Recent Advances in the Development of Magnesium-Based Alloy Guided Bone Regeneration (GBR) Membrane
Abstract
:1. Introduction
2. The Classification and Characteristics of Commercial GBR Membranes
3. The Feasibility of Utilizing Mg-Based Alloys as GBR Membrane
3.1. Biocompatibility
3.2. Biodegradation of Mg-Based Alloys
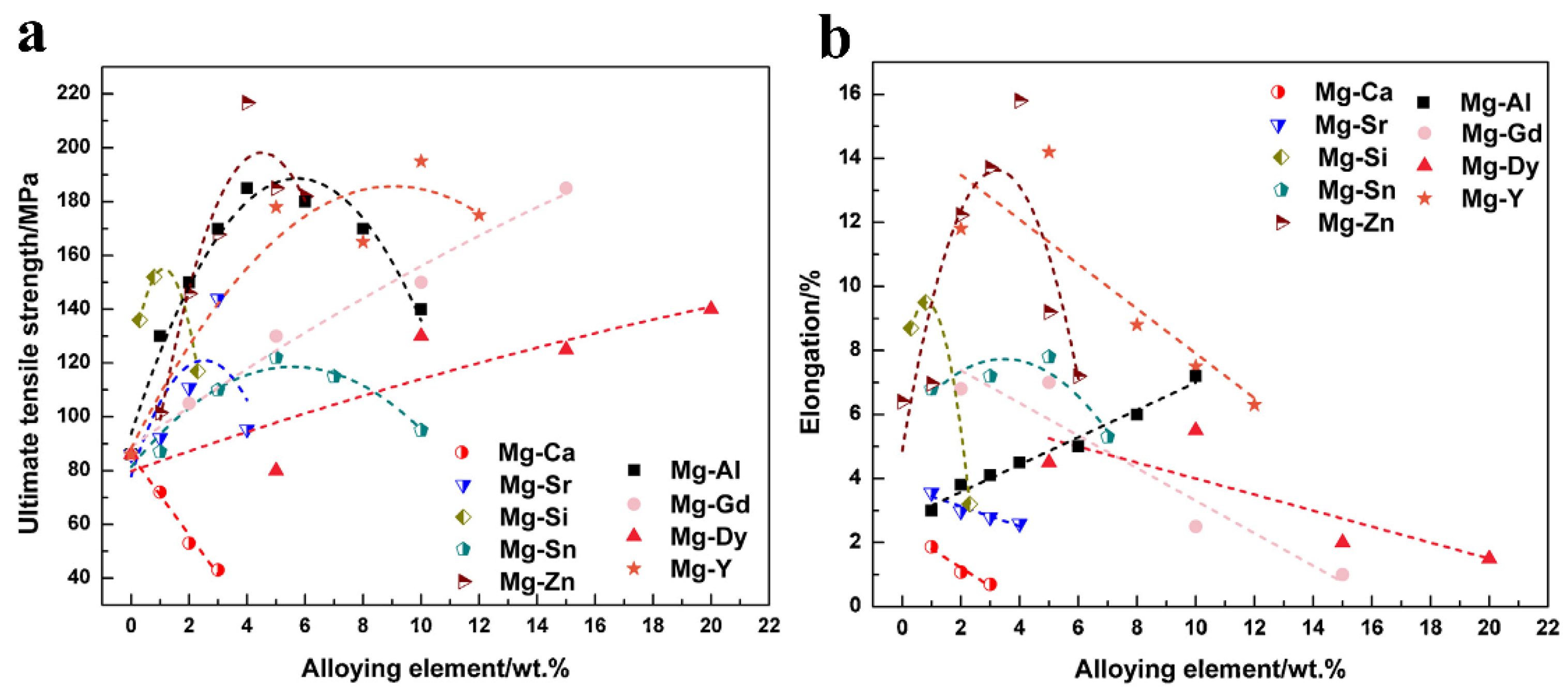
3.3. Mechanical Performance and Integrity
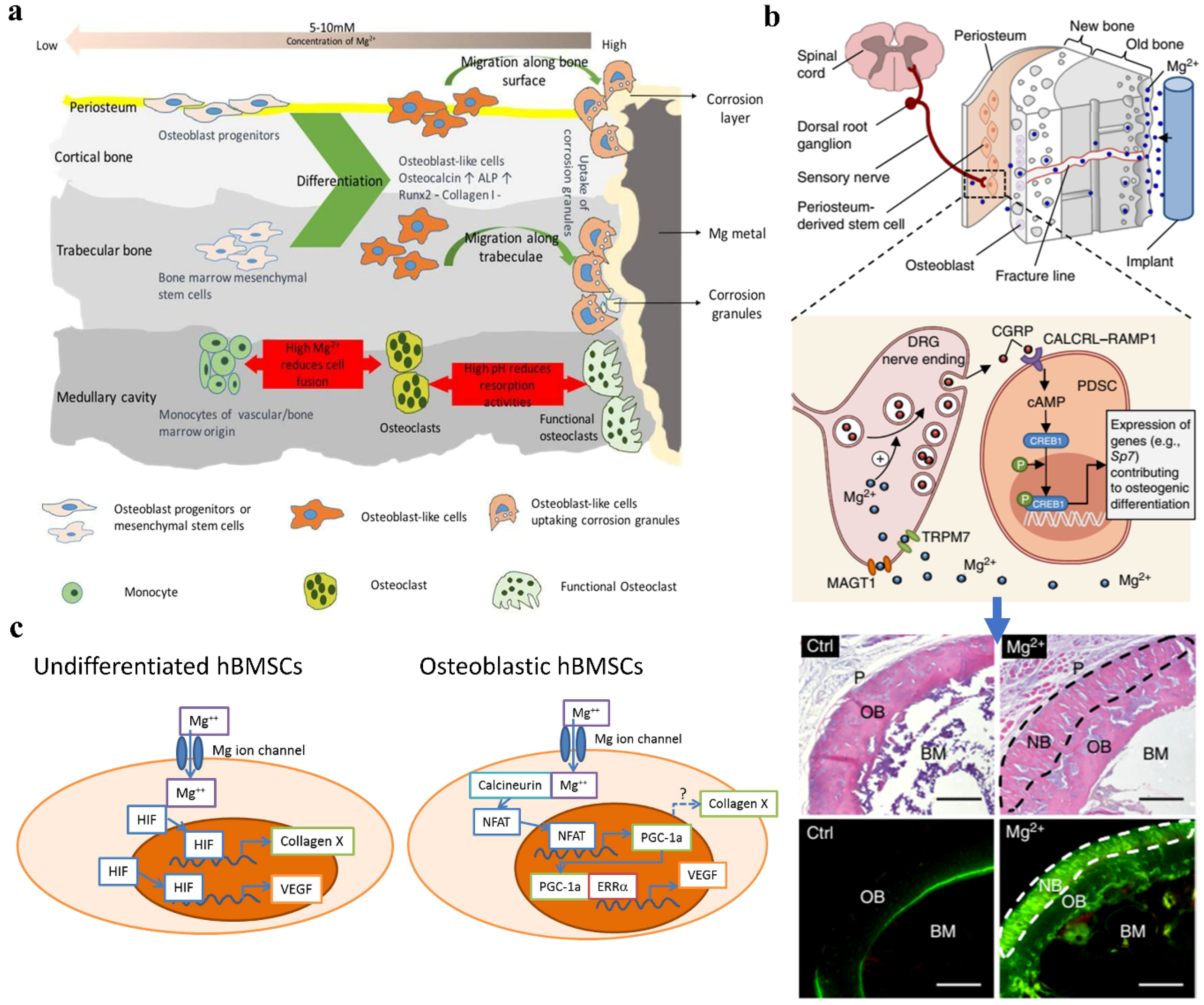
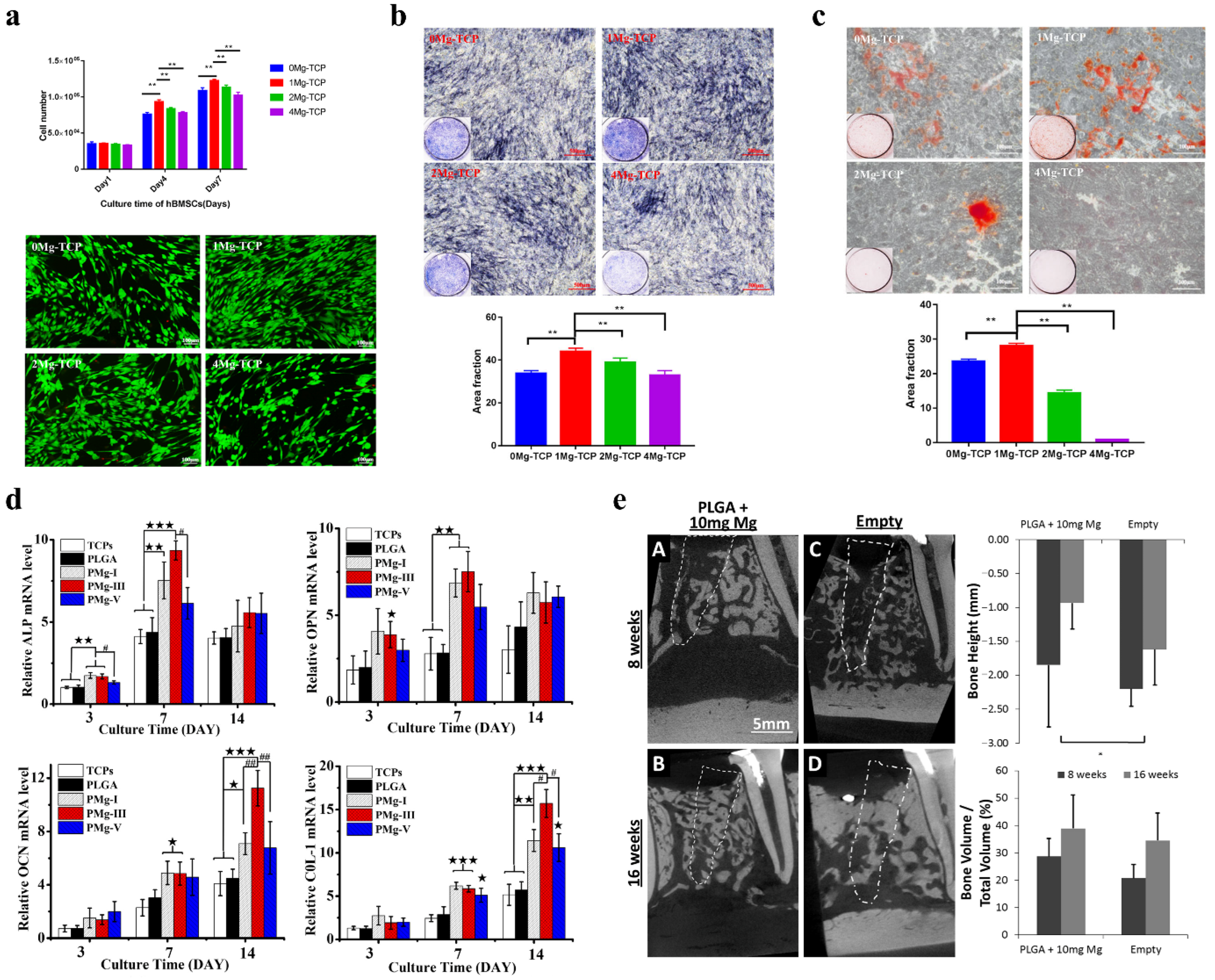
3.4. Osteopromotive Properties of Mg Alloys
3.5. Bacteriostatic Activity
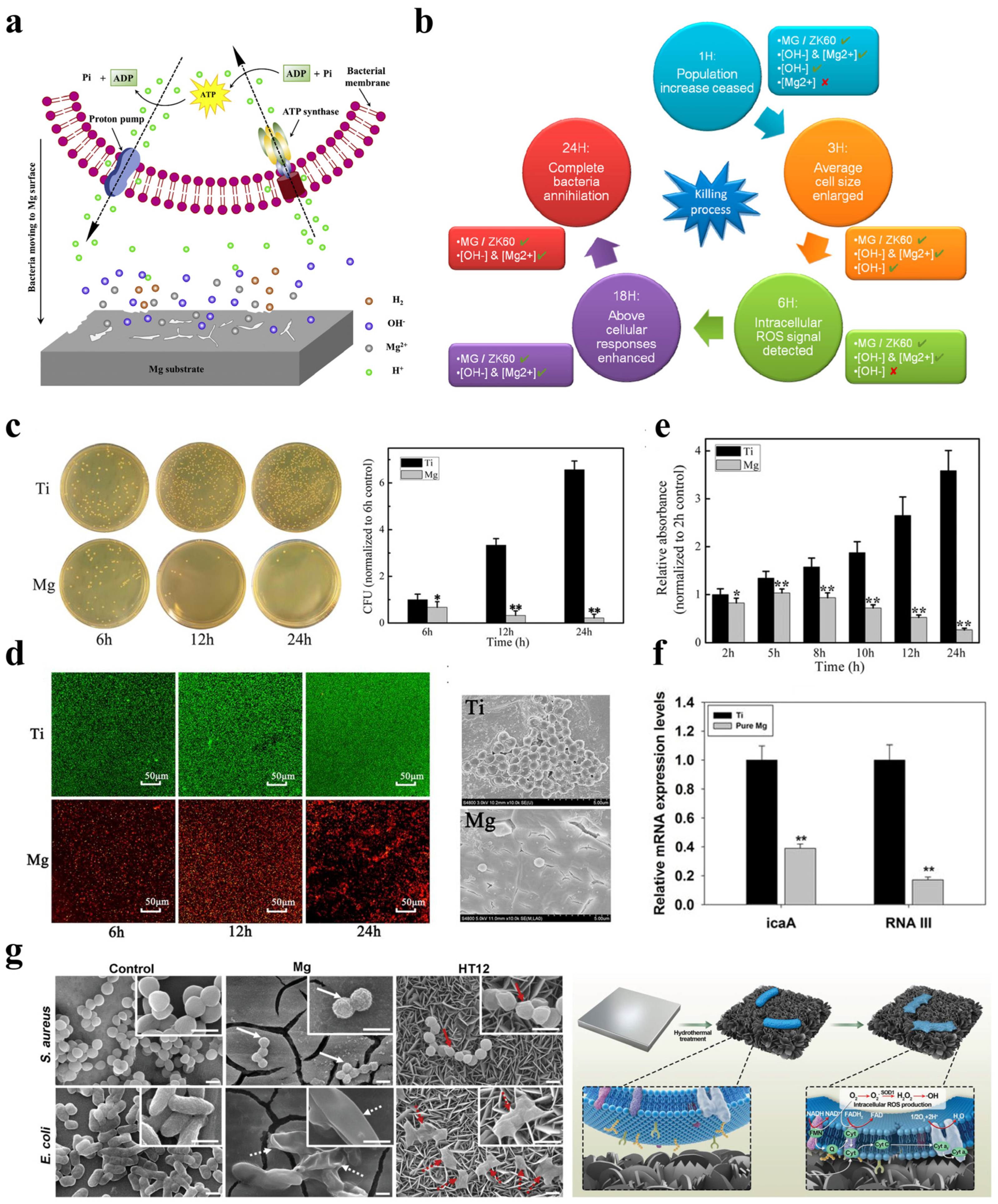
3.6. Wound-Healing Ability
4. The Challenges Faced with Mg-Based GBR Membranes
5. The Outlook for the Future
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Healthc. Mater. 2020, 9, 2000707. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzynski, M.; Rybak, Z.; Szymonowicz, M.; Wiglusz, R.J. Selected Nanomaterials’ Application Enhanced with the Use of Stem Cells in Acceleration of Alveolar Bone Regeneration during Augmentation Process. Nanomaterials 2020, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Ding, Y.; Hu, K.; Zhou, H.; Qin, R.; Hou, R. Influence of preservation of the alveolar ridge on delayed implants after extraction of teeth with different defects in the buccal bone. Br. J. Oral Maxillofac. Surg. 2016, 54, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Fok, M.R.; Pelekos, G.; Tonetti, M.S. Feasibility and needs for simultaneous or staged bone augmentation to place prosthetically guided dental implants after extraction or exfoliation of first molars due to severe periodontitis. J. Clin. Periodontol. 2020, 47, 1237–1247. [Google Scholar] [CrossRef]
- Watson, T.J. Distraction Osteogenesis. JAAOS J. Am. Acad. Orthop. Surg. 2006, 14, S168–S174. [Google Scholar] [CrossRef]
- Chen, F.-M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.-F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Han, L.; Ma, S.; Zhao, J.; Chen, H.; Yang, Z.; Zhang, F.; Xia, Y.; Zhou, Y. Biocompatibility and osteogenic activity of guided bone regeneration membrane based on chitosan-coated magnesium alloy. Mater. Sci. Eng. C. 2019, 100, 226–235. [Google Scholar] [CrossRef]
- Saima, S.; Jan, S.; Shah, A.; Yousuf, A.; Batra, M. Bone grafts and bone substitutes in dentistry. J. Oral Res. Rev. 2016, 8, 36–38. [Google Scholar]
- Orsini, M.; Orsini, G.; Benlloch, D.; Aranda, J.J.; Sanz, M. Long-Term Clinical Results on the Use of Bone-Replacement Grafts in the Treatment of Intrabony Periodontal Defects. Comparison of the Use of Autogenous Bone Graft Plus Calcium Sulfate to Autogenous Bone Graft Covered with a Bioabsorbable Membrane. J. Periodontol. 2008, 79, 1630–1637. [Google Scholar] [CrossRef]
- Rodriguez, I.A.; Selders, G.S.; Fetz, A.E.; Gehrmann, C.J.; Stein, S.H.; Evensky, J.A.; Green, M.S.; Bowlin, G.L. Barrier membranes for dental applications: A review and sweet advancement in membrane developments. Mouth Teeth 2018, 2, 1–9. [Google Scholar]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Chiapasco, M.; Zaniboni, M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: A systematic review. Clin. Oral Implant. Res. 2009, 20, 113–123. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Chitosan as a vehicle for growth factor delivery: Various preparations and their applications in bone tissue regeneration. Int. J. Biol. Macromol. 2017, 104, 1383–1397. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46, 103–123. [Google Scholar] [CrossRef] [Green Version]
- Leghissa, G.C.; Zaffe, D.; Assenza, B.; Botticelli, A.R. Guided bone regeneration using titanium grids: Report of 10 cases. Clin. Oral Implant. Res. 1999, 10, 62–68. [Google Scholar] [CrossRef]
- Mamalis, A.A.; Cochran, D.L. The therapeutic potential of oxygen tension manipulation via hypoxia inducible factors and mimicking agents in guided bone regeneration. A review. Arch. Oral Biol. 2011, 56, 1466–1475. [Google Scholar] [CrossRef]
- Milstein, D.M.J.; Mathura, K.R.; Lindeboom, J.A.H.; Ramsoekh, D.; Lindeboom, R.; Ince, C. The temporal course of mucoperiosteal flap revascularization at guided bone regeneration-treated implant sites: A pilot study. J. Clin. Periodontol. 2009, 36, 892–897. [Google Scholar] [CrossRef]
