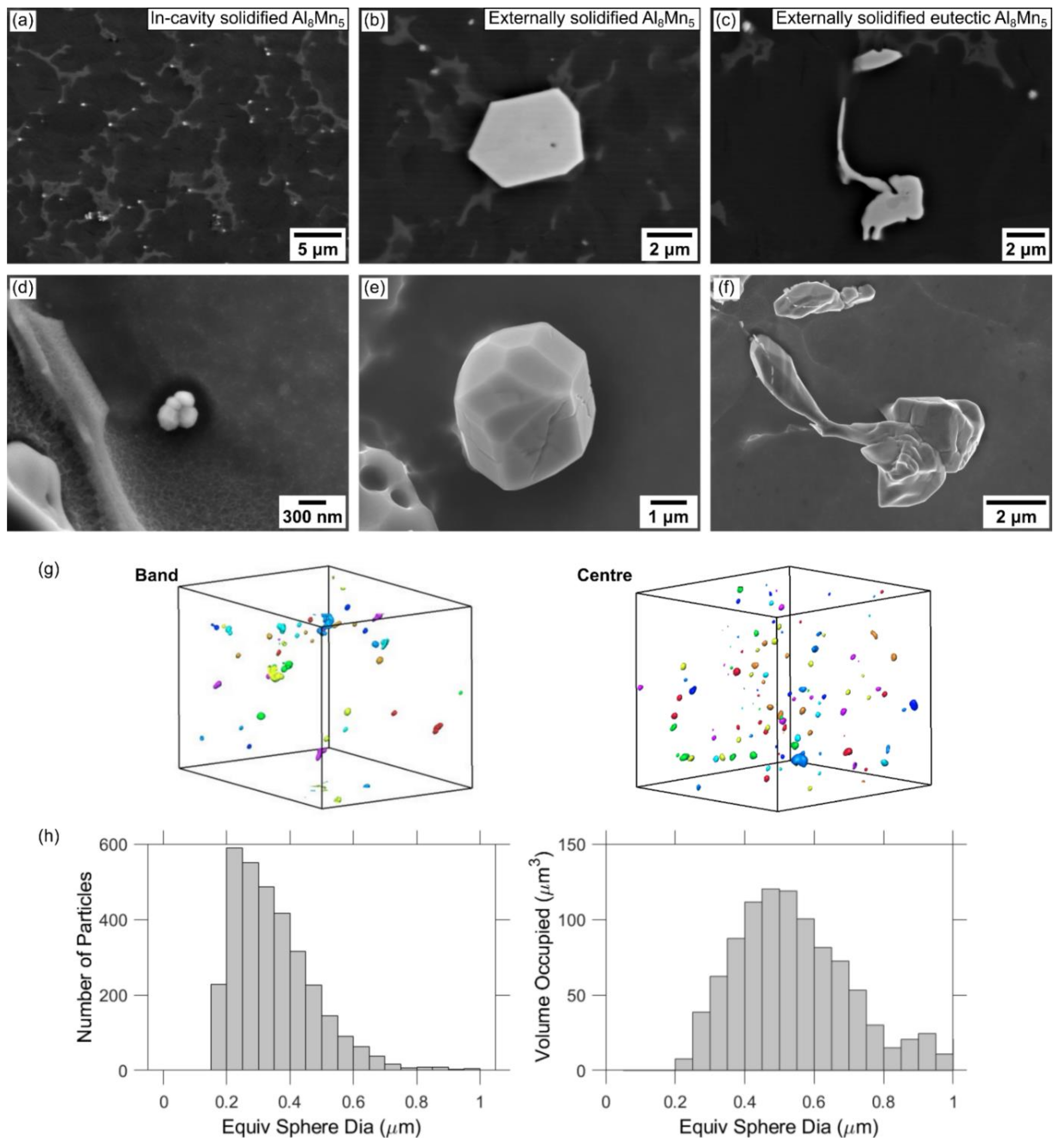
3.2.3. Al-Mn IMC Formation in HPDC AZ91D
3.3. Al-Mn IMCs in DC-Cast AZ80A
3.3.1. Microstructure of Defect and Defect-Free Region
3.3.2. Microstructure of Al-Mn IMCs in Heat-Treated DC-Cast AZ80A
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blawert, C.; Hort, N.; Kainer, K. Automotive applications of magnesium and its alloys. Trans. Indian Inst. Met. 2004, 57, 397–408. [Google Scholar]
- Luo, A.A. Magnesium casting technology for structural applications. J. Magnes. Alloys 2013, 1, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Brady, M.P.; Joost, W.J.; Warren, C.D. Insights from a recent meeting: Current status and future directions in magnesium corrosion research. Corrosion 2017, 73, 452–462. [Google Scholar] [CrossRef]
- Weiler, J. A review of magnesium die-castings for closure applications. J. Magnes. Alloys 2019, 7, 297–304. [Google Scholar] [CrossRef]
- American Society for Testing and Materials, ASTM. B94-18, Standard Specification for Magnesium-Alloy Die Castings; ASTM-International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- American Society for Testing and Materials, ASTM. B80-15, Standard Specification for Magnesium-Alloy Sand Castings; ASTM-International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- American Society for Testing and Materials, ASTM. B403-20, Standard Specification for Magnesium-Alloy Investment Castings; ASTM-International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- American Society for Testing and Materials, ASTM. B91-17, Standard Specification for Magnesium-Alloy Forgings; ASTM-International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Agnew, S.R. Wrought magnesium: A 21st century outlook. JOM 2004, 56, 20–21. [Google Scholar] [CrossRef]
- He, H.; Huang, S.; Yi, Y.; Guo, W. Simulation and experimental research on isothermal forging with semi-closed die and multi-stage-change speed of large AZ80 magnesium alloy support beam. J. Mater. Process. Technol. 2017, 246, 198–204. [Google Scholar] [CrossRef]
- American Society for Testing and Materials, ASTM. B90-21, Standard Specification for Magnesium-Alloy Sheet and Plate; ASTM-International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Lunder, O.; Nordien, J.; Nisancioglu, K. Corrosion resistance of cast Mg-Al alloys. Corros. Rev. 1997, 15, 439–470. [Google Scholar] [CrossRef]
- Hanawalt, J. Corrosion studies of magnesium and its alloys. Trans. AIME 1942, 147, 273–299. [Google Scholar]
- Hanawalt, J.D.; Nelson, C.E.; Holdeman, G.E. Removal of iron from magnesiumbase alloys. U.S. Patent 2267862A, 30 December 1941. [Google Scholar]
- Esmaily, M.; Svensson, J.; Fajardo, S.; Birbilis, N.; Frankel, G.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Lunder, O.; Nisancioglu, K.; Hansen, R.S. Corrosion of Die Cast Magnesium-Aluminum Alloys; 0148-7191; SAE Technical Paper: 930755; SAE International: Warrendale, PA, USA, March 1993. [Google Scholar]
