Corrosion Behavior of Copper Exposed in Marine Tropical Atmosphere in Rapa Nui (Easter Island) Chile 20 Years after MICAT
Abstract
:1. Introduction
2. Materials and Methods
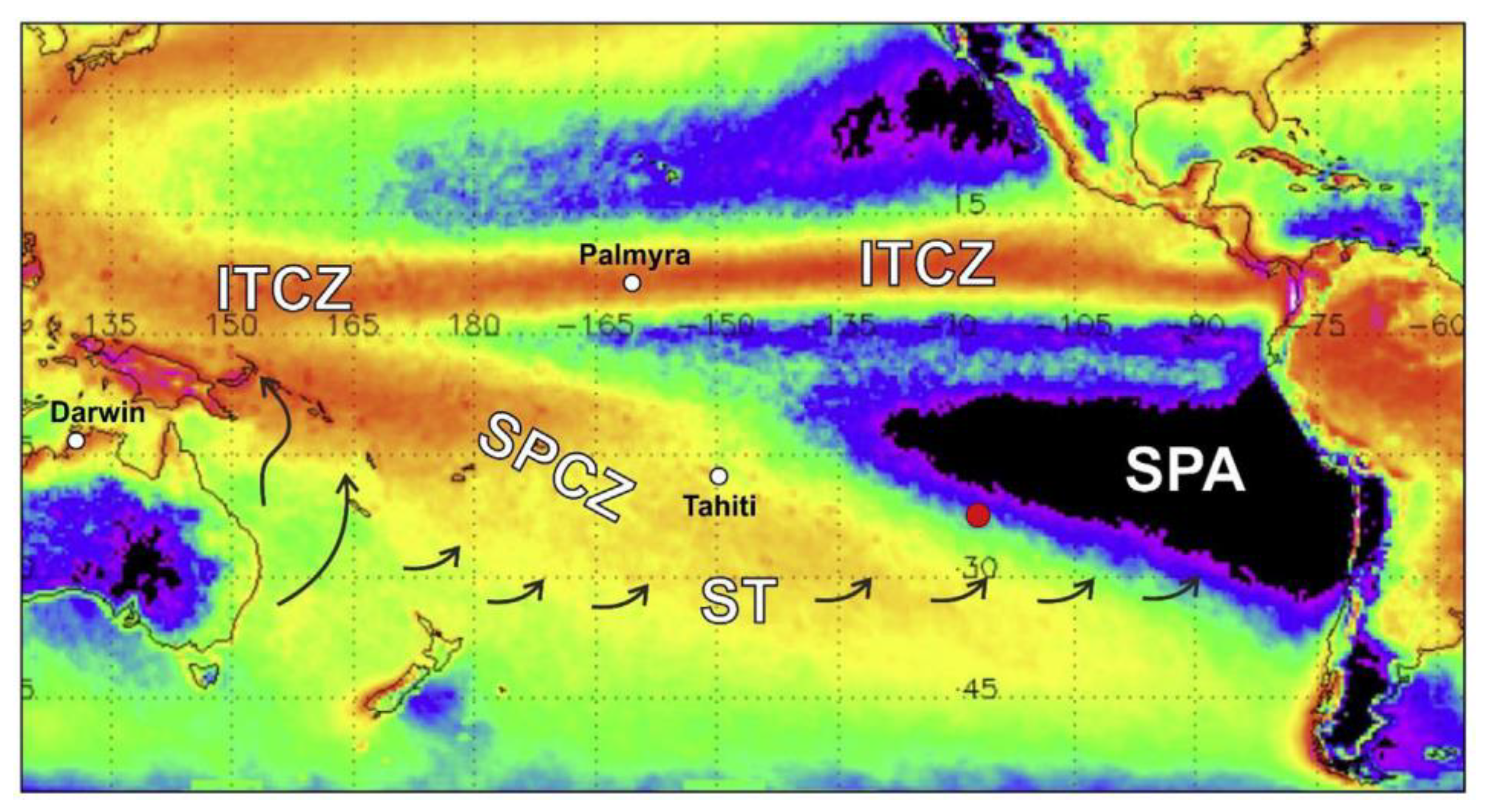
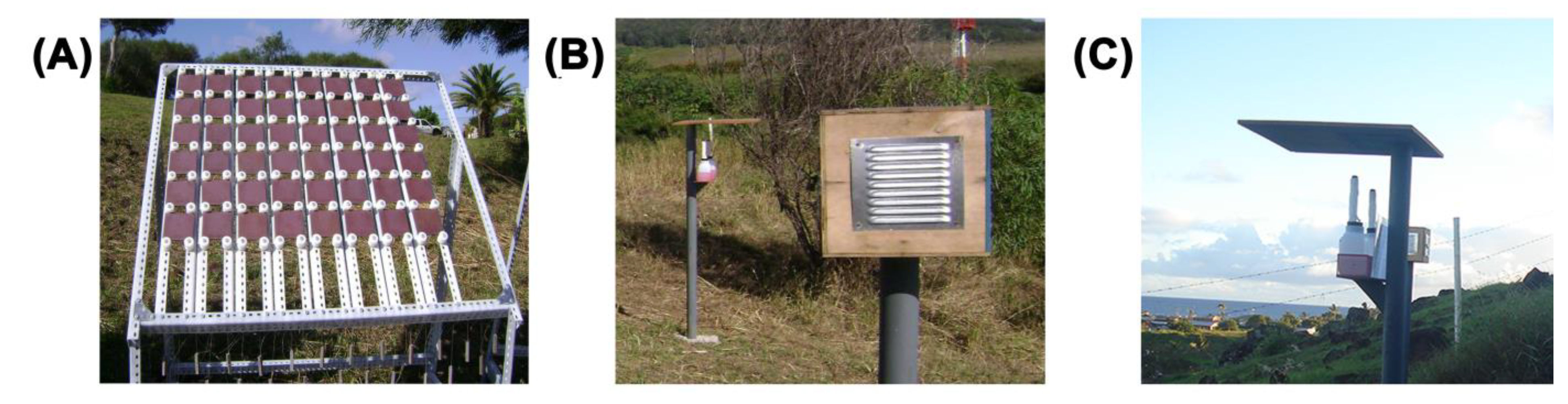
2.1. Place, Sample Preparation, Installation, and Meteorological Measurements
2.2. Corrosion Testing
3. Results and Discussion
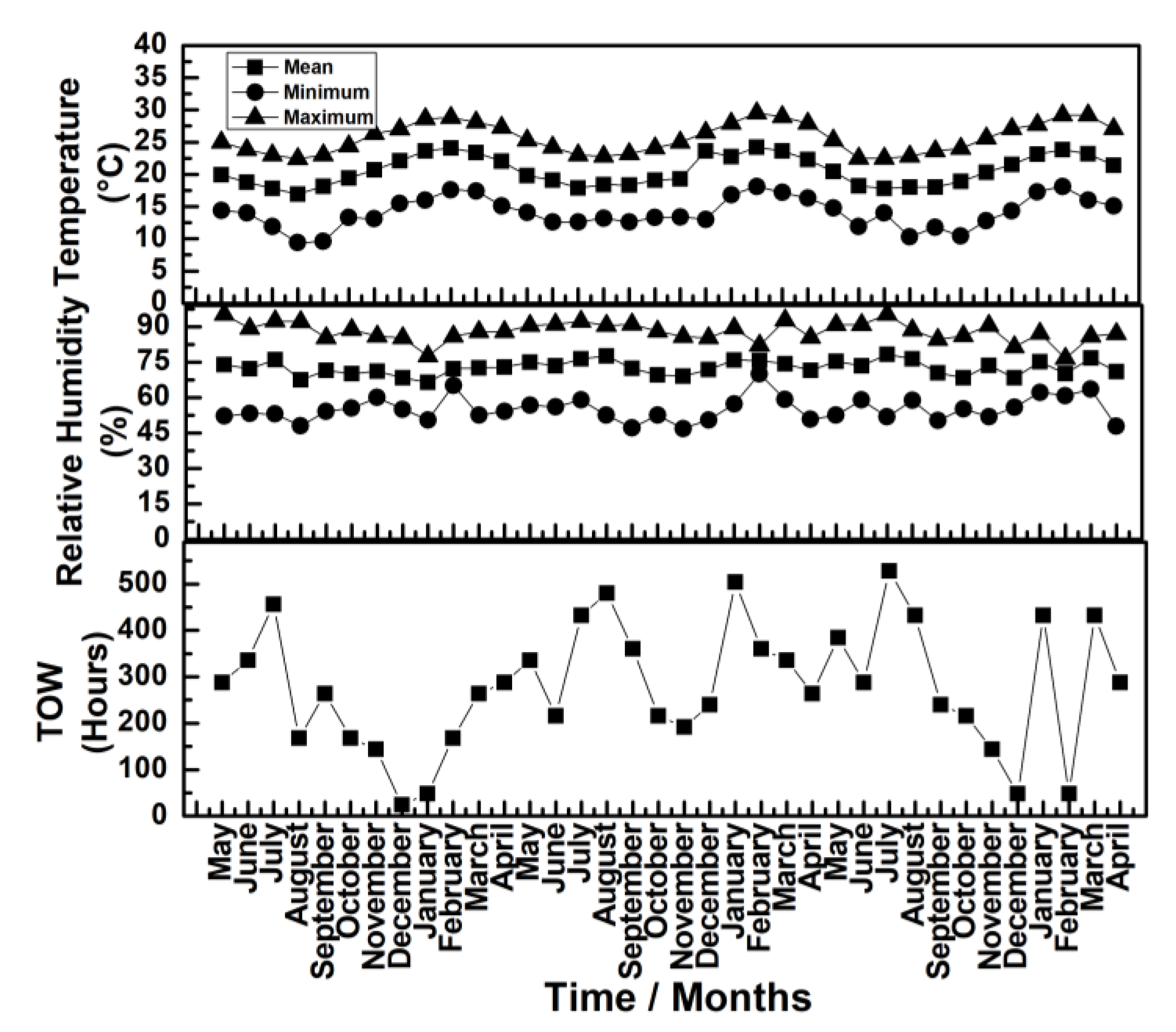
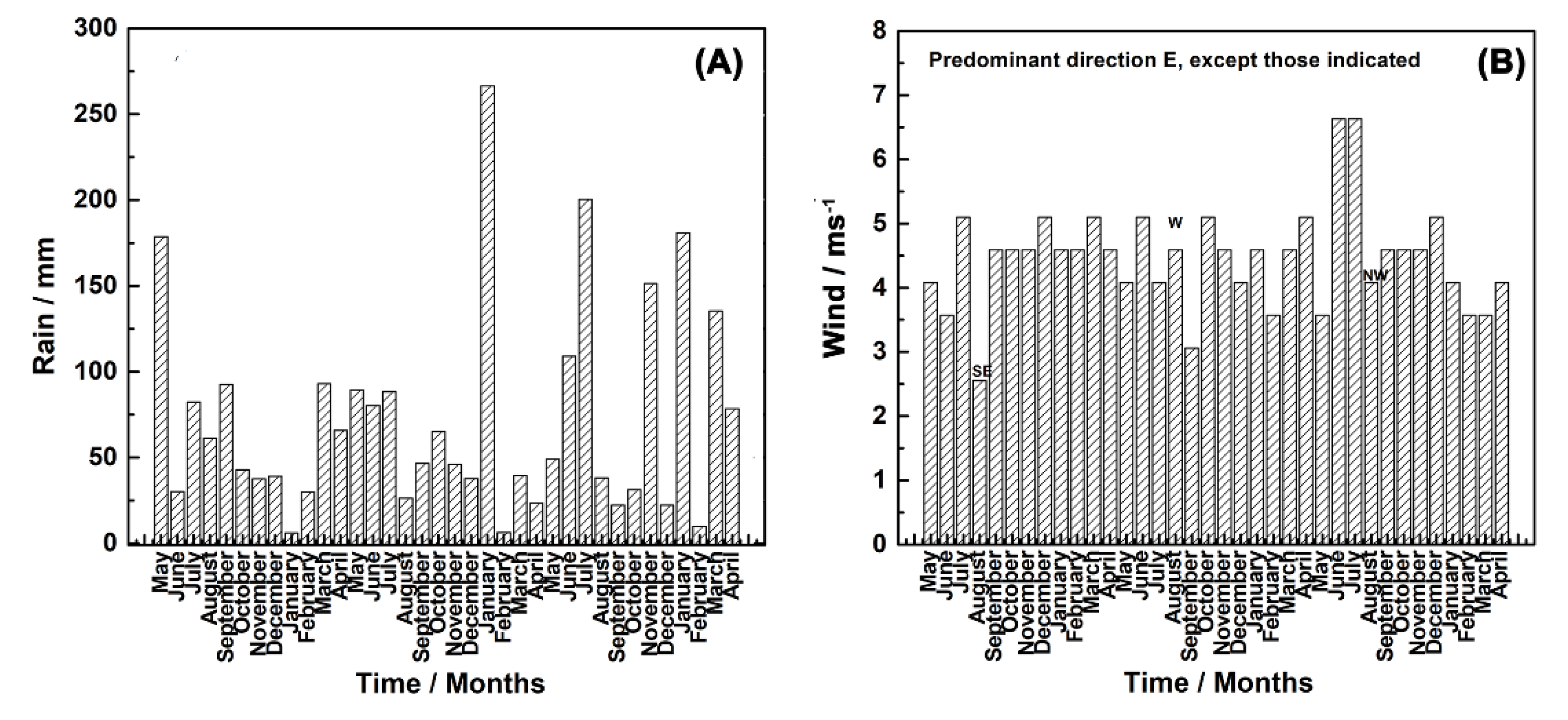
3.1. Characterization of the Test Atmosphere
3.2. Corrosion Rate and Characteristics of Corrosion Products
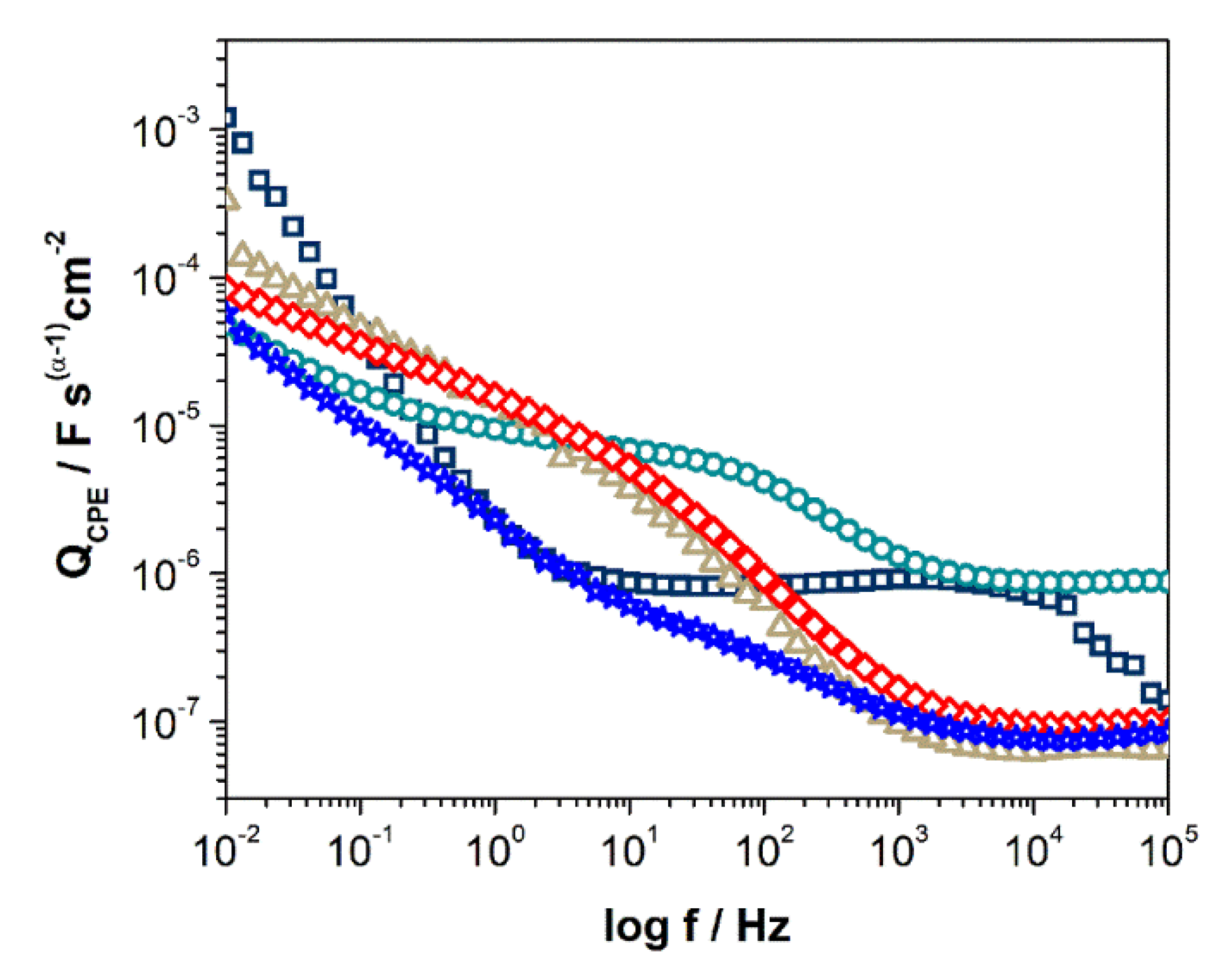
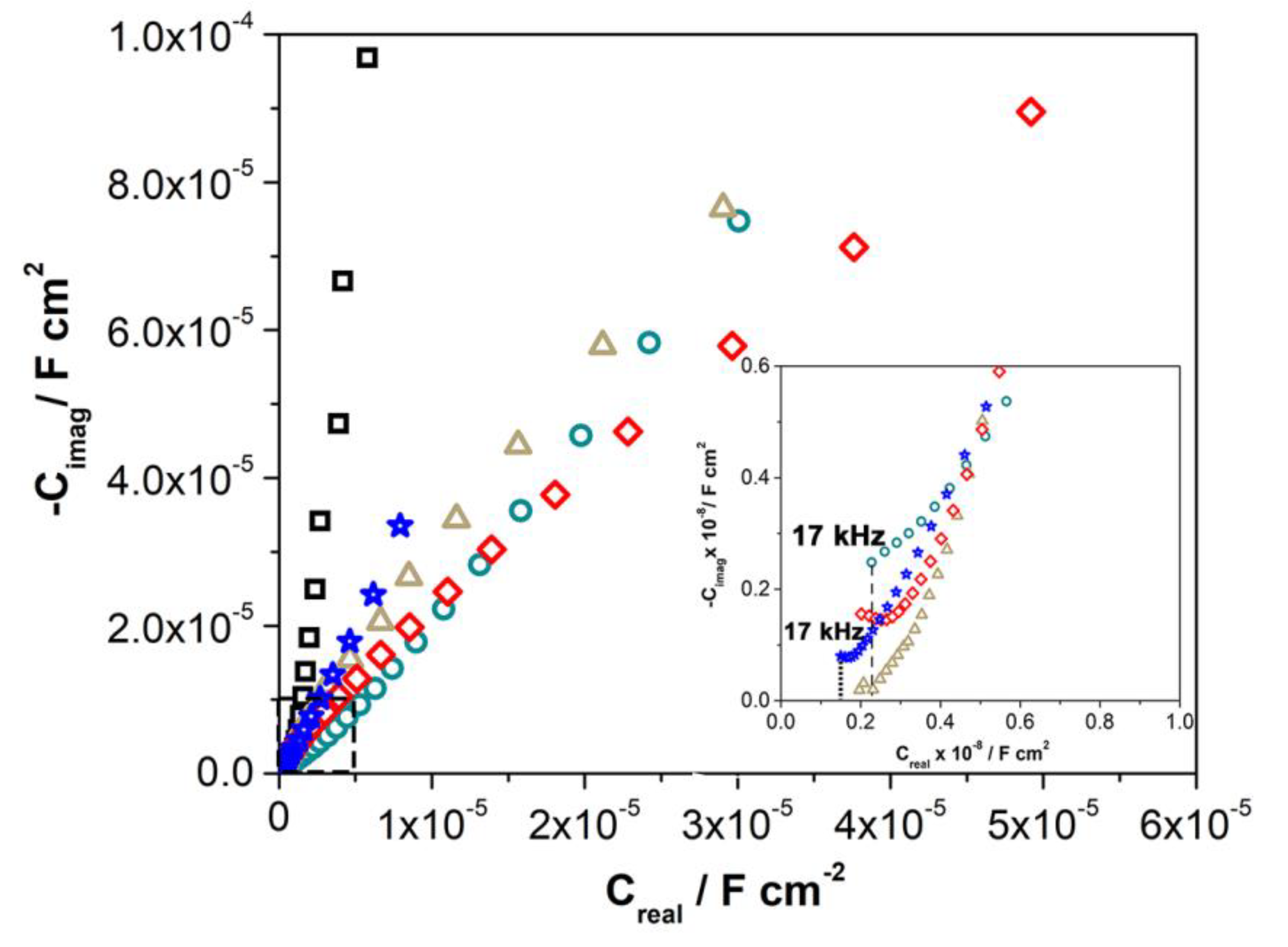
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morcillo, M.; Almeida, E.; Rosales, B.; Uruchurtu, J.; Marrocos, M. Corrosion y Proteccion de Metales En Las Atmosferas de Iberoamerica, Parte I; CYTED: Cambridge, UK, 1998; ISBN 8493044814. [Google Scholar]
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; de la Fuente, D. Atmospheric Corrosion of Weathering Steels. Overview for Engineers. Part I: Basic Concepts. Constr. Build. Mater. 2019, 213, 723–737. [Google Scholar] [CrossRef]
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; de la Fuente, D. Atmospheric Corrosion of Weathering Steels. Overview for Engineers. Part II: Testing, Inspection, Maintenance. Constr. Build. Mater. 2019, 222, 750–765. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; de la Fuente, D.; Almeida, E.; Joseph, G.; Rivero, S.; Rosales, B. Atmospheric Corrosion of Reference Metals in Antarctic Sites. Cold Reg. Sci. Technol. 2004, 40, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Morcillo, M.; Chico, B.; de la Fuente, D.; Simancas, J. Looking Back on Contributions in the Field of Atmospheric Corrosion Offered by the MICAT Ibero-American Testing Network. Int. J. Corros. 