The Preparation, Corrosion Resistance and Formation Mechanism of a New-Type Mo-Based Composite Conversion Coating on 6061 Aluminum Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of pH and CTI
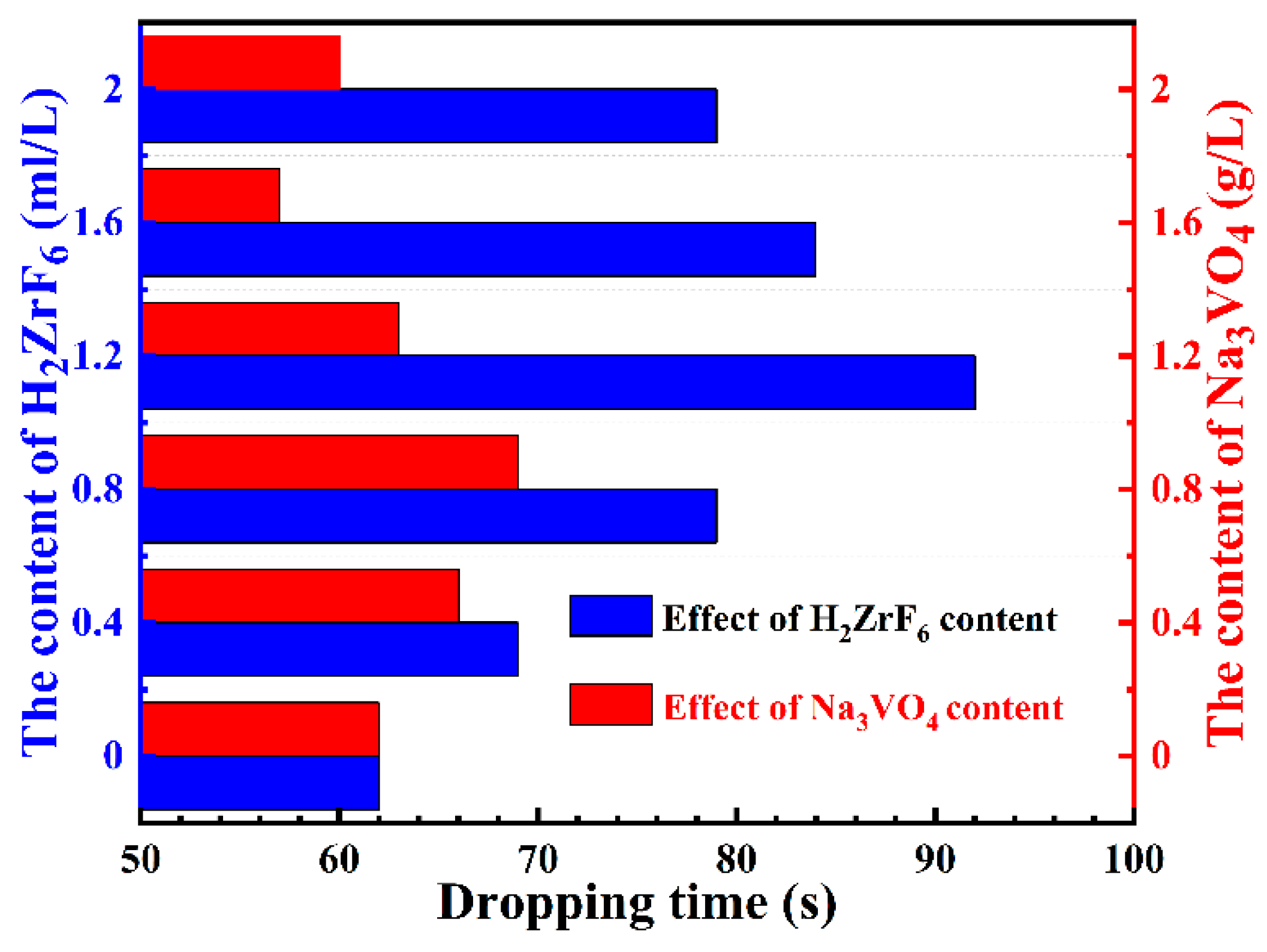
3.2. Effect of Na3VO4 and H2ZrF6 Content
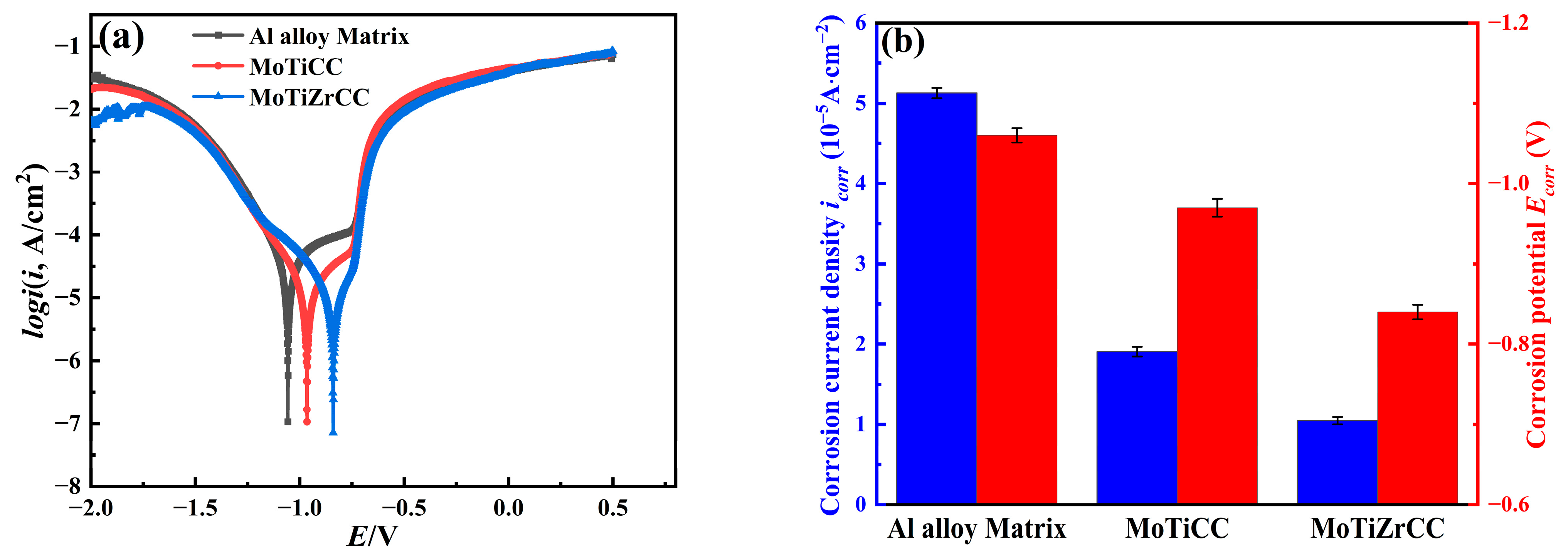
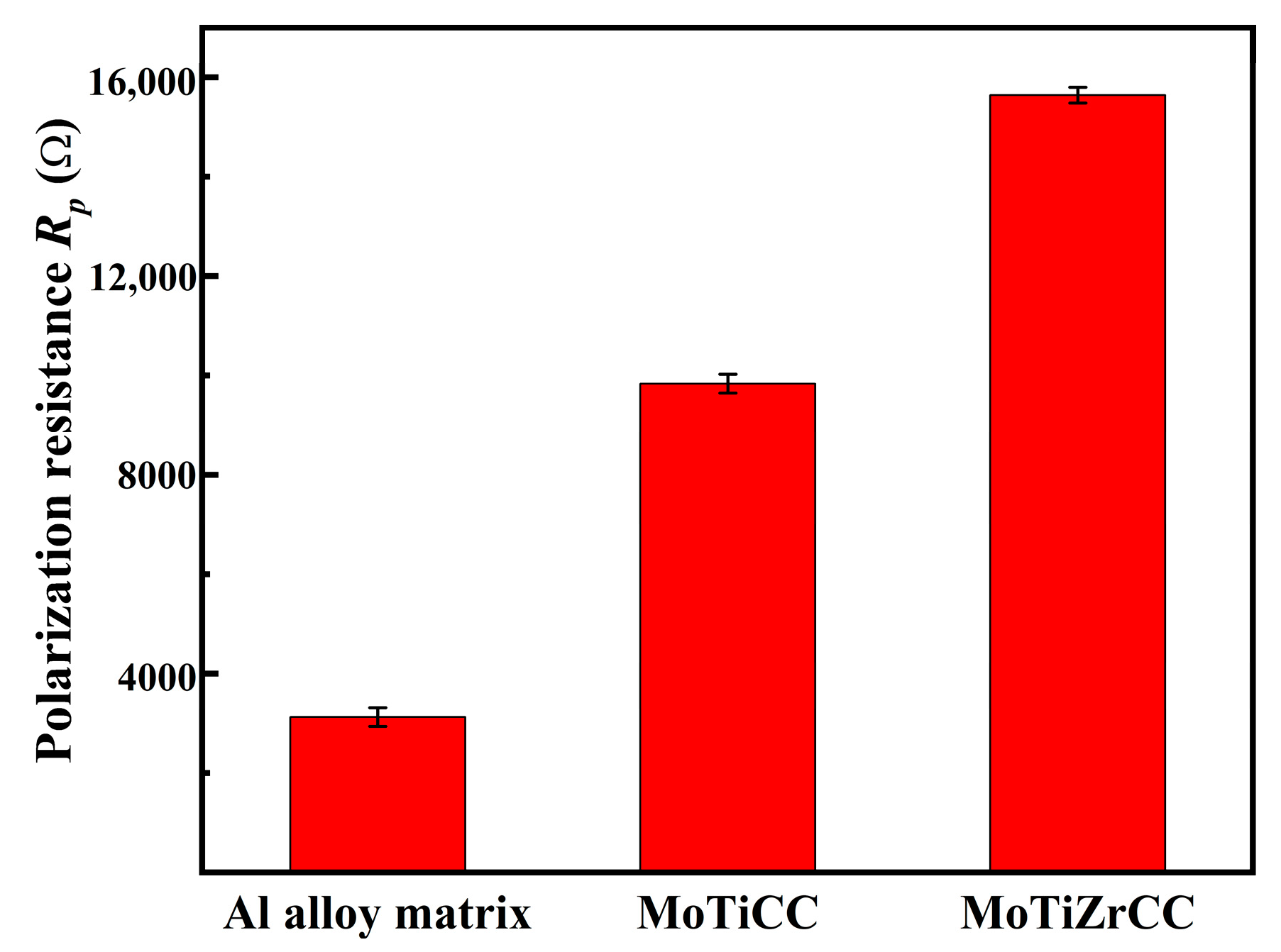
3.3. Electrochemical Analysis
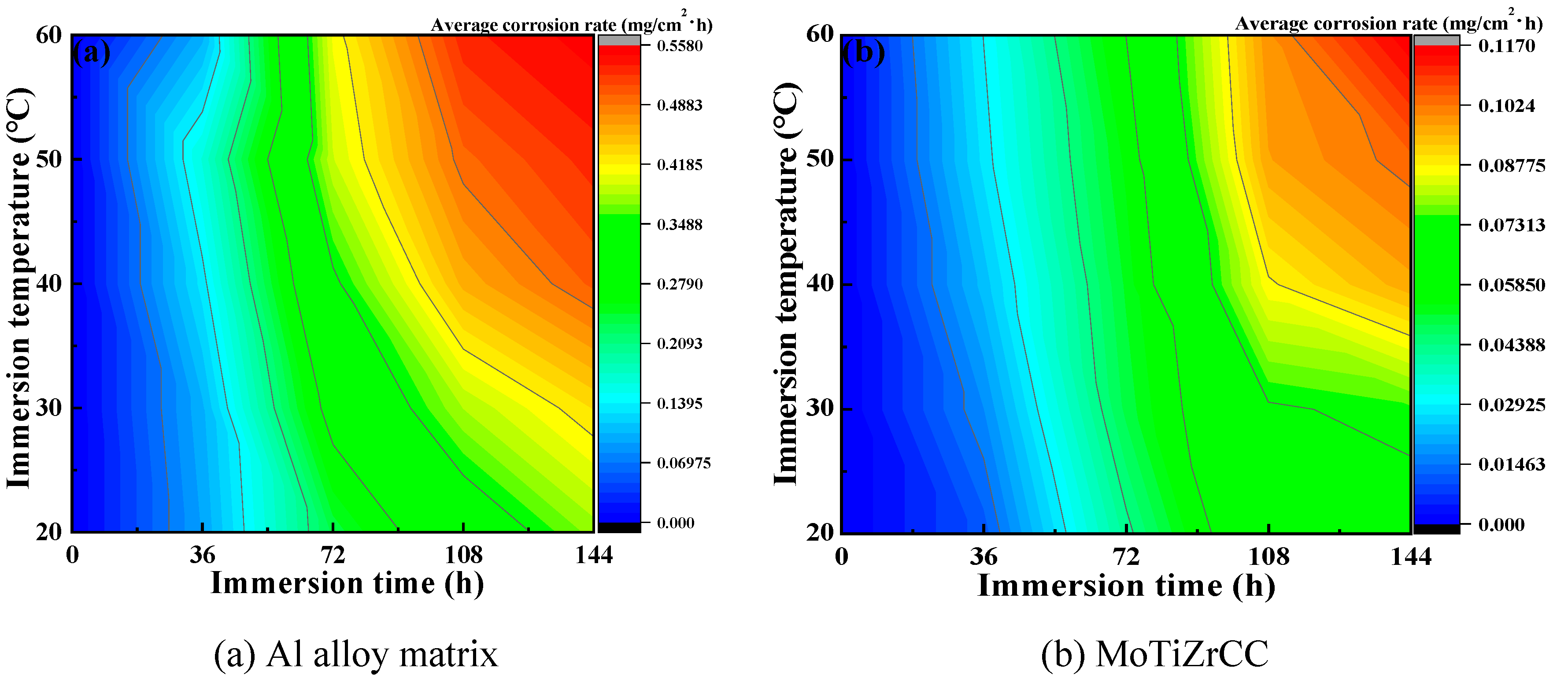
3.4. Immersion Test
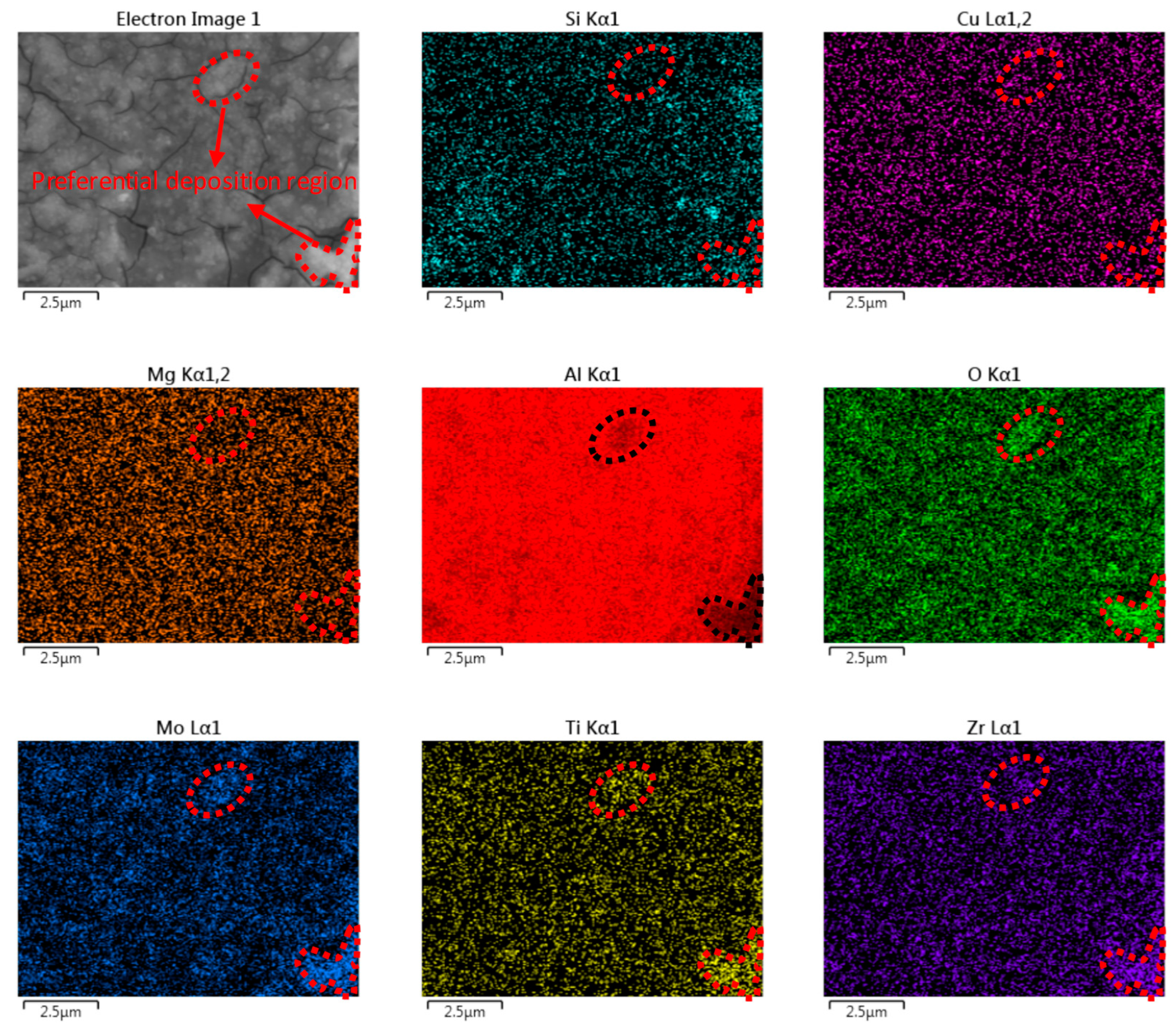
3.5. Morphology and Composition Analysis
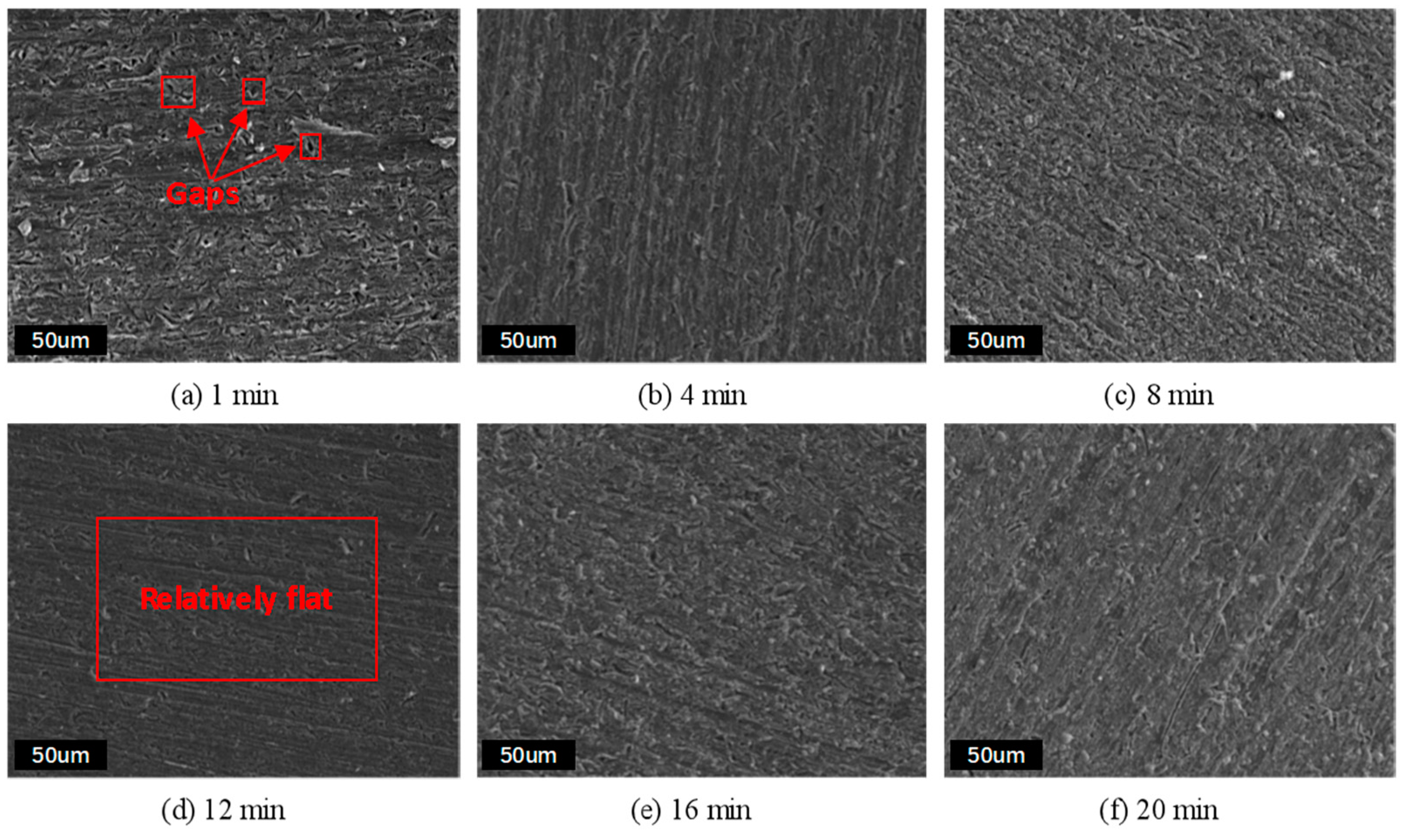
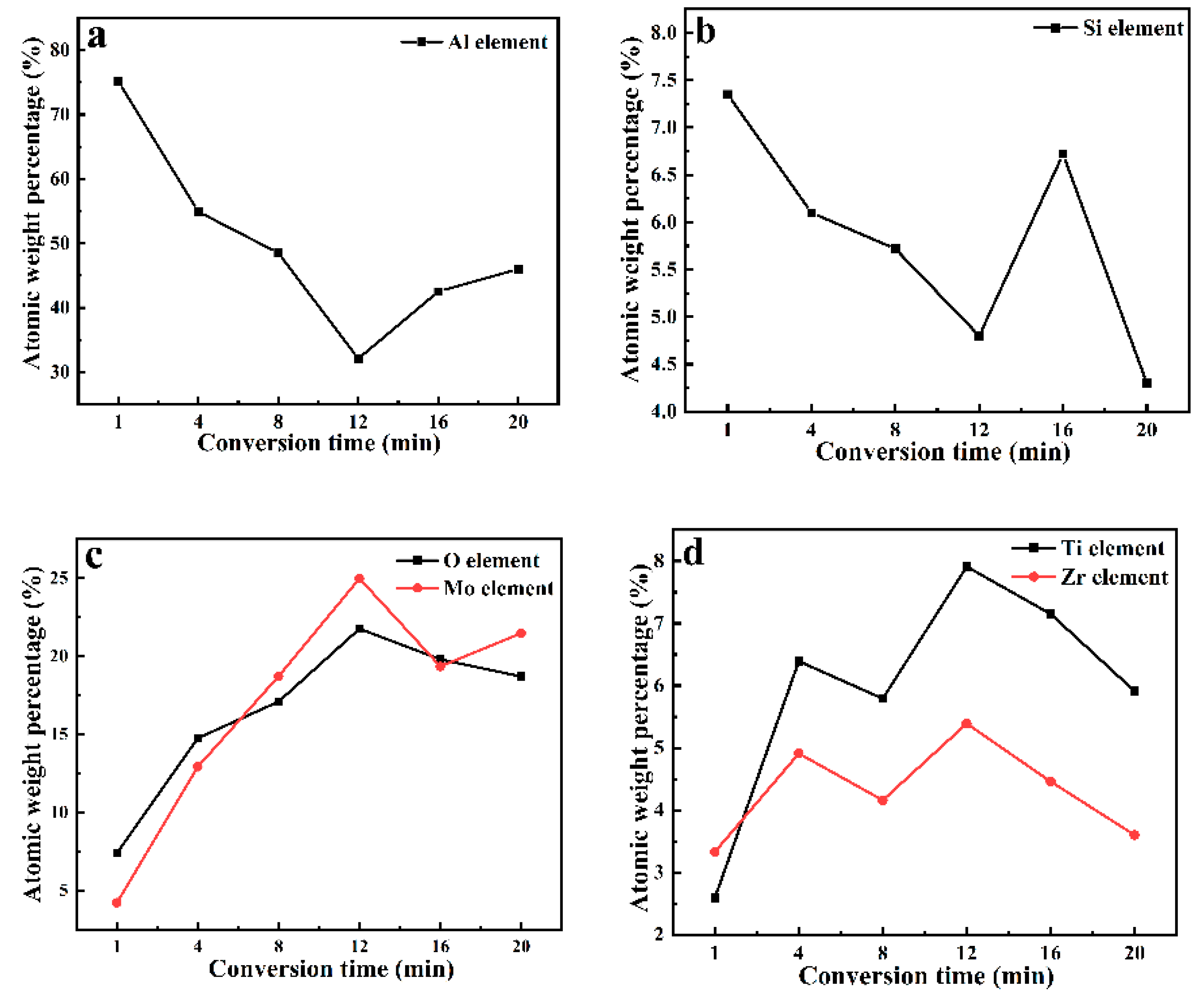
3.5.1. SEM and EDS Analysis
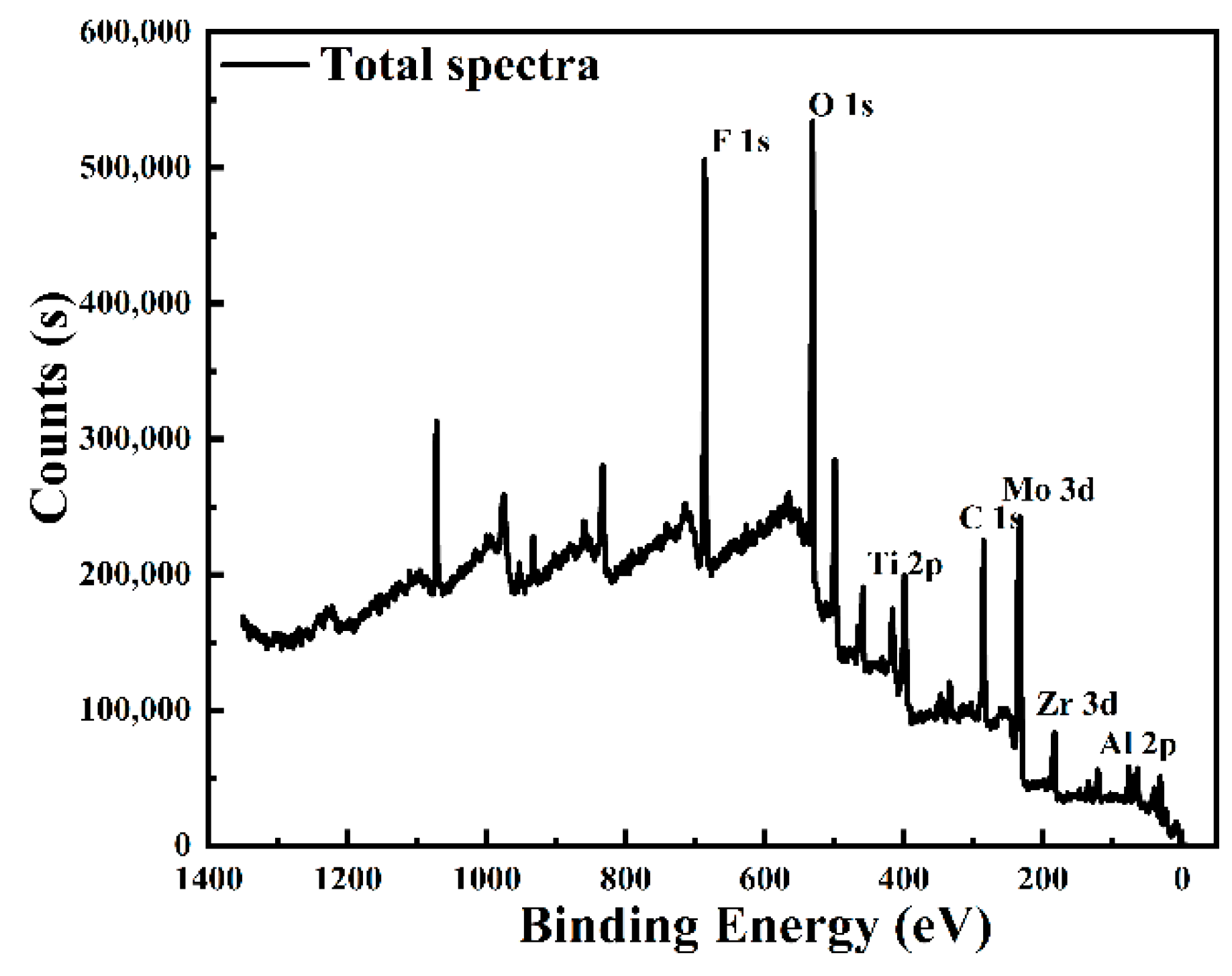
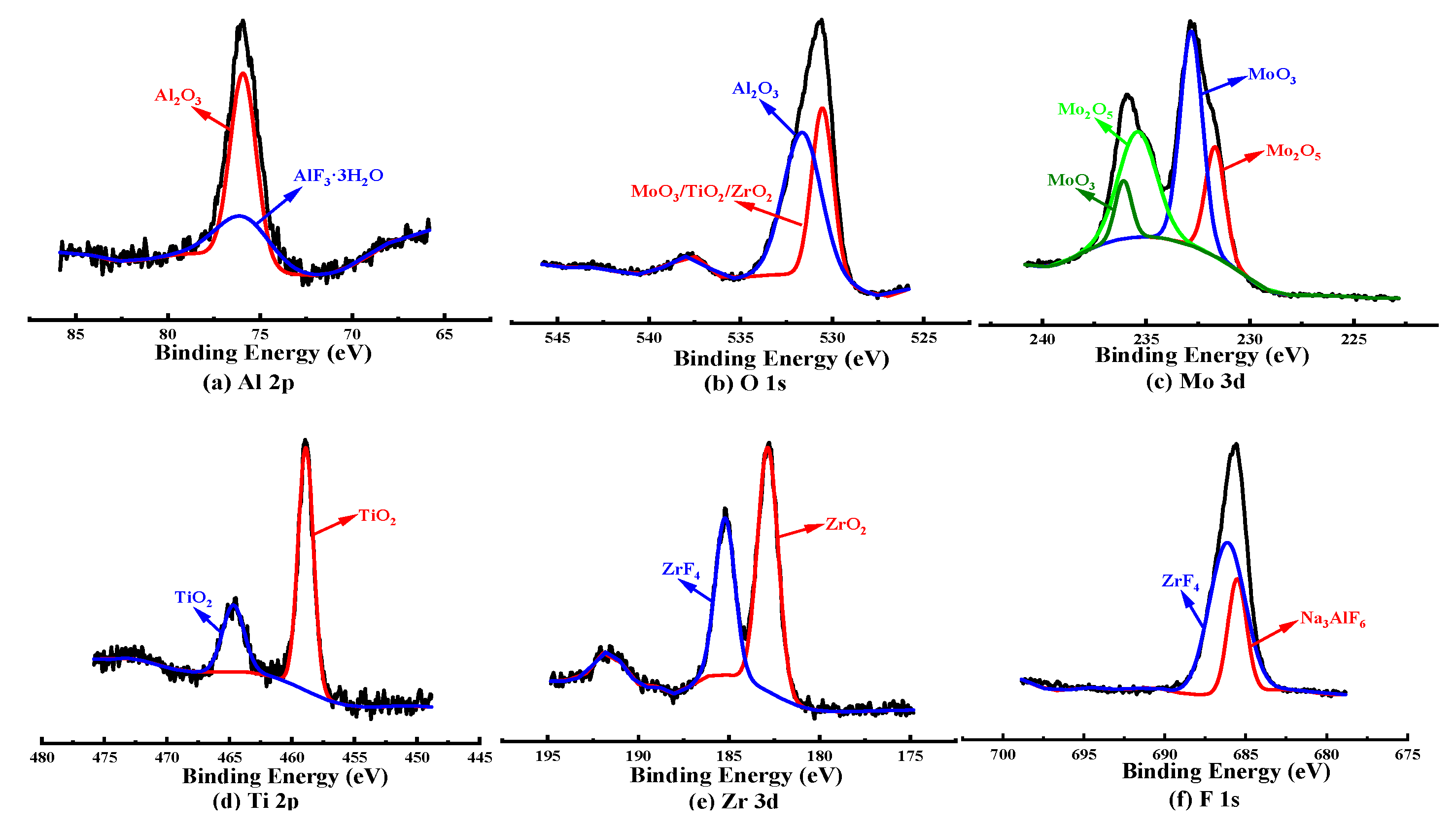
3.5.2. XPS Analysis
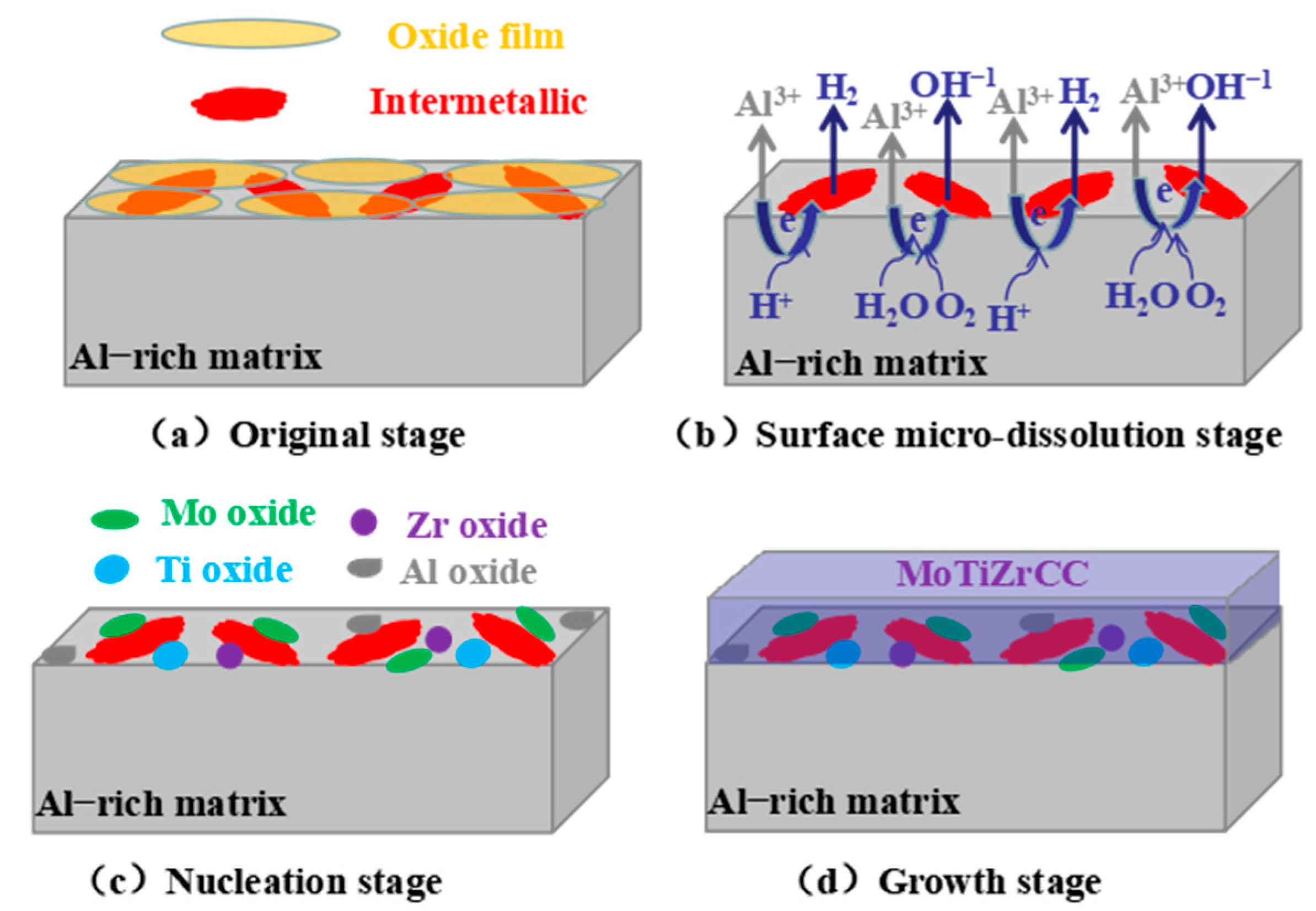
3.6. Formation Mechanism of MoTiZrCC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Ou, X.; Zhang, X. Mode, technology, energy consumption, and resulting CO2 emissions in China’s transport sector up to 2050. Energ. Policy 2017, 109, 719–733. [Google Scholar] [CrossRef]
- Hosseinzadeh, F.; Rastegar, S.; Ashengroph, M. Bioleaching of rare earth elements from spent automobile catalyst as pretreatment method to improve Pt and Pd recovery: Process optimization and kinetic study. Proc. Biochem. 2021, 105, 1–7. