Influence of the Gas Reaction Atmosphere on the Structure, Phase Composition, Functional Properties and Cytocompatibility of Porous Titanium–Nickel Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
- The results of the XRD and EBSD analyses showed that the porous NiTi-(N) alloy is characterized by an increase in the volume fraction of brittle interstitial Ti4Ni2O(N) phases and an appearance of a finely dispersed TiNi3 phase. The increase in the proportion of the Ti4Ni2O(N) phase as well as the increased nitrogen content in the surface layer found in the NiTi-(N) alloy favorably affect the cytocompatibility of the surface with bone marrow mesenchymal cells. The percentage of the cells covering the surface of NiTi–(N) is high and accounts for 90%, in contrast to NiTi–(Ar) where the percentage of the cell coverage is 75%.
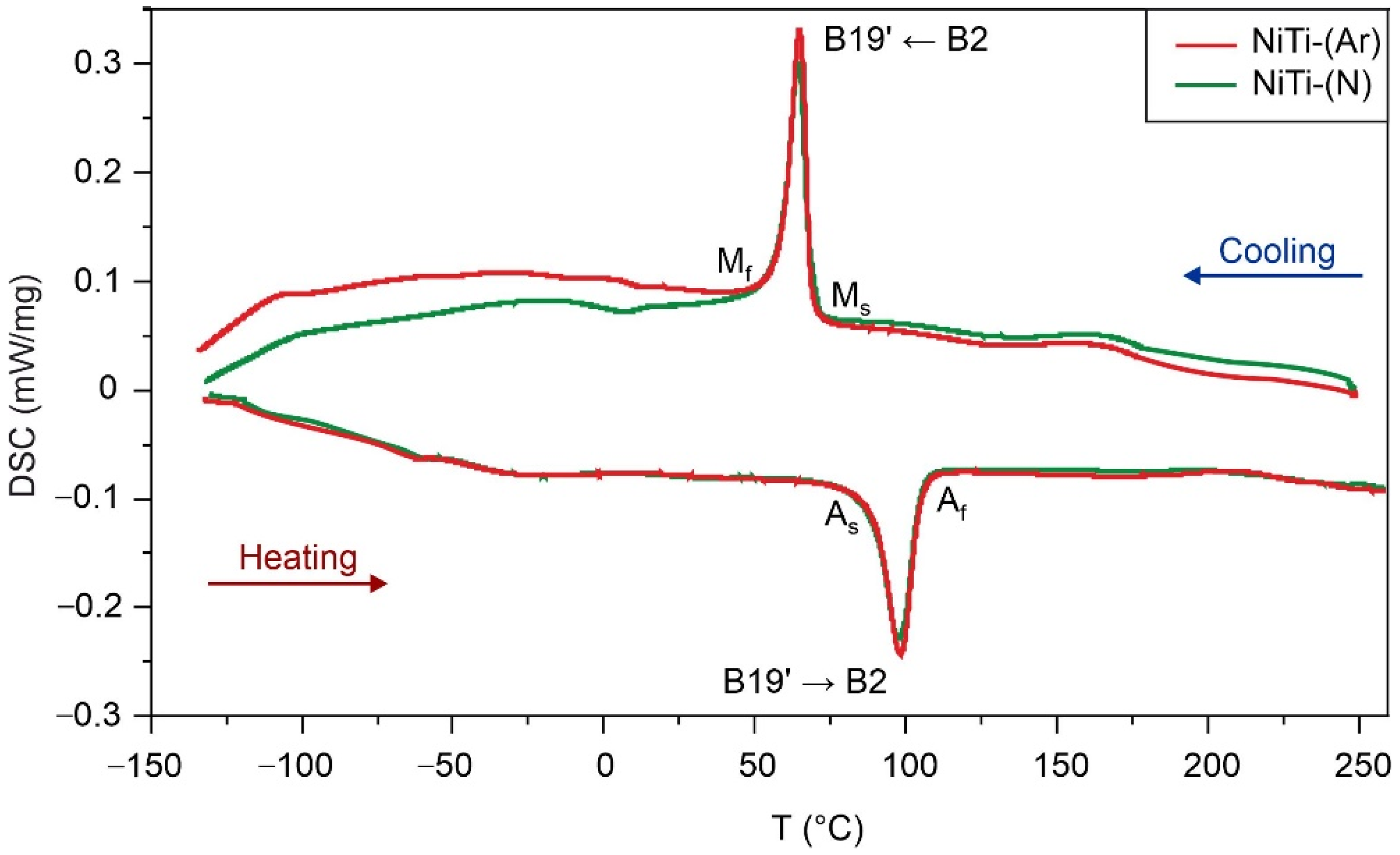
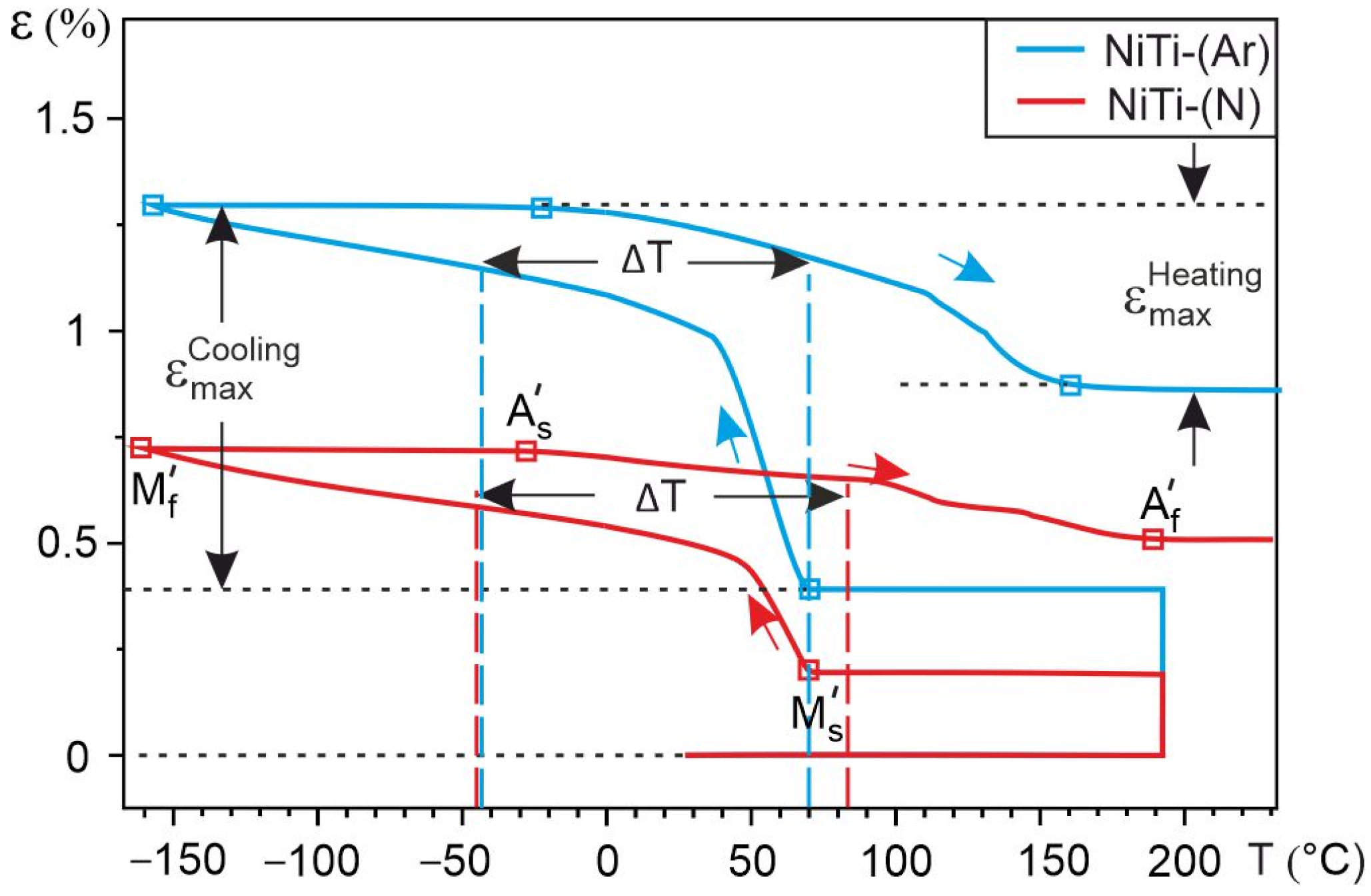
- DSC analysis shows that the reversible martensitic transition B2↔B19’ is typical of these two alloys and the gaseous atmosphere does not affect the characteristic martensitic transformation temperatures during