2012, 2012, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Morcillo, M.; Chico, B.; Díaz, I.; Cano, H.; de la Fuente, D. Atmospheric Corrosion Data of Weathering Steels. A Review. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.; Díaz, F.; Hernández-Borges, J.; González, S. Atmospheric Corrosion in Subtropical Areas: XRD and Electrochemical Study of Zinc Atmospheric Corrosion Products in the Province of Santa Cruz de Tenerife (Canary Islands, Spain). Corros. Sci. 2006, 48, 361–371. [Google Scholar] [CrossRef]
- Vidal, F.; Vicente, R.; Mendes Silva, J. Review of Environmental and Air Pollution Impacts on Built Heritage: 10 Questions on Corrosion and Soiling Effects for Urban Intervention. J. Cult. Herit. 2019, 37, 273–295. [Google Scholar] [CrossRef]
- de la Fuente, D.; Alcántara, J.; Chico, B.; Díaz, I.; Jiménez, J.A.; Morcillo, M. Characterisation of Rust Surfaces Formed on Mild Steel Exposed to Marine Atmospheres Using XRD and SEM/Micro-Raman Techniques. Corros. Sci. 2016, 110, 253–264. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Simancas, J.; Díaz, I.; de la Fuente, D.; Morcillo, M. An Attempt to Classify the Morphologies Presented by Different Rust Phases Formed during the Exposure of Carbon Steel to Marine Atmospheres. Mater. Charact. 2016, 118, 65–78. [Google Scholar] [CrossRef]
- Vera, R.; Rosales, B.M.M.; Tapia, C. Effect of the Exposure Angle in the Corrosion Rate of Plain Carbon Steel in a Marine Atmosphere. Corros. Sci. 2003, 45, 321–337. [Google Scholar] [CrossRef]
- Vera, R.; Rincón, O.T.; Ramirez, J.; Valverde, B.; Díaz-Gómez, A.; Sánchez-González, R. Effect of Height on the Corrosion of Steel and Galvanized Steel in Nontropical Coastal Marine Environments. Mater. Corros. 2020, 71, 1125–1137. [Google Scholar] [CrossRef]
