Control of Bismuth and Manganese Sulfide Inclusions in Free-Cutting Steels of Different Classes
Abstract
1. Introduction
2. Materials and Methods
- With different content of bismuth (lines 1 and 2);
- With bismuth and high sulfur content (line 3).
- With bismuth (line 4).
3. Results and Discussion
3.1. Description of the Model for the Formation of Bismuth Particles and Manganese Sulfides
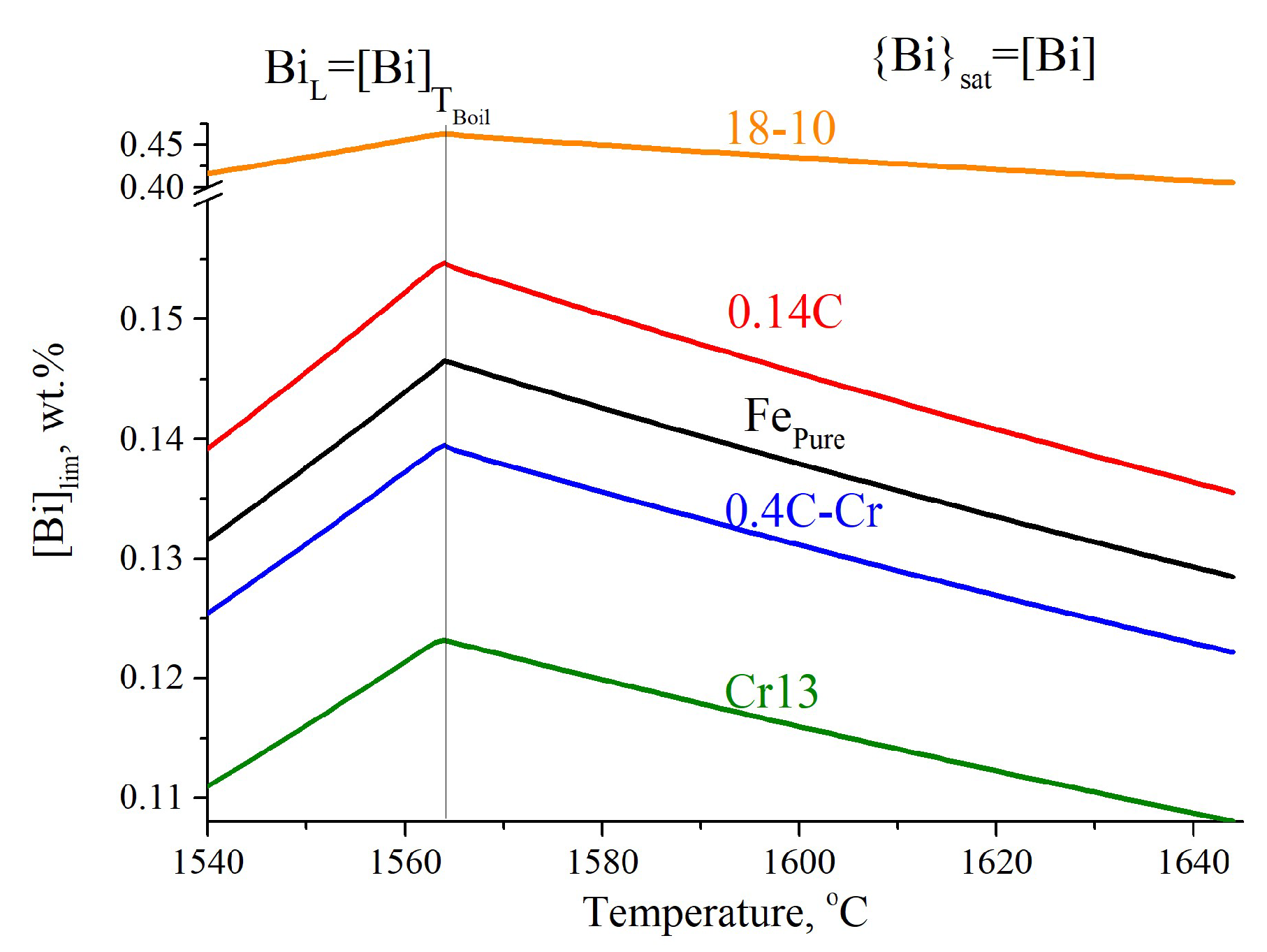
3.1.1. Thermodynamics of Formation of Bismuth Particles
3.1.2. Thermodynamics of the Formation of Manganese Sulfide
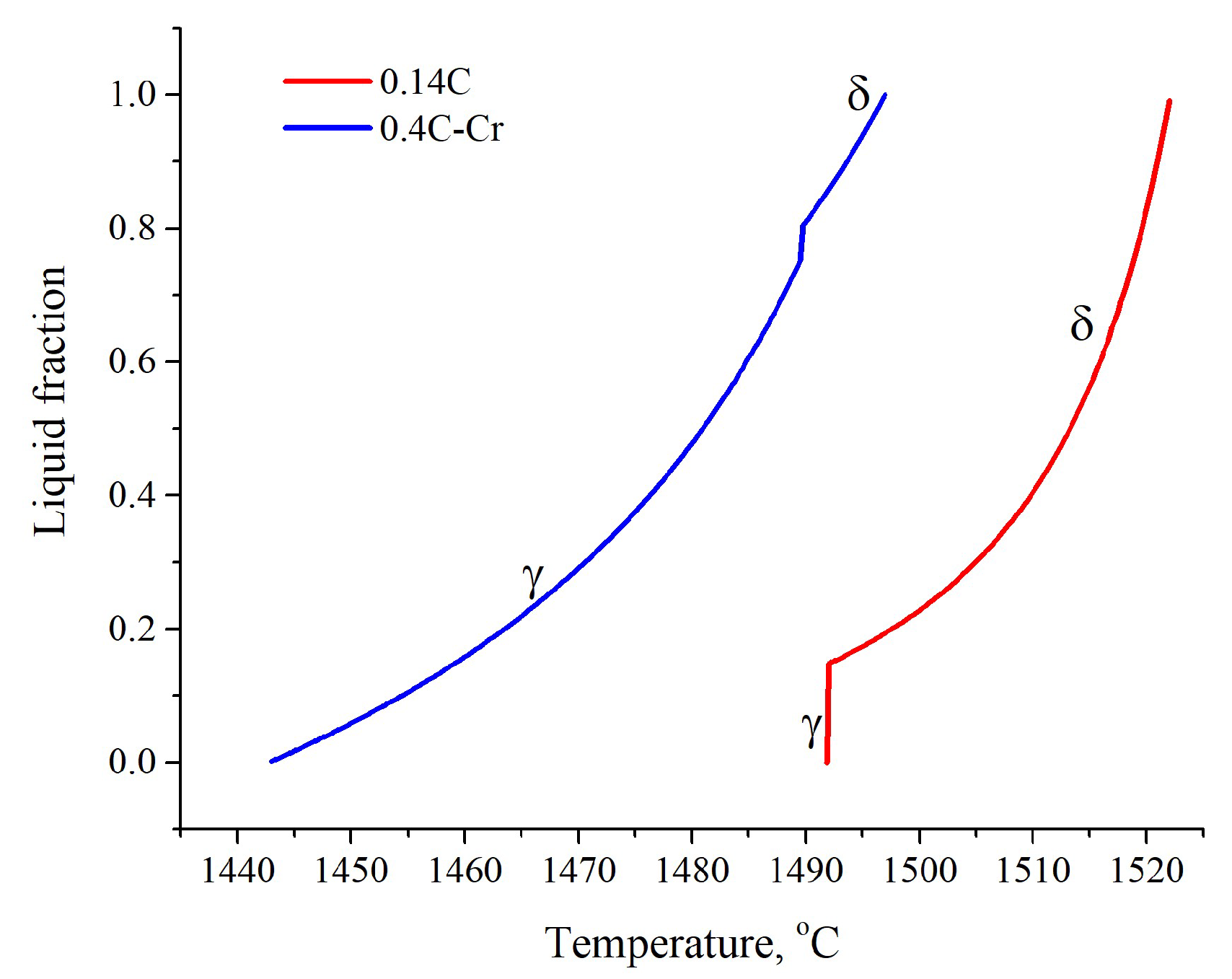
3.1.3. Calculation of Segregation during Solidification
