Preparation and Characterization of Synchronous Chemical Conversion Coating on 6061 Aluminum Alloy/7075 Aluminum Alloy/Galvanized Steel Substrates
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
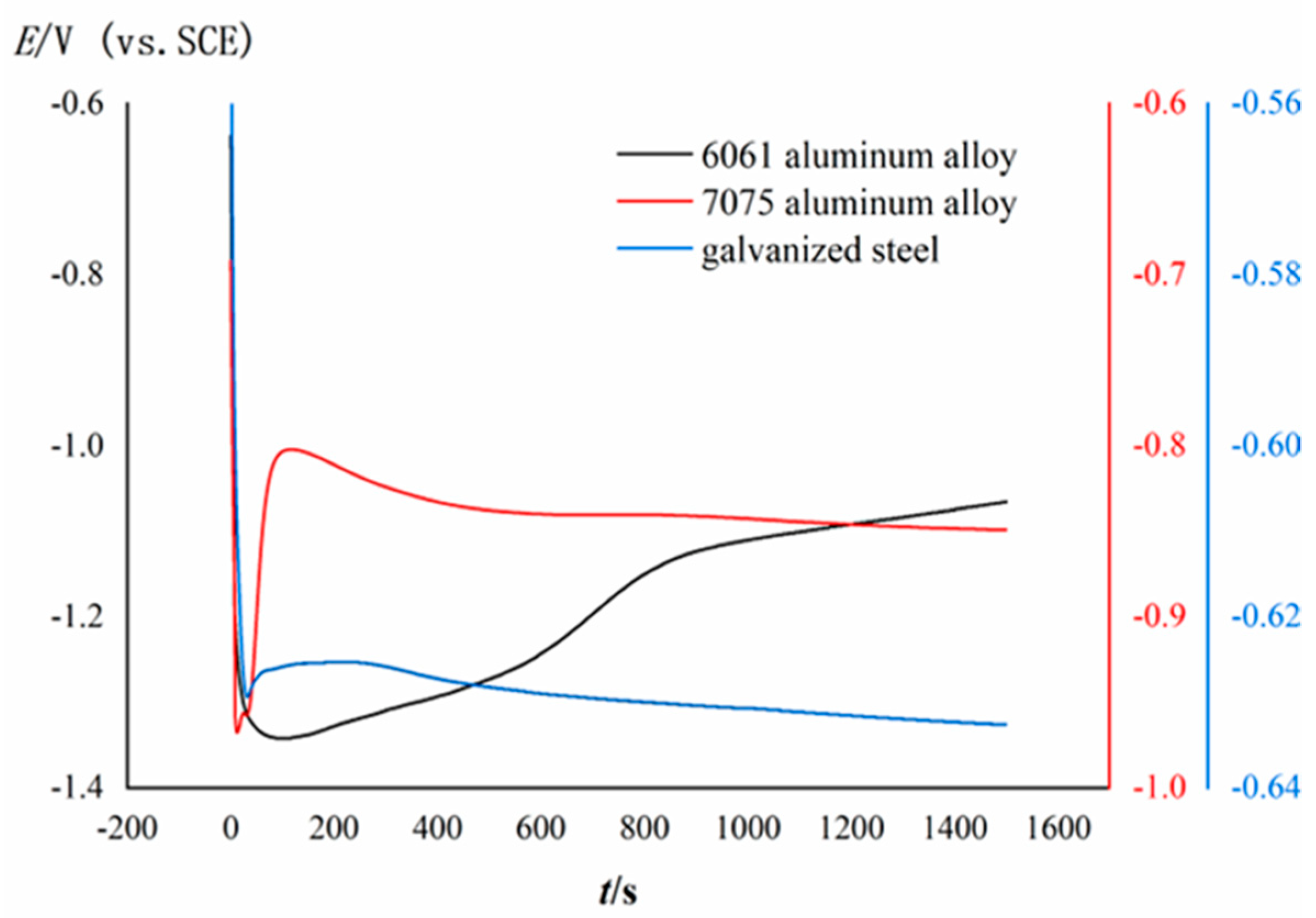
3.1. OCP Measurements during the Formation of Conversion Coating
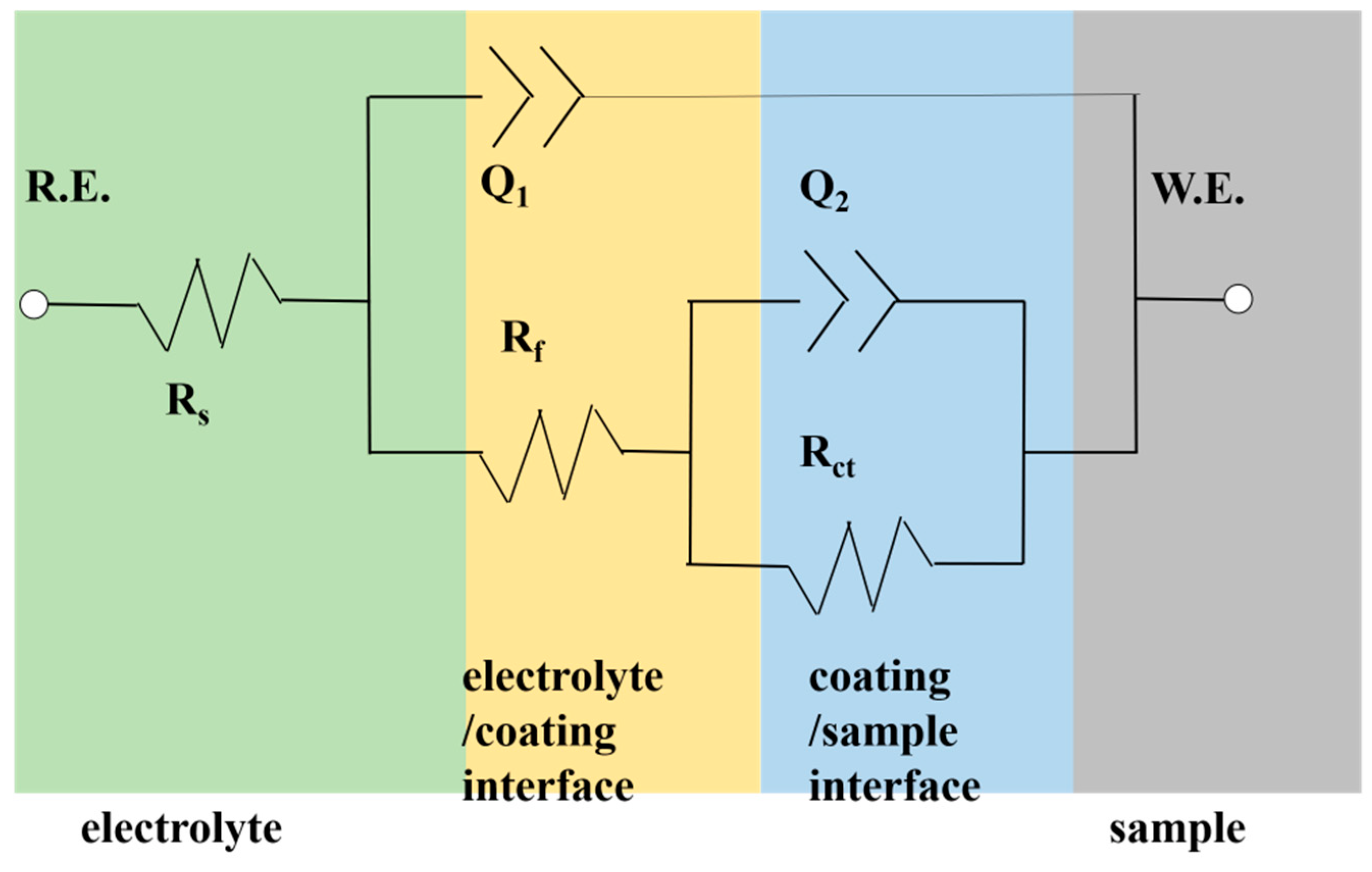
3.2. Electrochemical Analysis
3.3. Surface Characterization
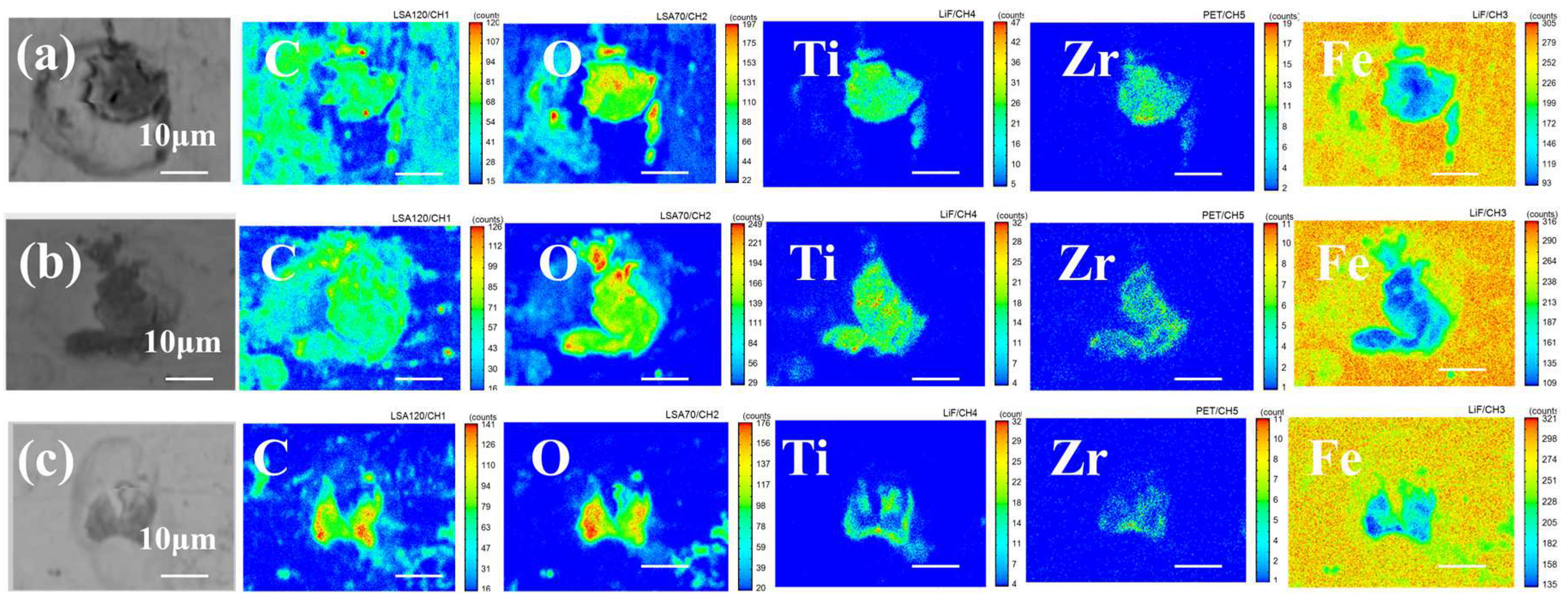
3.4. EPMA Analysis
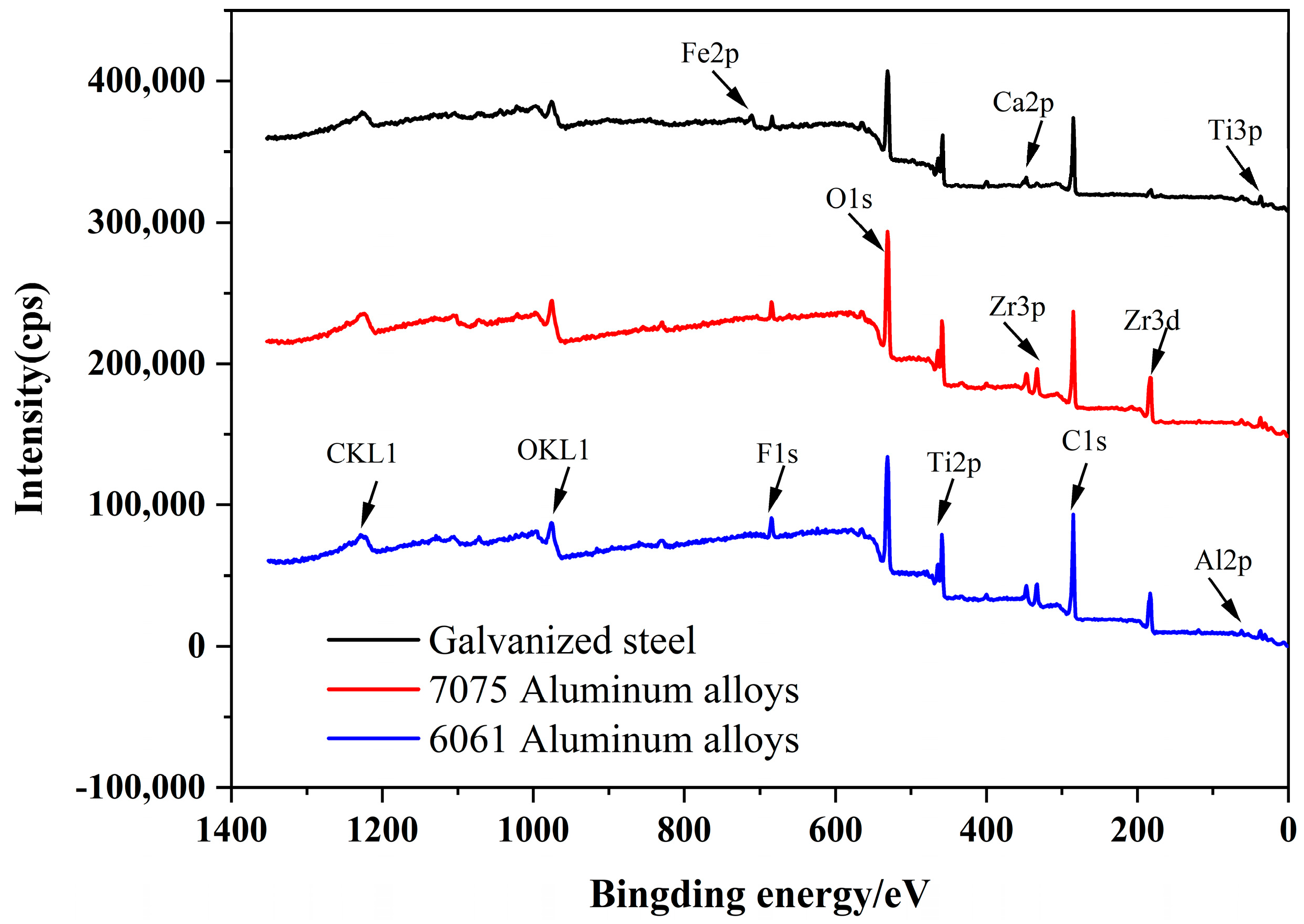
3.5. XPS Analysis
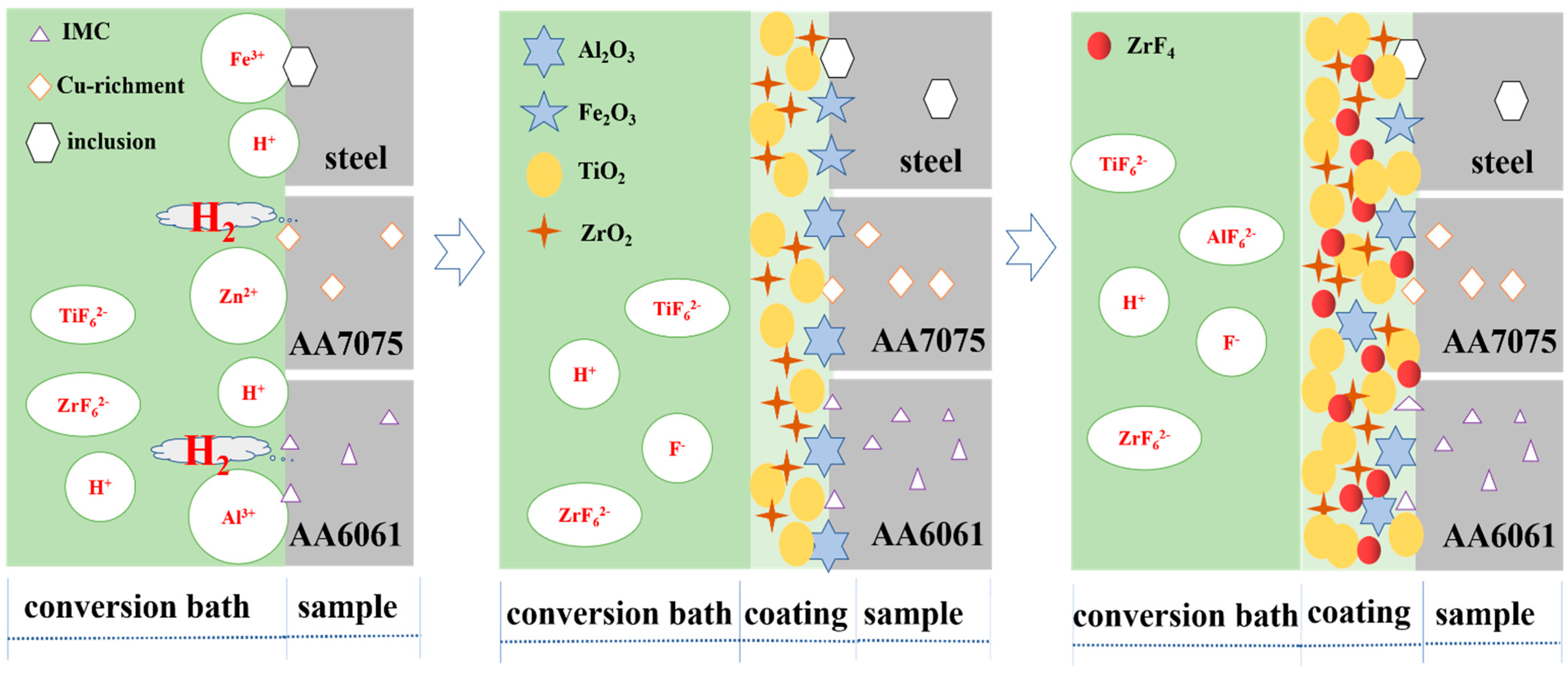
3.6. Discussion of the Formation of Synchronous Chemical Conversion Coatings
- A.
- The dissolution of Al or Zn/Fe on the substrate surface in a conversion bath [45].
- B.
- C.
- The dissolution of the micro anode and the formation of conversion coatings in the micro cathode region reach a dynamic equilibrium.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fockaert, L.; Pletincx, S.; Ganzinga-Jurg, D.; Boelen, B.; Hauffman, T.; Terryn, H.; Mol, J. Chemisorption of polyester coatings on zirconium-based conversion coated multi-metal substrates and their stability in aqueous environment. Appl. Surf. Sci. 2019, 508, 144771. [Google Scholar] [CrossRef]
- Hung, S.-M.; Lin, H.; Chen, H.-W.; Chen, S.-Y.; Lin, C.-S. Corrosion resistance and electrical contact resistance of a thin permanganate conversion coating on dual-phase LZ91 Mg–Li alloy. J. Mater. Res. Technol. 2021, 11, 1953–1968. [Google Scholar] [CrossRef]
- Riyas, A.H.; Geethanjali, C.V.; Arathy, S.; Anil, A.; Shibli, S.M.A. Exploration and tuning of Al2O3/Mo composite for enhancement of anti-corrosion and tribological characteristics in zinc phosphate conversion coatings. Appl. Surf. Sci. 2022, 593, 153370. [Google Scholar] [CrossRef]