- Vera, R.; Puentes, M.; Araya, R.; Rojas, P.; Carvajal, A. Mapa de Corrosión Atmosférica de Chile : Resultados Después de Un Año de Exposición. Revista de la Construcción 2012, 11, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Vera, R.; Delgado, D.; Araya, R.; Puentes, M.; Guerrero, I.; Rojas, P.; Cabrera, G.; Erazo, S.; Carvajal, A.M. Construcción de Mapas de Corrosión Atmosférica de Chile: Resultados Preliminares. Rev. Latinoam. Metal. y Mater. 2012, 32, 269–276. [Google Scholar]
- Carvajal, M.; Winckler, P.; Garreaud, R.; Igualt, F.; Contreras-López, M.; Averil, P.; Cisternas, M.; Gubler, A.; Breuer, W.A. Extreme Sea Levels at Rapa Nui (Easter Island) during Intense Atmospheric Rivers. Nat. Hazards 2021, 106, 1619–1637. [Google Scholar] [CrossRef]
- An, S.-I.; Park, H.-J.; Kim, S.-K.; Shin, J.; Yeh, S.-W.; Kug, J.-S. Intensity Changes of Indian Ocean Dipole Mode in a Carbon Dioxide Removal Scenario. npj Clim. Atmos. Sci. 2022, 5, 20. [Google Scholar] [CrossRef]
- Rull, V. Contributions of Paleoecology to Easter Island’s Prehistory: A Thorough Review. Quat. Sci. Rev. 2021, 252, 106751. [Google Scholar] [CrossRef]
- Castañeda, A.; Valdés, C.; Corvo, F. Atmospheric Corrosion Study in a Harbor Located in a Tropical Island. Mater. Corros. 2018, 69, 1462–1477. [Google Scholar] [CrossRef]
- Corvo, F.; Perez, T.; Dzib, L.R.; Martin, Y.; Castañeda, A.; Gonzalez, E.; Perez, J. Outdoor–Indoor Corrosion of Metals in Tropical Coastal Atmospheres. Corros. Sci. 2008, 50, 220–230. [Google Scholar] [CrossRef]
- de la Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-Term Atmospheric Corrosion of Mild Steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef] [Green Version]