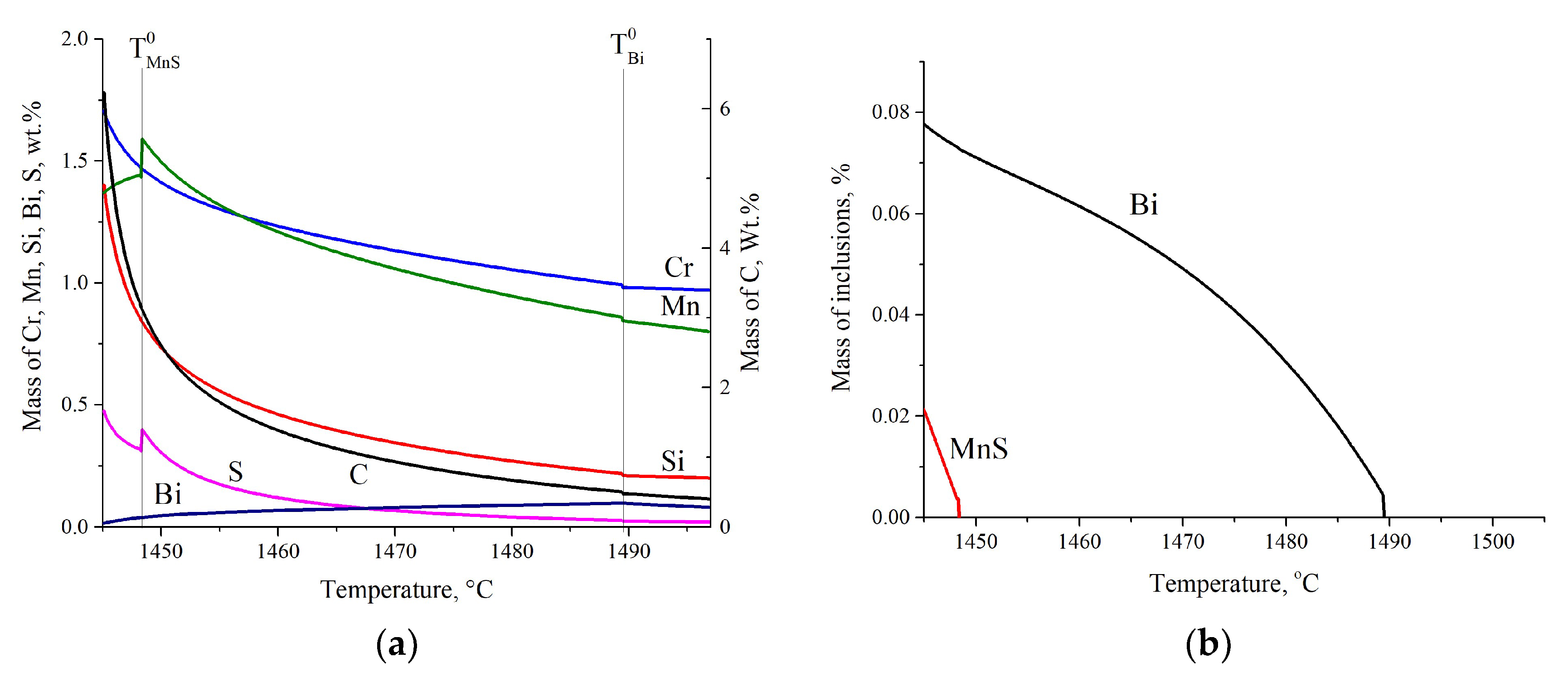
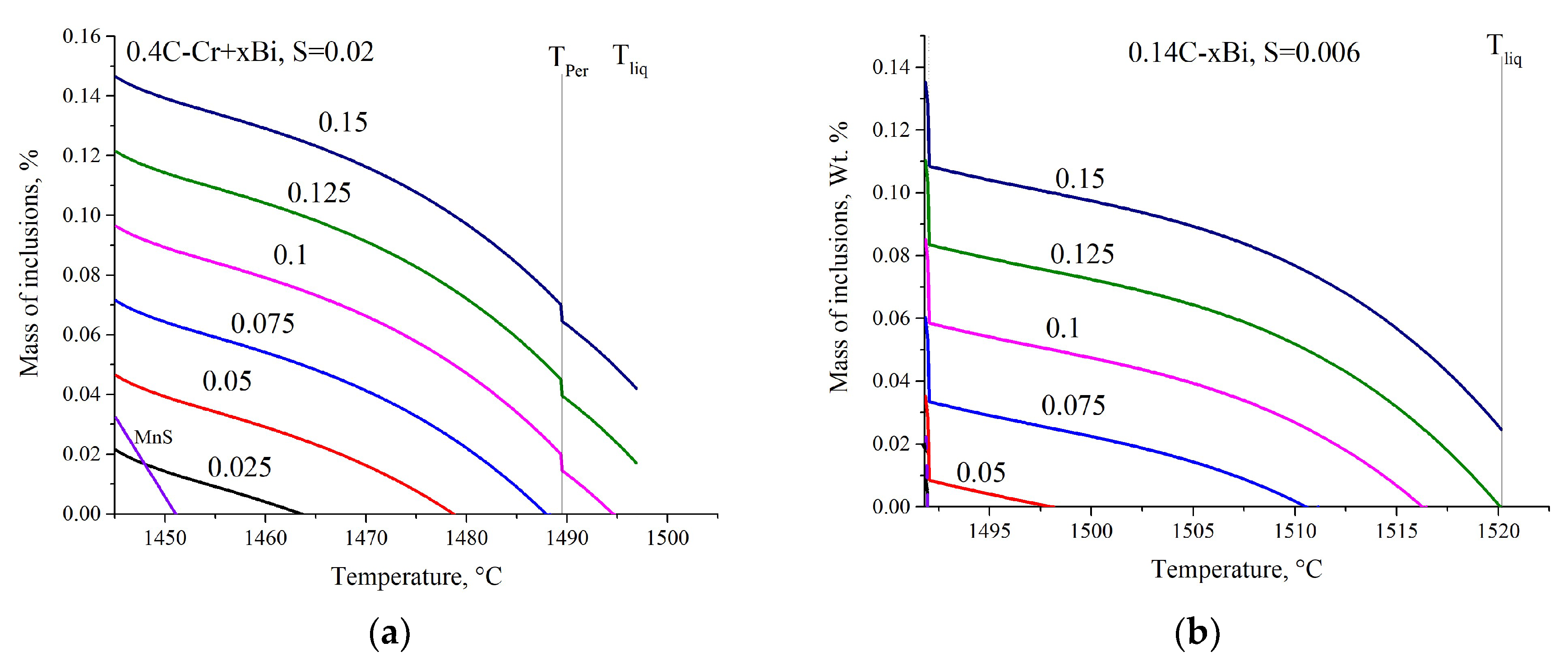
3.1.4. Modeling Results
3.2. Investigation of Laboratory Ingots
4. Conclusions
- An analysis of the known thermodynamic data on the solubility of bismuth and on the influence of other elements included in steels with improved machinability was carried out. Based on this analysis, mutually consistent thermodynamic data were obtained, making it possible to predict the solubility of bismuth not only in pure iron, but also in multicomponent steel. In addition, the partition coefficient of bismuth during solidification was estimated.
- These data were used to create a thermodynamic model for the formation of bismuth into an independent phase during solidification in the Ohnaka model for steel components and in the Sheil equation for bismuth. The model also takes into account the formation of manganese sulfide.
- The series of calculations carried out shows how the solubility of bismuth and the amount of bismuth formed as an independent phase change depending on the composition of the steel, on the amount of alloying elements, and on the amount of added bismuth and sulfur.
- An experiment was carried out to obtain steels with bismuth and sulfur, and a comparison of the results of this experiment with the forecast of the developed model was performed.