cooling and heating without load. However, the study of martensitic transformation under load during SME showed a significant difference in the deformation behavior of porous NiTi-(Ar) and NiTi-(N) alloys. It has been established that the austenitic B2 phase in porous NiTi-(Ar) alloys accumulates up to 1% deformation while cooling, and in porous NiTi-(N) alloys it does not exceed 0.5%. It was found that there is an increase in the width of the temperature hysteresis and irreversible deformation in NiTi-(N) alloy.
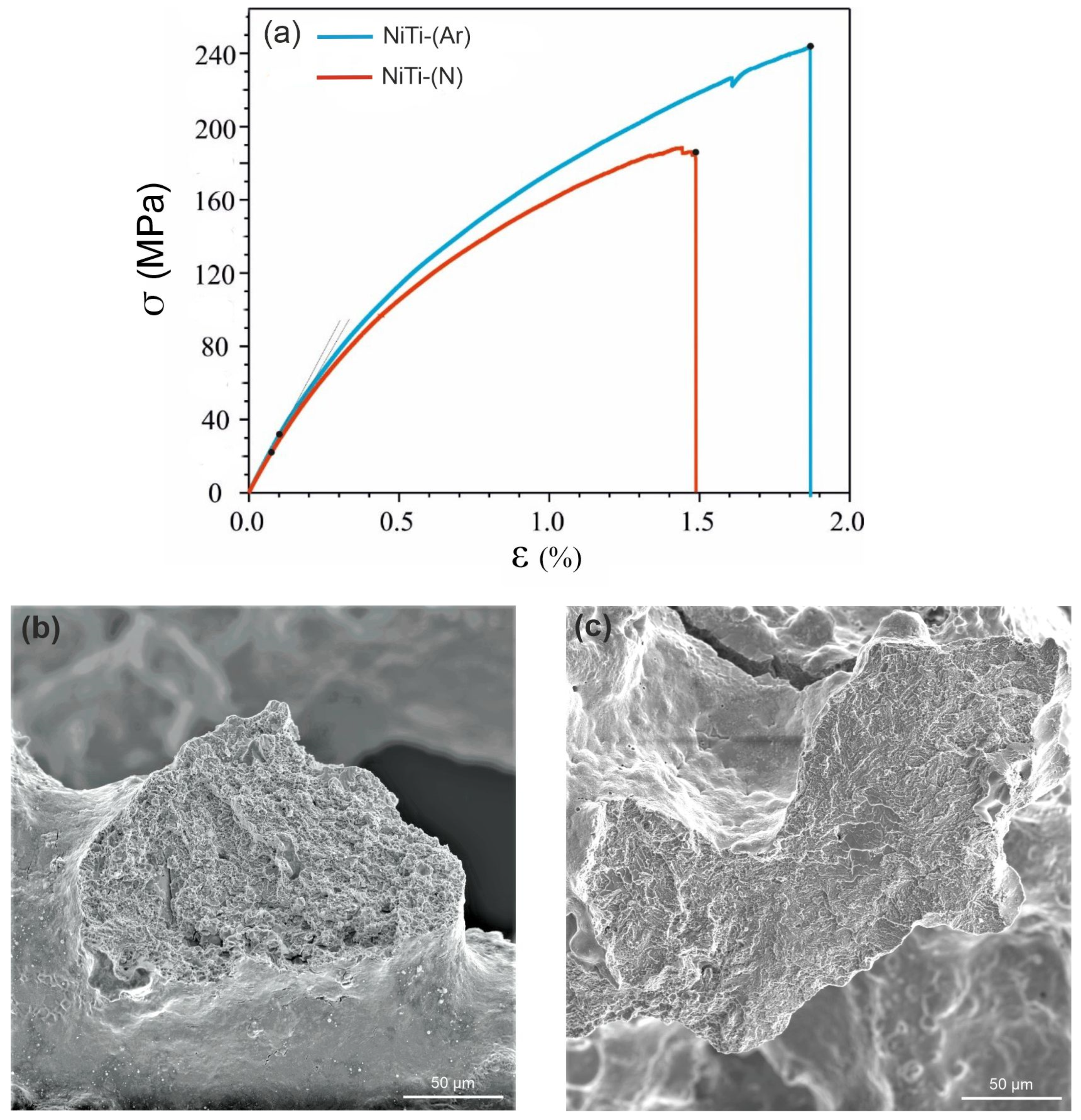
- Uniaxial tension of porous NiTi-(Ar) and NiTi-(N) plates to fracture showed that the alloys are characterized by an elastic–plastic mechanism of strain hardening. A large proportion of brittle fracture areas was found on the fractograms of the NiTi-(N) alloy, which indicates the presence of a larger volume fraction of brittle Ti2Ni + Ti4Ni2O(N) phases. All mechanical characteristics of NiTi-(N) alloys are noticeably lower than those of NiTi-(Ar) alloys. The ultimate strength of the NiTi-(Ar) alloy was 250 MPa, whereas that of the NiTi-(N) alloy was 190 MPa.