- Verbruggen, H.; Baert, K.; Terryn, H.; De Graeve, I. Molybdate-phosphate conversion coatings to protect steel in a simulated concrete pore solution. Surf. Coat. Technol. 2019, 361, 280–291. [Google Scholar] [CrossRef]
- Xian, Z.; Gui, Y.; Yan, J.; Zhao, X.; Lu, Y. Facile preparation of hopeite coating on stainless steel by chemical conversion method. Surf. Coat. Technol. 2014, 240, 361–364. [Google Scholar]
- Qi, J.-T.; Hashimoto, T.J.R.; Walton, X.; Zhou, P.; Skeldon, G.E. Thompson. Trivalent chromium conversion coating formation on aluminium. Surf. Coat. Technol. 2015, 280, 317–329. [Google Scholar] [CrossRef]
- Munson, C.A.; McFall-Boegeman, S.A.; Swain, G.M. Cross comparison of TCP conversion coating performance on aluminum alloys during neutral salt-spray and thin-layer mist accelerated degradation testing. Electrochim. Acta 2018, 282, 171–184. [Google Scholar] [CrossRef]
- Bouali, A.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.; Zheludkevich, M. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Cho, S.; Cubides, Y.; Castaneda, H. Probing the degradation mechanism of a Cr(VI) coating/aluminum alloy 2024-T3 system based on dynamic mechanisms and a 2D deterministic-probabilistic approach. Electrochim. Acta 2017, 236, 82–96. [Google Scholar] [CrossRef]
- Mohammadloo, H.E.; Sarabi, A. Titanium-phytic acid nano structured conversion coating formation on CRS substrate. Prog. Org. Coat. 2016, 101, 391–399. [Google Scholar] [CrossRef]