- Modeling adequately describes the behavior of bismuth during solidification and makes it possible to predict its amount. However, for the upper part of the ingot, where the formation of macrosegregation occurs, the model is not suitable, but the experimental data obtained make it possible to predict the appearance of large bismuth inclusions in industrial continuously cast billets.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grigoriev, S.N.; Bobrovskij, N.M.; Melnikov, P.A.; Bobrovskij, I.N.; Levitskih, O.O. Ecological and Toxicological Characteristics of Metalworking Fluids Used in Finishing Processing in Russian Federation. IOP Conf. Ser. Earth Environ. Sci. 2017, 66, 012012. [Google Scholar] [CrossRef]
- Luiz, N.E.; Machado, A.R. Development trends and review of free-machining steels. J. Eng. Manuf. 2008, 222, 347–360. [Google Scholar] [CrossRef]
- Xie, J.; Fan, T.; Zeng, Z.; Sun, H.; Fu, J. Bi-Sulfide Existence in 0Cr18Ni9 Steel: Correlation with Machinability and Mechanical Properties. J. Mater. Res. Technol. 2020, 9, 9142–9152. [Google Scholar] [CrossRef]
- Hashimura, M.; Mizuno, A.; Miyanishi, K. Development of Low-Carbon Lead-Free Free-Cutting Steel Friendly to Environment. Nippon. Steel Tech. Rep. 2007, 96, 45–49. [Google Scholar]
- Shen, P.; Yang, Q.; Zhang, D.; Yang, S.; Fu, J. The Effect of Tellurium on the Formation of MnTe-MnS Composite Inclusions in Non-Quenched and Tempered Steel. Metals 2018, 8, 639. [Google Scholar] [CrossRef]
- Wang, F.; Guo, H.; Liu, W.; Yang, S.; Zhang, S.; Li, J. Control of MnS Inclusions in High- and Low-Sulfur Steel by Tellurium Treatment. Materials 2019, 12, 1034. [Google Scholar] [CrossRef]
- Zivkovic, D.; Štrbac, N.; Ekinovic, S.; Begović, E. Lead-free free-cutting steels as modern environmentally friendly materials. Ecologica 2011, 18, 451–456. [Google Scholar]
- POSCO Succeeds in Mass-Producing Clean Steel. Available online: https://www.azovpromstal.com/news/one/id/8299 (accessed on 25 August 2022).
- You, D.; Michelic, S.; Wieser, G.; Bernhard, C. Modeling of Manganese Sulfide Formation during the Solidification of Steel. J. Mater. Sci. 2017, 52, 1797–1812. [Google Scholar] [CrossRef]
- Kontanistov, M.E.; Terletskii, S.V.; Shatilo, V.A. The influence of the chemical composition of steel, the state of melts equipment and casting process parameters on the occurrence of surface and internal defects of continuous casting billets. Litye I Met. Foundry Prod. Metall. 2013, 1, 28–31. [Google Scholar]
- Maciejewski, J. The Effects of Sulfide Inclusions on Mechanical Properties and Failures of Steel Components. J. Fail. Anal. Preven 2015, 15, 169–178. [Google Scholar] [CrossRef]
- Biswas, D.K.; Venkatraman, M.; Narendranath, C.S.; Chatterjee, U.K. Influence of Sulfide Inclusion on Ductility and Fracture Behavior of Resulfurized HY-80 Steel. Metall. Mater. Trans. A 1992, 23, 1479–1492. [Google Scholar] [CrossRef]
- DeArdo, A.J.; Hamburg, E.G. Influence of elongated inclusions on the mechanical properties of high strength steel plate, in Sulfide Inclusions in Steel. In Proceedings of an International Symposium; American Society for Metals: Novelty, OH, USA, 1975. [Google Scholar]
- Bellot, J.; Gantois, M. The influence of ‘sulfide-type’ inclusions on the mechanical properties of construction steels. Trans. Iron Steel Inst. 1978, 18, 546–553. [Google Scholar] [CrossRef]
- Spitzig, W.A. Effect of Sulfides and Sulfide Morphology on Anisotropy of Tensile Ductility and Toughness of Hot-Rolled C-Mn Steels. Metall. Mater. Trans. A 1983, 14, 471–484. [Google Scholar] [CrossRef]
- Xiao, G.; Dong, H.; Wang, M.; Hui, W. Effect of Sulfur Content and Sulfide Shape on Fracture Ductility in Case Hardening Steel. J. Iron Steel Res. 2011, 18, 58–64. [Google Scholar] [CrossRef]
- For instance, “Guideline for Honda Green Purchase”. Purchase Center, Honda Motor Co., Ltd.: Tokyo, Japan, 2001.
- Tellurium-Futures Contract-Prices|2017-2022 Data|2023-2024 Forecast. Available online: https://ru.tradingeconomics.com/commodity/tellurium (accessed on 25 August 2022).