- Thus, according to the data obtained, porous NiTi-(N) alloys under low physiological load can be considered more biocompatible. To use porous NiTi-(N) alloys under high physiological load, it is necessary to increase their reversible deformation value and tensile strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wisutmethangoon, S.; Denmud, N.; Sikong, L. Characteristics and compressive properties of porous NiTi alloy synthesized by SHS technique. Mater. Sci. Eng. A 2009, 515, 93–97. [Google Scholar] [CrossRef]
- Saedi, S.; Saghaian, S.E.; Jahadakbar, A.; Moghaddam, N.S.; Andani, M.T.; Saghaian, S.M.; Lu, Y.C.; Elahinia, M.; Karaca, H.E. Shape memory response of porous NiTi shape memory alloys fabricated by selective laser melting. J. Mater. Sci. Mater. Med. 2018, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Superelastic behaviors of biomedical porous NiTi alloy with high porosity and large pore size prepared by spark plasma sintering. J. Alloys Compd. 2015, 44, 513–522. [Google Scholar] [CrossRef]
- Sevilla, P.; Aparicio, C.; Planell, J.A.; Gil, F.J. Comparison of the mechanical properties between tantalum and nickel-titanium foams implant materials for bone ingrowth applications. J. Alloys Compd. 2007, 439, 67–73. [Google Scholar] [CrossRef]
- Ipek, N.G.; Dericioglu, A.F.; Bor, S. Fatigue behavior of TiNi foams processed by the magnesium space holder technique. J. Mech. Behav. Biomed. Mater. 2011, 4, 2017–2023. [Google Scholar] [CrossRef]