- Xia, D.; Yang, F.; Zheng, Y.; Liu, Y.; Zhou, Y. Research status of biodegradable metals designed for oral and maxillofacial applications: A review. Bioact. Mater. 2021, 6, 4186–4208. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Geng, X.; Jiang, J.; Zhang, X. Corrosion Behavior of Mg-xGd-1Zn-0.4Zr Alloys with Different Gd Additions for Biomedical Application. Metals 2022, 12, 1763. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, J.; Dong, Q.; Ba, Z.; Wu, Y. Corrosion behavior and mechanical degradation of as-extruded Mg–Gd–Zn–Zr alloys for orthopedic application. J. Biomed. Mater. Res. B. 2020, 108, 698–708. [Google Scholar] [CrossRef]
- Hong, L.; Wang, R.; Zhang, X. Effects of Nd on microstructure and mechanical properties of as-cast Mg-12Gd-2Zn-xNd-0.4Zr alloys with stacking faults. Int. J. Miner. Metall. Mater. 2022, 29, 1570–1577. [Google Scholar] [CrossRef]
- Nie, Y.; Dai, J.; Li, X.; Zhang, X. Recent developments on corrosion behaviors of Mg alloys with stacking fault or long period stacking ordered structures. J. Magnes. Alloy 2021, 9, 1123–1146. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, J.; Zhang, R.; Ba, Z.; Birbilis, N. Corrosion behavior of Mg–3Gd–1Zn–0.4Zr alloy with and without stacking faults. J. Magnes. Alloy 2019, 7, 240–248. [Google Scholar] [CrossRef]
- Nie, Y.-J.; Dai, J.-W.; Zhang, X.-B. Effect of Ag Addition on Microstructure, Mechanical and Corrosion Properties of Mg–Nd–Zn–Zr Alloy for Orthopedic Application. Acta Metall. Sin. 2022; preprint. [Google Scholar] [CrossRef]
- Zhang, X.; Kairy, S.K.; Dai, J.; Birbilis, N. A Closer Look at the Role of Nanometer Scale Solute-Rich Stacking Faults in the Localized Corrosion of a Magnesium Alloy GZ31K. J. Electrochem. Soc. 2018, 165, C310–C316. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Liu, Q.; Yang, S.; Wang, Z. Effects of Load on Dry Sliding Wear Behavior of Mg–Gd–Zn–Zr Alloys. J. Mater. Sci. Technol. 2017, 33, 645–651. [Google Scholar] [CrossRef]
- Chen, K.; Lu, Y.; Tang, H.; Gao, Y.; Zhao, F.; Gu, X.; Fan, Y. Effect of strain on degradation behaviors of WE43, Fe and Zn wires. Acta Biomater. 2020, 113, 627–645. [Google Scholar] [CrossRef]
- Chen, K.; Xie, X.; Tang, H.; Sun, H.; Qin, L.; Zheng, Y.; Gu, X.; Fan, Y. In vitro and in vivo degradation behavior of Mg–2Sr–Ca and Mg–2Sr–Zn alloys. Bioact. Mater. 2020, 5, 275–285. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Song, G.; Ghali, E.; Dietzel, W.; Kainer, K.U.; Hort, N.; Blawert, C. A Critical Review of the Stress Corrosion Cracking (SCC) of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 659–693. [Google Scholar] [CrossRef]
- Kasaj, A.; Reichert, C.; Götz, H.; Röhrig, B.; Smeets, R.; Willershausen, B. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head Face Med. 2008, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Milella, E.; Ramires, P.A.; Brescia, E.; la Sala, G.; di Paola, L.; Bruno, V. Physicochemical, mechanical, and biological properties of commercial membranes for GTR. J. Biomed. Mater. Res. 2001, 58, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Kostopoulos, L.; Karring, T. Alveolar ridge augmentation using a resorbable copolymer membrane and autogenous bone grafts. Clin. Oral Implant. Res. 2002, 13, 203–213. [Google Scholar] [CrossRef]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [Green Version]
- Klinge, U.; Klosterhalfen, B.; Müller, M.; Schumpelick, V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur. J. Surg. 1999, 165, 665–673. [Google Scholar]
- Hou, L.-T.; Yan, J.-J.; Tsai, A.Y.M.; Lao, C.S.; Lin, S.J.; Liu, C.-M. Polymer-assisted regeneration therapy with Atrisorb® barriers in human periodontal intrabony defects. J. Clin. Periodontol. 2004, 31, 68–74. [Google Scholar] [CrossRef]
- Coonts, B.A.; Whitman, S.L.; O’Donnell, M.; Polson, A.M.; Bogle, G.; Garrett, S.; Swanbom, D.D.; Fulfs, J.C.; Rodgers, P.W.; Southard, G.L.; et al. Biodegradation and biocompatibility of a guided tissue regeneration barrier membrane formed from a liquid polymer material. J. Biomed. Mater. Res. 1998, 42, 303–311. [Google Scholar] [CrossRef]
- Bottino, M.C.; Jose, M.V.; Thomas, V.; Dean, D.R.; Janowski, G.M. Freeze-dried acellular dermal matrix graft: Effects of rehydration on physical, chemical, and mechanical properties. Dent. Mater. 2009, 25, 1109–1115. [Google Scholar] [CrossRef]
- Owens, K.W.; Yukna, R.A. Collagen Membrane Resorption in Dogs: A Comparative Study. Implant. Dent. 2001, 10, 49–58. [Google Scholar] [CrossRef]
- Coïc, M.; Placet, V.; Jacquet, E.; Meyer, C. Propriétés mécaniques des membranes de collagène. Rev. Stomatol. Chir. Maxillo-Faciale 2010, 111, 286–290. [Google Scholar] [CrossRef]
