Stress Corrosion Analysis and Direct Cell Viability of Biodegradable Zn-Fe-Ca Alloy in In-Vitro Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alloy Preparation
2.2. Microstructure Characterization
2.3. Cyclic Potentiodynamic Polarization
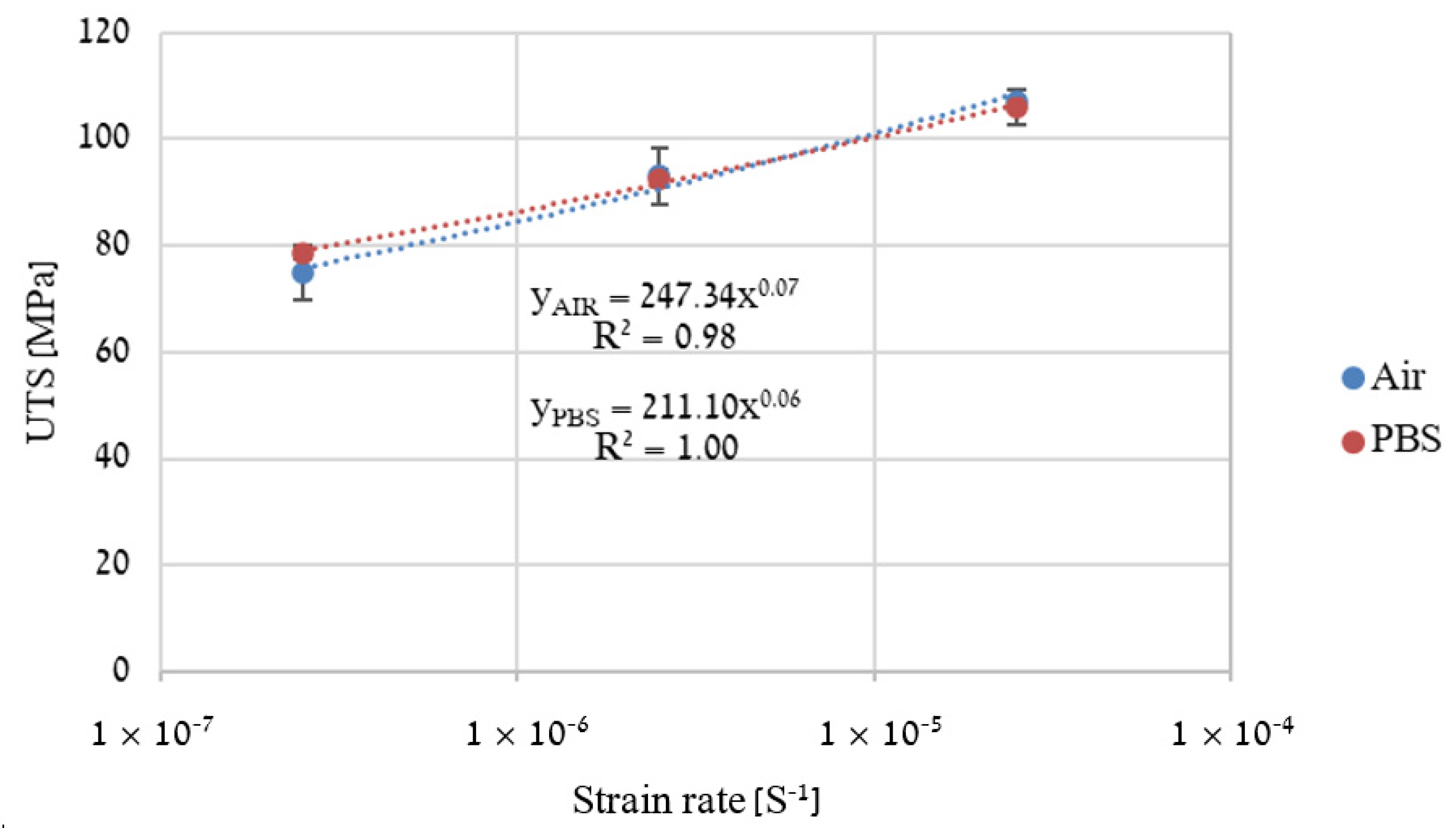
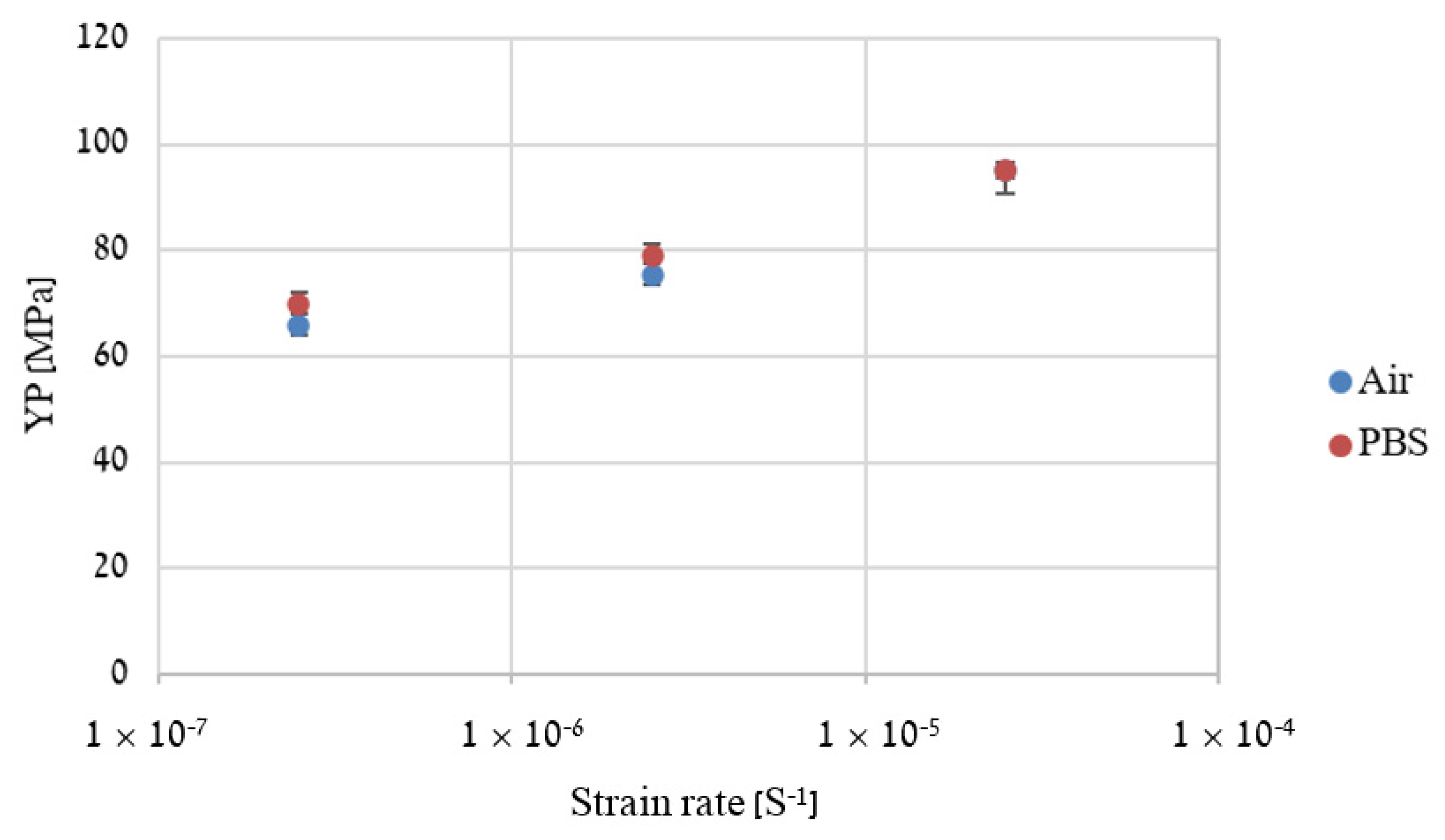
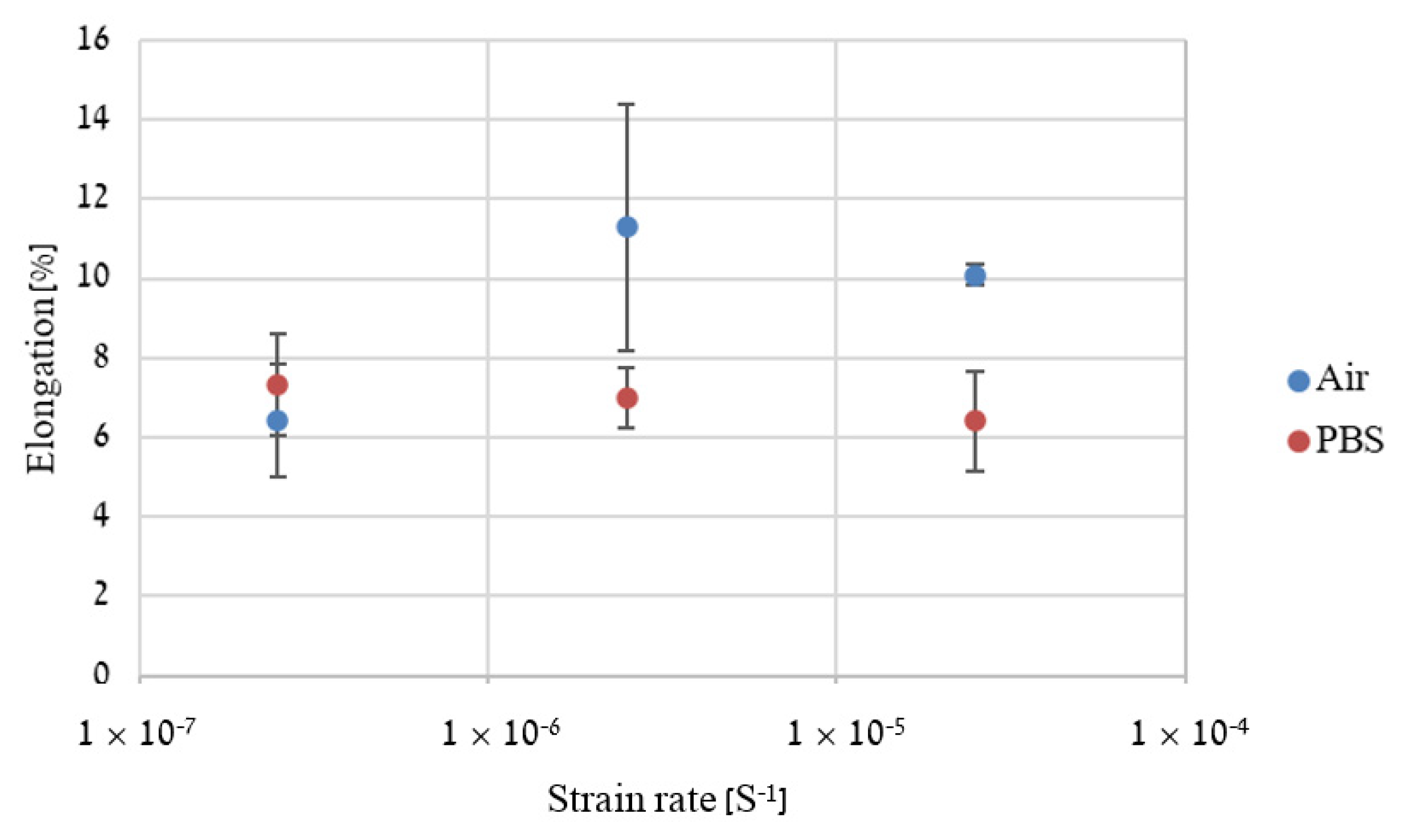
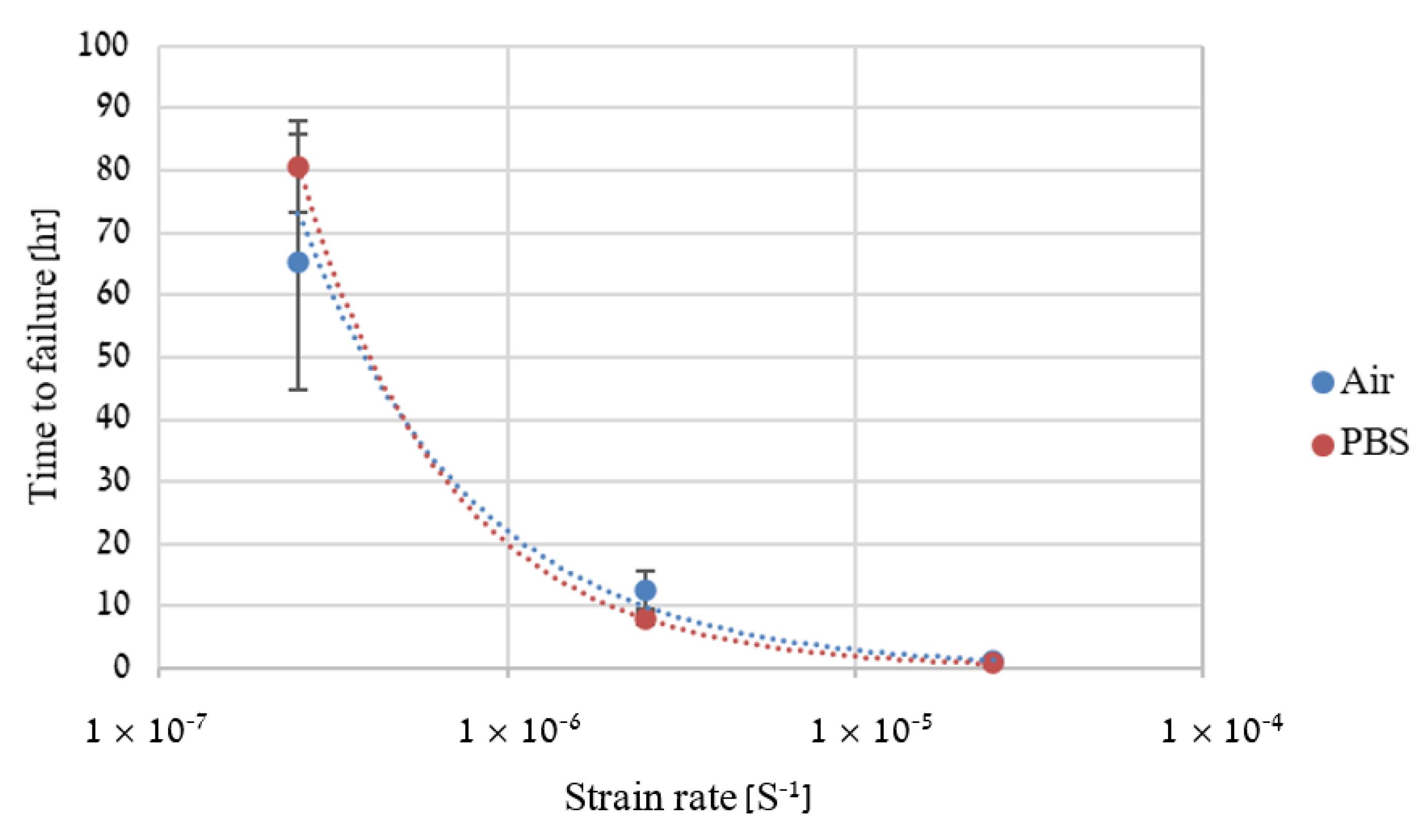
2.4. Slow Strain Rate Tensile (SSRT)
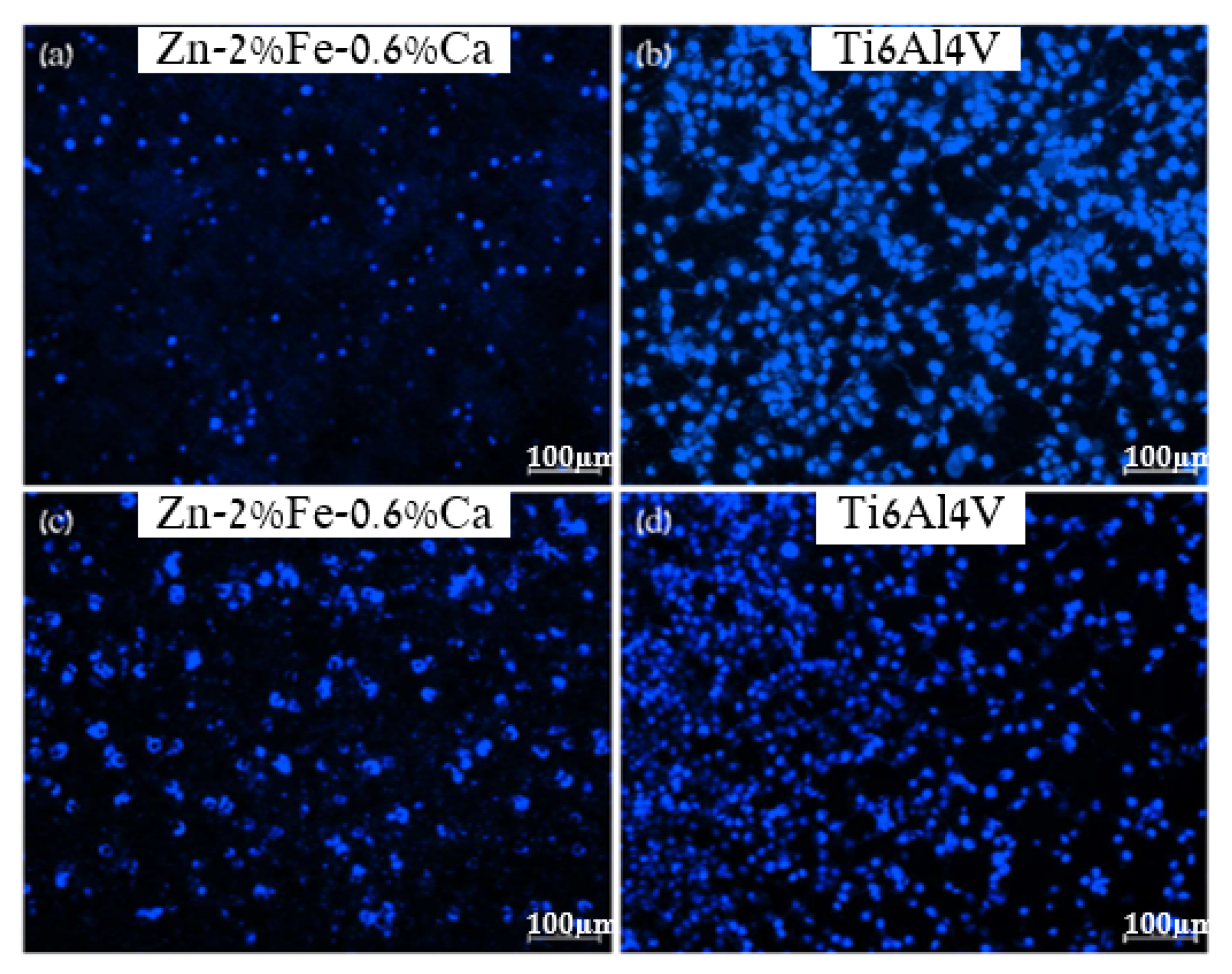
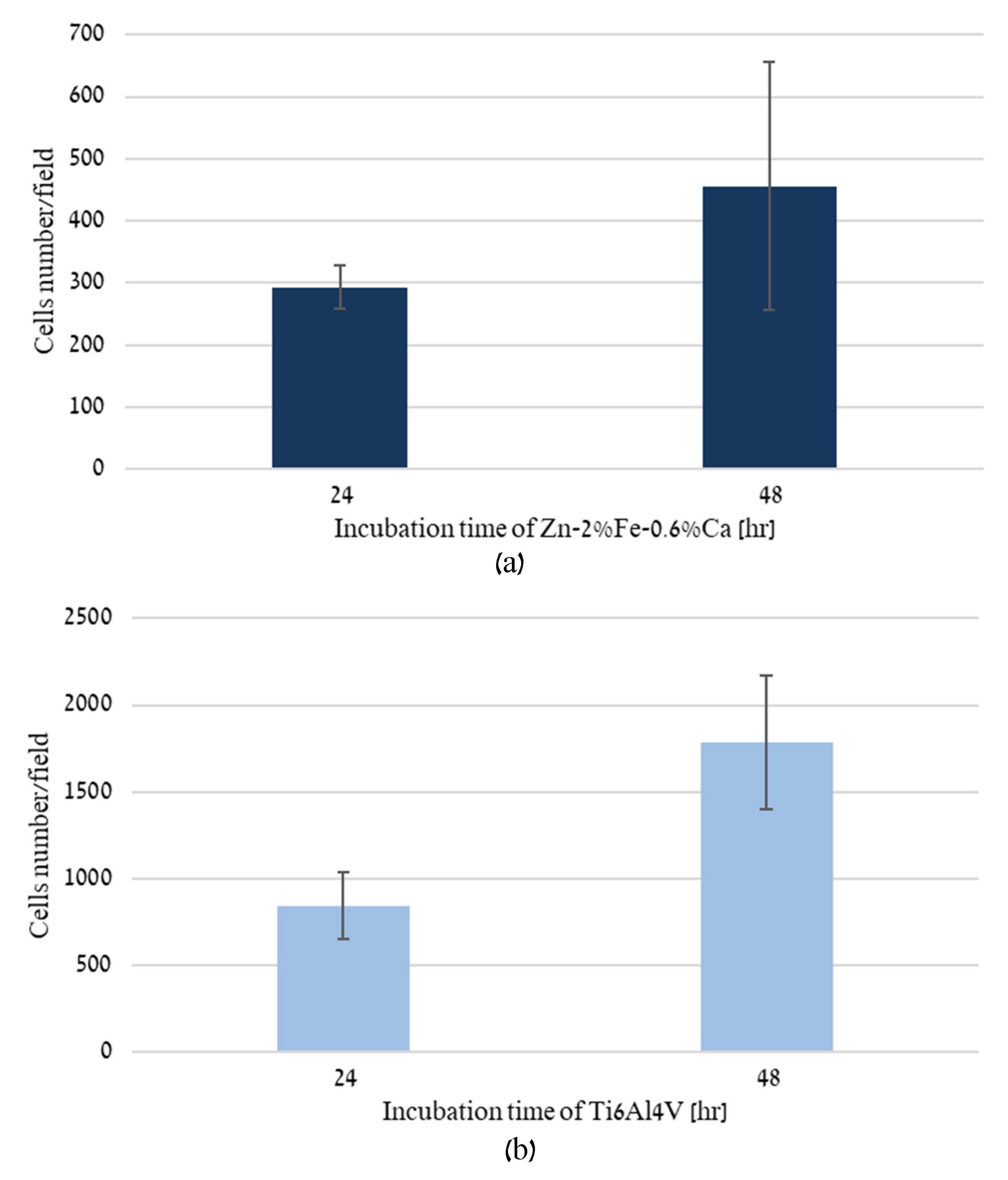
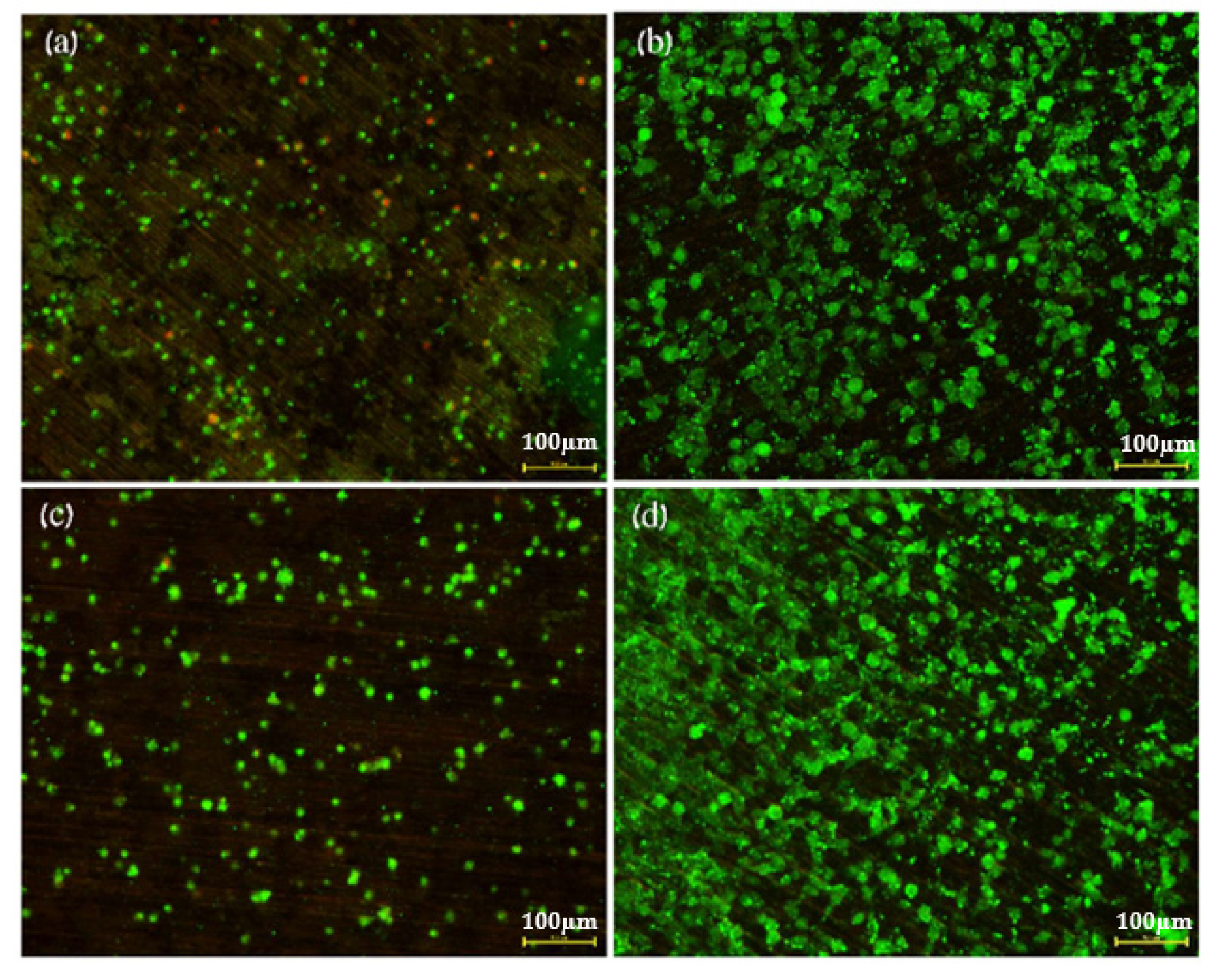
2.5. Direct Incubation of Cells on Zn-2%Fe-0.6%Ca Alloy
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Seitz, J.M.; Durisin, M.; Goldman, J.; Drelich, J.W. Recent advances in biodegradable metals for medical sutures: A critical review. Adv. Healthc. Mater. 2015, 4, 1915–1936. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.; Li, X.; Zheng, Y. In vitro evaluation of the feasibility of commercial Zn alloys as biodegradable metals. J. Mater. Sci. Technol. 2016, 32, 909–918. [Google Scholar] [CrossRef]
