Coarsening Kinetics of Y2O3 Dispersoid in New Grade of Fe-Al-Cr-Based ODS Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results
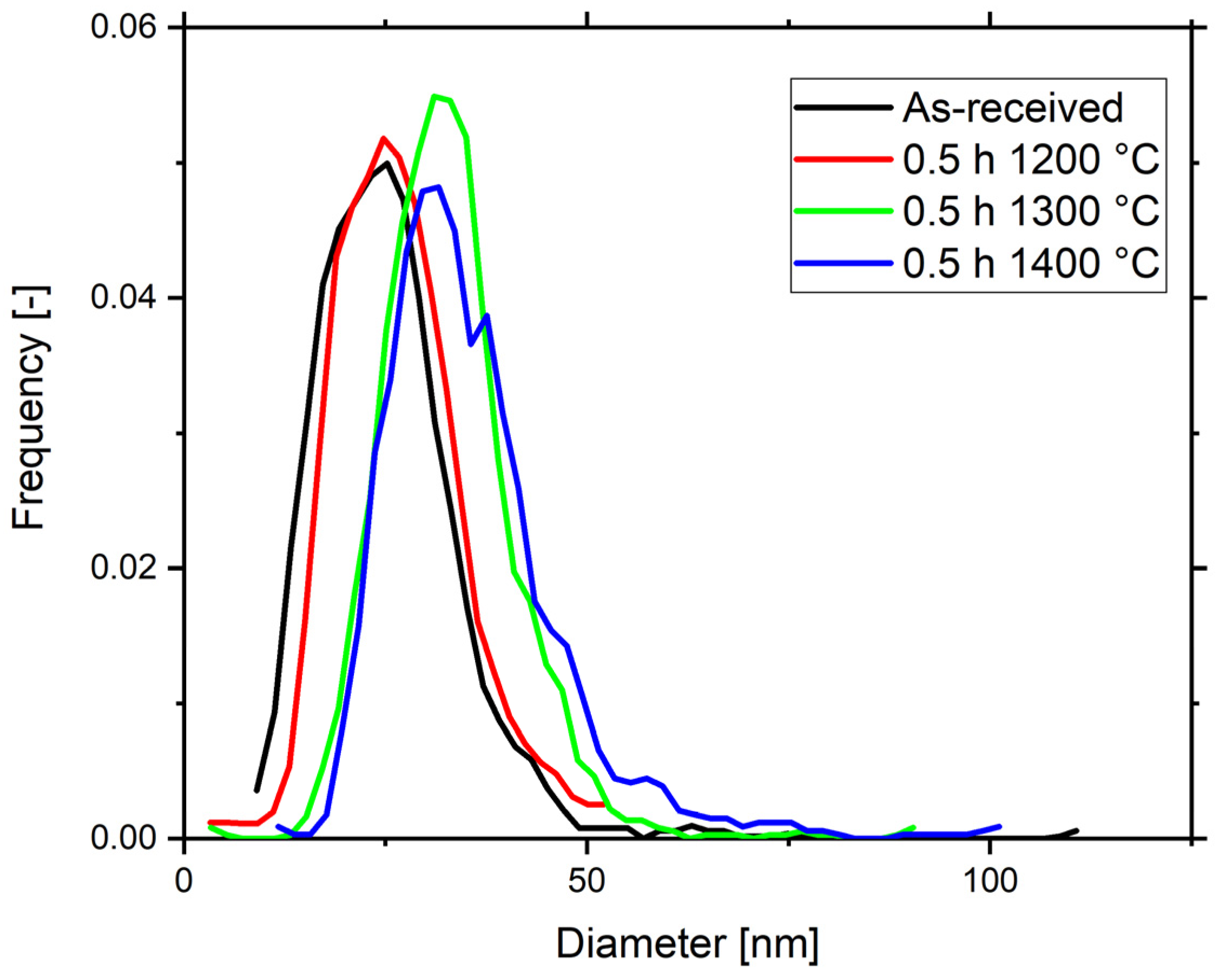
3.1. Microstructure and Particle Analysis of Y2O3 Dispersoid