- Santana, J.J.; Ramos, A.; Rodriguez-Gonzalez, A.; Vasconcelos, H.C.; Mena, V.; Fernández-Pérez, B.M.; Souto, R.M. Shortcomings of International Standard Iso 9223 for the Classification, Determination, and Estimation of Atmosphere Corrosivities in Subtropical Archipelagic Conditions—The Case of the Canary Islands (Spain). Metals 2019, 9, 1105. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.M.; Tsai, L.H.; La, H.H.; Wu, W.T.; Lin, M.D. A Study on the Metallic Corrosion Characteristics under Atmospheric Environment in Taiwan: Taking Taichung Harbor as an Example. Mater. Corros. 2018, 69, 251–265. [Google Scholar] [CrossRef]
- Vera, R.; Araya, R.; Bagnara, M.; Díaz-Gómez, A.; Ossandón, S. Atmospheric Corrosion of Copper Exposed to Different Environments in the Region of Valparaiso, Chile. Mater. Corros. 2017, 68, 316–328. [Google Scholar] [CrossRef]
- Lopesino, P.; Alcántara, J.; de la Fuente, D.; Chico, B.; Jiménez, J.; Morcillo, M. Corrosion of Copper in Unpolluted Chloride-Rich Atmospheres. Metals 2018, 8, 866. [Google Scholar] [CrossRef] [Green Version]
- Rosales, B.M.; Vera, R.M.; Hidalgo, J.P. Characterisation and Properties of Synthetic Patina on Copper Base Sculptural Alloys. Corros. Sci. 2010, 52, 3212–3224. [Google Scholar] [CrossRef]
- Franey, J.P.; Davis, M.E. Metallographic Studies of the Copper Patina Formed in the Atmosphere. Corros. Sci. 1987, 27, 659–668. [Google Scholar] [CrossRef]
- Chang, T.; Wallinder, I.O.; de la Fuente, D.; Chico, B.; Morcillo, M.; Welter, J.M.; Leygraf, C. Analysis of Historic Copper Patinas. Influence of Inclusions on Patina Uniformity. Materials 2017, 10, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Odnevall Wallinder, I.; Leygraf, C. Mechanistic Studies of Corrosion Product Flaking on Copper and Copper-Based Alloys in Marine Environments. Corros. Sci. 2014, 85, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Morcillo, M.; Chang, T.; Chico, B.; de la Fuente, D.; Odnevall Wallinder, I.; Jiménez, J.A.; Leygraf, C. Characterisation of a Centuries-Old Patinated Copper Roof Tile from Queen Anne’s Summer Palace in Prague. Mater. Charact. 2017, 133, 146–155. [Google Scholar] [CrossRef]
