Structure and Oxidation Behavior of NiAl-Based Coatings Produced by Non-Vacuum Electron Beam Cladding on Low-Carbon Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Structure of NiAl Coatings on Steel Substrate
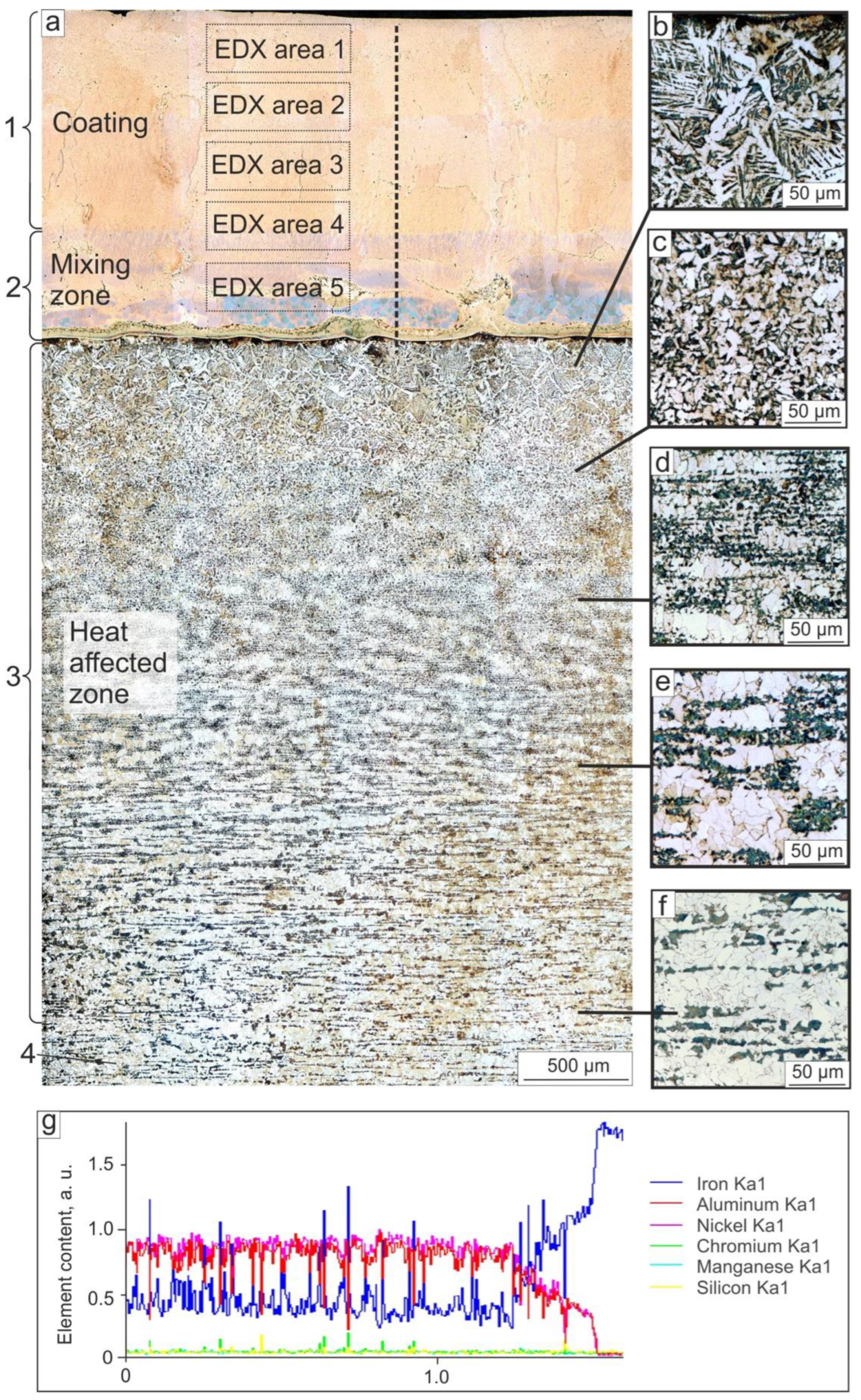
3.1.1. Structure of the Coating and Substrate in the Cross-Section
3.1.2. Structure of NiAl-Based Coatings
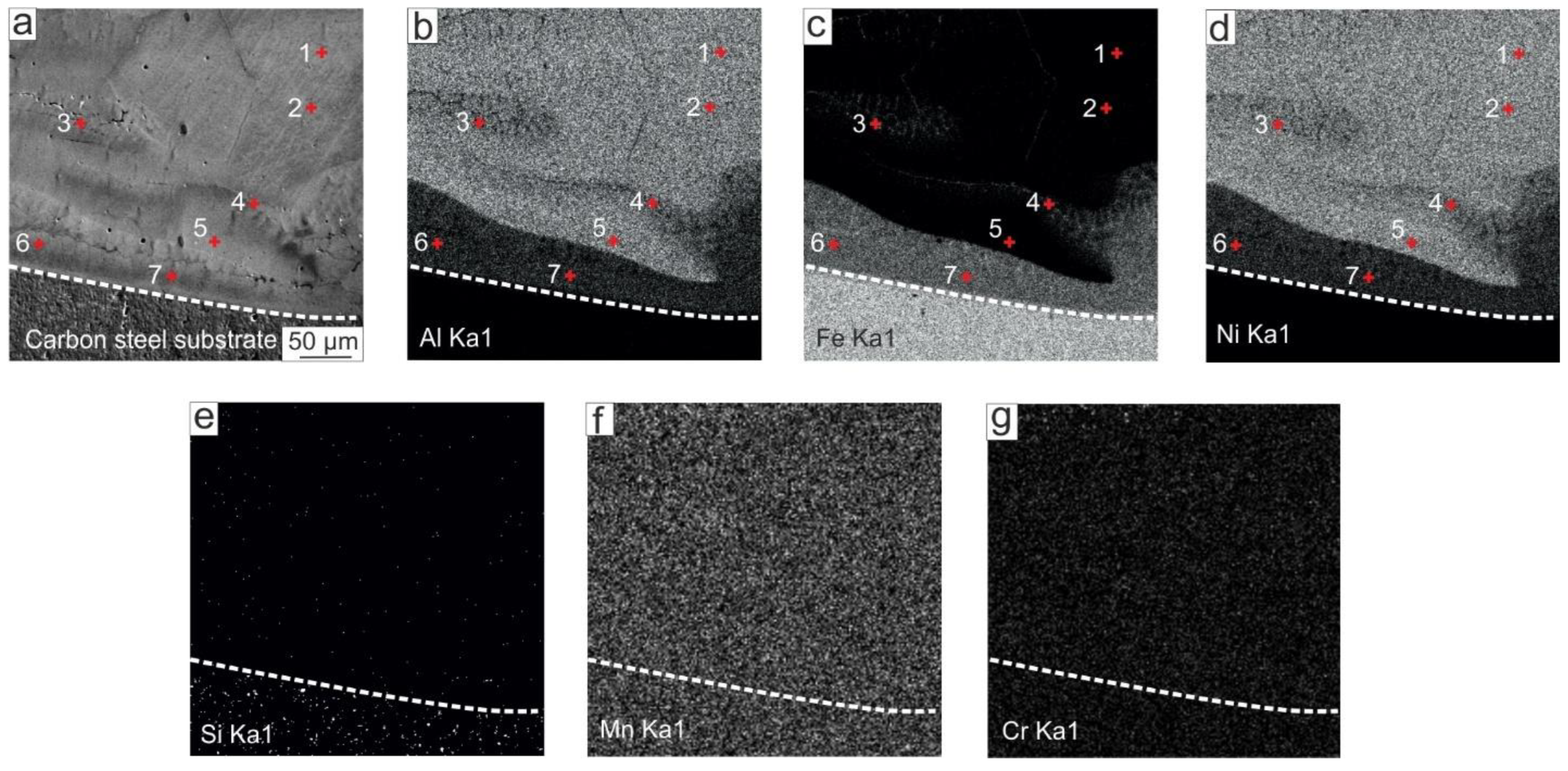
3.1.3. Structure of NiAl-Based Coatings near the “Coating–Substrate” Interface
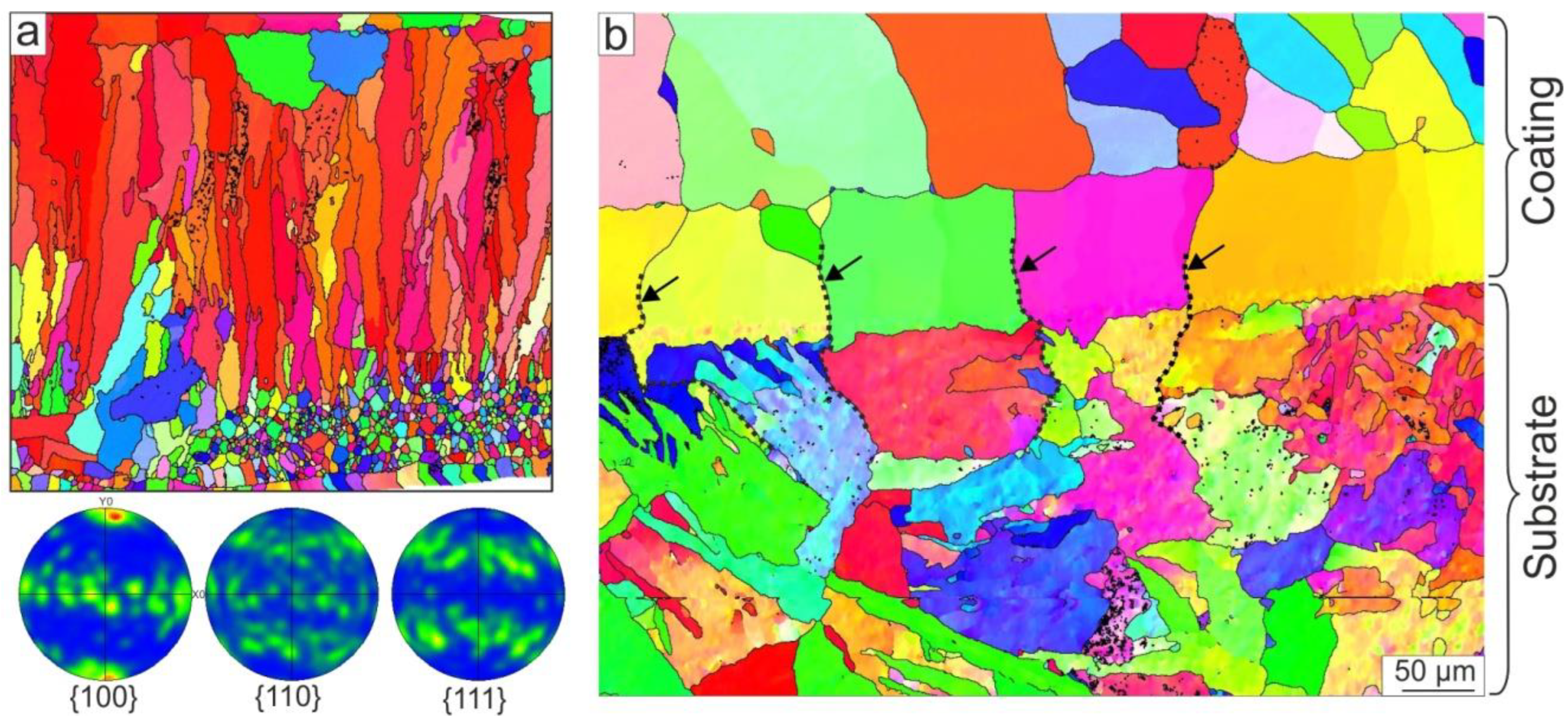
3.1.4. EBSD Analysis Results
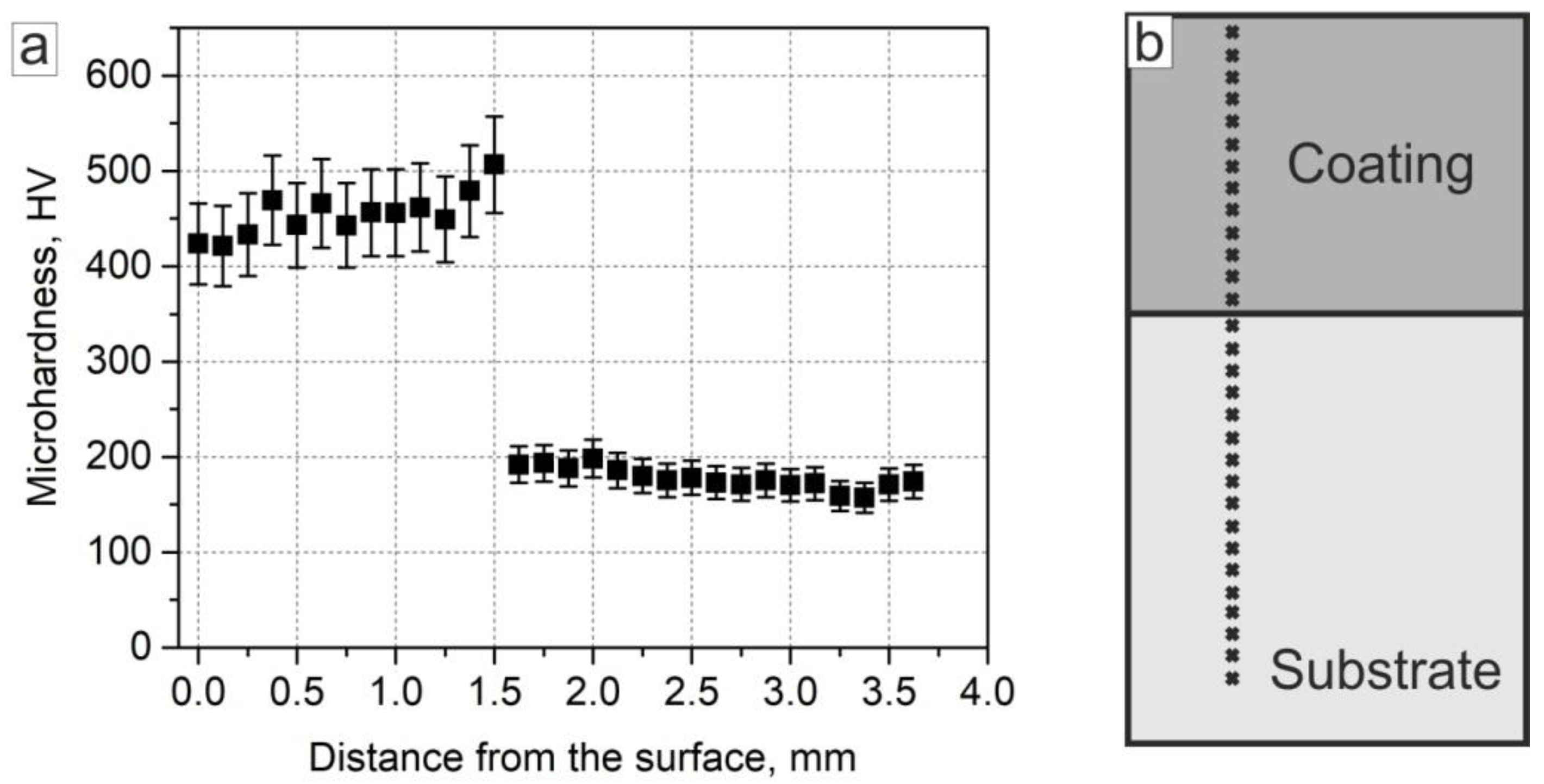