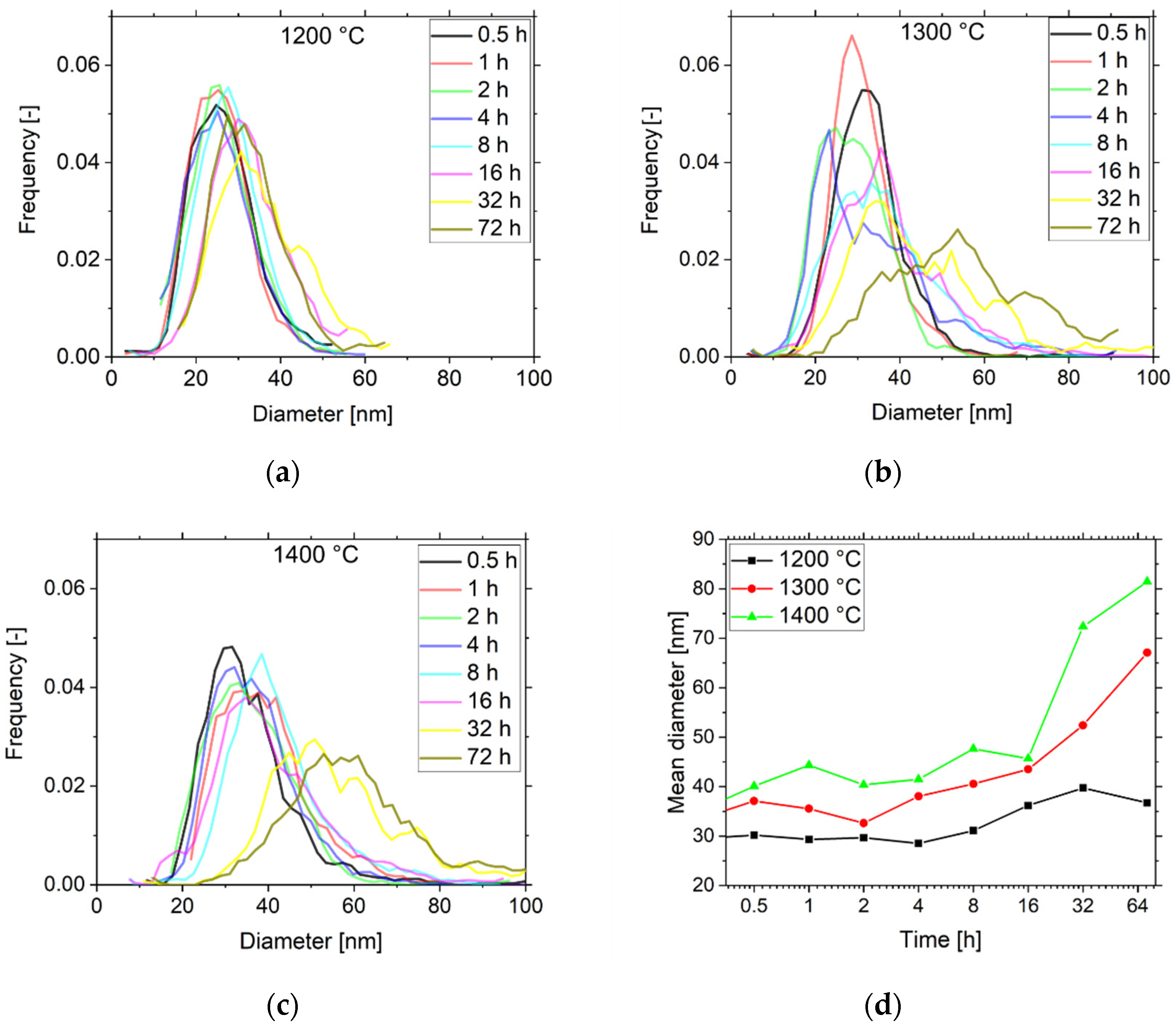
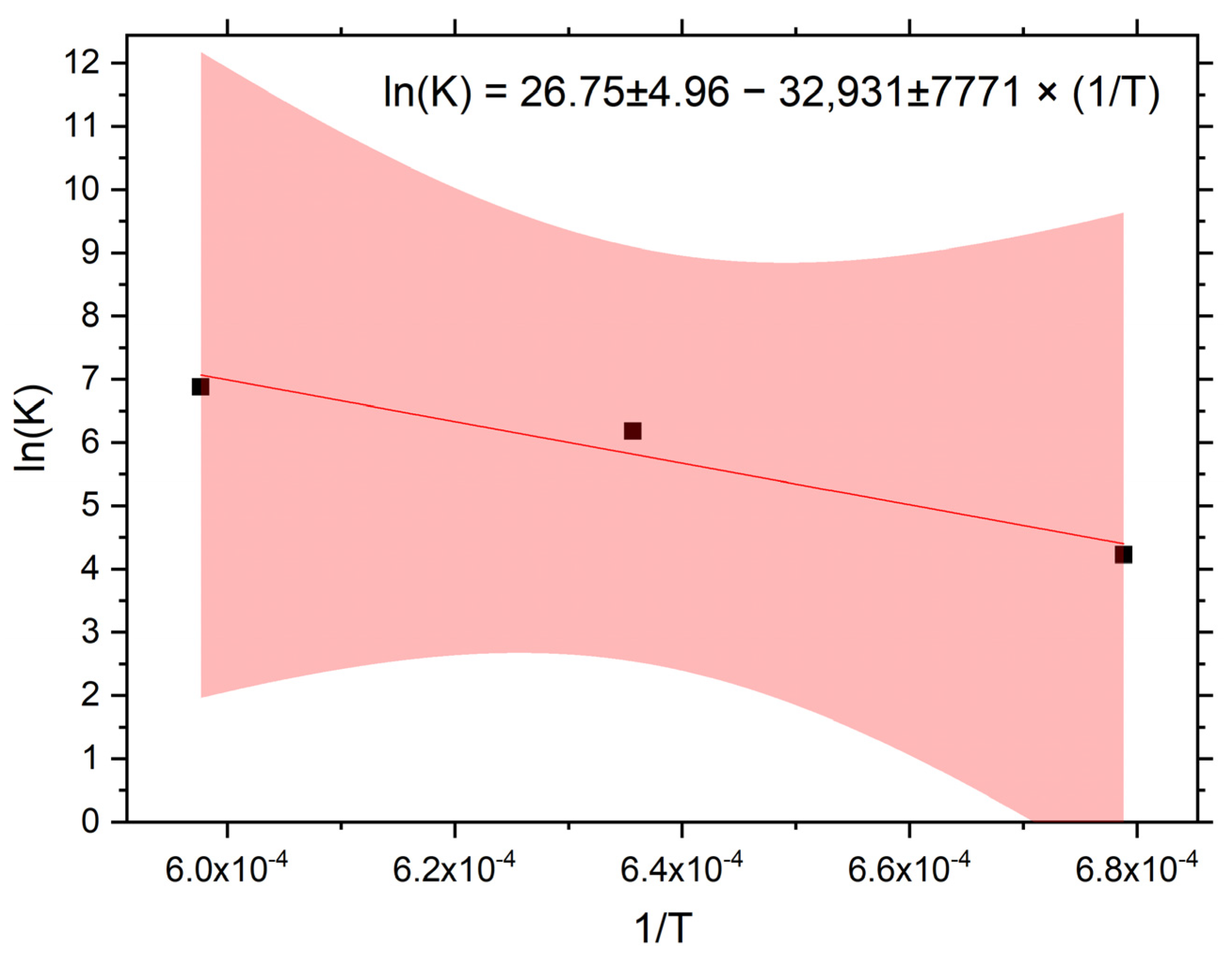
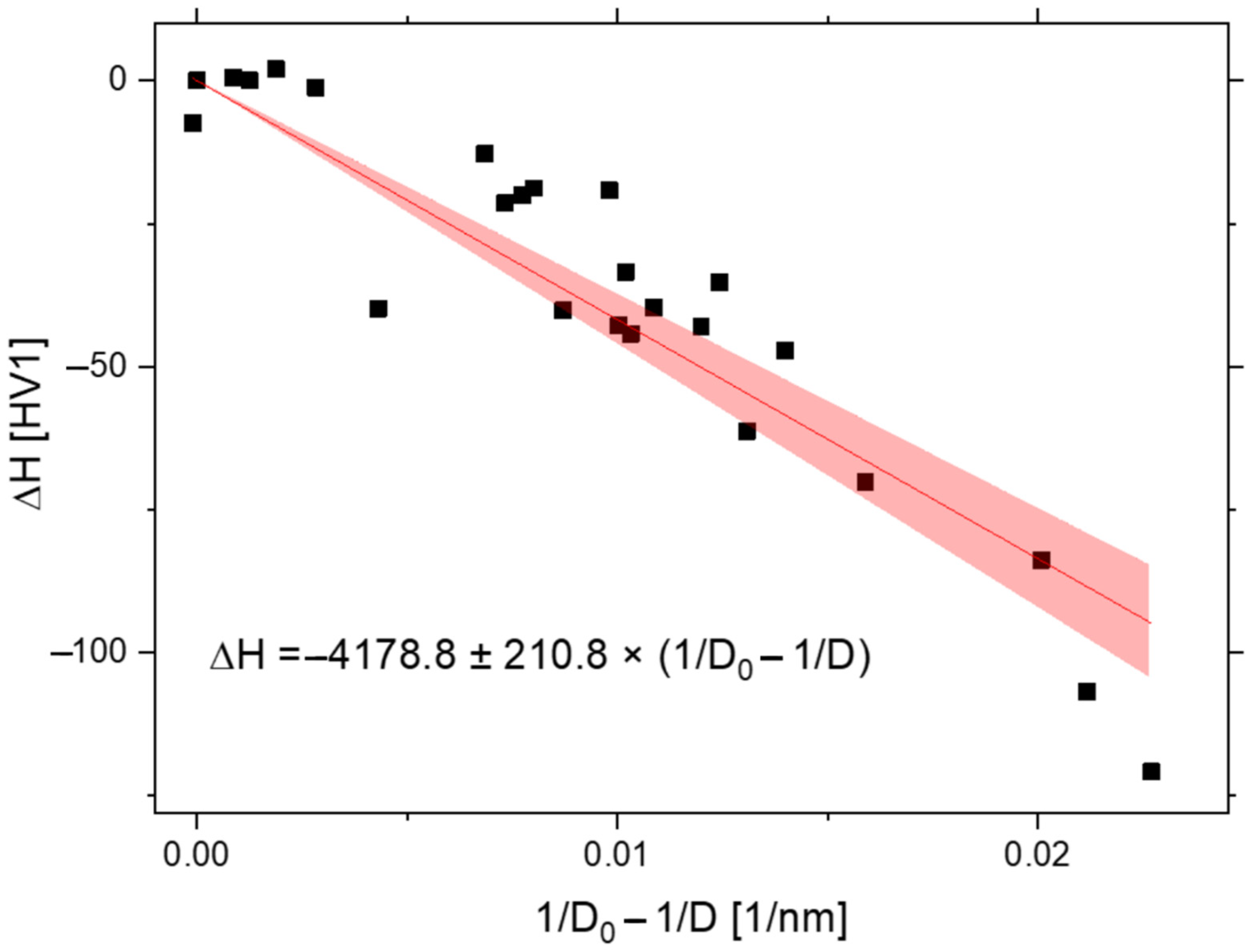
3.2. Dispersoid Coarsening and Calculation of Activation Energy of Y2O3
3.3. Hardness Correlation
4. Discussion
5. Conclusions
- The measured coarsening kinetics of Y2O3 nanoprecipitates in the Fe-10Al-4Cr matrix at temperatures 1200–1400 °C and times up to 72 h indicates an unprecedented resistance of the dispersoid against coarsening.
- The coarsening kinetics of the dispersoid obeys cubic law with the activation energy Q = 274 ± 65 kJ.
- The size distribution of the dispersoid exhibits a right skewness that increases with the amount of coarsening. This phenomenon is explained by different interface energies of individual precipitates.