- Bunyaratavej, P.; Wang, H.-L. Collagen Membranes: A Review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Kojima, N.; Att, W.; Minamikawa, H.; Sakurai, K.; Ogawa, T. Improvement in the osteoblastic cellular response to a commercial collagen membrane and demineralized freeze-dried bone by an amino acid derivative: An in vitro study. Clin. Oral Implant. Res. 2011, 22, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhao, L.; Sun, J.; Gu, X.; Huang, C.; Su, H.; Fan, Y. Utilizing biodegradable alloys as guided bone regeneration (GBR) membrane: Feasibility and challenges. Sci. China. Mater. 2022, 65, 2627–2646. [Google Scholar] [CrossRef]
- Fischerauer, S.F.; Kraus, T.; Wu, X.; Tangl, S.; Sorantin, E.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J.; Weinberg, A.M. In vivo degradation performance of micro-arc-oxidized magnesium implants: A micro-CT study in rats. Acta Biomater. 2013, 9, 5411–5420. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Dai, J.; Zhang, X. Improvement of corrosion resistance of magnesium alloys for biomedical applications. Corros. Rev. 2015, 33, 101–117. [Google Scholar] [CrossRef]
- Haude, M.; Erbel, R.; Erne, P.; Verheye, S.; Degen, H.; Böse, D.; Vermeersch, P.; Wijnbergen, I.; Weissman, N.; Prati, F.; et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet 2013, 381, 836–844. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral Sci. 2020, 12, 37. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.; Dodge, A.; Luepke, P.; Wang, H.-L.; Kapila, Y.; Lin, G.-H. Effect of membrane exposure on guided bone regeneration: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Jung, R.E.; Fenner, N.; Hämmerle, C.H.F.; Zitzmann, N.U. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin. Oral Implant. Res. 2013, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Zhou, G.; Li, Q.; Tang, H.; Wang, S.; Li, P.; Gu, X.; Fan, Y. In vitro degradation, biocompatibility and antibacterial properties of pure zinc: Assessing the potential of Zn as a guided bone regeneration membrane. J. Mater. Chem. B 2021, 9, 5114–5127. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Qureshi, J.; Alshahrani, A.M.; Nassar, H.; Ikeda, Y.; Glogauer, M.; Ganss, B. Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology 2017, 105, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Joop, A.; Rahlf, B.; Gellrich, N.-C.; Kampmann, A.; von See, C.; Stoetzer, M. Examination of Local Periosteal Microcirculation After Application of Absorbable and Nonabsorbable Membranes. J. Oral Implantol. 2017, 43, 462–467. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Chen, X.H.; Yang, J.A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental Theory of Biodegradable Metals—Definition, Criteria, and Design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Knowles, A.F.; Leng, L. Purification of a low affinity Mg2+ (Ca2+)-ATPase from the plasma membranes of a human oat cell carcinoma. J. Biol. Chem. 1984, 259, 10919–10924. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Guangyin, Y.; Jia, Z.; Wenjiang, D. Research Progress of Mg-Based Alloys as Degradable Biomedical Materials. Mater. China 2011, 30, 44–50. [Google Scholar]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Mauskop, A.; Varughese, J. Why all migraine patients should be treated with magnesium. J. Neural Transm. 2012, 119, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, D. Thiamine and magnesium deficiencies: Keys to disease. Med. Hypotheses 2015, 84, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.G. Magnesium metabolism in health and disease. Int. Urol. Nephrol. 2009, 41, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.F.; Staiger, M.P.; Dias, G.J. The in vitro and in vivo evaluation of the biocompatibility of Mg alloys. Biomed. Mater. 2014, 9, 015006. [Google Scholar] [CrossRef]
- Tang, H.; Wang, F.; Li, D.; Gu, X.; Fan, Y. Mechanical properties, degradation behaviors and biocompatibility of micro-alloyed Mg-Sr-RE alloys for stent applications. Mater. Lett. 2020, 264, 127285. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, Y.; Liu, C.; Li, Q.; Bai, Y.; Li, P.; Wang, C.; Gu, X.; Fan, Y. Novel Mg-Ca-La alloys for guided bone regeneration: Mechanical performance, stress corrosion behavior and biocompatibility. Mater. Today Commun. 2022, 32, 103949. [Google Scholar] [CrossRef]
- Gu, X.; Wang, F.; Xie, X.; Zheng, M.; Li, P.; Zheng, Y.; Qin, L.; Fan, Y. In vitro and in vivo studies on as-extruded Mg-5.25wt.%Zn-0.6wt.%Ca alloy as biodegradable metal. Sci. China. Mater. 2018, 61, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Medhekar, N.V.; Frankel, G.S.; Birbilis, N. Corrosion mechanism and hydrogen evolution on Mg. Curr. Opin. Solid State Mater. Sci. 2015, 19, 85–94. [Google Scholar] [CrossRef]