- Nakas, G.I.; Aşik, E.E.; Tunca, B.; Bor, S. Fatigue and Fracture Behavior of Porous TiNi. Alloys. Mater. Sci. Forum. 2014, 783, 591–596. [Google Scholar] [CrossRef]
- Yasenchuk, Y.; Marchenko, E.; Gunther, V.; Radkevich, A.; Kokorev, O.; Gunther, S.; Baigonakova, G.; Hodorenko, V.; Chekalkin, T.; Kang, J.H.; et al. Biocompatibility and clinical application of porous TiNi alloys made by self-propagating high-temperature synthesis (SHS). Materials 2019, 12, 2405. [Google Scholar] [CrossRef] [Green Version]
- Marchenko, E.S.; Baigonakova, G.A.; Yasenchuk, Y.F.; Chekalkin, T.L.; Volinsky, A.A. Structure, biocompatibility and corrosion resistance of the ceramic-metal surface of porous nitinol. Ceram. Int. 2022, 48, 33514–33523. [Google Scholar] [CrossRef]
- Starosvetsky, D.; Gotman, I. TiN coating improves the corrosion behavior of superelastic NiTi surgical alloy. Surf. Coat. Technol. 2001, 148, 268–276. [Google Scholar] [CrossRef]
- Soltanalipour, M.; Khalil-Allafi, J.; Ghareshabani, E.; Khalili, V. Electrochemical behaviour of NiTi alloy coated with TiN using DPF. Surf. Eng. 2017, 33, 474–481. [Google Scholar] [CrossRef]
- Novak, P.; Mejzlikova, L.; Michalcova, A.; Capek, J.; Beran, P.; Vojtech, D. Effect of SHS conditions on microstructure of NiTi shape memory alloy. Intermetallics 2013, 42, 85–91. [Google Scholar] [CrossRef]
- Tay, B.Y.; Goh, C.W.; Gu, Y.W.; Lim, C.S.; Yong, M.S.; Ho, M.K.; Myint, M.H. Porous NiTi fabricated by self-propagating high-temperature synthesis of elemental powders. J. Mater. Process. Technol. 2008, 202, 359–364. [Google Scholar] [CrossRef]