- The softening of the ODS alloy due to coarsening of the dispersoid is in agreement with the Orowan theory of strengthening.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montealegre, M.; González-Carrasco, J.L.; Morris-Muñoz, M.; Chao, J.; Morris, D. The high temperature oxidation behaviour of an ODS FeAl alloy. Intermetallics 2000, 8, 439–446. [Google Scholar] [CrossRef]
- Stoloff, N. Iron aluminides: Present status and future prospects. Mater. Sci. Eng. A 1998, 258, 1–14. [Google Scholar] [CrossRef]
- Morris, D.; Morris-Munoz, M.A. The influence of microstructure on the ductility of iron aluminides. Intermetallics 1999, 7, 1121–1129. [Google Scholar] [CrossRef]
- Strothers, S.; Vedula, K. Hot extrusion of B2 iron aluminide powders. Prog. Powder Metall. 1987, 43, 597–610. [Google Scholar]
- Matysik, P.; Jozwiak, S.; Czujko, T. The kinetics of non-isothermal iron and aluminum powder mixtures sintering in protective atmosphere. J. Alloys Compd. 2012, 549, 92–99. [Google Scholar] [CrossRef]
- Lindau, R.; Möslang, A.; Rieth, M.; Klimiankou, M.; Materna-Morris, E.; Alamo, A.; Tavassoli, A.-A.F.; Cayron, C.; Lancha, A.-M.; Fernández, P.; et al. Present development status of EUROFER and ODS-EUROFER for application in blanket concepts. Fusion Eng. Des. 2005, 75–79, 989–996. [Google Scholar] [CrossRef]
- Jitsukawa, S.; Kimura, A.; Kohyama, A.; Klueh, R.; Tavassoli, A.; van der Schaaf, B.; Odette, G.; Rensman, J.; Victoria, M.; Petersen, C. Recent results of the reduced activation ferritic/martensitic steel development. J. Nucl. Mater. 2004, 329–333, 39–46. [Google Scholar] [CrossRef]
- Heintze, C.; Hernández-Mayoral, M.; Ulbricht, A.; Bergner, F.; Shariq, A.; Weissgärber, T.; Frielinghaus, H. Nanoscale characterization of ODS Fe–9%Cr model alloys compacted by spark plasma sintering. J. Nucl. Mater. 2012, 428, 139–146. [Google Scholar] [CrossRef]
- Ji, G.; Bernard, F.; Launois, S.; Grosdidier, T. Processing conditions, microstructure and mechanical properties of hetero-nanostructured ODS FeAl alloys produced by spark plasma sintering. Mater. Sci. Eng. A 2013, 559, 566–573. [Google Scholar] [CrossRef]