- Yoganandan, G.J.N.; Balaraju, V.K.; Grips, W. The surface and electrochemical analysis of permanganate based conversion coating on alclad and unclad 2024 alloy. Appl. Surf. Sci. 2012, 258, 8880–8888. [Google Scholar] [CrossRef]
- Mao, S.; Li, W.; Zeng, X.; Yi, A.; Liao, Z.; Zhu, W. Multiple transitional metal oxides conversion coating on AA6063 toward corrosion protection and electrical conductivity. Surf. Coat. Technol. 2020, 397, 125819. [Google Scholar] [CrossRef]
- Lakshmi, R.; Sampath, S.; Aruna, S. Silica-alumina based sol-gel coating containing cerium oxide nanofibers as a potent alternative to conversion coating for AA2024 alloy. Surf. Coat. Technol. 2021, 411, 127007. [Google Scholar] [CrossRef]
- Chen, L.-A.; Lu, Y.-S.; Lin, Y.-T.; Lee, Y.-L. Preparation and characterization of cerium-based conversion coating on a Fe50Mn30Co10Cr10 dual-phase high-entropy alloy. Appl. Surf. Sci. 2021, 562, 150200. [Google Scholar] [CrossRef]
- Gea, B.; Jk, C.; Im, A. Prolonged protection by zirconium conversion coatings of AlSi7Mg0.3 aluminium alloy in chloride solution. Corros. Sci. 2020, 169, 108615. [Google Scholar]
- Zhu, W.; Li, W.; Mu, S.; Fu, N.; Liao, Z. Comparative study on Ti/Zr/V and chromate conversion treated aluminum alloys: Anti-corrosion performance and epoxy coating adhesion properties. Appl. Surf. Sci. 2017, 405, 157–168. [Google Scholar] [CrossRef]
- Mohammadloo, H.E.; Sarabi, A. Titanium composite conversion coating formation on CRS In the presence of Mo and Ni ions: Electrochemical and microstructure characterizations. Appl. Surf. Sci. 2016, 387, 252–259. [Google Scholar] [CrossRef]
- Coloma, P.S.; Izagirre, U.; Belaustegi, Y.; Jorcin, J.; Cano, F.; Lapeña, N. Chromium-free conversion coatings based on inorganic salts (Zr/Ti/Mn/Mo) for aluminum alloys used in aircraft applications. Appl. Surf. Sci. 2015, 345, 24–35. [Google Scholar] [CrossRef]