- Li, B.Y.; Rong, L.J.; Li, Y.Y.; Gjunter, V.E. Synthesis of porous Ni–Ti shape-memory alloys by self-propagating high-temperature synthesis: Reaction mechanism and anisotropy in pore structure. Acta Mater. 2000, 48, 389–3904. [Google Scholar] [CrossRef]
- Novak, P.; Vesely, T.; Marek, I.; Dvorak, P.; Vojtech, V.; Salvetr, P.; Hausild, P.; Kopecek, J. Effect of Particle Size of Titanium and Nickel on the Synthesis of NiTi by TE-SHS. Metall. Mater. Trans. B. 2016, 47, 932–938. [Google Scholar] [CrossRef]
- Resnina, N.; Rubanik, V., Jr.; Rubanik, V.; Kulak, M.; Belyaev, S.; Liulchak, P.; Chepela, D.; Kalganov, V. Influence of the Ar pressure on the structure of the NiTi foams produced by self-propagating high-temperature synthesis. Mater. Lett. 2021, 299, 130047. [Google Scholar] [CrossRef]
- Novak, P.; Kadlecova, B.; Salvetr, P.; Knaislova, A.; Skolakova, A.; Karlik, M.; Kopecek, J. Effect of Reaction Atmosphere and Heating Rate During Reactive Sintering of Ni-Ti Intermetallics. Procedia Eng. 2017, 184, 681–686. [Google Scholar] [CrossRef]
- Gunther, V.; Yasenchuk, Y.; Chekalkin, T.; Marchenko, E.; Gunther, S.; Baigonakova, G.; Hodorenko, V.; Chekalkin, T.; Kang, J.H.; Weiss, S.; et al. Formation of pores and amorphous-nanocrystalline phases in porous TiNi alloys made by self-propagating high-temperature synthesis (SHS). Adv. Powder Technol. 2019, 30, 673–680. [Google Scholar] [CrossRef]