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Hao, J.; Liu, H.; Wu, X.; Hu, J.; Walsh, M.; Wallington, T.; Zhang, K.; Stevanovic, S. On-road vehicle emissions and their control in China: A review and outlook. Sci. Total. Environ. 2017, 574, 332–349. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, F.; Wan, X. Optimal Design and Experimental Investigations of Aluminium Sheet for Lightweight of Car Hood. Mater. Today Proc. 2015, 2, 5029–5036. [Google Scholar] [CrossRef]
- Iqbal, M.; Sun, L.; Lachance, A.; Ding, H.; Fedel, M. In situ growth of a CaAl-NO3-layered double hydroxide film directly on an aluminum alloy for corrosion resistance. Dalton. Trans. 2020, 49, 3956–3964. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, B.; Wang, K.; Li, S.; Zhang, Y. Friction behaviors of 6061 aluminum alloy sheets in hot stamping under dry and lubricated conditions based on hot strip drawing test. Tribol. Int. 2020, 151, 106504. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y. Corrosion Resistance of Aluminum Fluoride Modified 6061 Aluminum Alloy. Mater. Lett. 2021, 298, 129932. [Google Scholar] [CrossRef]
- El-Meligi, A.; Sanad, S.; Ismail, A.; Barakat, A. Corrosion penetration and crystal structure of AA5022 in HCl solution and rare earth elements. J. Mater. Sci. Technol. 2005, 21, 324–330. [Google Scholar] [CrossRef]