- Nordlien, J.; Walmsley, J.; Østerberg, H.; Nisancioglu, K. Formation of a zirconium-titanium based conversion layer on AA 6060 aluminium. Surf. Coat. Technol. 2002, 153, 72–78. [Google Scholar] [CrossRef]
- Lunder, O.; Simensen, C.; Yu, Y.; Nisancioglu, K. Formation and characterisation of Ti–Zr based conversion layers on AA6060 aluminium. Surf. Coat. Technol. 2004, 184, 278–290. [Google Scholar] [CrossRef]
- Andreatta, F.; Turco, A.; de Graeve, I.; Terryn, H.; de Wit, J.; Fedrizzi, L. SKPFM and SEM study of the deposition mechanism of Zr/Ti based pre-treatment on AA6016 aluminum alloy. Surf. Coat. Technol. 2007, 201, 7668–7685. [Google Scholar] [CrossRef]
- Salil, S.; Caterina, Z. A localized study on the influence of surface preparation on the reactivity of cast Al-7Si-1Fe and Al-7Si-2Cu-1Fe alloys and their effect on cerium conversion coating deposition. Appl. Surf. Sci. 2022, 585, 152730. [Google Scholar]
- Zhang, S.-H.; Kong, G.; Lu, J.-T.; Che, C.-S.; Liu, L.-Y. Growth behavior of lanthanum conversion coating on hot-dip galvanized steel. Surf. Coat. Technol. 2014, 259, 654–659. [Google Scholar] [CrossRef]
- Boyer, Q.; Ortega Vega, M.R.; De Fraga Malfatti, C.; Duluard, S.; Ansart, F. Correlation between morphology and electrochemical behavior of chromium-free conversion coatings for aluminum alloys corrosion protection. Surf. Coat. Technol. 2018, 351, 115–127. [Google Scholar] [CrossRef]
- Zhan, W.; Qian, X.; Gui, B.; Liu, L.; Hu, L. Preparation and corrosion resistance of titanium–zirconium–cerium based conver-sion coating on 6061 aluminum alloy. Mater. Corros. 2019, 71, 1–11. [Google Scholar]