- Zeng, R.-C.; Zhang, J.; Huang, W.-J.; Dietzel, W.; Kainer, K.; Blawert, C.; Ke, W. Review of studies on corrosion of magnesium alloys. Trans. Nonferrous Met. Soc. China 2006, 16, s763–s771. [Google Scholar] [CrossRef]
- Pawar, S.; Zhou, X.; Thompson, G.; Scamans, G.; Fan, Z. The role of intermetallics on the corrosion initiation of twin roll cast AZ31 Mg alloy. J. Electrochem. Soc. 2015, 162, C442–C448. [Google Scholar] [CrossRef]
- Sarvesha, R.; Chalapathi, D.; Yadava, M.; Jain, J.; Singh, S. In-situ studies on deformation and fracture characteristics of AZ91 Mg alloy. Materialia 2021, 18, 101177. [Google Scholar] [CrossRef]
- Mackie, D.; Robson, J.; Withers, P.; Turski, M. Characterisation and modelling of defect formation in direct-chill cast AZ80 alloy. Mater. Charact. 2015, 104, 116–123. [Google Scholar] [CrossRef]
- Zeng, G.; Nogita, K.; Belyakov, S.; Xian, J.; McDonald, S.; Yang, K.; Yasuda, H.; Gourlay, C. Real-Time Observation of AZ91 Solidification by Synchrotron Radiography. In Magnesium Technology 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 597–603. [Google Scholar]
- Zeng, G.; Xian, J.; Gourlay, C. Growth of Al8Mn5 Intermetallic in AZ91. In Magnesium Technology 2017; Springer: Berlin/Heidelberg, Germany, 2017; pp. 85–92. [Google Scholar]
- Zeng, G.; Xian, J.; Gourlay, C. Nucleation and growth crystallography of Al8Mn5 on B2-Al (Mn, Fe) in AZ91 magnesium alloys. Acta Mater. 2018, 153, 364–376. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Ma, G.; Liu, X. Effect of manganese on the microstructure of Mg–3Al alloy. J. Alloy Compd. 2009, 486, 136–141. [Google Scholar] [CrossRef]
- Han, G.; Liu, X. Phase control and formation mechanism of Al–Mn (–Fe) intermetallic particles in Mg–Al-based alloys with FeCl 3 addition or melt superheating. Acta Mater. 2016, 114, 54–66. [Google Scholar] [CrossRef]
- Yao, S.; Liu, S.; Zeng, G.; Li, X.; Lei, T.; Li, Y.; Du, Y. Effect of manganese on microstructure and corrosion behavior of the Mg-3Al alloys. Metals 2019, 9, 460. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Zeng, G.; Su, T.; Yasuda, H.; Nogita, K.; Gourlay, C. Al8Mn5 Particle Settling and Interactions with Oxide Films in Liquid AZ91 Magnesium Alloys. JOM 2019, 71, 2235–2244. [Google Scholar] [CrossRef] [Green Version]
- Xian, J.; Peng, L.; Zeng, G.; Wang, D.; Gourlay, C. Al11Mn4 formation on Al8Mn5 during the solidification and heat treatment of AZ-series magnesium alloys. Materialia 2021, 19, 101192. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, Y.; Liu, T.; Li, D.; Tang, A.; Chen, X.; Schmid-Fetzer, R.; Pan, F. Effect of Mn Addition on Melt Purification and Fe Tolerance in Mg Alloys. JOM 2021, 73, 892–902. [Google Scholar] [CrossRef]