- Wang, Z.; Shao, Z.; Li, Z.; Zhang, H.; Wang, J.; Zhang, H.; Yuan, J. Making of Free Cutting Austenitic Stainless Steels with Additions of Sulfur. Rare Earth Bismuth 2015. [Google Scholar] [CrossRef]
- Ryabov, A.V.; Povolotsky, D.Y.; Ryabov, V.V. Bismuth assimilation during alloying of machine steel in the process of siphon casting. Izv. Chelyabinskogo Nauchnogo Tsentra News Chelyabinsk Sci. Cent. 2001, 1, 81–90. [Google Scholar]
- Ryabov, A.V.; Chumanov, I.V. Possibility of Making New Easy-to-Cut Corrosion-Resistant Steel. Russ. Metall. 2012, 12, 1065–1067. [Google Scholar] [CrossRef]
- Ryabov, A.V. Solubility of fusible, oxidizing and volatile elements in iron-based alloys. Vestnik Yuzhno-Uralskogo gosudarstvennogo universiteta Seriya. Metallurgiya 2014, 14, 3. [Google Scholar]
- Liu, H.; Chen, W.; Li, W.; Yu, Y. Solubility of Bismuth in Liquid Bi-S Based Free Cutting Steel. High Temp. Mater. Process. 2014, 33, 187–191. [Google Scholar] [CrossRef]
- Kazakov, A.; Kiselev, D. Industrial application of Thixomet image analyzer for quantitative description of steel and alloy’s microstructure. Metallogr. Microstruct. Anal. 2016, 5, 294–301. [Google Scholar] [CrossRef]
- Grigoryan, V.A. Physical and Chemical Calculations of Electric Steelmaking Processes; Textbook for Universities; Metallurgiya (Metallurgy): Barnaul, Russia, 1989; p. 288. [Google Scholar]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification; Trans Tech Publications: Wollerau, Switzerland, 1984; ISBN 0-87849-523-3. [Google Scholar]
- Young-Mok, W.; Brian, G.T. Simple Model of Microsegregation during Solidification of Steels. Metall. Mater. Trans. 2001, 32, 1755. [Google Scholar]
- Zhitenev, A.; Salynova, M.; Shamshurin, A.; Ryaboshuk, S.; Kolnyshenko, V. Database Clustering after Automatic Feature Analysis of Nonmetallic Inclusions in Steel. Metals 2021, 11, 1650. [Google Scholar] [CrossRef]