- Amaya, J.M.S.; Blanco, G.; Garcia-Garcia, F.J.; Bethencourt, M.; Botana, F. XPS and AES analyses of cerium conversion coatings generated on AA5083 by thermal activation. Surf. Coat. Technol. 2012, 213, 105–116. [Google Scholar] [CrossRef]
- Duarte, T.; Meyer, Y.A.; Osório, W.R. The Holes of Zn Phosphate and Hot Dip Galvanizing on Electrochemical Behaviors of Multi–Coatings on Steel Substrates. Metals 2022, 12, 863. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. Electrochemical behavior and compressive strength of Al-Cu/xCu composites in NaCl solution. J. Solid State Electrochem. 2021, 25, 1303–1317. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Menezes, I.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. EIS investigation of the corrosion behavior of steel bars embedded into modified concretes with eggshell contentes. Metals 2022, 12, 417. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-Phase-Element Behavior Caused by Resistivity Distributions in Films. J. Electrochem. Soc. 2010, 157, C458–C463. [Google Scholar] [CrossRef]
- Valdez, B.; Kiyota, S.; Stoytcheva, M.; Zlatev, R.; Bastidas, J. Cerium-based conversion coatings to improve the corrosion resistance of aluminium alloy 6061-T6. Corros. Sci. 2014, 87, 141–149. [Google Scholar] [CrossRef]
- Jia, X.; Song, J.; Xiao, B.; Liu, Q.; Zhao, H.; Yang, Z.; Liao, J.; Wu, L.; Jiang, B.; Atrens, A.; et al. Influence of indentation size on the corrosion behaviour of a phosphate conversion coated AZ80 magnesium alloy. J. Mater. Res. Technol. 2021, 14, 1739–1753. [Google Scholar] [CrossRef]