- Nicolo, A.; Paussa, L.; Gobessi, A.; Lanzutti, A.; Cepek, C.; Andreatta, F.; Fedrizzi, L. Cerium conversion coating and sol–gel multilayer system for corrosion protection of AA6060. Surf. Coat. Tech. 2016, 287, 33–43. [Google Scholar] [CrossRef]
- Zhan, W.; Li, X.; Qian, X.; Li, Y.; Ding, Y.; Zu, Y.; Xie, F.; Tian, F. Preparation and Characterization of Synchronous Chemical Conversion Coating on 6061 Aluminum Alloy/7075 Aluminum Alloy/Galvanized Steel Substrates. Metals 2022, 12, 2011. [Google Scholar] [CrossRef]
- Díaz, B.; Freire, L.; Mojío, M.; Novoa, X. Optimization of conversion coatings based on zinc phosphate on high strength steels, with enhanced barrier properties. J. Electroanal. Chem. 2015, 737, 174–183. [Google Scholar] [CrossRef]
- Mahidashti, Z.; Shahrabi, T.; Ramezanzadeh, B. A new strategy for improvement of the corrosion resistance of a green cerium conversion coating through thermal treatment procedure before and after application of epoxy coating. Appl. Surf. Sci. 2016, 390, 623–632. [Google Scholar] [CrossRef]
- Roshan, S.; Sarabi, A. Improved performance of Ti-based conversion coating in the presence of Ce/Co ions: Surface characterization, electrochemical and adhesion study. Surf. Coat. Tech. 2021, 410, 126931. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, Z.; Buhe, B.; Wang, J.; Mao, L.; Liu, B.; Zhou, P.; Zhao, Y.; Zhang, T.; Wang, F. Effect of Temperature on Corrosion Resistance of Layered Double Hydroxides Conversion Coatings on Magnesium Alloys Based on a Closed-Cycle System. Metals 2021, 11, 1658. [Google Scholar] [CrossRef]
- Munson, C.; McFall-Boegeman, S.; Swain, G. Cross comparison of TCP conversion coating performance on aluminum alloys during neutral salt-spray and thin-layer mist accelerated degradation testing. Electrochim. Acta 2018, 282, 171–184. [Google Scholar] [CrossRef]
- Qian, X.; Zhao, W.; Zhan, W.; Zhang, T.; Pan, J.; Liu, Y. Formation and corrosion resistance of a novel Co-Ti-Mo composite chromium-free chemical conversion coating on LY12 aluminum alloy. Mater. Corros. 2022, 73, 710–719. [Google Scholar] [CrossRef]
- Bouali, A.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.; Zheludkevich, M. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, S.; Xu, S. Preparation and adhesive properties of colored Ti/Zr-based conversion coating on aluminum foil for lithium battery packaging. Surf. Interface. Anal. 2019, 51, 190–198. [Google Scholar] [CrossRef]
- Coloma, P.; Izagirre, U.; Belaustegi, Y.; Jorcin, J.; Canoa, F.; Lapeña, N. Chromium-free conversion coatings based on inorganic salts (Zr/Ti/Mn/Mo) for aluminum alloys used in aircraft applications. Appl. Surf. Sci. 2015, 345, 24–35. [Google Scholar] [CrossRef]