- Sarvesha, R.; Alam, W.; Gokhale, A.; Guruprasad, T.; Bhagavath, S.; Karagadde, S.; Jain, J.; Singh, S. Quantitative assessment of second phase particles characteristics and its role on the deformation response of a Mg-8Al-0.5 Zn alloy. Mater. Sci. Eng. A 2019, 759, 368–379. [Google Scholar] [CrossRef]
- Sarvesha, R.; Thirunavukkarasu, G.; Chiu, Y.L.; Jones, I.P.; Jain, J.; Singh, S. A study on the phase transformation of γ2-Al8Mn5 to LT-Al11Mn4 during solutionizing in AZ91 alloy. J. Alloy Compd. 2021, 873, 159836. [Google Scholar] [CrossRef]
- Sarvesha, R.; Bhagyaraj, J.; Bhagavath, S.; Karagadde, S.; Jain, J.; Singh, S. 2D and 3D characteristics of intermetallic particles and their role in fracture response of AZ91 magnesium alloy. Mater. Charact. 2021, 171, 110733. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, G.; Xian, J.; Gourlay, C.M. Al–Mn–Fe intermetallic formation in AZ91 magnesium alloys: Effects of impurity iron. Intermetallics 2022, 142, 110733. [Google Scholar] [CrossRef]
- Gryguc, A.; Shaha, S.K.; Behravesh, S.B.; Jahed, H.; Wells, M.; Williams, B.; Su, X. Monotonic and cyclic behaviour of cast and cast-forged AZ80 Mg. Int. J. Fatigue 2017, 104, 136–149. [Google Scholar] [CrossRef] [Green Version]
- Owen, E.; Pickup, L.; Roberts, I. Lattice constants of five elements possessing hexagonal structure. Z. Krist. Cryst. Mater. 1935, 91, 70–76. [Google Scholar] [CrossRef]
- Thimmaiah, S.; Tener, Z.; Lamichhane, T.N.; Canfield, P.C.; Miller, G.J. Crystal structure, homogeneity range and electronic structure of rhombohedral γ-Mn5Al8. Z. Krist. Cryst. Mater. 2017, 232, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Kontio, A.; Stevens, E.; Coppens, P.; Brown, R.; Dwight, A.; Williams, J. New investigation of the structure of Mn4Al11. Acta Crystallogr. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 435–436. [Google Scholar] [CrossRef]
- Braun, P.B.; Goedkoop, J.A. An x-ray and neutron diffraction investigation of the magnetic phase Al0.89Mn1.11. Acta Crystallogr. 1963, 16, 737–740. [Google Scholar] [CrossRef]
- Schobinger-Papamantellos, P.; Fischer, P. Neutronenbeugungsuntersuchung der Atomverteilung von Mg17Al12. Die Naturwissenschaften 1970, 57, 128–129. [Google Scholar] [CrossRef]
- Ellner, M. The structure of the high-temperature phase MnAl (h) and the displacive transformation from MnAl(h) into Mn5Al8. Metall. Trans. A 1990, 21, 1669–1672. [Google Scholar] [CrossRef]
- Sannes, S.; Westengen, H. The Influence of Process Conditions on the Microstructure and Mechanical Properties of Magnesium Die Castings. In Proceedings of the International conference and exhibition: Magnesium alloys and their applications, Wolfsburg, Germany, 28–30 April 1998; pp. 223–228. [Google Scholar]
- Dahle, A.; Sannes, S.; John, D.S.; Westengen, H. Formation of defect bands in high pressure die cast magnesium alloys. J. Light Met. 2001, 1, 99–103. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, G.; Wang, Y. Microstructure and mechanical properties of rheo-diecast AZ91D magnesium alloy. J. Mater. Sci. 2006, 41, 3631–3644. [Google Scholar] [CrossRef]