- Mihalache, V.; Mercioniu, I.; Velea, A.; Palade, P. Effect of the process control agent in the ball-milled powders and SPS-consolidation temperature on the grain refinement, density and Vickers hardness of Fe14Cr ODS ferritic alloys. Powder Technol. 2019, 347, 103–113. [Google Scholar] [CrossRef]
- Oksiuta, Z.; Baluc, N. Effect of mechanical alloying atmosphere on the microstructure and Charpy impact properties of an ODS ferritic steel. J. Nucl. Mater. 2009, 386–388, 426–429. [Google Scholar] [CrossRef]
- Ukai, S.; Fujiwara, M. Perspective of ODS alloys application in nuclear environments. J. Nucl. Mater. 2002, 307–311, 749–757. [Google Scholar] [CrossRef]
- Toualbi, L.; Cayron, C.; Olier, P.; Malaplate, J.; Praud, M.; Mathon, M.-H.; Bossu, D.; Rouesne, E.; Montani, A.; Logé, R.; et al. Assessment of a new fabrication route for Fe–9Cr–1W ODS cladding tubes. J. Nucl. Mater. 2012, 428, 47–53. [Google Scholar] [CrossRef]
- Tatlock, G.J.; Dawson, K.; Boegelein, T.; Moustoukas, K.; Jones, A.R. High resolution microstructural studies of the evolution of nano-scale, yttrium-rich oxides in ODS steels subjected to ball milling, selective laser melting or friction stir welding. Mater. Today Proc. 2016, 3, 3086–3093. [Google Scholar] [CrossRef]
- Stratil, L.; Horník, V.; Dymáček, P.; Roupcová, P.; Svoboda, J. The influence of aluminium content on oxidation resistance of new-generation ODS alloy at 1200 °C. Metals 2020, 10, 1478. [Google Scholar] [CrossRef]
- Svoboda, J.; Kunčická, L.; Luptáková, N.; Weiser, A.; Dymáček, P. Fundamental Improvement of Creep Resistance of New-Generation Nano-Oxide Strengthened Alloys via Hot Rotary Swaging Consolidation. Materials 2020, 13, 5217. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, J.; Luptáková, N.; Jarý, M.; Dymáček, P. Influence of Hot Consolidation Conditions and Cr-Alloying on Microstructure and Creep in New-Generation ODS Alloy at 1100 °C. Materials 2020, 13, 5070. [Google Scholar] [CrossRef] [PubMed]