- Alba-Galvín, J.J.; González-Rovira, L.; Botana, F.J.; Lekka, M.; Andreatta, F.; Fedrizzi, L.; Bethencourt, M. Application of Commercial Surface Pretreatments on the Formation of Cerium Conversion Coating (CeCC) over High-Strength Aluminum Alloys 2024-T3 and 7075-T6. Metals 2021, 11, 930. [Google Scholar] [CrossRef]
- Balaraju, J.; Srinivasan, A.; Yoganandan, G.; Grips, V.; Rajam, K. Effect of Mn/Mo incorporated oxide layer on the corrosion behavior of AA 2024 alloy. Corros. Sci. 2011, 53, 4084–4092. [Google Scholar] [CrossRef]
- Becker, M. Chromate-free chemical conversion coatings for aluminum alloys. Corros. Rev. 2019, 37, 321–342. [Google Scholar] [CrossRef]
- Qian, X.; Zhan, W.; Pan, J.; Liu, Y.; Huang, F.; Wang, B. Improving the corrosion resistance of LY12 aluminum alloy via a novel Mo-Zr-Ti composite conversion coating. Mater. Res. Express. 2021, 8, 036403. [Google Scholar] [CrossRef]
- Cerezo, J.; Taheri, P.; Vandendael, I.; Posner, R.; Lill, K.; Wit, J.; Mol, J.; Terryn, H. Influence of surface hydroxyls on the formation of Zr-based conversion coatings on AA6014 aluminum alloy. Surf. Coat. Tech. 2014, 254, 277–283. [Google Scholar] [CrossRef]