- Pan, C.; Lv, W.; Wang, Z.; Su, W.; Wang, C.; Liu, S. Atmospheric Corrosion of Copper Exposed in a Simulated Coastal-Industrial Atmosphere. J. Mater. Sci. Technol. 2017, 33, 587–595. [Google Scholar] [CrossRef]
- Mendoza, A.R.; Corvo, F.; Gómez, A.; Gómez, J. Influence of the Corrosion Products of Copper on Its Atmospheric Corrosion Kinetics in Tropical Climate. Corros. Sci. 2004, 46, 1189–1200. [Google Scholar] [CrossRef]
- ISO 9223:2012; Corrosion of Metals and Alloys. Corrosivity of Atmospheres. Classification, Determination and Estimation. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 9224:2012; Corrosion of Metals and Alloys-Corrosivity of Atmospheres-Guiding Values for the Corrosivity Categories. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 9225:2012; Corrosivity of Atmospheres-Measurement of Environmental Parameters Affecting Corrosivity of Atmospheres. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 9226:2012; Corrosion of Metals and Alloys-Corrosivity of Atmospheres-Determination of Corrosion Rate of Standard Specimens for the Evaluation of Corrosivity. International Organization for Standardization: Geneva, Switzerland, 2012.
- ASTM G1−03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM Special Technical Publication: West Conshohocken, PA, USA, 2017; pp. 505–510.
- ASTM G50-20; Standard Practice for Conducting Atmospheric Corrosion Tests on Metals. ASTM Special Technical Publication: West Conshohocken, PA, USA, 2020; Volume 1, pp. 1–6.
- Chilean Navy Hydrographic and Oceanografic Service. Available online: http://www.shoa.cl/php/index.php (accessed on 22 April 1999 and 15 May 2015).