- Chlupová, A.; Šulák, I.; Kunčická, L.; Kocich, R.; Svoboda, J. Microstructural aspects of new grade ODS alloy consolidated by rotary swaging. Mater. Charact. 2021, 181, 111477. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Gesmundo, F.; Hou, P.Y.; Niu, Y. Criteria for the formation of protective Al2O3 scales on Fe–Al and Fe–Cr–Al alloys. Corros. Sci. 2006, 48, 741–765. [Google Scholar] [CrossRef]
- Issartel, C.; Buscail, H.; Chevalier, S.; Favergeon, J. Effect of yttrium as alloying on a model alumina-forming alloy oxidation at 1100 °C. Oxid. Met. 2017, 88, 409–420. [Google Scholar] [CrossRef]
- Pedraza, F.; Grosseau-Poussard, J.; Dinhut, J. Evolution of oxide scales on an ODS FeAl intermetallic alloy during high temperature exposure in air. Intermetallics 2005, 13, 27–33. [Google Scholar] [CrossRef][Green Version]
- Montealegre, M.; Strehl, G.; González-Carrasco, J.; Borchardt, G. Oxidation behaviour of novel ODS FeAlCr intermetallic alloys. Intermetallics 2005, 13, 896–906. [Google Scholar] [CrossRef]
- Li, D.; Zhou, L.; Xi, Y.; Liu, L.; Liu, Z.; Si, J.; Zhu, K. Phase transformation behavior of alumina grown on FeAl alloys with reactive element dopants at 1273 K. J. Alloys Compd. 2017, 692, 427–433. [Google Scholar] [CrossRef]
- Svoboda, J.; Ecker, W.; Razumovskiy, V.; Zickler, G.; Fischer, F. Kinetics of interaction of impurity interstitials with dislocations revisited. Prog. Mater. Sci. 2019, 101, 172–206. [Google Scholar] [CrossRef]
- Svoboda, J.; Horník, V.; Riedel, H. Modelling of Processing Steps of New Generation ODS Alloys. Met. Mater. Trans. A 2020, 51, 5296–5305. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Oono, N.; Nakamura, K.; Ukai, S.; Kaito, T.; Torimaru, T.; Kimura, A.; Hayashi, S. Oxide particle coarsening at temperature over 1473 K in 9CrODS steel. Nucl. Mater. Energy 2016, 9, 342–345. [Google Scholar] [CrossRef]
- Svoboda, J.; Horník, V.; Stratil, L.; Hadraba, H.; Mašek, B.; Khalaj, O.; Jirková, H. Microstructure Evolution in ODS Alloys with a High-Volume Fraction of Nano Oxides. Metals 2018, 8, 1079. [Google Scholar] [CrossRef]