- Qi, J.; Němcová, A.; Walton, J.R.; Zhou, X.; Skeldon, P.; Thompson, G.E. Influence of pre-and post-treatments on formation of a trivalent chromium conversion coating on AA2024 alloy. Thin Solid Films 2016, 616, 270–278. [Google Scholar] [CrossRef]
- Ekularac, G.; Kova, J.; Milošev, I. Comparison of the Electrochemical Behaviour and Self-sealing of Zirconium Conversion Coatings Applied on Aluminium Alloys of series 1xxx to 7xxx. J. Electrochem. Soc. 2020, 167, 111506. [Google Scholar] [CrossRef]
- Campestrini, P.; Terryn, H.; Hovestad, A.; De Wit, J.H.W. Formation of a cerium-based conversion coating on AA2024: Rela-tionship with the microstructure. Surf. Coat. Technol. 2003, 176, 365–381. [Google Scholar] [CrossRef]
- Verdalet-Guardiola, X.; Bonino, J.-P.; Duluard, S.; Fori, B.; Blanc, C. Influence of the alloy microstructure and surface state on the protective properties of trivalent chromium coatings grown on a 2024 aluminium alloy. Surf. Coat. Technol. 2018, 344, 276–287. [Google Scholar] [CrossRef]
- Viroulaud, R.; Światowska, J.; Seyeux, A.; Zanna, S.; Tardelli, J.; Marcus, P. Influence of surface pretreatments on the quality of trivalent chromium process coatings on aluminum alloy. Appl. Surf. Sci. 2017, 423, 927–938. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; Mu, S.; Yang, Y.; Zuo, X. The adhesion performance of epoxy coating on AA6063 treated in Ti/Zr/V based solution. Appl. Surf. Sci. 2016, 384, 333–340. [Google Scholar] [CrossRef]