- Schindelholz, E.; Kelly, R.G.; Cole, I.S.; Ganther, W.D.; Muster, T.H. Comparability and Accuracy of Time of Wetness Sensing Methods Relevant for Atmospheric Corrosion. Corros. Sci. 2013, 67, 233–241. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I.; Igonin, T.N.; Kovtanyuk, V.V.; Nikolaeva, L.A. Long-Term Forecast of Corrosion Mass Losses of Technically Important Metals in Various World Regions Using a Power Function. Corros. Sci. 2014, 88, 306–316. [Google Scholar] [CrossRef]
- Kreislova, K.; Geiplova, H. Prediction of the Long-Term Corrosion Rate of Copper Alloy Objects. Mater. Corros. 2016, 67, 152–159. [Google Scholar] [CrossRef]
- Panchenko, Y.M.; Marshakov, A.I. Long-Term Prediction of Metal Corrosion Losses in Atmosphere Using a Power-Linear Function. Corros. Sci. 2016, 109, 217–229. [Google Scholar] [CrossRef]
- Ma, X.Z.; Meng, L.D.; Cao, X.K.; Zhang, X.X.; Dong, Z.H. Investigation on the Initial Atmospheric Corrosion of Mild Steel in a Simulated Environment of Industrial Coastland by Thin Electrical Resistance and Electrochemical Sensors. Corros. Sci. 2022, 204, 110389. [Google Scholar] [CrossRef]
- Gießgen, T.; Mittelbach, A.; Höche, D.; Zheludkevich, M.; Kainer, K.U. Enhanced Predictive Corrosion Modeling with Implicit Corrosion Products. Mater. Corros. 2019, 70, 2247–2255. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Zhao, H.; Pan, C.; Wang, Z. Corrosion Behavior of Copper in Extremely Harsh Marine Atmosphere in Nansha Islands, China. Trans. Nonferrous Met. Soc. China 2021, 31, 703–714. [Google Scholar] [CrossRef]
- Liao, X.; Cao, F.; Zheng, L.; Liu, W.; Chen, A.; Zhang, J.; Cao, C. Corrosion Behaviour of Copper under Chloride-Containing Thin Electrolyte Layer. Corros. Sci. 2011, 53, 3289–3298. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Feng, C.; Ding, Z.; Li, D.; Zhou, X.; Wu, T. Atmospheric Corrosion of Tin Coatings on H62 Brass and T2 Copper in an Urban Environment. Eng. Fail. Anal. 2022, 141, 106735. [Google Scholar] [CrossRef]