- Guo, G.; Song, D.; Jiang, J.; Ma, A.; Zhang, L.; Li, C. Effect of Synthesizing Temperature on Microstructure and Electrochemical Property of the Hydrothermal Conversion Coating on Mg-2Zn-0.5Mn-Ca-Ce Alloy. Metals 2016, 6, 44. [Google Scholar] [CrossRef]
- Song, D.; Lian, B.; Fu, Y.; Wang, G.; Qiao, Y.; Klu, E.E.; Gong, X.; Jiang, J. Dual-Layer Corrosion-Resistant Conversion Coatings on Mg-9Li Alloy via Hydrothermal Synthesis in Deionized Water. Metals 2021, 11, 1396. [Google Scholar] [CrossRef]
- Duan, H.; Yan, C.; Wang, F. Effect of electrolyte additives on performance of plasma electrolytic oxidation films formed on magnesium alloy AZ91D. Electrochim. Acta 2007, 52, 3785–3793. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, W.; Song, X.; Zhang, Q.; Zeng, R. Self-catalytic degradation of iron-bearing chemical conversion coating on magnesium alloys-Influence of Fe content. Front. Mater. Sci. 2020, 3, 296–313. [Google Scholar] [CrossRef]
- Cerezo, J.; Vandendael, I.; Posner, R.; Wit, J.; Mol, J.; Terryn, H. The effect of surface pre-conditioning treatments on the local composition of Zr-based conversion coatings formed on aluminium alloys. Appl. Surf. Sci. 2016, 366, 339–347. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Li, W.; Wang, Q.; Li, Y. A study on microstructure evolution and corrosion resistance of cutting layer metal of 7055 aluminum alloy based on extreme environment. Mater. Corros. 2017, 69, 1389–1397. [Google Scholar] [CrossRef]