- Mei, D.; Lamaka, S.V.; Lu, X.; Zheludkevich, M.L. Selecting medium for corrosion testing of bioabsorbable magnesium and other metals—A critical review. Corros. Sci. 2020, 171, 108722. [Google Scholar] [CrossRef]
- Chen, K.; Gu, X.; Sun, H.; Tang, H.; Yang, H.; Gong, X.; Fan, Y. Fluid-induced corrosion behavior of degradable zinc for stent application. J. Mater. Sci. Technol. 2021, 91, 134–147. [Google Scholar] [CrossRef]
- Kieke, M.; Feyerabend, F.; Lemaitre, J.; Behrens, P.; Willumeit-Römer, R. Degradation rates and products of pure magnesium exposed to different aqueous media under physiological conditions. BioNanoMaterials 2016, 17, 131–143. [Google Scholar] [CrossRef]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.S.; Willumeit-Romer, R.; Feyerabend, F. Magnesium degradation under physiological conditions—Best practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef]
- Jin, L.; Chen, C.; Jia, G.; Li, Y.; Zhang, J.; Huang, H.; Kang, B.; Yuan, G.; Zeng, H.; Chen, T. The bioeffects of degradable products derived from a biodegradable Mg-based alloy in macrophages via heterophagy. Acta Biomater. 2020, 106, 428–438. [Google Scholar] [CrossRef]
- Moses, O.; Pitaru, S.; Artzi, Z.; Nemcovsky, C.E. Healing of dehiscence-type defects in implants placed together with different barrier membranes: A comparative clinical study. Clin. Oral Implant. Res. 2005, 16, 210–219. [Google Scholar] [CrossRef]
- Matzeu, G.; Naveh, G.R.S.; Agarwal, S.; Roshko, J.A.; Ostrovsky-Snider, N.A.; Napier, B.S.; Omenetto, F.G. Functionalized Mouth-Conformable Interfaces for pH Evaluation of the Oral Cavity. Adv. Sci. 2021, 8, 2003416. [Google Scholar] [CrossRef]
- Zeng, R.C.; Li, X.T.; Liu, L.J.; Li, S.Q.; Zhang, F. In vitro Degradation of Pure Mg for Esophageal Stent in Artificial Saliva. J. Mater. Sci. Technol. 2016, 32, 437–444. [Google Scholar] [CrossRef]
- Erinc, M.; Sillekens, W.H.; Mannens, R.G.T.M.; Werkhoven, R.J. Applicability of existing magnesium alloys as biomedical implant materials. In Proceedings of the Magnesium Technology 2009, San Francisco, CA, USA, 15–19 February 2009; pp. 209–214. [Google Scholar]
- Wu, S.; Jang, Y.-S.; Lee, M.-H. Enhancement of Bone Regeneration on Calcium-Phosphate-Coated Magnesium Mesh: Using the Rat Calvarial Model. Front. Bioeng. Biotechnol. 2021, 9, 652334. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jang, Y.-S.; Kim, Y.-K.; Kim, S.-Y.; Ko, S.-O.; Lee, M.-H. Surface Modification of Pure Magnesium Mesh for Guided Bone Regeneration: In Vivo Evaluation of Rat Calvarial Defect. Materials 2019, 12, 2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, O.; Hesse, B.; Stojanovic, S.; Seim, C.; Weitkamp, T.; Batinic, M.; Goerke, O.; Kačarević, Ž.P.; Rider, P.; Najman, S.; et al. Biocompatibility Analyses of HF-Passivated Magnesium Screws for Guided Bone Regeneration (GBR). Int. J. Mol. Sci. 2021, 22, 12567. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Kühnel, L.; Witte, F.; Pissarek, J.; Precht, C.; Xiong, X.; Krastev, R.; Wegner, N.; Walther, F.; Jung, O. Degradation, Bone Regeneration and Tissue Response of an Innovative Volume Stable Magnesium-Supported GBR/GTR Barrier Membrane. Int. J. Mol. Sci. 2020, 21, 3098. [Google Scholar] [CrossRef]
- Yan, Z.-Y.; Zhu, J.-H.; Liu, G.-Q.; Liu, Z.-C.; Guo, C.-B.; Cui, N.-H.; Han, J.-M. Feasibility and Efficacy of a Degradable Magnesium-Alloy GBR Membrane for Bone Augmentation in a Distal Bone-Defect Model in Beagle Dogs. Bioinorg. Chem. Appl. 2022, 2022, 4941635. [Google Scholar] [CrossRef]
- Byun, S.-H.; Lim, H.-K.; Kim, S.-M.; Lee, S.-M.; Kim, H.-E.; Lee, J.-H. The Bioresorption and Guided Bone Regeneration of Absorbable Hydroxyapatite-Coated Magnesium Mesh. J. Craniofacial Surg. 2017, 28, 518–523. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.-X.; Shan, X.-F.; Wang, Y.-C.; He, F.; Wang, X.-J.; Tan, L.-L.; Yang, K. Mg-based absorbable membrane for guided bone regeneration (GBR): A pilot study. Rare Met. 2019, 38, 577–587. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, X.; Kang, C.; Yang, M.; Zhao, Y.; Wang, C. Study on repairing canine mandibular defect with porous Mg–Sr alloy combined with Mg–Sr alloy membrane. Regen. Biomater. 2020, 7, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Liu, W.; Ma, S.; Wang, J.; Zou, J.; Liu, Z.; Zhao, J.; Zhou, Y. A preliminary study for novel use of two Mg alloys (WE43 and Mg3Gd). J. Mater. Sci. Mater. Med. 2016, 27, 82. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, S.-H.; Sato, H.; Zhu, Y.; Shanov, V.; Tiasha, T.; D’Amore, A.; Luketich, S.; Wan, G.; Wagner, W.R. Hybrid scaffolds of Mg alloy mesh reinforced polymer/extracellular matrix composite for critical-sized calvarial defect reconstruction. J. Tissue Eng. Regen. Med. 2018, 12, 1374–1388. [Google Scholar] [CrossRef]