- Becker, J.; Pellé, J.; Rioual, S.; Lescop, B.; Le Bozec, N.; Thierry, D. Atmospheric Corrosion of Silver, Copper and Nickel Exposed to Hydrogen Sulphide: A Multi-Analytical Investigation Approach. SSRN Electron. J. 2022, 209, 110726. [Google Scholar] [CrossRef]
- Sun, B.; Ye, T.; Feng, Q.; Yao, J.; Wei, M. Accelerated Degradation Test and Predictive Failure Analysis of B10 Copper-Nickel Alloy under Marine Environmental Conditions. Materials 2015, 8, 6029–6042. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.; Sancy, M.; Guerra, C.; Flores, M.; Molina, P.; Muñoz, H.; Bruna, T.; Arcos, C.; Urzúa, M.; Encinas, M.V.; et al. Comparison of the Protective Efficiency of Polymethacrylates with Different Side Chain Length for AA2024 Alloy. J. Mater. Res. Technol. 2021, 15, 7125–7135. [Google Scholar] [CrossRef]
- Tribollet, B.; Vivier, V.; Orazem, M.E. EIS Technique in Passivity Studies: Determination of the Dielectric Properties of Passive Films; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128098943. [Google Scholar]
- Amand, S.; Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Constant-Phase-Element Behavior Caused by Inhomogeneous Water Uptake in Anti-Corrosion Coatings. Electrochim. Acta 2013, 87, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.S.; Causse, N.; Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Determination of Water Uptake in Organic Coatings Deposited on 2024 Aluminium Alloy: Comparison between Impedance Measurements and Gravimetry. Prog. Org. Coat. 2017, 112, 93–100. [Google Scholar] [CrossRef]



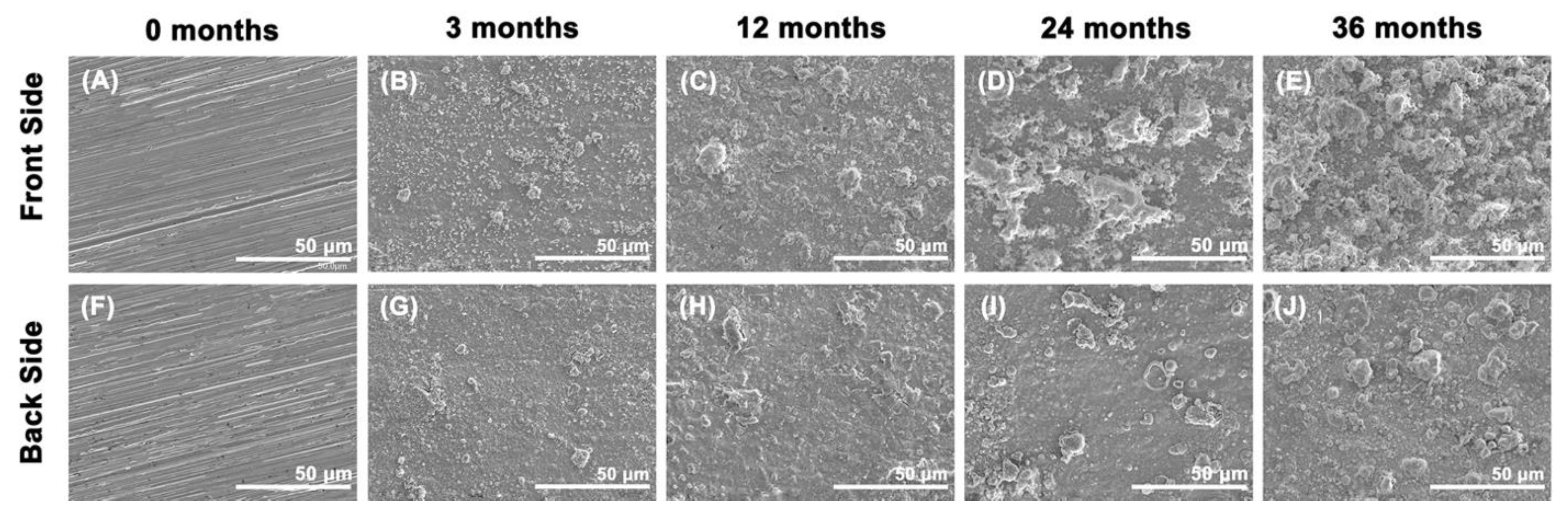
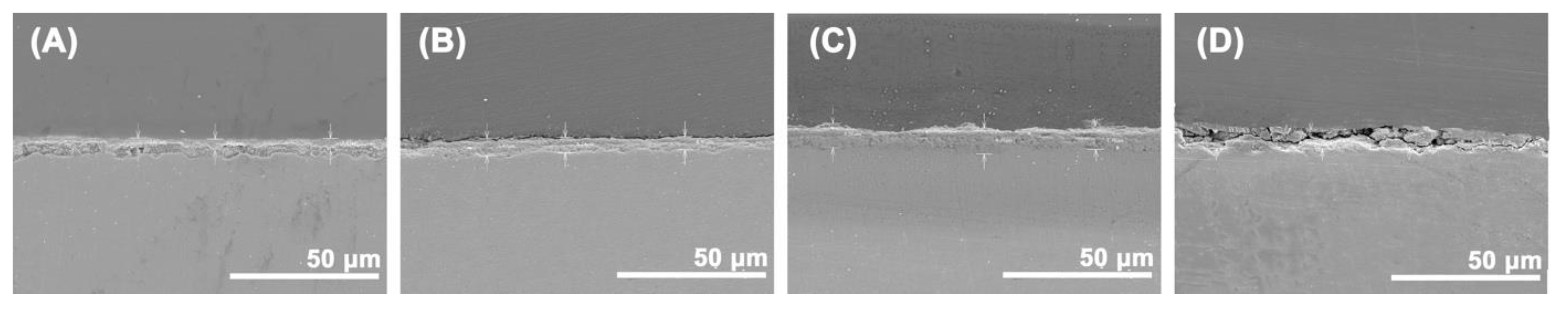
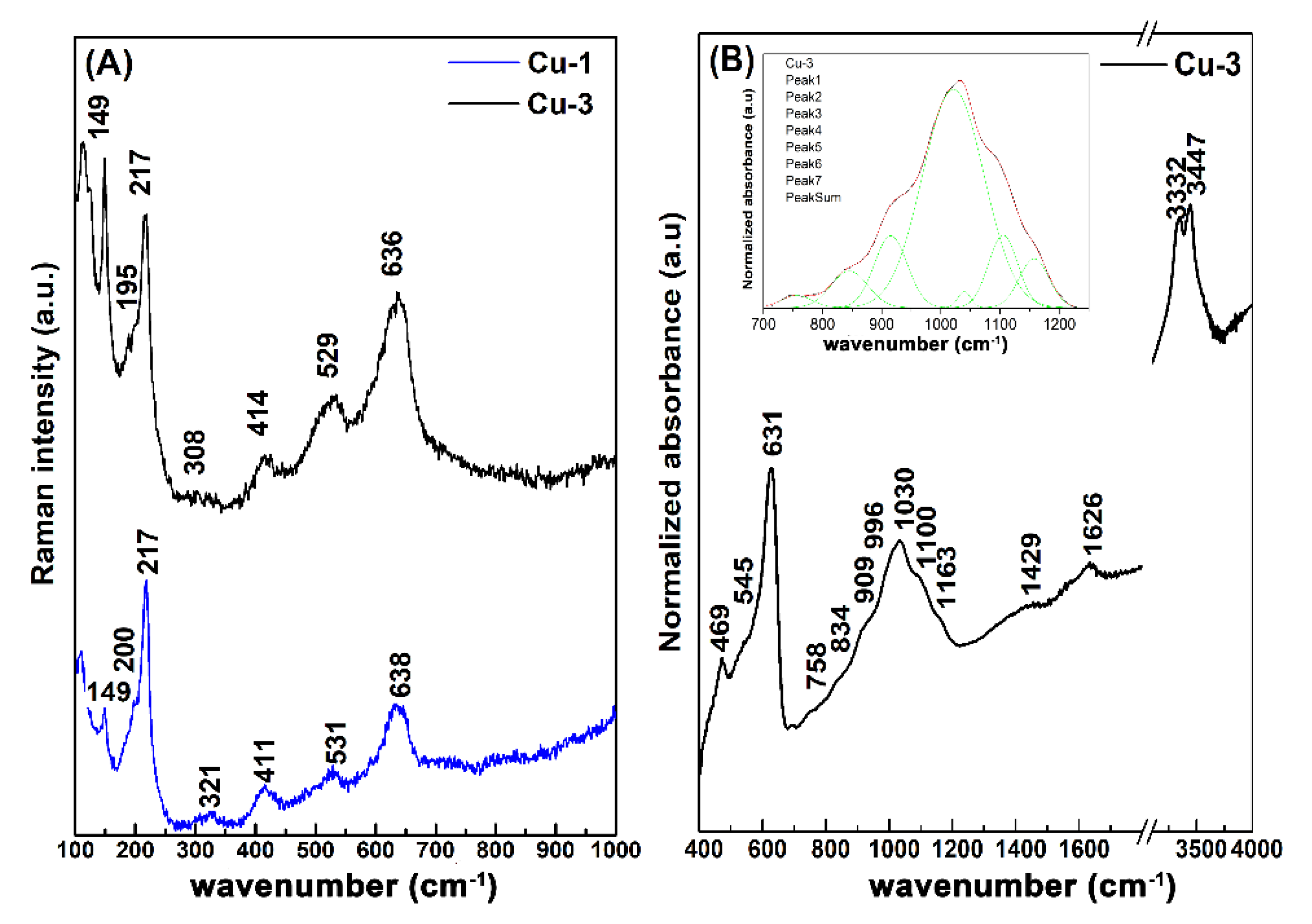

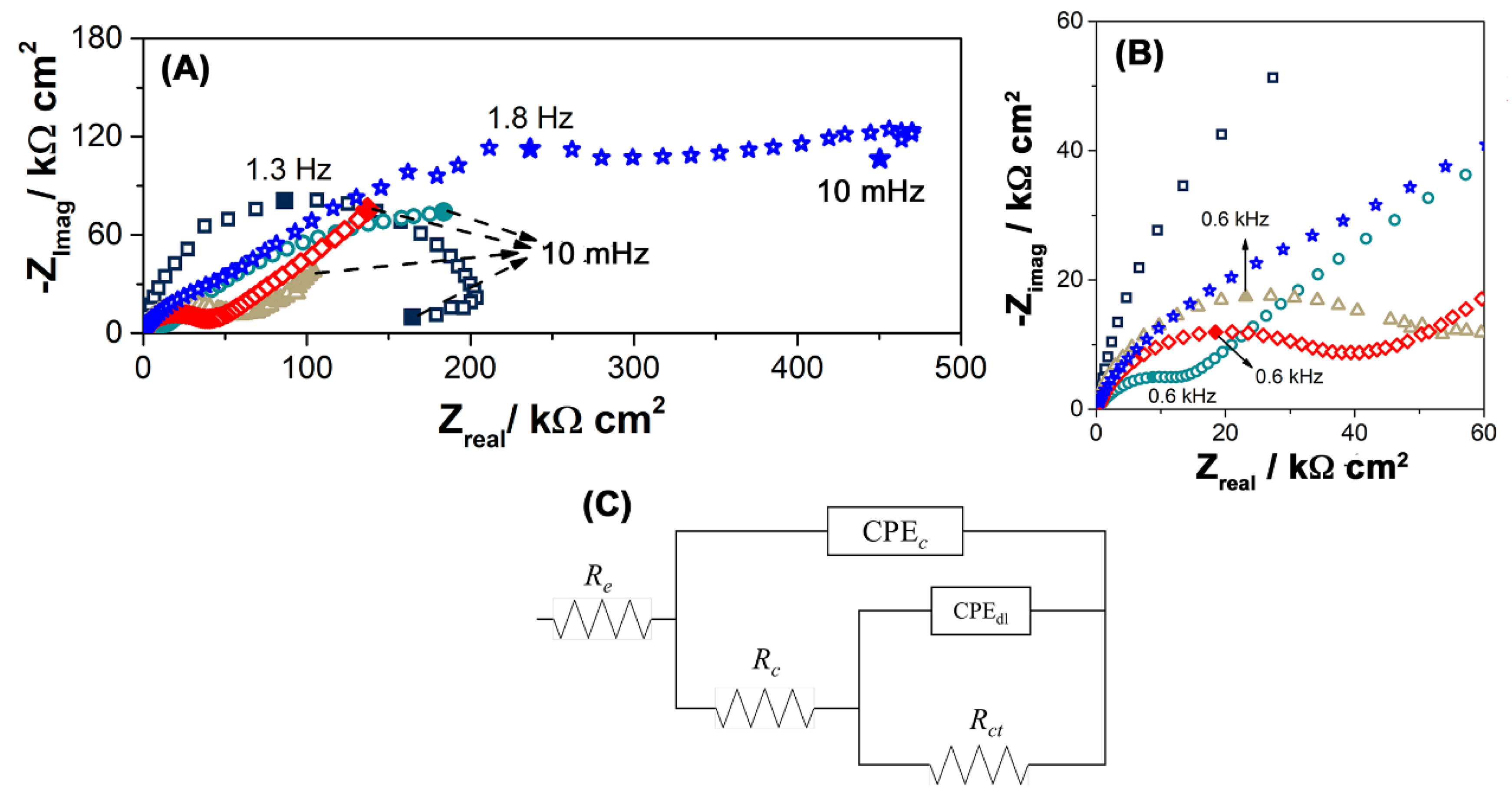

| Exposition (Year) | TOW (τ) (hour year−1) | Chloride (S) (mg m−2 d−1) | Sulfur Dioxide (P) (mg m−2 d−1) | Corrosiveness Category |
|---|---|---|---|---|
| 1st | 2616 | 53.04 | 1.89 | τ4S1P0/C3 |
| 2nd | 3936 | 51.13 | 5.41 | τ4S1P1/C3 |
| 3rd | 3480 | 48.37 | 3.30 | τ4S1P0/C3 |
| Atom % | 3 Months | 12 Months | 24 Months | 36 Months | ||||
|---|---|---|---|---|---|---|---|---|