- Oger, L.; Lafouresse, M.; Odemer, G.; Peguet, L.; Blanc, C. Hydrogen diffusion and trapping in a low copper 7xxx aluminium alloy investigated by Scanning Kelvin Probe Force Microscopy. Mat. Sci. Eng. A 2017, 706, 126–135. [Google Scholar] [CrossRef]
- Ahmadi, P.; Sarabi, A.; Mohammadloo, H.; Behgam, R. Effect of practical parameters on the structure and corrosion behavior of vanadium/zirconium conversion coating on AA 2024 aluminum alloy. J. Coat. Technol. Res. 2019, 6, 1503–1513. [Google Scholar] [CrossRef]
| Element | Cu | Mg | Mn | Fe | Si | Zn | Cr | Al |
|---|---|---|---|---|---|---|---|---|
| Content/% | 0.16 | 1.01 | 0.45 | 0.31 | 0.55 | 0.08 | 0.05 | Bal. |
| Sample | Na2MoO4∙2H2O (g/L) | H2TiF6 (mL/L) | (NaPO3)6 (g/L) | H2ZrF6 (mL/L) |
|---|---|---|---|---|
| MoTiCC | 2.8 | 1.8 | 0.5 | 0 |
| MoTiZrCC | 2.8 | 1.8 | 0.5 | 1.2 |
| CTI (min) | 1 | 4 | 8 | 12 | 16 | 20 | 24 | |
|---|---|---|---|---|---|---|---|---|
| PH | ||||||||
| 3 | 33 | 41 | 43 | 36 | 32 | 26 | 20 | |
| 3.5 | 29 | 36 | 40 | 33 | 44 | 35 | 27 | |
| 4 | 35 | 46 | 50 | 41 | 48 | 40 | 45 | |
| 4.5 | 28 | 43 | 58 | 62 | 54 | 50 | 52 | |