- Zuo, X.; Li, W.; Mu, S.; Du, J.; Yang, Y.; Tang, P. Investigation of composition and structure for a novel Ti–Zr chemical conversion coating on 6063 aluminum alloy. Prog. Org. Coat. 2015, 87, 61–68. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, X.; Jia, Y.; Liu, Y. Self-repairing vanadium–zirconium composite conversion coating for aluminum alloys. Appl. Surf. Sci. 2013, 280, 489–493. [Google Scholar] [CrossRef]
- Niu, L.; Guo, R.; Tang, C.; Guo, H.; Chen, J. Surface characterization and corrosion resistance of fluoferrite conversion coating on carbon steel. Surf. Coat. Technol. 2016, 300, 110–117. [Google Scholar] [CrossRef]
- Díaz, B.; Freire, L.; Mojío, M.; Nóvoa, X. Optimization of conversion coatings based on zinc phosphate on high strength steels, with enhanced barrier properties. J. Electroanal. Chem. 2015, 737, 174–183. [Google Scholar] [CrossRef]
- Yoganandan, G.; Premkumar, K.P.; Balaraju, J.N. Evaluation of corrosion resistance and self-healing behavior of zirconium-cerium conversion coating developed on AA2024 alloy. Surf. Coat. Technol. 2015, 270, 249–258. [Google Scholar] [CrossRef]
- Mohammadloo, H.E.; Sarabi, A.; Hosseini, R.M.; Sarayloo, M.; Sameie, H.; Salimi, R. A comprehensive study of the green hexafluorozirconic acid-based conversion coating. Prog. Org. Coat. 2014, 77, 322–330. [Google Scholar] [CrossRef]
- Cerezo, J.; Vandendael, I.; Posner, R.; Lill, K.; de Wit, J.; Mol, J.; Terryn, H. Initiation and growth of modified Zr-based conversion coatings on multi-metal surfaces. Surf. Coat. Technol. 2013, 236, 284–289. [Google Scholar] [CrossRef]






| Samples | icorr/μA·cm−2 | Ecorr/V | Rct/Ω | Rs/Ω | Rf/Ω | Q1 | Q2 |
|---|---|---|---|---|---|---|---|
| with conversion AA6061 | 0.174 | −1.084 | 84610 | 8.66 | 3675 | 6.317 × 10−5 | 9.334 × 10−5 |
| without conversion AA6061 | 1.096 | −1.151 | 0.268 | 12.97 | 919 | 8.661 × 10−4 | 6.221 × 10−4 |