- Si, J.; Shen, H.; Miao, H.; Tian, Y.; Huang, H.; Shi, J.; Yuan, G.; Shen, G. In vitro and in vivo evaluations of Mg-Zn-Gd alloy membrane on guided bone regeneration for rabbit calvarial defect. J. Magnes. Alloy 2021, 9, 281–291. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, G.; Li, Y.; Yu, X.; Yuan, S.; Nie, Z.; Wang, J.; Han, J.; Tan, C.; Guo, C. Degradation Behavior, Transport Mechanism and Osteogenic Activity of Mg–Zn–RE Alloy Membranes in Critical-Sized Rat Calvarial Defects. Coatings 2020, 10, 496. [Google Scholar] [CrossRef]
- Du, J.; Wang, G.; Song, D.; Jiang, J.; Jiang, H.; Gao, J. In-vitro degradation behavior and biocompatibility of superhydrophilic hydroxyapatite coating on Mg–2Zn–Mn–Ca–Ce alloy. J. Mater. Res. Technol. 2022, 17, 2742–2754. [Google Scholar] [CrossRef]
- Lin, D.-J.; Hung, F.-Y.; Lee, H.-P.; Yeh, M.-L. Development of a Novel Degradation-Controlled Magnesium-Based Regeneration Membrane for Future Guided Bone Regeneration (GBR) Therapy. Metals 2017, 7, 481. [Google Scholar] [CrossRef]
- Ge, W.; Chen, K.; Tang, H.; Arken, X.; Zhang, X.; Gu, X.; Zhu, C. Degradability and in vivo biocompatibility of micro-alloyed Mg-Ca-La alloys as orthopedic implants. Mater. Lett. 2022, 310, 131510. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar]
- Guo, H.; Xia, D.; Zheng, Y.; Zhu, Y.; Liu, Y.; Zhou, Y. A pure zinc membrane with degradability and osteogenesis promotion for guided bone regeneration: In vitro and in vivo studies. Acta Biomater. 2020, 106, 396–409. [Google Scholar] [CrossRef]
- Wang, J.-L.; Xu, J.-K.; Hopkins, C.; Chow, D.H.-K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xu, J.; Song, B.; Chow, D.H.; Yung, P.S.H.; Qin, L. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017, 63, 393–410. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Li, H.; Liu, Y.; Bai, X.; Chau, W.; Zheng, Y.; Qin, L. Magnesium alloy based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits. Biomaterials 2018, 157, 86–97. [Google Scholar] [CrossRef]
- Maradze, D.; Musson, D.; Zheng, Y.; Cornish, J.; Lewis, M.; Liu, Y. High Magnesium Corrosion Rate has an Effect on Osteoclast and Mesenchymal Stem Cell Role During Bone Remodelling. Sci. Rep. 2018, 8, 10003. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Hung, C.-C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019, 98, 246–255. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef]
- Wang, M.; Yu, Y.; Dai, K.; Ma, Z.; Liu, Y.; Wang, J.; Liu, C. Improved osteogenesis and angiogenesis of magnesium-doped calcium phosphate cement via macrophage immunomodulation. Biomater. Sci. 2016, 4, 1574–1583. [Google Scholar] [CrossRef]
- Zhai, Z.; Qu, X.; Li, H.; Yang, K.; Wan, P.; Tan, L.; Ouyang, Z.; Liu, X.; Tian, B.; Xiao, F.; et al. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling. Biomaterials 2014, 35, 6299–6310. [Google Scholar]
- Chen, Z.; Mao, X.; Tan, L.; Friis, T.; Wu, C.; Crawford, R.; Xiao, Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials 2014, 35, 8553–8565. [Google Scholar] [CrossRef]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar]
- Gu, Y.; Zhang, J.; Zhang, X.; Liang, G.; Xu, T.; Niu, W. Three-dimensional Printed Mg-Doped β-TCP Bone Tissue Engineering Scaffolds: Effects of Magnesium Ion Concentration on Osteogenesis and Angiogenesis In Vitro. Tissue Eng. Regen. Med. 2019, 16, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.M.; Wu, S.; Chu, P.K.; Cheng, S.H.; Luk, K.D.K.; Cheung, K.M.C.; Yeung, K.W.K. Low-modulus Mg/PCL hybrid bone substitute for osteoporotic fracture fixation. Biomaterials 2013, 34, 7016–7032. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wei, P.; Huang, Y.; Zhang, W.; Chen, F.; Zhang, X.; Mao, J.; Chen, D.; Cai, Q.; Yang, X. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019, 85, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Zaky, S.; Ray, H.; Sfeir, C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015, 11, 543–553. [Google Scholar] [CrossRef]