| 5 | 21 | 27 | 25 | 39 | 44 | 49 | 40 | |
| 5.5 | 18 | 22 | 33 | 31 | 38 | 43 | 47 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Huang, F.; Teng, X.; Wang, Y.; Fang, Y.; Pan, J.; Wang, W.; Li, Y.; Zhan, W. The Preparation, Corrosion Resistance and Formation Mechanism of a New-Type Mo-Based Composite Conversion Coating on 6061 Aluminum Alloy. Metals 2023, 13, 168. https://doi.org/10.3390/met13010168
Qian X, Huang F, Teng X, Wang Y, Fang Y, Pan J, Wang W, Li Y, Zhan W. The Preparation, Corrosion Resistance and Formation Mechanism of a New-Type Mo-Based Composite Conversion Coating on 6061 Aluminum Alloy. Metals. 2023; 13(1):168. https://doi.org/10.3390/met13010168
Chicago/Turabian StyleQian, Xuzheng, Feng Huang, Xu Teng, Yiqun Wang, Yingsong Fang, Jingjing Pan, Wenhao Wang, Yingpeng Li, and Wen Zhan. 2023. "The Preparation, Corrosion Resistance and Formation Mechanism of a New-Type Mo-Based Composite Conversion Coating on 6061 Aluminum Alloy" Metals 13, no. 1: 168. https://doi.org/10.3390/met13010168
APA StyleQian, X., Huang, F., Teng, X., Wang, Y., Fang, Y., Pan, J., Wang, W., Li, Y., & Zhan, W. (2023). The Preparation, Corrosion Resistance and Formation Mechanism of a New-Type Mo-Based Composite Conversion Coating on 6061 Aluminum Alloy. Metals, 13(1), 168. https://doi.org/10.3390/met13010168