- Fischer, F.D.; Svoboda, J.; Fratzl, P. A thermodynamic approach to grain growth and coarsening. Philos. Mag. 2003, 83, 1075–1093. [Google Scholar] [CrossRef]
- Lifshitz, I.; Slyozov, V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Svoboda, J.; Bořil, P.; Holzer, J.; Luptáková, N.; Jarý, M.; Mašek, B.; Dymáček, P. Substantial Improvement of High Temperature Strength of New-Generation Nano-Oxide-Strengthened Alloys by Addition of Metallic Yttrium. Materials 2022, 15, 504. [Google Scholar] [CrossRef]
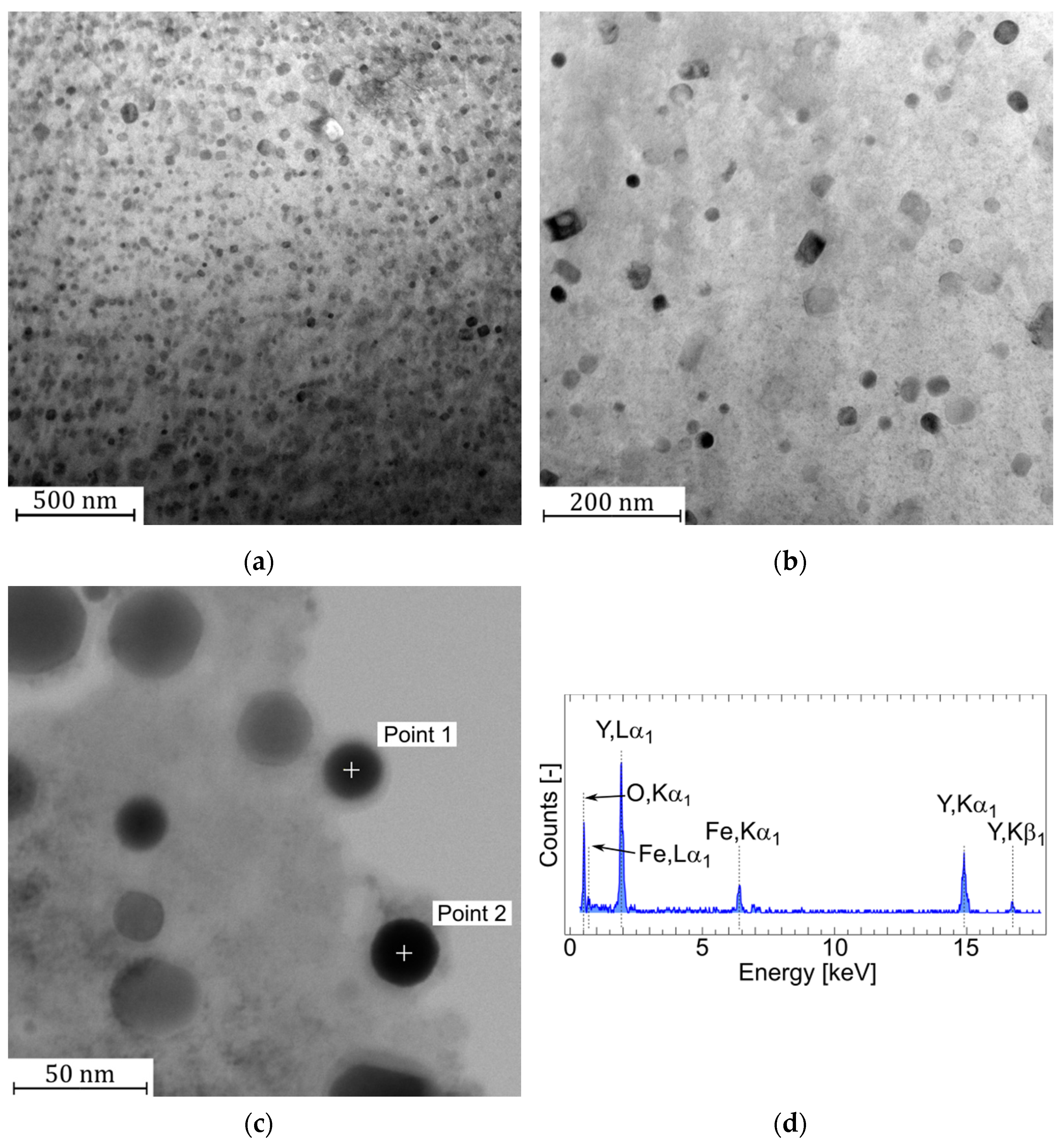



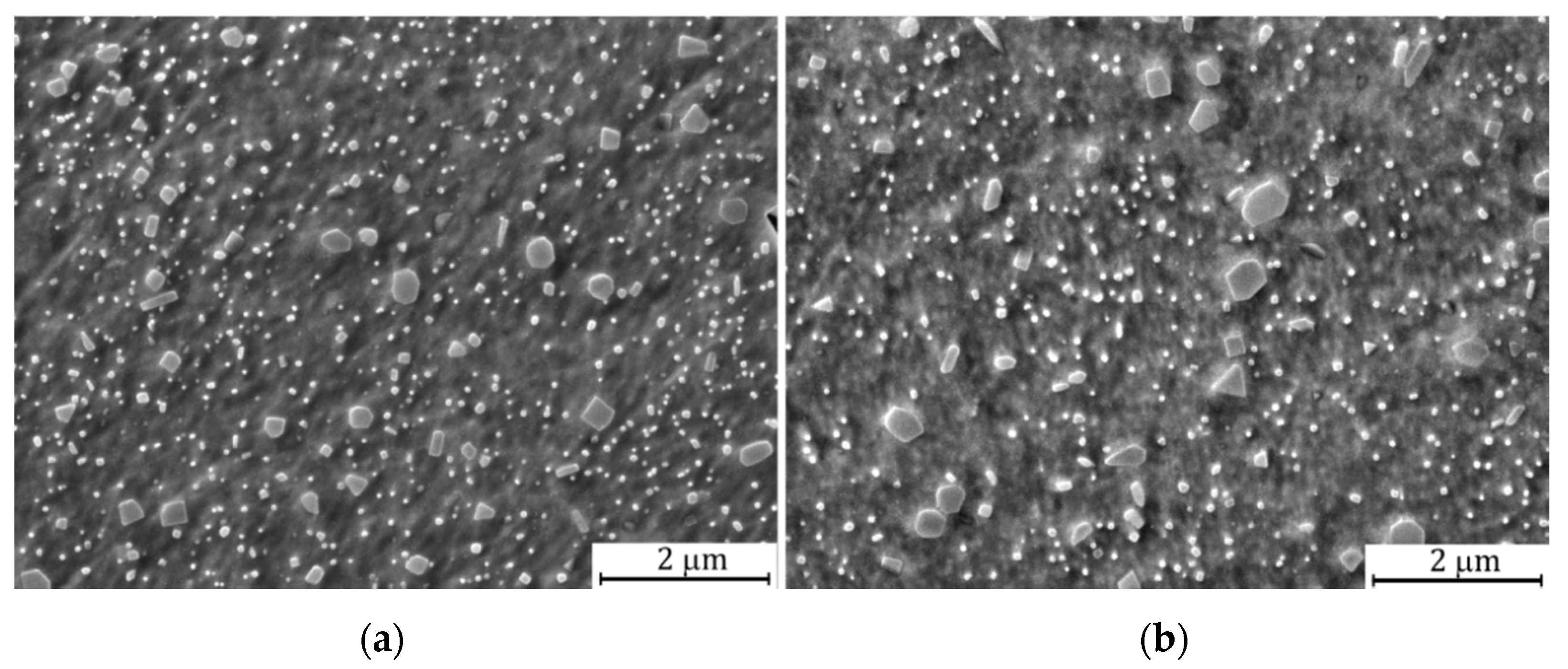
| Element | Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | Point 6 |
|---|---|---|---|---|---|---|
| O | 41.2 | 50.3 | 42.6 | 40.4 | 49.2 | 52.4 |
| Al | 1.6 | 1.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| Cr | 2.7 | 0.0 | 0.9 | 0.6 | 0.7 | 1.2 |
| Fe | 5.1 | 6.2 | 8.1 | 8.6 | 8.0 | 9.6 |
| Y | 49.4 | 42.4 | 48.3 | 50.4 | 42.1 | 36.8 |
| Specimen | Mean [nm] | Median [nm] |
|---|---|---|
| As-received | 28.6 ± 0.7 | 27.70 |
| 1200 °C | 30.2 ± 0.7 | 29.49 |
| 1300 °C | 37.08 ± 0.7 | 36.36 |
| 1400 °C | 40.09 ± 1 | 37.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzer, J.; Gamanov, Š.; Luptáková, N.; Dlouhý, A.; Svoboda, J. Coarsening Kinetics of Y2O3 Dispersoid in New Grade of Fe-Al-Cr-Based ODS Alloy. Metals 2022, 12, 210. https://doi.org/10.3390/met12020210
Holzer J, Gamanov Š, Luptáková N, Dlouhý A, Svoboda J. Coarsening Kinetics of Y2O3 Dispersoid in New Grade of Fe-Al-Cr-Based ODS Alloy. Metals. 2022; 12(2):210. https://doi.org/10.3390/met12020210
Chicago/Turabian StyleHolzer, Jakub, Štěpán Gamanov, Natália Luptáková, Antonín Dlouhý, and Jiří Svoboda. 2022. "Coarsening Kinetics of Y2O3 Dispersoid in New Grade of Fe-Al-Cr-Based ODS Alloy" Metals 12, no. 2: 210. https://doi.org/10.3390/met12020210
APA StyleHolzer, J., Gamanov, Š., Luptáková, N., Dlouhý, A., & Svoboda, J. (2022). Coarsening Kinetics of Y2O3 Dispersoid in New Grade of Fe-Al-Cr-Based ODS Alloy. Metals, 12(2), 210. https://doi.org/10.3390/met12020210