- Ryabov, A.V. Solubility of bismuth and lead in liquid and solid iron. Vestnik YuUrGU. Seriya Metall. 2013, 13, 2. [Google Scholar]
- Liu, H.; Hu, D.; Fu, J. Analysis of MnS Inclusions Formation in Resulphurised Steel via Modeling and Experiments. Materials 2019, 12, 2028. [Google Scholar] [CrossRef]
- Shu, Q.; Visuri, V.-V.; Alatarvas, T.; Fabritius, T. Model for Inclusion Precipitation Kinetics During Solidification of Steel Applications in MnS and TiN Inclusions. Metall. Mater. Trans. B 2020, 51, 2905–2916. [Google Scholar] [CrossRef]
- Shmrga, L. Solidification and crystallization of steel ingots; Metallurgiya (Metallurgy): Barnaul, Russia, 1985. [Google Scholar]
- FactSage.Com. Available online: https://www.factsage.com/ (accessed on 25 August 2022).
- Kazakov, A.A.; Zhitenev, A.I.; Kovalev, P.V. Distribution Pattern of Nonmetallic Inclusions on a Cross Section of Continuous-Cast Steel Billets for Rails. Microsc. Microanal. 2015, 21 (Suppl. 3), 1751–1752. [Google Scholar] [CrossRef]
- Golod, V.M.; Emelyanov, K.I. System analysis of morphological evolution of dendritic structure of steel. Chernyye Met. 2014, 4, 988. [Google Scholar]
- Golod, V.M. Evolution of filtration permeability of dendrite structure in the conditions of capillary-diffusion coalescency of the secondary side branches. Eur. Phys. J. Spec. Top. 2020, 229, 225–237. [Google Scholar] [CrossRef]
- Luo, S.; Wang, B.; Wang, Z.; Jiang, D.; Wang, W.; Zhu, M. Morphology of Solidification Structure and MnS Inclusion in High Carbon Steel Continuously Cast Bloom. ISIJ Int. 2017, 57, 2000–2009. [Google Scholar] [CrossRef]
- You, D.; Michelic, S.K.; Bernhard, C.; Loder, D.; Wieser, G. Modeling of Inclusion Formation during the Solidification of Steel. ISIJ Int. 2016, 56, 1770–1778. [Google Scholar] [CrossRef]