3.1.5. Microhardness Measurements
3.2. Oxidation Test Results
3.2.1. XRD Analysis of the Oxide Scales
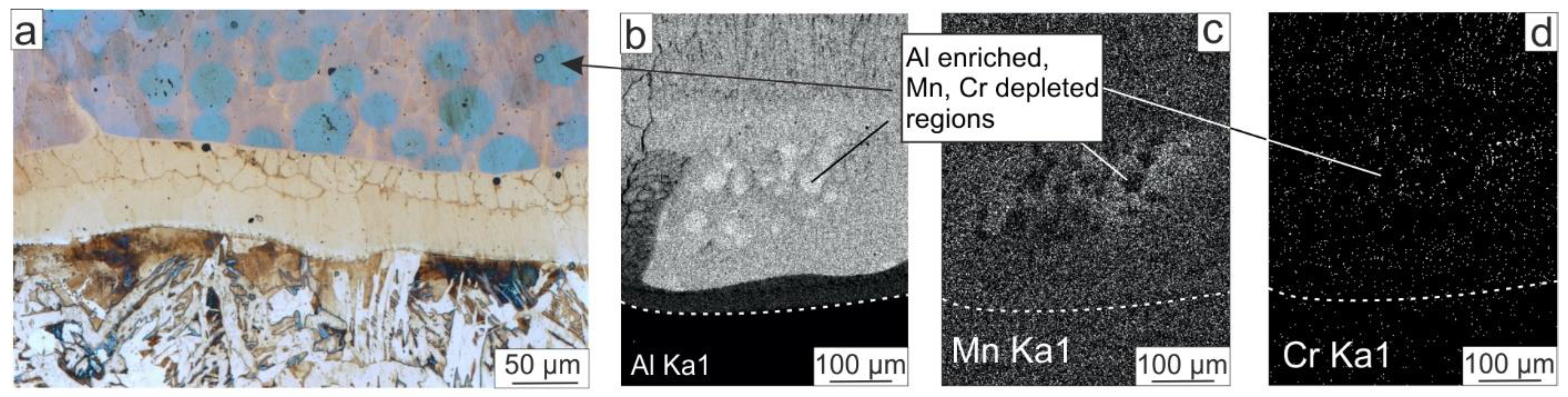
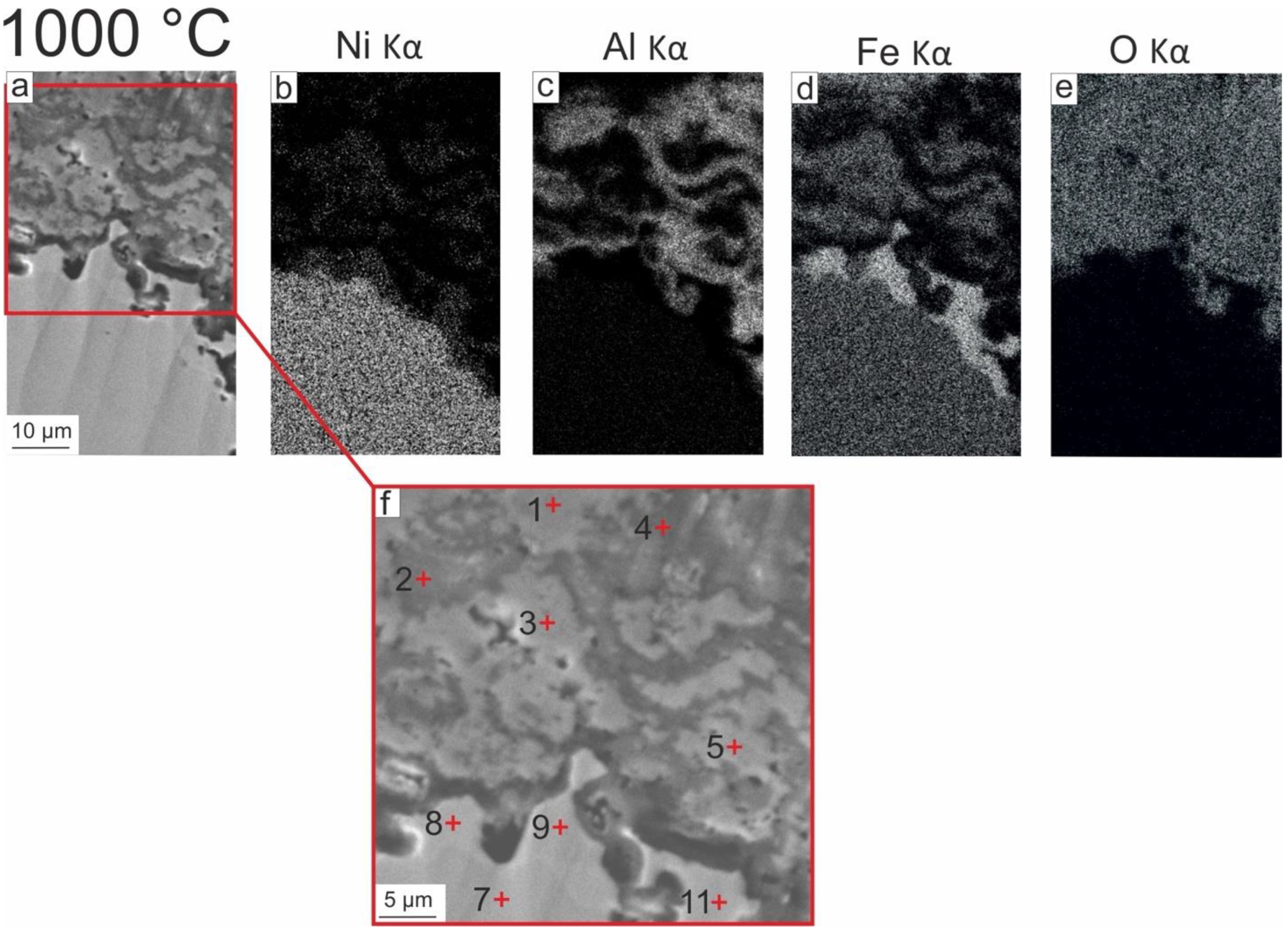
3.2.2. EDX Analysis of Oxide Scales
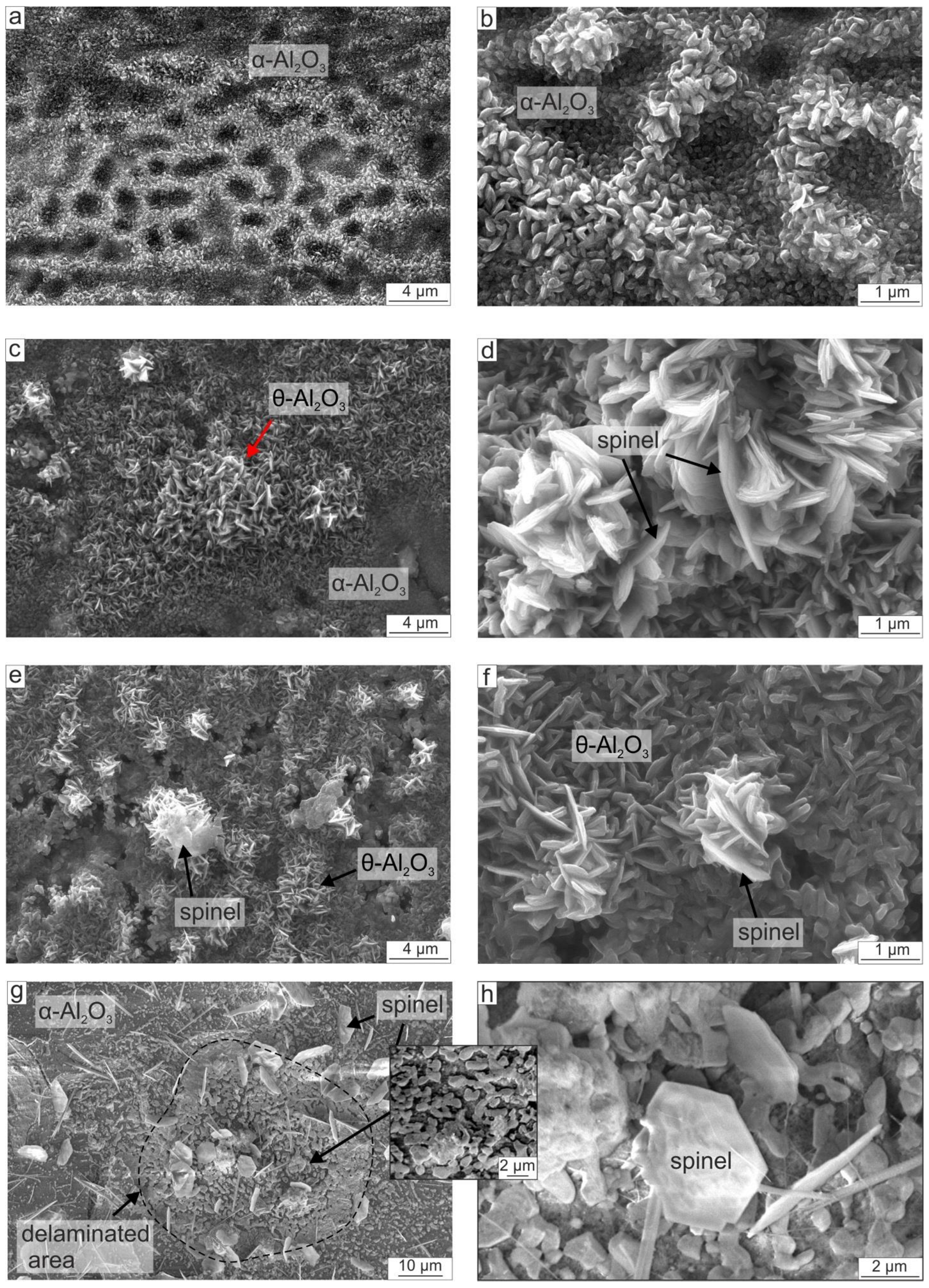
3.2.3. Top Surface Morphology of the Oxide Films
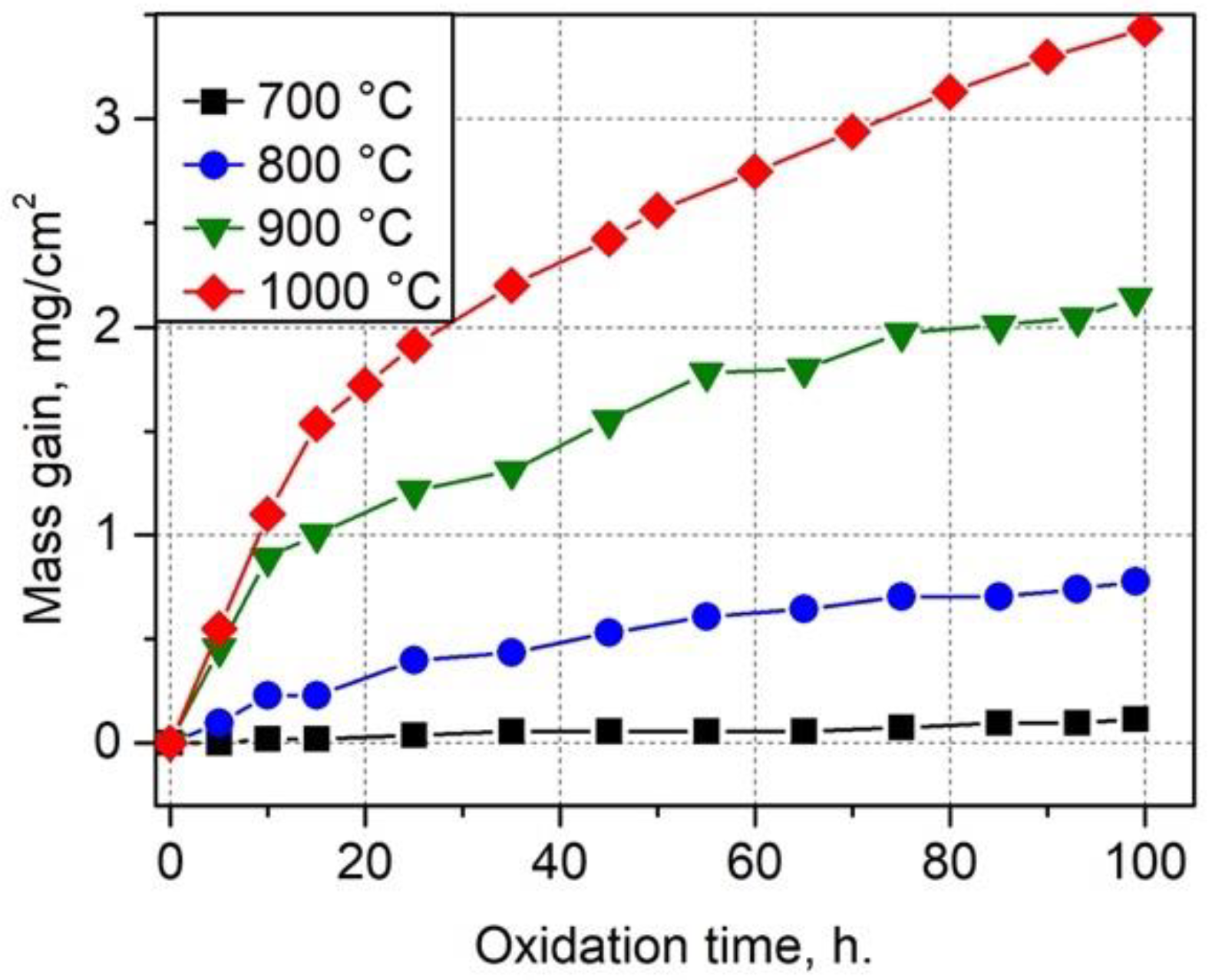
3.2.4. The Oxidation Rate of the NiAl-Based Coatings
4. Conclusions
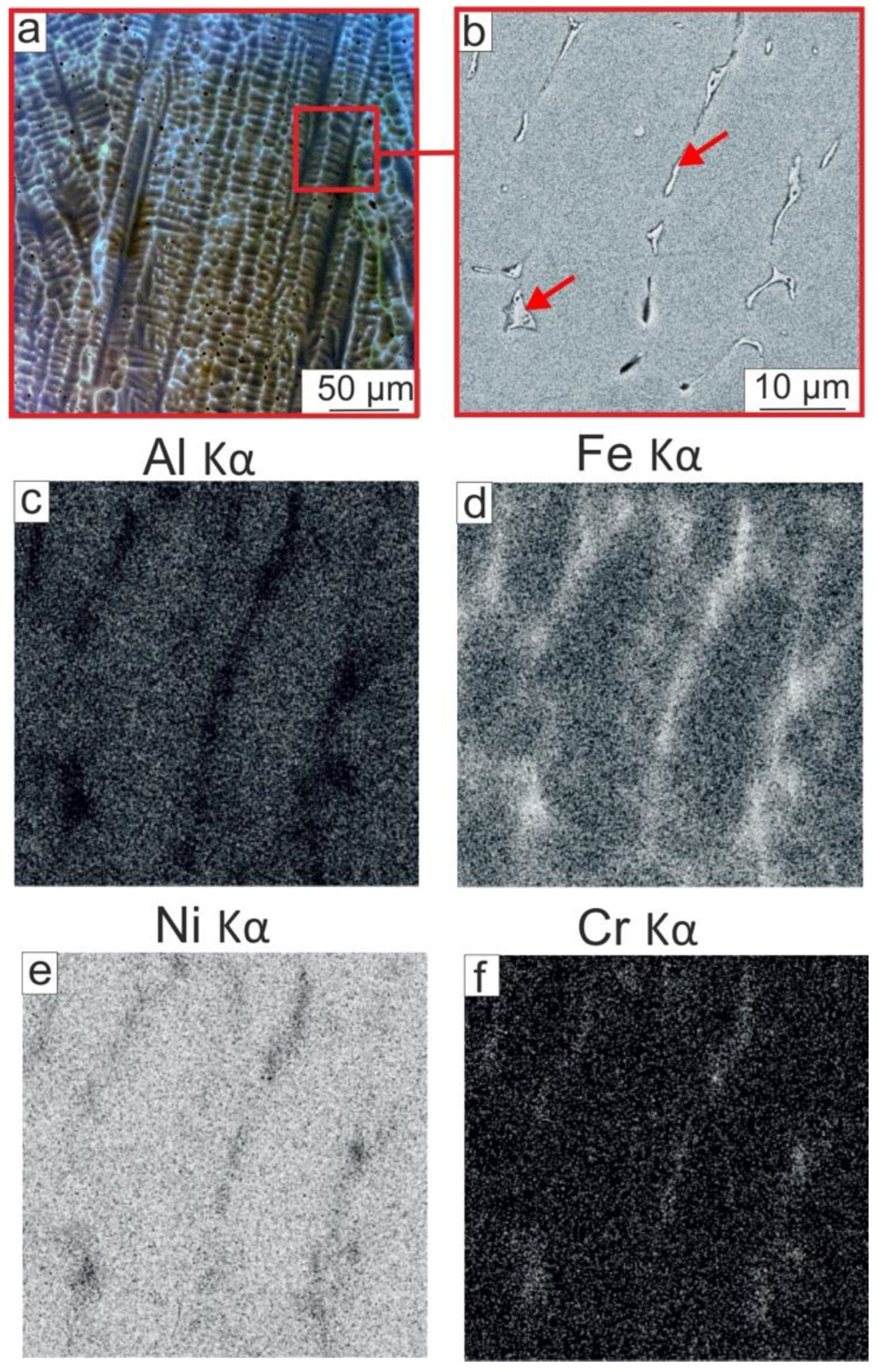
- NiAl-based coatings of 1.5 mm thickness were produced by non-vacuum electron beam cladding NiAl powder on