| with conversion AA7075 | 0.018 | −0.603 | 23250 | 7.03 | 2876 | 9.112 × 10−5 | 3.666 × 10−5 |
| without conversion AA7075 | 1.470 | −0.653 | 19330 | 11.64 | 799 | 7.663 × 10−4 | 2.729 × 10−4 |
| with conversion galvanized steel | 1.012 | −0.838 | 13010 | 6.07 | 2017 | 1.117 × 10−4 | 3.915 × 10−4 |
| without conversion galvanized steel | 6.312 | −0.938 | 5441 | 9.88 | 366 | 7.664 × 10−4 | 9.226 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, W.; Li, X.; Qian, X.; Li, Y.; Ding, Y.; Zu, Y.; Xie, F.; Tian, F. Preparation and Characterization of Synchronous Chemical Conversion Coating on 6061 Aluminum Alloy/7075 Aluminum Alloy/Galvanized Steel Substrates. Metals 2022, 12, 2011. https://doi.org/10.3390/met12122011
Zhan W, Li X, Qian X, Li Y, Ding Y, Zu Y, Xie F, Tian F. Preparation and Characterization of Synchronous Chemical Conversion Coating on 6061 Aluminum Alloy/7075 Aluminum Alloy/Galvanized Steel Substrates. Metals. 2022; 12(12):2011. https://doi.org/10.3390/met12122011
Chicago/Turabian StyleZhan, Wen, Xinxiang Li, Xuzhen Qian, Yingpeng Li, Yunhu Ding, Yunhe Zu, Fan Xie, and Feng Tian. 2022. "Preparation and Characterization of Synchronous Chemical Conversion Coating on 6061 Aluminum Alloy/7075 Aluminum Alloy/Galvanized Steel Substrates" Metals 12, no. 12: 2011. https://doi.org/10.3390/met12122011
APA StyleZhan, W., Li, X., Qian, X., Li, Y., Ding, Y., Zu, Y., Xie, F., & Tian, F. (2022). Preparation and Characterization of Synchronous Chemical Conversion Coating on 6061 Aluminum Alloy/7075 Aluminum Alloy/Galvanized Steel Substrates. Metals, 12(12), 2011. https://doi.org/10.3390/met12122011