- Doel, J.J.; Benjamin, N.; Hector, M.P.; Rogers, M.; Allaker, R.P. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005, 113, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Sun, X.; Yang, H. The Role of Antibacterial Metallic Elements in Simultaneously Improving the Corrosion Resistance and Antibacterial Activity of Magnesium Alloys. Mater. Des. 2021, 198, 109350. [Google Scholar] [CrossRef]
- Padan, E.; Bibi, E.; Ito, M.; Krulwich, T.A. Alkaline pH homeostasis in bacteria: New insights. Biochim. Biophys. Acta Biomembr. 2005, 1717, 67–88. [Google Scholar] [CrossRef]
- Robinson, D.A.; Griffith, R.W.; Shechtman, D.; Evans, R.B.; Conzemius, M.G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010, 6, 1869–1877. [Google Scholar] [CrossRef]
- Rahim, M.I.; Eifler, R.; Rais, B.; Mueller, P.P. Alkalization is responsible for antibacterial effects of corroding magnesium. J. Biomed. Mater. Res. A 2015, 103, 3526–3532. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Zhai, Z.; Liu, L.; Li, H.; Yang, K.; Tan, L.; Wan, P.; Liu, X.; Ouyang, Z.; et al. Antibacterial properties of magnesium in vitro and in an in vivo model of implant-associated methicillin-resistant Staphylococcus aureus infection. Antimicrob. Agents Chemother. 2014, 58, 7586–7591. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Zhao, Y.; Cheng, M.; Wang, Q.; Wang, Q.; Wang, J.; Jiang, Y.; An, Z.; Zhang, X. Anti-biofilm properties of magnesium metal via alkaline pH. RSC Adv. 2015, 5, 21434–21444. [Google Scholar] [CrossRef]
- Virtanen, S. Biodegradable Mg and Mg alloys: Corrosion and biocompatibility. Mater. Sci. Eng. B 2011, 176, 1600–1608. [Google Scholar] [CrossRef]
- Demishtein, K.; Reifen, R.; Shemesh, M. Antimicrobial Properties of Magnesium Open Opportunities to Develop Healthier Food. Nutrients 2019, 11, 2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matevosyan, L.; Bazukyan, I.; Trchounian, A. Comparative analysis of the effect of Ca and Mg ions on antibacterial activity of lactic acid bacteria isolates and their associations depending on cultivation conditions. AMB Express 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Sánchez, J.; Pacha-Olivenza, M.Á.; González-Martín, M.L. Bactericidal effect of magnesium ions over planktonic and sessile Staphylococcus epidermidis and Escherichia coli. Mater. Chem. Phys. 2019, 221, 342–348. [Google Scholar] [CrossRef]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K.; Zhao, Y.; Yang, K.; Chu, P.K. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci. Rep. 2016, 6, 27374. [Google Scholar] [CrossRef] [Green Version]
- Wolf, F.I.; Torsello, A.; Fasanella, S.; Cittadini, A. Cell physiology of magnesium. Mol. Asp. Med. 2003, 24, 11–26. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Y.; Chen, Z.; Pan, D.; Cheng, Y.; Liu, Z.; Lin, Z.; Guan, X. Investigation of Antibacterial Activity and Related Mechanism of a Series of Nano-Mg(OH)2. ACS Appl. Mater. Inter. 2013, 5, 1137–1142. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, W.; Mo, S.; Xie, L.; Liao, Q.; Hu, L.; Ruan, Q.; Tang, K.; Mehrjou, B.; Liu, M.; et al. Nonleaching Antibacterial Concept Demonstrated by In Situ Construction of 2D Nanoflakes on Magnesium. Adv. Sci. 2020, 7, 1902089. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Wang, G.; Jin, W.; Zhang, X.; Huang, Y.; Gao, A.; Wu, H.; Wu, G.; Chu, P.K. Systematic Study of Inherent Antibacterial Properties of Magnesium-based Biomaterials. ACS Appl. Mater. Inter. 2016, 8, 9662–9673. [Google Scholar] [CrossRef]
- Gao, Z.; Song, M.; Liu, R.-L.; Shen, Y.; Ward, L.; Cole, I.; Chen, X.-B.; Liu, X. Improving in vitro and in vivo antibacterial functionality of Mg alloys through micro-alloying with Sr and Ga. Mater. Sci. Eng. C 2019, 104, 109926. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, P.; Wan, P.; Pei, Y.; Shi, L.; Fan, B.; Shen, C.; Xiao, X.; Yang, K.; Guo, Z. Novel Bio-functional Magnesium Coating on Porous Ti6Al4V Orthopaedic Implants: In vitro and In vivo Study. Sci. Rep. 2017, 7, 40755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.-J.; Hung, F.-Y.; Yeh, M.-L.; Lui, T.-S. Microstructure-modified biodegradable magnesium alloy for promoting cytocompatibility and wound healing in vitro. J. Mater. Sci. Mater. Med. 2015, 26, 248. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Z.; Zhang, N.; Gao, W.; Li, J.; Pu, Y.; He, B.; Xie, J. A Mg2+/polydopamine composite hydrogel for the acceleration of infected wound healing. Bioact. Mater. 2022, 15, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Sathi, G.A.; Yamamoto, O. Wound healing effect of bioactive ion released from Mg-smectite. Mater. Sci. Eng. C 2017, 77, 52–57. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C.-n.; Gu, Y.-x.; Shi, J.-y.; Mo, J.-j.; Qian, S.-j.; Qiao, S.-c.; Lai, H.-c. The responses of human gingival fibroblasts to magnesium-doped titanium. J. Biomed. Mater. Res. A 2020, 108, 267–278. [Google Scholar] [CrossRef]