low-carbon steel substrates. The coating had a dendritic structure with elongated grains with a <100> crystallographic direction oriented along the growth direction and perpendicular to the surface of the substrate. The partial melting of the steel substrate and its mixing with the coating led to the dissolution of about 14 at. % Fe in NiAl. In addition to Fe, a minor amount of Cr, Mn, and Si passed out of the substrate to the coating. In the main part of the coating, Fe was predominantly concentrated in the interdendritic space due to the lower crystallization temperature of Fe-containing NiAl compounds. During the annealing of the coating at 900 °C for 100 h, Fe-enriched areas dissolved in NiAl, and the homogenization of the NiAl-based coating occurred.
- Closer to the bottom part of the coating, the Fe content gradually increased; the average Fe content in the area within the distance 350 μm to the substrate was ~27 at. %. Along the “coating–substrate” interface, the layer of Fe50-Al25-Ni25 at. % a 50–100 μm thickness was formed, separated from the rest of the coating by a clearly distinguished boundary. The average microhardness in the area with increased Fe content was 500 HV, which was 50 HV higher than in the main part of the coating.
- The mass gains of the coatings after 100 h of oxidation at 700, 800, 900, and 1000 °C were 0.1, 0.8, 2.14, and 3.4 mg/cm2, respectively. Due to the presence of Fe, the oxidation rate of the obtained Fe-alloyed NiAl was several times higher compared with the pure NiAl oxidation rate observed by other researchers. Oxide scales predominantly consisted of α-Al2O3 nanograins and a certain amount of θ-Al2O3; the presence of Fe also contributed to the formation of a small amount of spinel.