- Balogh, Z.; Schmitz, G. Physical Metallurgy, 1st ed.; Laughlin, D.E., Hono, K., Eds.; Elsevier: Pittsburgh, PA, USA, 2015; pp. 387–559. [Google Scholar]
- Zeng, K.; Schmid-Fetzer, R.; Rogl, P. A thermodynamic analysis of cermet sintering of TiN-Ni powder mixtures. J. Phase Equilib. 1997, 19, 124–135. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Novelty, OH, USA, 1990; p. 3589. [Google Scholar]
- Qiu, A.T.; Liu, L.J.; Pang, W.; Lu, X.G.; Li, C.H. Calculation of phase diagram of Ti-Ni-O system and application to deoxidation of TiNi alloy. Trans. Nonfer. Met. Soc. China 2011, 21, 1808–1816. [Google Scholar] [CrossRef]
- Mehrer, H. Diffusion in Solid Metals and Alloys; Springer: Berlin/Heidelberg, Germany, 1990; p. 752. [Google Scholar]
- Vykhodets, V.B.; Klotsman, S.M.; Kurennykh, T.Y.; Levin, A.D.; Pavlov, V.A. Diffusion of oxygen in α-Ti. V. temperature dependence coefficients of oxygen diffusion. Phys. Met. Metallogr. 1989, 68, 94–97. [Google Scholar]
- Kaya, M.; Orhan, N.; Kurt, B.; Khan, T.I. The effect of solution treatment under loading on the microstructure and phase transformation behavior of porous NiTi shape memory alloy fabricated by SHS. J. Alloys Compd. 2009, 475, 378–382. [Google Scholar] [CrossRef]
- Jiang, H.C.; Rong, L.J. Ways to lower transformation temperatures of porous NiTi shape memory alloy fabricated by self-propagating high-temperature synthesis. Mater. Sci. Eng. A 2006, 438, 883–886. [Google Scholar] [CrossRef]