- Törne, K.; Larsson, M.; Norlin, A.; Weissenrieder, J. Degradation of zinc in saline solutions, plasma, and whole blood. J. Biomed. Mater. Res. Part B 2016, 104, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Bolz, A.; Popp, T. Implantable, Bioresorbable VesselWall Support, in Particular Coronary Stent. U.S. Patent 6,287,332 B1, 11 September 2001. [Google Scholar]
- Zhou, G.; Gongqi, Q.; Gong, H. Kind of Absorbable High Strength and Toughness Corrosion-Resistant Zinc Alloy Implant Material for Human Body. U.S. Patent 20,170,028,107 A1, 2 February 2017. [Google Scholar]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 1–16. [Google Scholar]
- Aghion, E.; Bronfin, B.; Eliezer, D.; Von Buch, F.; Schumann, S.; Friedrich, H. The Art of Developing New Magnesium Alloys for High Temperature Applications. Mater. Sci. Forum 2003, 419–422, 407–418. [Google Scholar] [CrossRef]
- Aghion, E.; Gueta, Y.; Moscovitch, N.; Bronfin, B. Effect of yttrium additions on the properties of grain-refined Mg–3% Nd alloy. J. Mater. Sci. 2008, 43, 4870–4875. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Qiu, K.; Yang, Y.; Pu, Z.; Li, L.; Zheng, Y. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–1.5Mg alloy. J. Alloy. Compd. 2016, 664, 444–452. [Google Scholar] [CrossRef]
- Niu, J.; Tang, Z.; Huang, H.; Pei, J.; Zhang, H.; Yuan, G.; Ding, W. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater. Sci. Eng. C 2016, 69, 407–413. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. C 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Zheng, Y.; Zhou, F.; Qiu, K.; Wang, X. Design and characterizations of novel biodegradable ternary Zn-based alloys with iia nutrient alloying elements mg, ca and sr. Mater. Des. 2015, 83, 95–102. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Izman, S.; Kurniawan, D.; Farahany, S.; Yahaya, B.; Hermawan, H. Influence of thermal treatment on microstructure, mechanical and degradation properties of Zn–3Mg alloy as potential biodegradable implant material. Mater. Des. 2015, 85, 431–437. [Google Scholar] [CrossRef]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Qin, L. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 1–14. [Google Scholar]
- Wang, C.; Yu, Z.; Cui, Y.; Zhang, Y.; Yu, S.; Qu, G.; Gong, H. Processing of a novel Zn alloy micro-tube for biodegradable vascular stent application. J. Mater. Sci. Technol. 2016, 32, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, J.; Yang, Y.; Zhou, F.; Pu, Z.; Li, L.; Zheng, Y. Microstructure, mechanical properties, in vitro degradation behavior and hemocompatibility of novel Zn–Mg–Sr alloys as biodegradable metals. Mater. Lett. 2016, 162, 242–245. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhou, F.; Yang, Y.; Chang, R.; Qiu, K.; Zheng, Y. Micro-alloying with Mn in Zn–Mg alloy for future biodegradable metals application. Mater. Des. 2016, 94, 95–104. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. The Effects of 4%Fe on the Performance of Pure Zinc as Biodegradable Implant Material. Ann. Biomed. Eng. 2019, 47, 1400–1408. [Google Scholar] [CrossRef]
- Guillory, R.J.; Bowen, P.K.; Hopkins, S.P.; Shearier, E.R.; Earley, E.J.; Gillette, A.A.; Aghion, E.; Bocks, M.L.; Drelich, J.W.; Goldman, J. Corrosion characteristics dictate the long-term inflammatory profile of degradable zinc arterial implants. ACS Biomater. Sci. Eng. 2016, 2, 2355–2364. [Google Scholar] [CrossRef]
- Aksakal, B.; Yildirim, Ö.S.; Gul, H. Metallurgical failure analysis of various implant materials used in orthopedic applications. J. Fail. Anal. Prev. 2004, 4, 17–23. [Google Scholar] [CrossRef]
- Li, H.F.; Shi, Z.Z.; Wang, L.N. Opportunities and challenges of biodegradable Zn-based alloys. J. Mater. Sci. Technol. 2020, 46, 136–138. [Google Scholar] [CrossRef]