- The porosity typical of oxidized pure NiAl was not observed at the “oxide scale–Fe-alloyed NiAl” interface due to the presence of Fe. It was found that, after oxidation at 1000 °C, a layer consisting of 60 at. % Fe, 20 at. % Ni, and 7 at. % Al formed in the coating on the side adjacent to the oxide scale. Its formation is explained by a stronger chemical bond between Fe and Al atoms compared with Ni and Al ones. Thus, the outward diffusion of Fe atoms at the “oxide scale–Fe-alloyed NiAl” interface occurred less intensively than that of the Ni atoms. This discovered feature confirms the suggestion of other researchers that the addition of Fe to NiAl reduces Kirkendall drift during oxidation, and the tendency to form Kirkendall voids in the “oxide scale—NiAl” interface decreases. Thus, the presence of Fe in NiAl is a positive factor in terms of improving the spallation resistance of the oxide scales.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simões, S.; Viana, F.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. Anisothermal Solid-State Reactions of Ni/Al Nanometric Multilayers. Intermetallics 2011, 19, 350–356. [Google Scholar] [CrossRef]
- Miracle, D.B. Overview No. 104 The Physical and Mechanical Properties of NiAl. Acta Metall. Mater. 1993, 41, 649–684. [Google Scholar] [CrossRef]
- Mi, D.-M.; Zhu, S.-L.; Liang, Y.-Q.; Li, Z.-Y.; Cui, Z.-D.; Yang, X.-J.; Inoue, A. Zr55Al10Ni5Cu30 Amorphous Alloy Film Prepared by Magnetron Sputtering Method. Rare Met. 2021, 40, 2237–2243. [Google Scholar] [CrossRef]
- Lee, J.; Hui, K.N.; Hui, K.S.; Cho, Y.R.; Chun, H.-H. Low Resistivity of Ni-Al Co-Doped ZnO Thin Films Deposited by DC Magnetron Sputtering at Low Sputtering Power. Appl. Surf. Sci. 2014, 293, 55–61. [Google Scholar] [CrossRef]
- Edelstein, A.S.; Everett, R.K.; Richardson, G.Y.; Qadri, S.B.; Altman, E.I.; Foley, J.C.; Perepezko, J.H. Intermetallic Phase Formation during Annealing of Al/Ni Multilayers. J. Appl. Phys. 1994, 76, 7850–7859. [Google Scholar] [CrossRef]
- Zhong, D.; Moore, J.J.; Sutter, E.; Mishra, B. Microstructure, Composition and Oxidation Resistance of Nanostructured NiAl and Ni–Al–N Coatings Produced by Magnetron Sputtering. Surf. Coat. Technol. 2005, 200, 1236–1241. [Google Scholar] [CrossRef]
- Brunetti, C.; Belotti, L.P.P.; Miyoshi, M.H.H.; Pintaúde, G.; D’Oliveira, A.S.C.M.S.C.M. Influence of Fe on the Room and High-Temperature Sliding Wear of NiAl Coatings. Surf. Coat. Technol. 2014, 258, 160–167. [Google Scholar] [CrossRef]
- Javadi, M.M.; Edris, H.; Salehi, M. Plasma Sprayed NiAl Intermetallic Coating Produced with Mechanically Alloyed Powder. J. Mater. Sci. Technol. 2011, 27, 816–820. [Google Scholar] [CrossRef]
- Poliarus, O.; Morgiel, J.; Umanskyi, O.; Pomorska, M.; Bobrowski, P.; Szczerba, M.J.; Kostenko, O. Microstructure and Wear of Thermal Sprayed Composite NiAl-Based Coatings. Arch. Civ. Mech. Eng. 2019, 19, 1095–1103. [Google Scholar] [CrossRef]
- Dudina, D.V.; Batraev, I.S.; Ulianitsky, V.Y.; Bulina, N.V.; Korchagin, M.A.; Lomovsky, O.I. Detonation Spraying of Ti–Al Intermetallics: Phase and Microstructure Development of the Coatings. Mater. Manuf. Proc. 2015, 30, 724–729. [Google Scholar] [CrossRef]
- Senderowski, C.; Bojar, Z.; Wołczyński, W.; Pawłowski, A. Microstructure Characterization of D-Gun Sprayed Fe–Al Intermetallic Coatings. Intermetallics 2010, 18, 1405–1409. [Google Scholar] [CrossRef]
- Dudina, D.V.; Korchagin, M.A.; Zlobin, S.B.; Ulianitsky, V.Y.; Lomovsky, O.I.; Bulina, N.V.; Bataev, I.A.; Bataev, V.A. Compositional Variations in the Coatings Formed by Detonation Spraying of Ti3Al at Different O2/C2H2 Ratios. Intermetallics 2012, 29, 140–146. [Google Scholar] [CrossRef]
- Ulianitsky, V.Y.; Dudina, D.V.; Shtertser, A.A.; Smurov, I. Computer-Controlled Detonation Spraying: Flexible Control of the Coating Chemistry and Microstructure. Metals 2019, 9, 1244. [Google Scholar] [CrossRef]
- Xu, L.; Jia, Y.D.; Zhang, L.B.; Wang, G. Fabrication of NiAl Intermetallic Alloy Integrated Materials Chips with Continuous One-Dimensional Composition Gradients by Plasma Spray Deposition and Laser Remelting. Mater. Lett. 2021, 284, 128944. [Google Scholar] [CrossRef]
- Jia, Q.; Li, D.; Li, S.; Zhang, Z.; Zhang, N. High-Temperature Oxidation Resistance of NiAl Intermetallic Formed in Situ by Thermal Spraying. Coatings 2018, 8, 292. [Google Scholar] [CrossRef]
- Guan, H.; Chai, L.; Wang, Y.; Xiang, K.; Wu, L.; Pan, H.; Yang, M.; Teng, C.; Zhang, W. Microstructure and Hardness of NbTiZr and NbTaTiZr Refractory Medium-Entropy Alloy Coatings on Zr Alloy by Laser Cladding. Appl. Surf. Sci. 2021, 549, 149338. [Google Scholar] [CrossRef]
- Xiang, K.; Chai, L.; Zhang, C.; Guan, H.; Wang, Y.; Ma, Y.; Sun, Q.; Li, Y. Investigation of Microstructure and Wear Resistance of Laser-Clad CoCrNiTi and CrFeNiTi Medium-Entropy Alloy Coatings on Ti Sheet. Opt. Laser Technol. 2022, 145, 107518. [Google Scholar] [CrossRef]
- Dolique, V.; Thomann, A.L.; Brault, P.; Tessier, Y.; Gillon, P. Thermal Stability of AlCoCrCuFeNi High Entropy Alloy Thin Films Studied by In-Situ XRD Analysis. Surf. Coat. Technol. 2010, 204, 1989–1992. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Alferova, G.I.; Golkovsky, M.G.; Emurlaev, K.I.; Emurlaeva, Y.Y.; Bataev, I.A.; Ogneva, T.S.; Ruktuev, A.A.; Stepanova, N.V.; Bataev, A.A. Formation of Wear-Resistant Copper-Bearing Layers on the Surfaces of Steel Substrates by Non-Vacuum Electron Beam Acladding Using Powder Mixtures. Surf. Coat. Technol. 2020, 395, 125927. [Google Scholar] [CrossRef]