- Cao, H.; Wessén, M. Characteristics of microstructure and banded defects in die cast AM50 magnesium components. Int. J. Cast Met. Res. 2005, 18, 377–384. [Google Scholar] [CrossRef]
- Cáceres, C.; Poole, W.; Bowles, A.; Davidson, C. Section thickness, macrohardness and yield strength in high-pressure diecast magnesium alloy AZ91. Mater. Sci. Eng. A 2005, 402, 269–277. [Google Scholar] [CrossRef]
- Gourlay, C.; Laukli, H.; Dahle, A. Defect band characteristics in Mg-Al and Al-Si high-pressure die castings. Metall. Mater. Trans. A 2007, 38, 1833–1844. [Google Scholar] [CrossRef]
- Li, X.; Yu, W.; Wang, J.; Xiong, S. Influence of melt flow in the gating system on microstructure and mechanical properties of high pressure die casting AZ91D magnesium alloy. Mater. Sci. Eng. A 2018, 736, 219–227. [Google Scholar] [CrossRef]
- Bowles, A.; Nogita, K.; Dargusch, M.; Davidson, C.; Griffiths, J. Grain size measurements in Mg-Al high pressure die castings using electron back-scattered diffraction (EBSD). Mater. Trans. 2004, 45, 3114–3119. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.V.; Easton, M.A.; Caceres, C.H. The development of the skin in HPDC Mg–Al alloys. Mater. Sci. Eng. A 2013, 580, 191–195. [Google Scholar] [CrossRef]
- Yu, W.; Cao, Y.; Li, X.; Guo, Z.; Xiong, S. Determination of interfacial heat transfer behavior at the metal/shot sleeve of high pressure die casting process of AZ91D alloy. J. Mater. Sci. Technol. 2017, 33, 52–58. [Google Scholar] [CrossRef]
- Wang, L.; Nguyen, T.; Savage, G.; Davidson, C. Thermal and flow modelling of ladling and injection in high pressure die casting process. Int. J. Cast Met. Res. 2003, 16, 409–417. [Google Scholar] [CrossRef]
- Li, X.-b.; Xiong, S.; Guo, Z.-p. On the porosity induced by externally solidified crystals in high-pressure die-cast of AM60B alloy and its effect on crack initiation and propagation. Mater. Sci. Eng. A 2015, 633, 35–41. [Google Scholar] [CrossRef]
- Bi, C.; Xiong, S.; Li, X.; Guo, Z. Development of a fluid-particle model in simulating the motion of external solidified crystals and the evolution of defect bands in high-pressure die casting. Metall. Mater. Trans. B 2016, 47, 939–947. [Google Scholar] [CrossRef]
- Li, X.-b.; Xiong, S.; Guo, Z.-p. Improved mechanical properties in vacuum-assist high-pressure die casting of AZ91D alloy. J. Mater. Process. Technol. 2016, 231, 1–7. [Google Scholar] [CrossRef]
- Zeng, G.; Zhu, X.; Ji, S.; Gourlay, C. The Morphology and Distribution of Al8Mn5 in High Pressure Die Cast AM50 and AZ91. In Magnesium Technology 2018, TMS 2018, Proceedings of the TMS Annual Meetings & Exhibition, Phoenix, AZ, USA, 11–15 March 2018; The Minerals, Metals & Materials Series; Orlov, D., Joshi, V., Solanki, K., Neelameggham, N., Eds.; Springer: Cham, Switzerland, 2018; pp. 137–144. [Google Scholar]