- Huang, T.; Cheng, J.; Zheng, Y.F. In vitro degradation and biocompatibility of Fe–Pd and Fe–Pt composites fabricated by spark plasma sintering. Mater. Sci. Eng. C 2014, 35, 43–53. [Google Scholar] [CrossRef]
- Kwok, S.C.H.; Ha, P.C.T.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Biocompatibility of calcium and phosphorus doped diamond-like carbon thin films synthesized by pla sma immersion ion implantation and deposition. Diam. Relat. Mater. 2006, 15, 893–897. [Google Scholar] [CrossRef]
- Avior, O.; Ben Ghedalia-Peled, N.; Ron, T.; Vago, R.; Aghion, E. The Effect of Ca on In Vitro Behavior of Biodegradable Zn-Fe Alloy in Simulated Physiological Environments. Metals 2020, 10, 1624. [Google Scholar] [CrossRef]
- Kaya, A.; Uzan, P.; Eliezer, D.; Aghion, E. Electron microscopical investigation of as cast AZ91D alloy. Mater. Sci. Technol. 2000, 16, 1001–1006. [Google Scholar] [CrossRef]
- ISO-10993-5. Biological Evaluation of Medical Devices, Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization. ISO Central Secretaria. 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 28 April 2021).
- ISO-10993-12. Biological Evaluation of Medical Devices, Part 12: Sample Preparation and Reference Materials. International Organization for Standardization, ISO Central Secretaria. 2012. Available online: https://www.iso.org/standard/53468.html (accessed on 28 April 2021).
- Levy, G.K.; Ventura, Y.; Goldman, J.; Vago, R.; Aghion, E. Cytotoxic characteristics of biodegradable EW10X04 Mg alloy after Nd coating and subsequent heat treatment. Mater. Sci. Eng. C 2016, 62, 752–761. [Google Scholar] [CrossRef]
- Liva and Dead Assay Manufacturer’s Protocol. Abcam. 2019. Available online: https://www.abcam.com/ps/products/115/ab115347/documents/ab115347%20Live%20and%20Dead%20Cell%20Assay_30%20Jun%2015b%20(website).pdf (accessed on 28 April 2021).