- Santana, D.A.; Koga, G.Y.; Wolf, W.; Bataev, I.A.; Ruktuev, A.A.; Bolfarini, C.; Kiminami, C.S.; Botta, W.J.; Jorge, A.M. Wear-Resistant Boride Reinforced Steel Coatings Produced by Non-Vacuum Electron Beam Cladding. Surf. Coat. Technol. 2020, 386, 125466. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Bataev, I.A.; Laptev, I.S.; Ruktuev, A.A.; Maliutina, I.N.; Golkovsky, M.G.; Bataev, A.A. Formation of Ti-Al Intermetallics on a Surface of Titanium by Non-Vacuum Electron Beam Treatment. Mater. Charact. 2017, 134, 202–212. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Q.; Liu, X.; Lou, S.; Li, Q.; Wang, Z. Microstructures and High-Temperature Wear Behavior of NiAl/WC-Fex Coatings on Carbon Steel by Plasma Cladding. J. Alloys Compd. 2020, 842, 155850. [Google Scholar] [CrossRef]
- Chen, W.; Shan, X.; Li, J.; Guo, Y.; Guo, F.; Zhao, X.; Ni, N.; Xiao, P. Effects of Iron and Platinum on the Isothermal Oxidation of β-NiAl Overlay Coatings Fabricated by Spark Plasma Sintering. Surf. Coat. Technol. 2020, 382, 125178. [Google Scholar] [CrossRef]
- Ruktuev, A.A.; Golkovski, M.G.; Lazurenko, D.V.; Bataev, V.A.; Ivanov, I.V.; Thömmes, A.; Bataev, I.A. TiTaNb Clads Produced by Electron Beam Surface Alloying in Regular Air at Atmospheric Pressure: Fabrication, Structure, and Properties. Mater. Charact. 2021, 179, 111375. [Google Scholar] [CrossRef]
- Golkovsky, M. Hardening and Surfacing with a Beam of Relativistic Electrons; LAP LAMBERT Academic Publishing: Sunnyvale, CA, USA, 2013. (In Russian) [Google Scholar]
- Hao, S.M.; Takayama, T.; Ishida, K.; Nishizawa, T. Miscibility Gap in Fe-Ni-Al and Fe-Ni-Al-Co Systems. Metall. Trans. A 1984, 15, 1819–1828. [Google Scholar] [CrossRef]
- Grabke, H.J. Oxidation of NiAl and FeAl. Intermetallics 1999, 7, 1153–1158. [Google Scholar] [CrossRef]
- Kaplin, C.; Ivanov, R.; Paliwal, M.; Jung, I.H.; Brochu, M. The Effect of Nanostructure on the Oxidation of NiAl. Intermetallics 2014, 54, 209–217. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J.; Wagemann, B. The Oxidation of NiAl-III. Internal and Intergranular Oxidation. Corros. Sci. 1994, 36, 37–53. [Google Scholar] [CrossRef]
- Doychak, J.; Smialek, J.L.; Mitchell, T.E. Transient Oxidation of Single-Crystal β-NiAl. Metall. Trans. A 1989, 20, 499–518. [Google Scholar] [CrossRef]
- Yang, J.C.; Schumann, E.; Levin, I.; Rühle, M. Transient Oxidation of NiAl. Acta Mater. 1998, 46, 2195–2201. [Google Scholar] [CrossRef]
- Smialek, J.L. Oxide Morphology and Spalling Model for NiAl. Metall Trans. A 1978, 9A, 309–320. [Google Scholar] [CrossRef]
- Grabke, H.J.; Brumm, M.W.; Wagemann, B. The Oxidation of NiAl. Mater. Corros. 1996, 677, 675–677. [Google Scholar] [CrossRef]
- Pint, B.A. The Oxidation Behavior of Oxide-Dispersed β-NiAl: I. Short-Term Performance at 1200 °C. Oxid. Met. 1998, 49, 531–559. [Google Scholar] [CrossRef]
- Zhang, Z.; Gleeson, B.; Jung, K.; Li, L.; Yang, J.C. A Diffusion Analysis of Transient Subsurface Γ′-Ni3Al Formation during β-NiAl Oxidation. Acta Mater. 2012, 60, 5273–5283. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. Oxidation Behaviour of NiAl—II. Cavity Formation beneath the Oxide Scale on NiAl of Different Stoichiometries. Corros. Sci. 1993, 34, 547–561. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. The Oxidation Behaviour of NiAl-I. Phase Transformations in the Alumina Scale during Oxidation of NiAl and NiAl-Cr Alloys. Corros. Sci. 1992, 33, 1677–1690. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Jeong, S.; Lee, D.; Yang, S.; Wang, F. Effect of Microcrystallization on the Oxidation Behavior of β-NiAl at 1000 °C in Air. Jinshu Xuebao/Acta Metall. Sin. 2000, 36, 511–516. [Google Scholar]
- Gleeson, B.; Wang, W.; Hayashi, S.; Sordelet, D.J. Effects of Platinum on the Interdiffusion and Oxidation Behavior of Ni-Al-Based Alloys. Mater. Sci. Forum 2004, 461–464, 213–222. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, J.; Li, J.; Yang, Z. B2-Ordered NiAl Content on Microstructure, Mechanical and Oxidation Properties of FeAl Intermetallic Compounds. J. Mater. Res. Technol. 2022, 16, 1875–1888. [Google Scholar] [CrossRef]
- Ford, S.; Kartono, R.; Young, D.J. Oxidation Resistance of Pt-Modified γ/Γ′ Ni-Al at 1150 °C. Surf. Coat. Technol. 2010, 204, 2051–2054. [Google Scholar] [CrossRef]
- Svensson, H.; Christensen, M.; Knutsson, P.; Wahnström, G.; Stiller, K. Influence of Pt on the Metal–Oxide Interface during High Temperature Oxidation of NiAl Bulk Materials. Corros. Sci. 2009, 51, 539–546. [Google Scholar] [CrossRef]
- Pint, B.A. Experimental Observations in Support of the Dynamic-Segregation Theory to Explain the Reactive-Element Effect. Oxid. Met. 1996, 45, 1–37. [Google Scholar] [CrossRef]
- Grabke, H.J.; Wiemer, D.; Viefhaus, H. Segregation of Sulfur during Growth of Oxide Scales. Appl. Surf. Sci. 1991, 47, 243–250. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. ChemInform Abstract: Oxidation Behavior of NiAl. Part 2. Cavity Formation Beneath the Oxide Scale on NiAl of Different Stoichiometries. ChemInform 2010, 24, 541–547. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, D. Effect of Fe on the High Temperature Oxidation of NiAl Alloys. Met. Mater. Int. 2005, 11, 313–317. [Google Scholar] [CrossRef]
- Schumann, E. The Effect of Y-Ion Implantation on the Oxidation of β-NiAl. Oxid. Met. 1995, 43, 157–172. [Google Scholar] [CrossRef]