- Griffiths, W.; Lai, N.-W. Double oxide film defects in cast magnesium alloy. Metall. Mater. Trans. A 2007, 38, 190–196. [Google Scholar] [CrossRef]
- Mirak, A.; Divandari, M.; Boutorabi, S.; Campbell, J. Oxide film characteristics of AZ91 magnesium alloy in casting conditions. Int. J. Cast Met. Res. 2007, 20, 215–220. [Google Scholar] [CrossRef]
- Wang, L.; Rhee, H.; Felicelli, S.D.; Sabau, A.S.; Berry, J.T. Oxide Film and Porosity Defects in Magnesium Alloy AZ91. In Proceedings of the Shape Casting: 3rd International Symposium, San Francisco, CA, USA, 15–19 February 2009; p. 348. [Google Scholar]
- Mirak, A.; Divandari, M.; Boutorabi, S.; Taylor, J. Effect of oxide film defects generated during mould filling on mechanical strength and reliability of magnesium alloy castings (AZ91). Int. J. Cast Met. Res. 2012, 25, 188–194. [Google Scholar] [CrossRef]
- Li, T.; Davies, J.; Zhu, X. Effect of carrier gases on the entrainment defects within AZ91 alloy castings. J. Magnes. Alloy 2021. [Google Scholar] [CrossRef]
- Campbell, J. Castings; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]






| Alloy | Composition (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mg | Al | Zn | Mn | Cu | Si | Ni | Fe | |
| AZ91 | Bal. | 8.95 | 0.72 | 0.19 | 0.001 | 0.039 | <0.001 | <0.001 |
| AZ80 | Bal. | 8.75 | 0.42 | 0.21 | <0.001 | 0.01 | <0.001 | 0.004 |
| Phase | Symbol | Space Group | Pearson Symbol | Lattice Parameters | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| a [Å] | b [Å] | c [Å] | α [°] | β [°] | γ [°] | |||||
| Mg | α | hP2 | 3.209 | 3.209 | 5.211 | 90.0 | 90.0 | 120.0 | [36] | |
| Al8Mn5 | γ2 | hR26 | 12.674 | 12.674 | 7.946 | 90.0 | 90.0 | 120.0 | [37] | |
| Al11Mn4 | ν | aP15 | 5.095 | 8.879 | 5.051 | 89.4 | 100.0 | 105.0 | [38] | |
| Al0.89Mn1.11 | τ | tP2 | 2.770 | 2.770 | 3.540 | 90.0 | 90.0 | 90.0 | [39] | |
| Mg17Al12 | β | cI58 | 10.544 | 10.544 | 10.544 | 90.0 | 90.0 | 90.0 | [40] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Zeng, G.; Wang, D.; Xian, J.; Ji, S.; Zhan, H.; Gourlay, C.M. Al-Mn Intermetallics in High Pressure Die Cast AZ91 and Direct Chill Cast AZ80. Metals 2022, 12, 266. https://doi.org/10.3390/met12020266
Peng L, Zeng G, Wang D, Xian J, Ji S, Zhan H, Gourlay CM. Al-Mn Intermetallics in High Pressure Die Cast AZ91 and Direct Chill Cast AZ80. Metals. 2022; 12(2):266. https://doi.org/10.3390/met12020266
Chicago/Turabian StylePeng, Liuqing, Guang Zeng, Di Wang, Jingwei Xian, Shouxun Ji, Hongyi Zhan, and Christopher M. Gourlay. 2022. "Al-Mn Intermetallics in High Pressure Die Cast AZ91 and Direct Chill Cast AZ80" Metals 12, no. 2: 266. https://doi.org/10.3390/met12020266
APA StylePeng, L., Zeng, G., Wang, D., Xian, J., Ji, S., Zhan, H., & Gourlay, C. M. (2022). Al-Mn Intermetallics in High Pressure Die Cast AZ91 and Direct Chill Cast AZ80. Metals, 12(2), 266. https://doi.org/10.3390/met12020266