- Bahmani, A.; Arthanari, S.; Shin, K.S. Formulation of corrosion rate of magnesium alloys using microstructural parameters. J. Magnes. Alloy 2020, 8, 134–149. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, Y.; Wu, J.; Liu, T.; Chen, X.; Tang, A.; Pan, F. Alloy Design Strategies of the Native Anti-corrosion Magnesium Alloy. In Magnesium Technology 2019; Joshi, V.V., Jordon, J.B., Orlov, D., Neelameggham, N.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 169–173. [Google Scholar]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Jafari, S.; Harandi, S.E.; Raman, R.K.S. A Review of Stress-Corrosion Cracking and Corrosion Fatigue of Magnesium Alloys for Biodegradable Implant Applications. J. Miner. 2015, 67, 1143–1153. [Google Scholar] [CrossRef]
- Wang, B.J.; Wang, S.D.; Xu, D.K.; Han, E.H. Recent progress in fatigue behavior of Mg alloys in air and aqueous media: A review. J. Mater. Sci. Technol. 2017, 33, 1075–1086. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Jafari, S.; Harandi, S.E. Corrosion fatigue fracture of magnesium alloys in bioimplant applications: A review. Eng. Fract. Mech. 2015, 137, 97–108. [Google Scholar] [CrossRef]





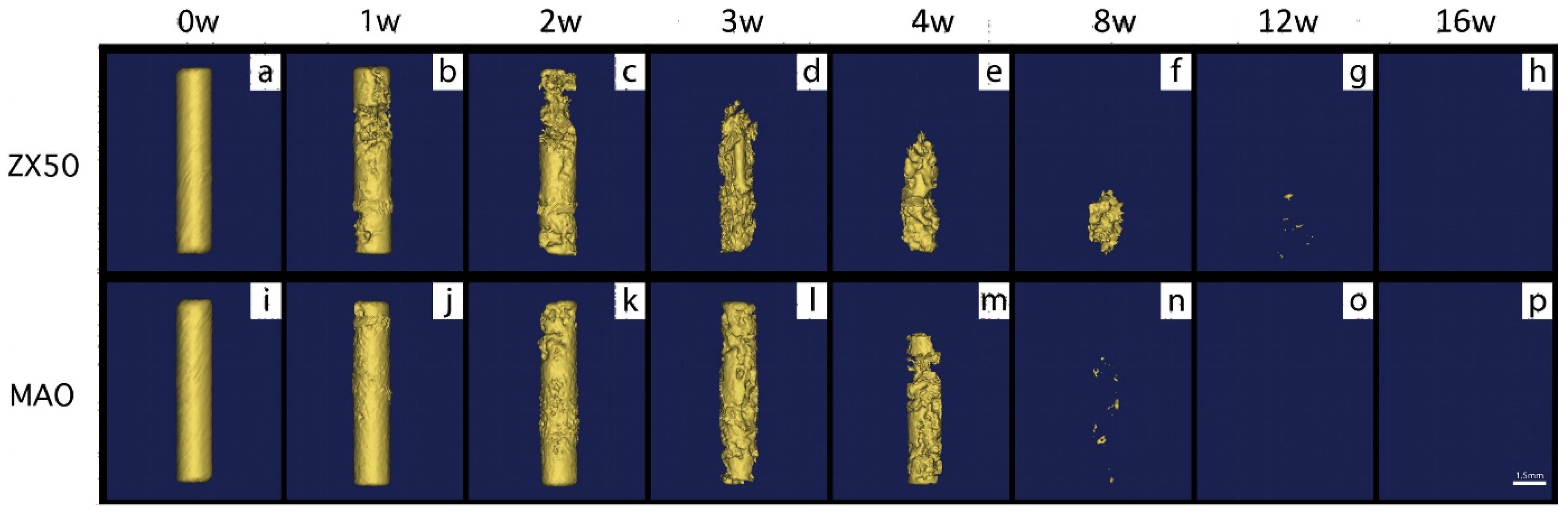

| Degradability | Technic | Commercial Name | Materials | Mechanical Strength | Degradation rate | Ref. |
|---|---|---|---|---|---|---|
| Non-resorbable | Synthetic Metallic | Cytoplast® TXT-200 Cytoplast® Ti-250 Frios® BoneShields | High-density polytetrafluoroethylene (d-PTFE) Titanium-reinforced PTFE Titanium film | N/A N/A N/A | Non-degradable Non-degradable Non-degradable | [11,32] |
| Resorbable | Synthetic | Resolut LT® Vicryl® Atrisorb® | Poly-dl-lactic/co-glycolic acid (PLGA) Polyglactin 910 Poly-dl-lactide and solvent (N-methyl-2-pyrrolidone) | 11.7–50 MPa N/A N/A | 5–6 months ~9 months 6–12 months | [33,34,35,36,37,38] |
| Collagen-based | ALLoDerm® Bio-Gide® BioMend Extend® Cytoplast® RTM | Collagen Type-I derived from cadaveric human skin Collagen derived from porcine skin (Types I and III) Collagen Type-I derived from bovine tendon Collagen Type-I derived from bovine tendon | 9.4–21.5 MPa 7.75 MPa 3.5–22.5 MPa N/A | ~16 weeks 24 weeks 18 weeks 26–38 weeks | [39,40,41,42,43] | |
| Resorbable | Metallic | N/A | Magnesium and its alloys | 87–560 MPa | 3–12 months | [44,45,46,47] |
| Materials | Ultimate Tensile Strength/MPa | Elongation/% | In Vitro Degradation Rate/(mm/yr) | Cytocompatibility | Pathological Model | Implantation Time/Week | Implanted Sample Size/mm | Therapeutic Effect | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| High pure Mg mesh Ca-P-coated Mg mesh | More mouse osteoblastic cells (MC3T3-E1) could be found on the surface of Ca-P-coated Mg mesh | Rat cranial bone defect model | 8 | Φ10 × 0.4 | Ca-P coating can endow Mg with higher surface energy and osteogenesis capability and lower degradation than pure Mg mesh | [82] | |||
| Pure Mg mesh PEO+HT-treated pure Mg mesh | Rat cranial bone defect model | 8 | Φ10 × 0.1 | The volume and mineral density around the PEO+HT-treated alloys are higher than that of untreated alloys | [83] | ||||
| HF-coated NovaMag® fixation screw | HF-treated Mg screws showed better cytocompatibility with L-929 mouse fibroblasts and mouse osteoblast precursor cells (MC3T3) than untreated screws | Rabbit femoral condyle defect model | 6 | Φ1.0 × 3.5 | HF-treated Mg screws implanted showed a reduction in gas formation, slower biodegradation, and better bony integration in comparison to the untreated Mg screws | [84] | |||
| MgF2-coated AZ31 mesh | MgF2-coated alloy showed higher cytocompatibility with L-929 mouse fibroblasts and mouse osteoblast precursor cells (MC3T3) than untreated alloy | Rabbit cranial bone defect model | 18 | i. HF-Mg shows less corrosion and is degraded by phagocytosis ii. The application of membranes did not result in higher bone regeneration | [85] | ||||
| MgF2-coated Mg-2Zn-0.46Y-0.5Nd Bio-Gide membrane | Distal bone-defect model in beagle dogs | 42 | 5 × 5 × 5 | i. Mg alloy did not increase the prevalence of infection, wound dehiscence, or subcutaneous emphysema compared with those using commercial Bio-Gide membrane ii. Higher trabecular bone volume could be inspected in the Mg alloy group | [86] | ||||