| Front Side | Back Side | Front Side | Back Side | Front Side | Back Side | Front Side | Back Side | |
| Copper | 69.65 | 64.74 | 47.11 | 56.23 | 34.88 | 41.41 | 27.09 | 43.38 |
| Oxygen | 32.81 | 29.01 | 47.36 | 38.61 | 54.56 | 46.76 | 62.84 | 50.53 |
| Chlorine | 1.34 | 2.45 | 5.53 | 5.16 | 10.07 | 8.08 | 10.56 | 9.86 |
| t (months) | Re (Ωcm2) | −αHF | ||
|---|---|---|---|---|
| 0 | 338 | 0.89 | 89.1 | 12.1 |
| 3 | 188 | 0.55 | 87.1 | 1.35 |
| 12 | 245 | 0.73 | 6.2 | 2.16 |
| 24 | 336 | 0.71 | 9.6 | 1.38 |
| 36 | 244 | 0.69 | 7.4 | 1.17 |
| Exposure Condition, Months | 3 | 12 | 24 | 36 |
|---|---|---|---|---|
| Thickness (µm) by GD-OES | 1.6 | --- | --- | 6.3 |
| Thickness (µm) by SEM | 3.5 | 5.2 | 8.3 | 9.1 |
| Thickness (µm), Graphically | 4.9 | 3.1 | 4.9 | 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera, R.; Valverde, B.; Olave, E.; Díaz-Gómez, A.; Sánchez-González, R.; Muñoz, L.; Martínez, C.; Rojas, P. Corrosion Behavior of Copper Exposed in Marine Tropical Atmosphere in Rapa Nui (Easter Island) Chile 20 Years after MICAT. Metals 2022, 12, 2082. https://doi.org/10.3390/met12122082
Vera R, Valverde B, Olave E, Díaz-Gómez A, Sánchez-González R, Muñoz L, Martínez C, Rojas P. Corrosion Behavior of Copper Exposed in Marine Tropical Atmosphere in Rapa Nui (Easter Island) Chile 20 Years after MICAT. Metals. 2022; 12(12):2082. https://doi.org/10.3390/met12122082
Chicago/Turabian StyleVera, Rosa, Bárbara Valverde, Elizabeth Olave, Andrés Díaz-Gómez, Rodrigo Sánchez-González, Lisa Muñoz, Carola Martínez, and Paula Rojas. 2022. "Corrosion Behavior of Copper Exposed in Marine Tropical Atmosphere in Rapa Nui (Easter Island) Chile 20 Years after MICAT" Metals 12, no. 12: 2082. https://doi.org/10.3390/met12122082
APA StyleVera, R., Valverde, B., Olave, E., Díaz-Gómez, A., Sánchez-González, R., Muñoz, L., Martínez, C., & Rojas, P. (2022). Corrosion Behavior of Copper Exposed in Marine Tropical Atmosphere in Rapa Nui (Easter Island) Chile 20 Years after MICAT. Metals, 12(12), 2082. https://doi.org/10.3390/met12122082