- Zu, X.T.; Zhang, C.F.; Zhu, S.; Huo, Y.; Wang, Z.G.; Wang, L.M. Electron irradiation-induced changes of martensitic transformation characteristics in a TiNiCu shape memory alloy. Mater. Lett. 2003, 57, 2099–2103. [Google Scholar] [CrossRef]
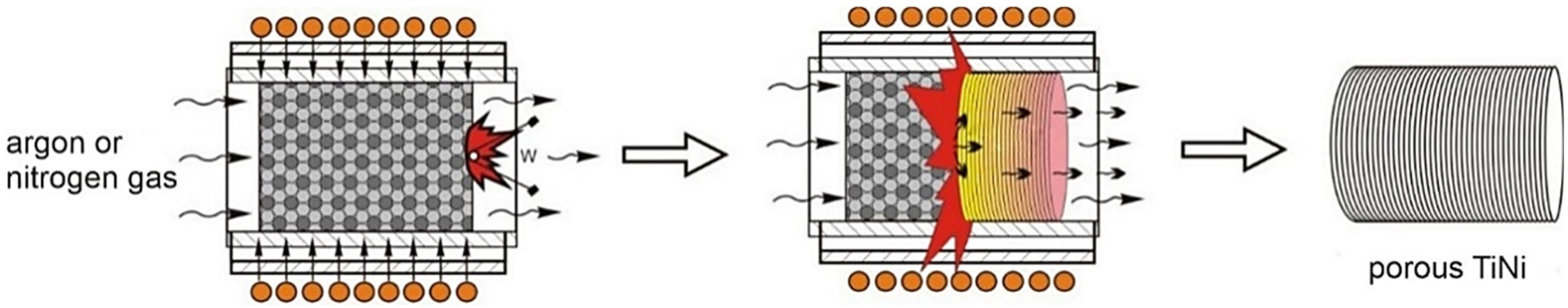
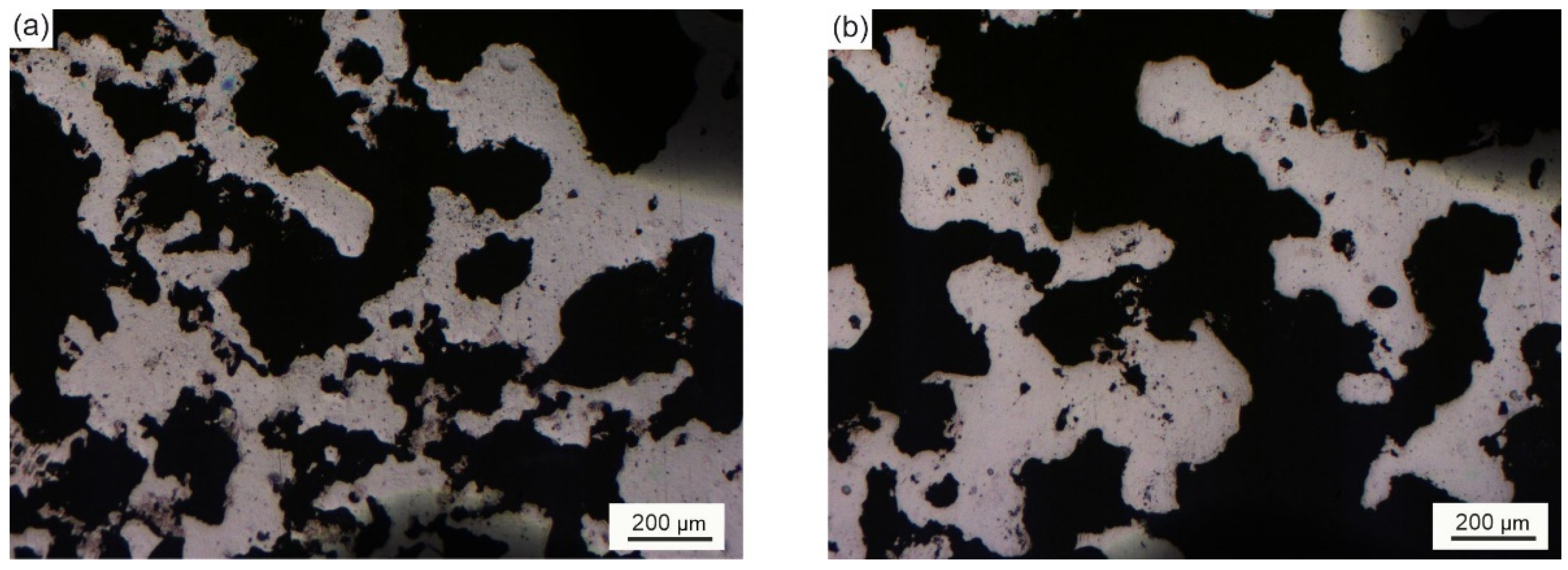
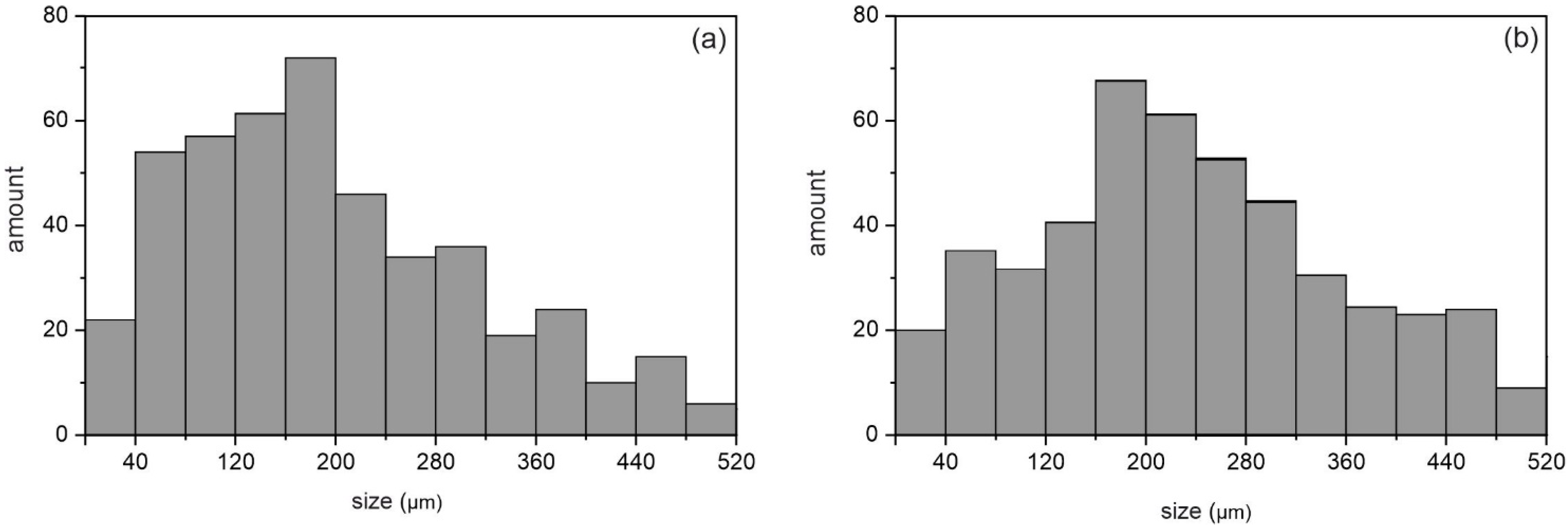
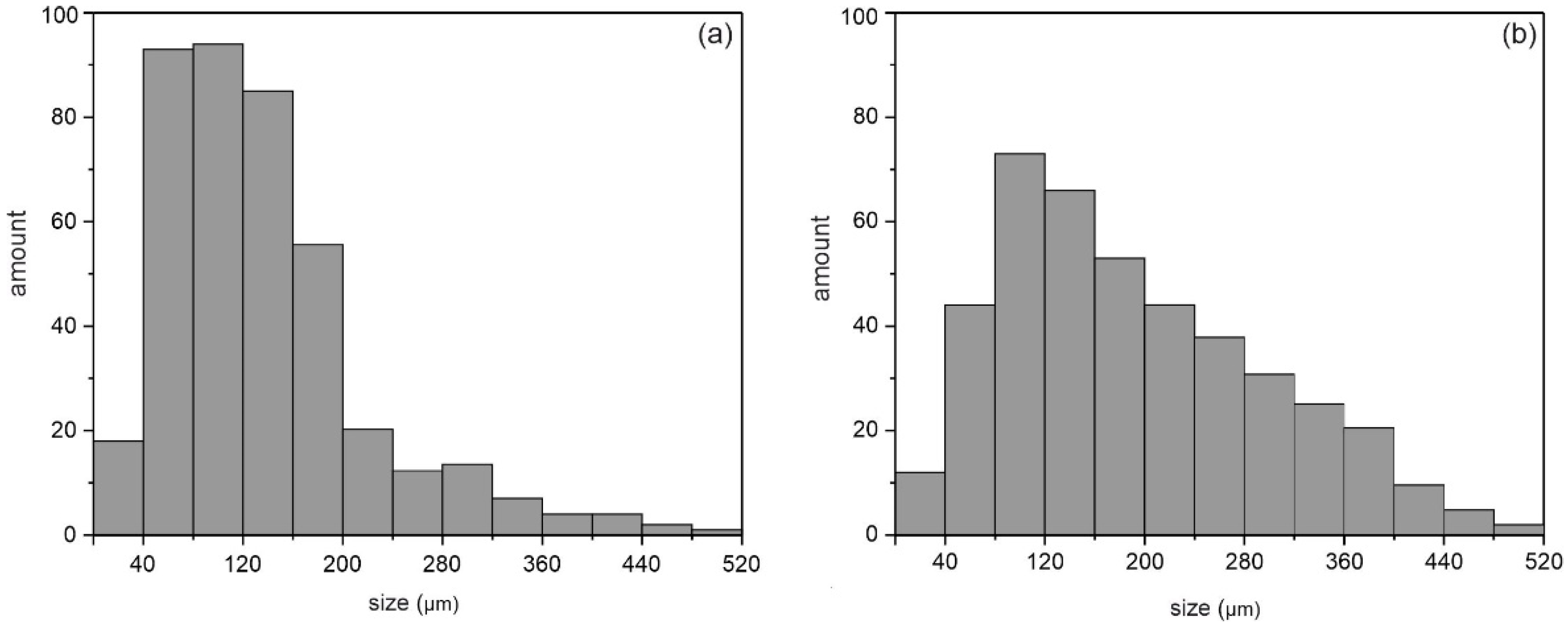
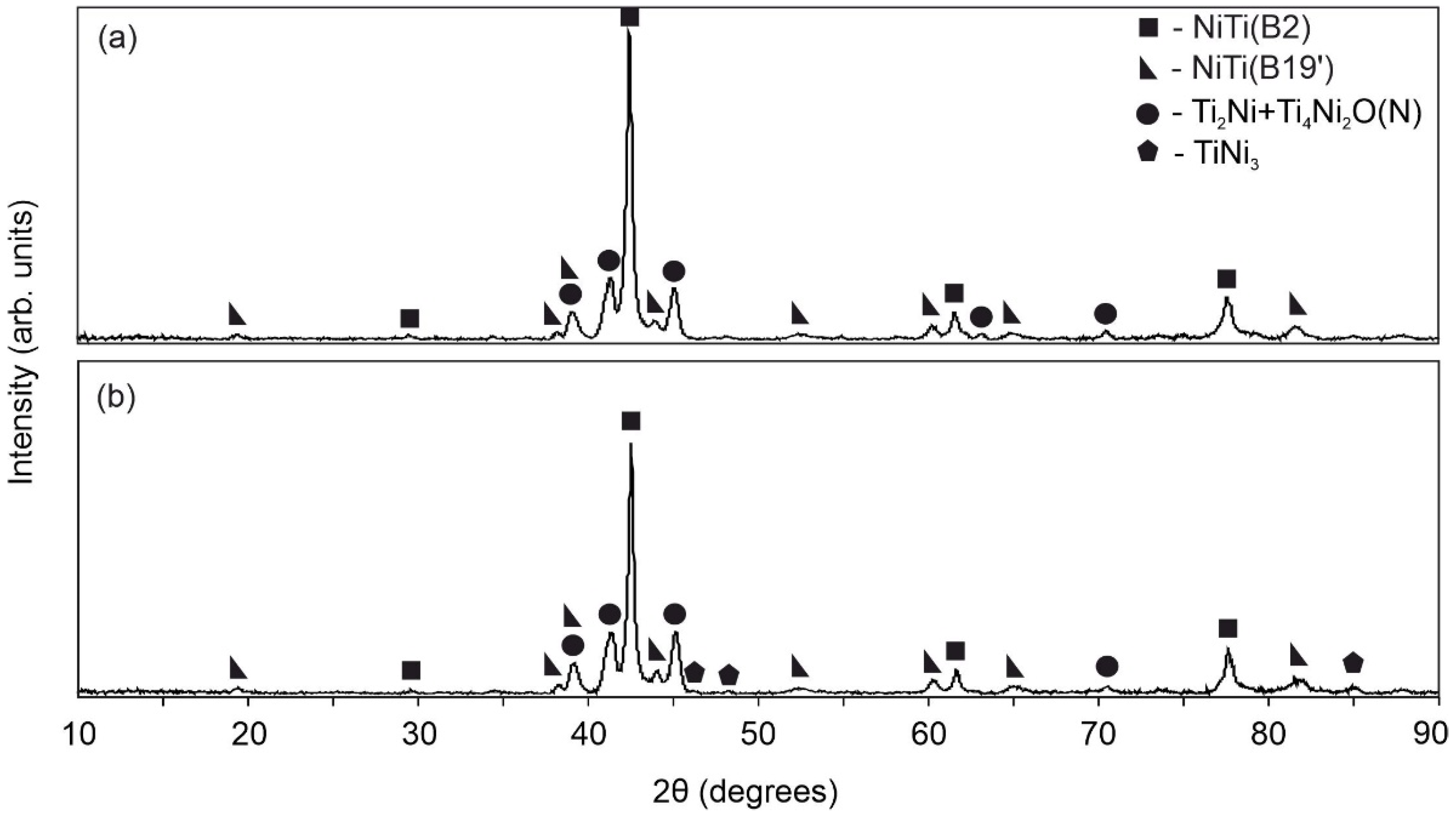

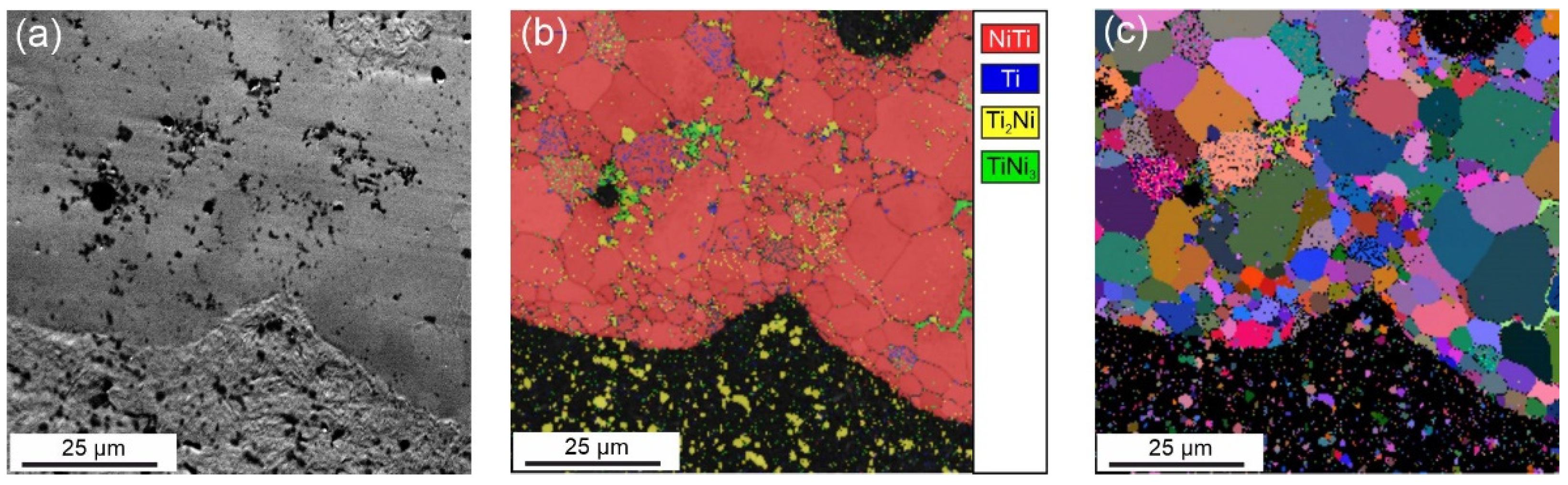
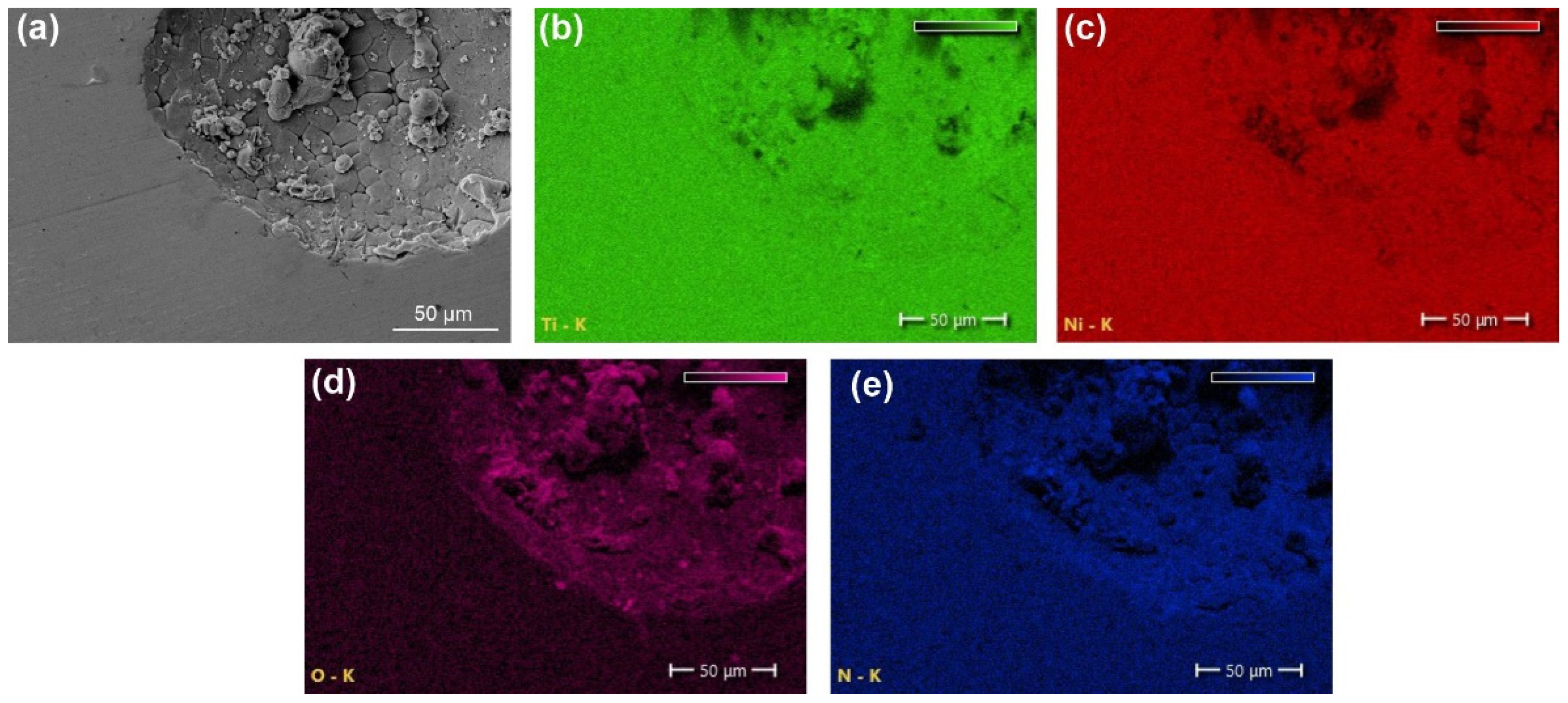
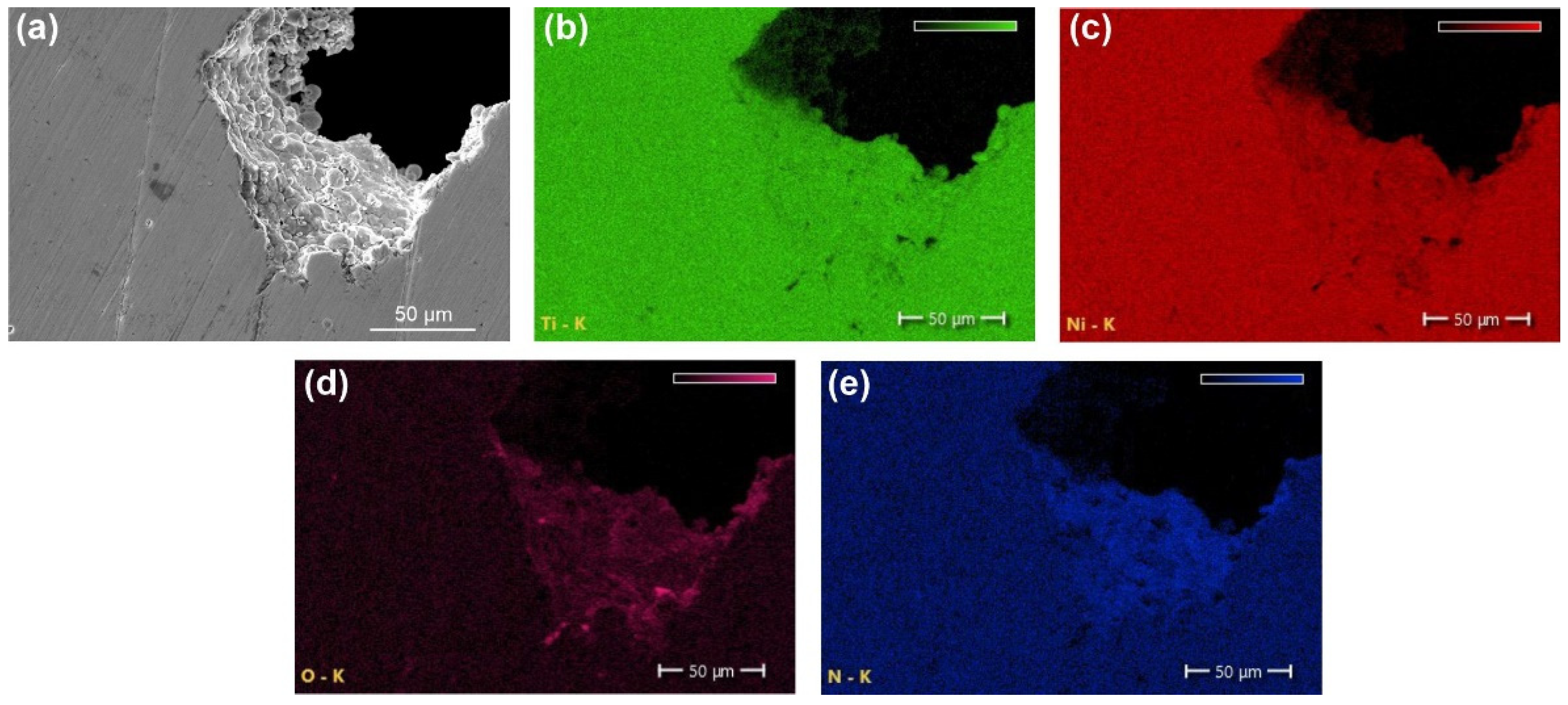

| Sample | Phase | Vol. Fraction, vol.% | Lattice Parameter, Å | CSR, nm | Δd/d × 10−3 |
|---|---|---|---|---|---|
| NiTi-(Ar) | NiTi (B2) | 66.2 | a = 3.0169 | 43 | 0.6 |
| NiTi (B19′) | 9.4 | a = 4.5470 b = 4.0784 c = 2.8848 β = 97 | 20 | 5.6 | |
| Ti2Ni + Ti4Ni2O | 24.4 | a = 11.3852 | 24 | 0.1 | |
| NiTi-(N) | NiTi (B2) | 55.8 | a = 3.0167 | 11 | 6.3 |
| NiTi (B19′) | 11.8 | a = 4.5768 b = 4.0697 c = 2.8908 β = 97 | 17 | 3.1 | |
| Ti2Ni + Ti4Ni2O (N) | 32.4 | a = 11.3967 | 24 | 0.8 | |
| TiNi3 | traces | - | - | - |
| Sample | Ms, °C | Mf, °C | As, °C | Af, °C | ΔHA→M, J/g | ΔHM→A, J/g |
|---|---|---|---|---|---|---|
| NiTi-(Ar) | 69 | 59 | 90 | 105 | −12.54 | 10.95 |
| NiTi-(N) | 69 | 58 | 88 | 105 | −11.65 | 10.67 |
| Sample | T0, °C | ΔSA→M, J/gK | ΔSM→A, J/gK | ΔGA→M, J/g |
|---|---|---|---|---|
| NiTi-(Ar) | 87 | −0.035 | 0.03 | −0.54 |
| NiTi-(N) | 87 | −0.032 | 0.029 | −0.52 |
| Sample | ΔT, °C | ||||||