- Gu, J.P.; Beckermann, C. Simulation of Convection and Macrosegregation in a Large Steel Ingot. Metall. Mater. Trans. Vol. 1999, 30, 1357. [Google Scholar] [CrossRef]
- Kazakov, A.A.; Zhitenev, A.I.; Salynova, M.A.; Kolpishon, E.Y. Nonmetallic inclusions quantitative assessment for forgings made from super large steel ingot. Chernye Met. 2018, 12, 50–56. [Google Scholar]
- Zhang, C.; Loucif, A.; Jahazi, M.; Morin, J.-B. FE Modelling and Prediction of Macrosegregation Patterns in Large Size Steel Ingots: Influence of Filling Rate. Metals 2022, 12, 29. [Google Scholar] [CrossRef]

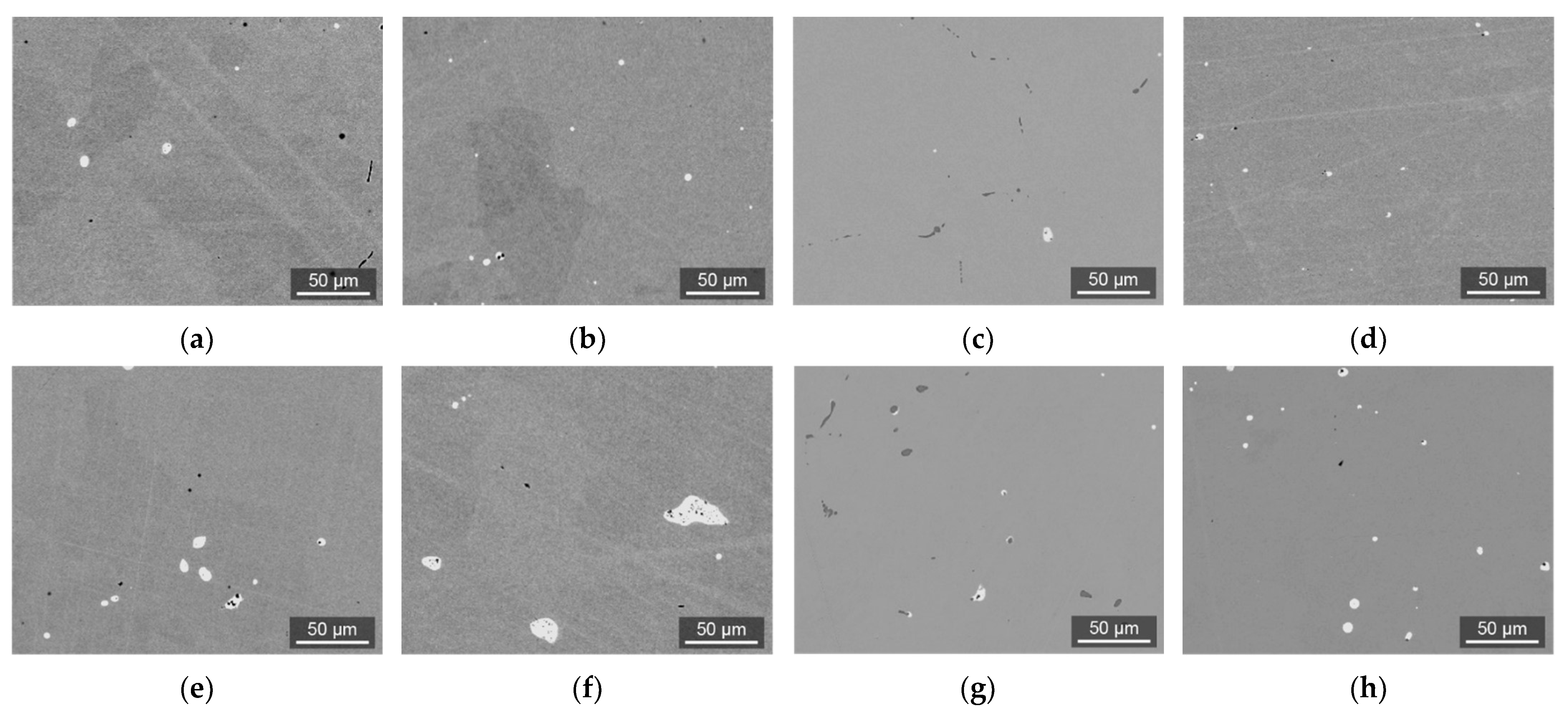
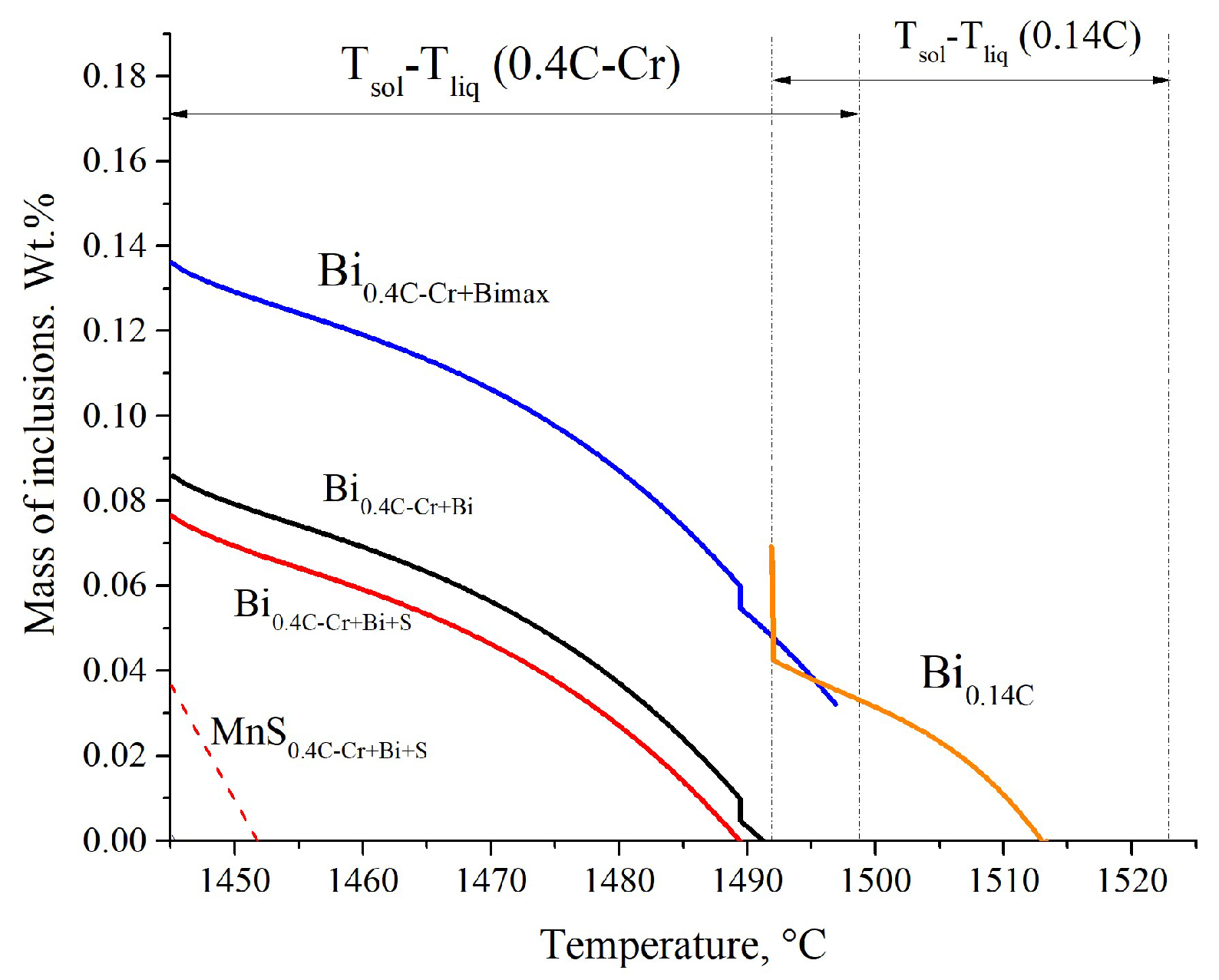
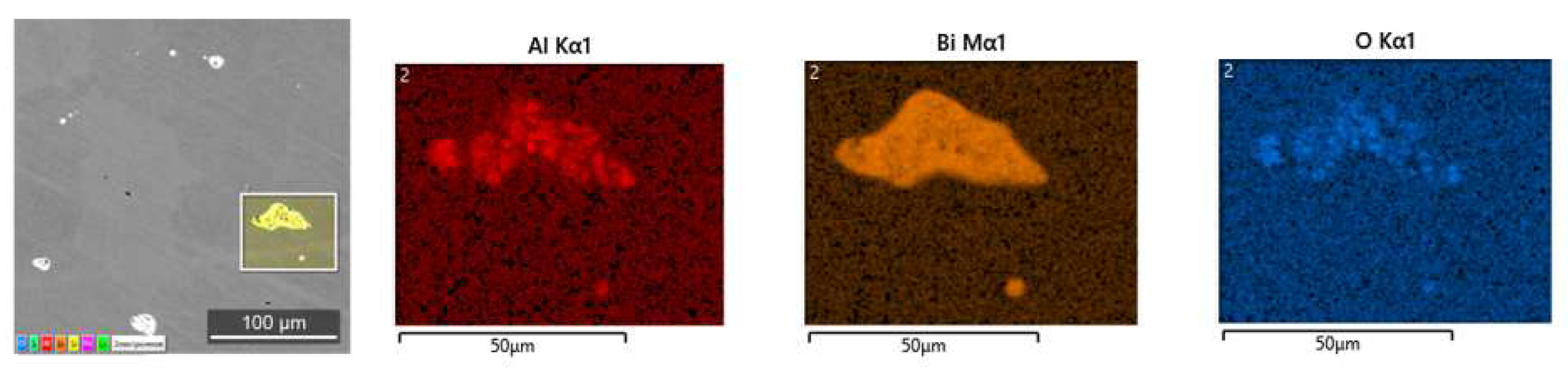
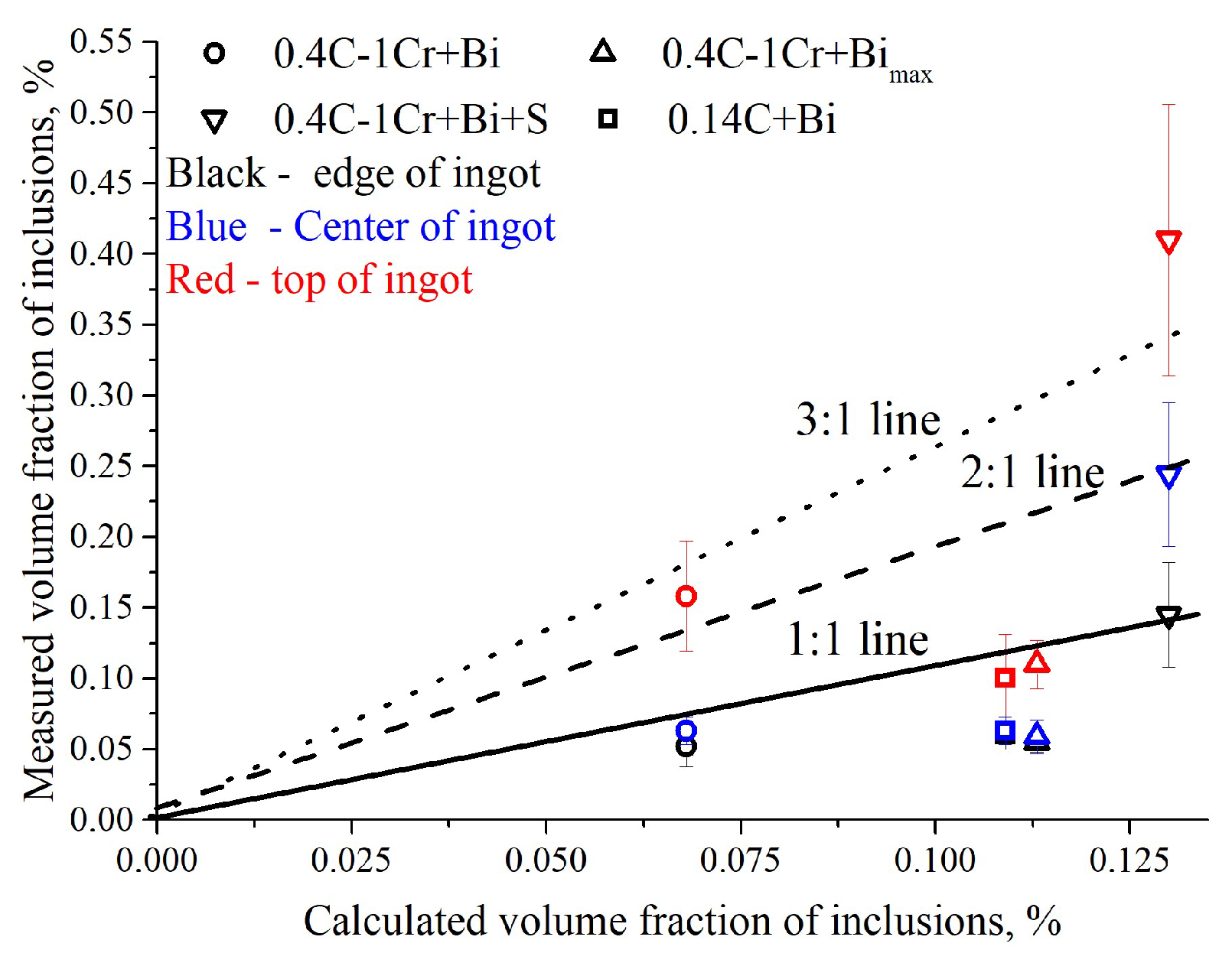
| Steel | Grade | Element, wt.% | ||||||
|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | Cr | Al | S | Bi | ||
| 0.4C-Cr + Bi | 37Cr4 (EN 10083) | 0.36 | 0.19 | 0.71 | 0.94 | 0.06 | 0.0048 | 0.09 |
| 0.4C-Cr + Bimax | 0.36 | 0.27 | 0.74 | 0.93 | 0.037 | 0.0055 | 0.14 | |
| 0.4C-Cr + Bi + S | 0.40 | 0.25 | 0.78 | 0.97 | 0.05 | 0.022 | 0.08 | |
| 0.14C + Bi | 9SMn28 (EN10277-3) | 0.13 | 0.07 | 0.78 | 0.1 | 0.03 | 0.0058 | 0.08 |
| Heading | e(i, j) | Element | |||||
|---|---|---|---|---|---|---|---|
| C | Si | Mn | Cr | Ni | S | ||
| 1 | Bi | 0.0884 1 | 0.1148 2 | −0.0512 2 | 0.0011 2 | −0.052 2 | - |
| 2 | S | 0.11 3 | 0.063 3 | −0.026 3 | −0.011 3 | 0 3 | −0.028 3 |
| 3 | Mn | −0.07 3 | 0 3 | 0 3 | - | - | −0.048 3 |
| 1—[21], 2—[23], 3—[30] | |||||||
| Element | kδ | kƳ | Dδ (10−4m2/s) | DƳ (10−4m2/s) |
|---|---|---|---|---|
| C | 0.20 | 0.35 | 0.0127exp(−81 379/RT) | 0.15exp(−143 511/RT) |
| Si | 0.77 | 0.52 | 8.0exp(−248 700/RT) | 0.30exp(−251 218/RT) |
| Mn | 0.76 | 0.78 | 0.76exp(−224 430/RT) | 0.055exp(−249 366/RT) |
| S | 0.05 | 0.035 | 4.56exp(−214 434/RT) | 2.4exp(−212 232/RT) |