| HA-coated Mg mesh | 200 | 11.8 | Rat cranial bone defect model | 18 | Φ12 × 0.5 | i. The results show no quantitative difference in bone regeneration between the control and experimental groups ii. Sufficient mechanical stability of HA-coated magnesium mesh for supporting overlying tissue and protecting bony defect during the resorption period | [87] | ||
| Ca-P-coated Mg | 1.76 | Rabbit cranial bone defect model | 12 | 8 × 8 × 0.05 | Ca–P-coated Mg membrane exhibited better bioactivity than pure Mg and blank group | [88] | |||
| Mg-1.5Sr | Dog mandible buccal fenestration bone defects model | 12 | 20 × 20 × 0.38 | After 12 weeks, the Mg-Sr alloy group showed a better osteogenesis property than that of the mineralized collagen group | [89] | ||||
| WE43 | 0.91 | i. Cell viability is influenced by the concentration of the extract ii. Good cytocompatibility with human osteoblast-like MG63 cells | [90] | ||||||
| Mg3Gd | 0.98 | i. Cell viability is influenced by the concentration of the extract ii. Good cytocompatibility with human osteoblast-like MG63 cells | [90] | ||||||
| Chitosan-coated Mg3Gd | Chitosan-coated alloys showed better biocompatibility with human osteoblast-like MG63 cells than uncoated alloys | New Zealand white rabbit calvarial critical-sized bone defect model | 12 | 10 × 10 × 1 | Chitosan-coated alloys showed more newly formed trabecular and woven bone matrix | [7] | |||
| Mg mesh (AZ31)-reinforced PLGA/DBM hybrid scaffold | Rat calvarial bone defect model | 12 | Φ8 × 0.25 | The Mg-mesh-reinforced PLGA/DBM hybrid scaffold promoted in vitro osteogenic differentiation of BMSCs as well as stimulated bone regeneration in rat calvarial defects as compared with the other control scaffolds | [91] | ||||
| Mg-2.0Zn-1.0Gd Ca-P-coated Mg-2.0Zn-1.0Gd | 235 | 22.5 | 0.26 0.13 | i. Diluted alloy extracts showed good cytocompatibility with mouse osteoblastic cells (MC3T3-E1) ii. Ca-P-coated alloys showed better cytocompatibility | New Zealand white rabbit calvarial critical-sized bone defect model | 12 | 10 × 10 × 0.6 | Ca-P-coated alloy displayed the highest amount of new bone formation compared to all the other groups at 3 months after the surgery | [92] |
| Mg-6.0Zn-2.7RE | Rat calvarial bone defect model | 8 | Φ5 × 0.11 | The new bone thickness and volume in the alloy group were significantly higher than those of the blank group | [93] | ||||
| Mg–2Zn–Mn–Ca–Ce alloy HA-coated Mg–2Zn–Mn–Ca–Ce alloy | 0.63 0.02 | HA-coated Mg–2Zn–Mn–Ca–Ce alloy further promoted cell adhesion and proliferation of murine monocyte-macrophage cell line (Raw 264.7), human bone mesenchymal stem cells (hBMSCs), and murine aortic endothelial cells (MAECs). | [94] | ||||||
| Mg-5Zn-0.45Zr HT-treated Mg-5Zn-0.45Zr HT+ Surface fluoride-treated Mg-5Zn-0.45Zr | 268 256 | 8.5 20.2 | 0.144 0.117 0.038 | Rat calvarial bone defect model | 12 | Φ5 × 0.4 | HT-treated alloy presented the highest bone-regeneration capability | [95] | |
| Mg-0.2La Mg-0.2Ca-0.2La Mg-0.8Ca-0.2La | 231.2 175.9 179.7 | 9.82 32.53 11.12 | 0.356 0.119 0.224 | Mg-0.2Ca-0.2La alloys display a favorable human gingival fibroblast (HGF) cellular response with benign cell adhesion, subsequent proliferation, and cell migration, as well as a good MC3T3-E1 cytocompatibility in vitro, demonstrating the promising potential for GBR membrane application | Rat femoral condyle bone defect model | 12 | Φ2×4 | Mg-0.2Ca-0.2La promote new bone formation adjacent to the implant, suggesting good osteogenesis capability. | [70,96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Zhao, L.; Huang, C.; Yin, X.; Zhang, X.; Li, P.; Gu, X.; Fan, Y. Recent Advances in the Development of Magnesium-Based Alloy Guided Bone Regeneration (GBR) Membrane. Metals 2022, 12, 2074. https://doi.org/10.3390/met12122074
Chen K, Zhao L, Huang C, Yin X, Zhang X, Li P, Gu X, Fan Y. Recent Advances in the Development of Magnesium-Based Alloy Guided Bone Regeneration (GBR) Membrane. Metals. 2022; 12(12):2074. https://doi.org/10.3390/met12122074
Chicago/Turabian StyleChen, Kai, Li Zhao, Chenyang Huang, Xiaofei Yin, Xiaobo Zhang, Ping Li, Xuenan Gu, and Yubo Fan. 2022. "Recent Advances in the Development of Magnesium-Based Alloy Guided Bone Regeneration (GBR) Membrane" Metals 12, no. 12: 2074. https://doi.org/10.3390/met12122074
APA StyleChen, K., Zhao, L., Huang, C., Yin, X., Zhang, X., Li, P., Gu, X., & Fan, Y. (2022). Recent Advances in the Development of Magnesium-Based Alloy Guided Bone Regeneration (GBR) Membrane. Metals, 12(12), 2074. https://doi.org/10.3390/met12122074