|---|---|---|---|---|---|---|---|
| NiTi-(Ar) | 70 | −158 | −22 | 160 | 0.9 | 52 | 113 |
| NiTi-(N) | 70 | −160 | −28 | 190 | 0.51 | 59 | 126 |
| Sample | σy, MPa | σB, MPa | E, GPa | εmax, % |
|---|---|---|---|---|
| NiTi-(Ar) | 32 ± 0.2 | 242 ± 23 | 31 ± 0.3 | 1.88 ± 0.1 |
| NiTi-(N) | 22 ± 0.2 | 187 ± 17 | 31 ± 0.4 | 1.49 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchenko, E.; Baigonakova, G.; Shishelova, A. Influence of the Gas Reaction Atmosphere on the Structure, Phase Composition, Functional Properties and Cytocompatibility of Porous Titanium–Nickel Alloys. Metals 2022, 12, 2170. https://doi.org/10.3390/met12122170
Marchenko E, Baigonakova G, Shishelova A. Influence of the Gas Reaction Atmosphere on the Structure, Phase Composition, Functional Properties and Cytocompatibility of Porous Titanium–Nickel Alloys. Metals. 2022; 12(12):2170. https://doi.org/10.3390/met12122170
Chicago/Turabian StyleMarchenko, Ekaterina, Gulsharat Baigonakova, and Arina Shishelova. 2022. "Influence of the Gas Reaction Atmosphere on the Structure, Phase Composition, Functional Properties and Cytocompatibility of Porous Titanium–Nickel Alloys" Metals 12, no. 12: 2170. https://doi.org/10.3390/met12122170
APA StyleMarchenko, E., Baigonakova, G., & Shishelova, A. (2022). Influence of the Gas Reaction Atmosphere on the Structure, Phase Composition, Functional Properties and Cytocompatibility of Porous Titanium–Nickel Alloys. Metals, 12(12), 2170. https://doi.org/10.3390/met12122170