| Cr | 0.95 | 0.86 | 2.4exp(−239 800/RT) | 0.0012exp(−219 000/RT) |
| Bi | 0.14 | Diffusion suppressed | ||
| # | Steel Grade | Edge | Center | Top | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V, % | N, 1/mm2 | d, μm | dmax, μm | V, % | N, 1/mm2 | d, μm | dmax, μm | V, % | N, 1/mm2 | d, μm | dmax, μm | ||
| λ2 = 218 μm | λ2 = 293 μm | λ2 = 316 μm | |||||||||||
| 1 | 0.4C-Cr + Bi | 0.05 | 117 | 2.6 | 5,8 | 0.06 | 115 | 2.9 | 6.9 | 0.16 | 589 | 1.7 | 27 |
| 2 | 0.4C-Cr + Bimax | 0.06 | 125 | 2.3 | 14 | 0.06 | 106 | 2.5 | 16 | 0.11 | 457 | 1.7 | 17 |
| 3 | 0.4C-Cr + Bi + S | 0.15 | 244 | 2.7 | 21 | 0.24 | 668 | 2.1 | 19 | 0.41 | 6412 | 0.8 | 14 |
| λ2 = 88 μm | λ2 = 132 μm | λ2 = 146 μm | |||||||||||
| 4 | 0.14C + Bi | 0.06 | 151 | 2 | 15 | 0.06 | 150 | 2 | 16 | 0.10 | 460 | 1.4 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhitenev, A.; Rovbo, A.; Nechaev, D.; Shaposhnikov, N.; Ryaboshuk, S.; Alkhimenko, A. Control of Bismuth and Manganese Sulfide Inclusions in Free-Cutting Steels of Different Classes. Metals 2022, 12, 2076. https://doi.org/10.3390/met12122076
Zhitenev A, Rovbo A, Nechaev D, Shaposhnikov N, Ryaboshuk S, Alkhimenko A. Control of Bismuth and Manganese Sulfide Inclusions in Free-Cutting Steels of Different Classes. Metals. 2022; 12(12):2076. https://doi.org/10.3390/met12122076
Chicago/Turabian StyleZhitenev, Andrey, Anna Rovbo, Daniil Nechaev, Nikita Shaposhnikov, Sergey Ryaboshuk, and Alexey Alkhimenko. 2022. "Control of Bismuth and Manganese Sulfide Inclusions in Free-Cutting Steels of Different Classes" Metals 12, no. 12: 2076. https://doi.org/10.3390/met12122076
APA StyleZhitenev, A., Rovbo, A., Nechaev, D., Shaposhnikov, N., Ryaboshuk, S., & Alkhimenko, A. (2022). Control of Bismuth and Manganese Sulfide Inclusions in Free-Cutting Steels of Different Classes. Metals, 12(12), 2076. https://doi.org/10.3390/met12122076