- Smeltzer, W.W. Growth and Microstructure of A-ALOa on B -NiAI. Solid State Sci. Technol. 1980, 127, 5–10. [Google Scholar]
- Tolpygo, V.K.; Clarke, D.R. Microstructural Evidence for Counter-Diffusion of Aluminum and Oxygen during the Growth of Alumina Scales. Mater. High Temp. 2003, 20, 261–271. [Google Scholar] [CrossRef]
- Pint, B.A.; Treska, M.; Hobbs, L.W. The Effect of Various Oxide Dispersions on the Phase Composition and Morphology of Al2O3 Scales Grown on β-NiAl. Oxid. Met. 1997, 47, 1–20. [Google Scholar] [CrossRef]
- Schumann, E.; Sarioglu, C.; Blachere, J.R.; Pettit, F.S.; Meier, G.H. High-Temperature Stress Measurements during the Oxidation of NiAl. Oxid. Met. 2000, 53, 259–272. [Google Scholar] [CrossRef]
- Pint, B.A.; More, K.L.; Wright, I.G. Effect of Quaternary Additions on the Oxidation Behavior of Hf-Doped NiAl. Oxid. Met. 2003, 59, 257–283. [Google Scholar] [CrossRef]
- Li, D.; Guo, H.; Wang, D.; Zhang, T.; Gong, S.; Xu, H. Cyclic Oxidation of β-NiAl with Various Reactive Element Dopants at 1200 °C. Corros. Sci. 2013, 66, 125–135. [Google Scholar] [CrossRef]
- Chen, W.; Shan, X.; Guo, Y.; Li, J.; Zou, Z.; Guo, F.; Zhao, X.; Xiao, P. The Effect of Reactive Element Species and Concentrations on the Isothermal Oxidation of β-NiAl Coating Fabricated by Spark Plasma Sintering. Surf. Coat. Technol. 2019, 357, 841–848. [Google Scholar] [CrossRef]





| C | Si | Mn | Ni | S | P | Cr | Cu | As |
|---|---|---|---|---|---|---|---|---|
| 0.17–0.24 | 0.17–0.37 | 0.35–0.65 | up to 0.3 | up to 0.04 | up to 0.035 | up to 0.25 | up to 0.3 | up to 0.08 |
| Content of Powder Mixture | Mass of Powder Mixture per Unit Area (g/cm2) | Beam Current, (mA) | Table Velocity, (cm/s) | Energy Density (J/cm2) | ||
|---|---|---|---|---|---|---|
| Powder | Mass (g) | wt. % | ||||
| NiAl | 28 | 70 | 0.8 | 32 | 15 | 6.0 |
| CaF2 | 12 | 30 | ||||
| Area # | Ni, at. % | Al, at. % | Fe, at. % | Mn, at. % | Cr, at. % | Si, at. % |
|---|---|---|---|---|---|---|
| Average value for areas #1–4 | 43.5 | 41.2 | 14.1 | 0.4 | 0.3 | 0.5 |
| Area #5 | 36.5 | 34.7 | 27.1 | 0.5 | 0.3 | 0.9 |
| # of Point | Ni, at. % | Al, at. % | Fe, at. % | Mn, at. % | Cr, at. % | Si, at. % |
|---|---|---|---|---|---|---|
| 1 | 48.2 | 51.5 | 0.3 | - | - | - |
| 2 | 49.5 | 50 | 0.5 | - | - | - |
| 3 | 45.8 | 42.9 | 10.5 | 0.6 | 0.2 | - |
| 4 | 41.5 | 37.5 | 20.0 | 0.6 | 0.3 | 0.3 |
| 5 | 47.0 | 50.3 | 1.0 | 0.7 | 0.3 | 0.7 |
| 6 | 21.8 | 20.3 | 56.3 | 0.6 | 0.3 | 0.7 |
| 7 | 25.4 | 23.6 | 49.7 | 0.5 | 0.2 | 0.6 |
| EDX Point # | Corresponding Phase | Ni, at. % | Al, at. % | Fe, at. % | Mn, at. % | Cr, at. % | Si, at. % | O, at. % | C, at. % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | spinel | 6.9 | 8.8 | 16 | 0.7 | 0.3 | 0.7 | 62.5 | 4.1 |
| 2 | Al2O3 | 1.4 | 30.5 | 3.9 | - | - | - | 62.4 | 1.8 |
| 3 | spinel | 7.9 | 8.8 | 16.2 | 0.6 | 0.5 | - | 61.9 | 4.1 |
| 4 | Al2O3 | 0.8 | 34.1 | 2.7 | - | - | - | 60.2 | 2.2 |
| 5 | spinel | 6.0 | 9.7 | 15.9 | 0.5 | 0.6 | - | 62.8 | 4.5 |
| 7 | (Ni,Fe)3Al | 40.1 | 28.2 | 23.3 | 0.6 | 0.2 | 0.5 | - | 7.1 |
| 8 | Fe-rich layer | 21.0 | 7.7 | 59.3 | 0.5 | 1.3 | 0.9 | - | 9.3 |
| 9 | Fe-rich layer | 20.6 | 7.4 | 58.6 | 0.5 | 1.3 | 0.9 | - | 10.7 |
| 11 | Fe-rich layer | 19.84 | 6.18 | 62.09 | 0.49 | 1.4 | 0.83 | - | 9.17 |
| Test Mode (Cyclic/Isothermal) | Composition of the Material | Oxidation Temperature, °C | Oxidation Time, h | Weight Gain, mg/cm2 | Reference |
|---|---|---|---|---|---|
| Isothermal | NiAl + 14 at. % Fe | 700 800 900 1000 | 50 | 0.05 0.6 1.6 2.3 | This study |
| Isothermal | NiAl + 14 at. % Fe | 700 800 900 1000 | 100 | 0.1 0.8 2.1 3.4 | This study |
| Isothermal | NiAl | 1000 1200 1400 | 48 | 0.2 0.5 3.8 | [46] |
| Isothermal | NiAl + 3.5 at. % Fe | 1000 1200 1400 | 48 | 0.3 0.7 4.8 | [46] |
| Cyclic (cycle 1 h) | NiAl (magnetron sputtering) | 1000 | 0.7 0.8 | [38] | |
| Isothermal | NiAl | 1150 | 15 | 1.5 | [54] |
| Cyclic (cycle 1 h) | NiAl | 1200 | 100 300 | 2.8 7.5 | [53] |
| Cyclic (cycle 1 h)/Isothermal | NiAl Different Ni/Al ratios 1000–1400 °C | 900 1200 | 20 60 | 0.1 0.6 | [45] |
| Isothermal | FeAl 60% NiAl FeAl 100% | 600 | 100 | 0.2 1 | [40] |
| Isothermal | NiAl | 1050 | 100 | 0.27 | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogneva, T.S.; Ruktuev, A.A.; Lazurenko, D.V.; Emurlaev, K.I.; Malyutina, Y.N.; Golkovsky, M.G.; Egoshin, K.D.; Bataev, I.A. Structure and Oxidation Behavior of NiAl-Based Coatings Produced by Non-Vacuum Electron Beam Cladding on Low-Carbon Steel. Metals 2022, 12, 1679. https://doi.org/10.3390/met12101679
Ogneva TS, Ruktuev AA, Lazurenko DV, Emurlaev KI, Malyutina YN, Golkovsky MG, Egoshin KD, Bataev IA. Structure and Oxidation Behavior of NiAl-Based Coatings Produced by Non-Vacuum Electron Beam Cladding on Low-Carbon Steel. Metals. 2022; 12(10):1679. https://doi.org/10.3390/met12101679
Chicago/Turabian StyleOgneva, Tatiana S., Alexey A. Ruktuev, Daria V. Lazurenko, Kemal I. Emurlaev, Yulia N. Malyutina, Mikhail G. Golkovsky, Kirill D. Egoshin, and Ivan A. Bataev. 2022. "Structure and Oxidation Behavior of NiAl-Based Coatings Produced by Non-Vacuum Electron Beam Cladding on Low-Carbon Steel" Metals 12, no. 10: 1679. https://doi.org/10.3390/met12101679
APA StyleOgneva, T. S., Ruktuev, A. A., Lazurenko, D. V., Emurlaev, K. I., Malyutina, Y. N., Golkovsky, M. G., Egoshin, K. D., & Bataev, I. A. (2022). Structure and Oxidation Behavior of NiAl-Based Coatings Produced by Non-Vacuum Electron Beam Cladding on Low-Carbon Steel. Metals, 12(10), 1679. https://doi.org/10.3390/met12101679