- Khamaj, J.A. Cyclic polarization analysis of corrosion behavior of ceramic coating on 6061 Al/SiC p composite for marine applications. Prot. Met. Phys. Chem. Surf. 2016, 52, 886–893. [Google Scholar] [CrossRef]
- Arnon, A.; Aghion, E. Stress Corrosion Cracking of Nano/Sub-micron E906 Magnesium Alloy. Adv. Eng. Mater. 2008, 10, 742–745. [Google Scholar] [CrossRef]
- Shearier, E.R.; Bowen, P.K.; He, W.; Drelich, A.; Drelich, J.; Goldman, J.; Zhao, F. In vitro cytotoxicity, adhesion, and proliferation of human vascular cells exposed to zinc. ACS Biomater. Sci. Eng. 2016, 2, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wei, M.; Yang, J.; Zhao, Z.; Ma, J.; Liu, D.; Lan, Y. Effect of Ca addition on the microstructure, mechanical properties and corrosion rate of degradable Zn-1Mg alloys. J. Alloy. Compd. 2021, 887, 161255. [Google Scholar] [CrossRef]
- Chen, L.; Sheng, Y.; Wang, X.; Zhao, X.; Liu, H.; Li, W. Effect of Microstructure and Distribution of the Second Phase on the Stress Corrosion Cracking of Biodegradable Mg-Zn-Zr-xSr Alloys. Materials 2018, 11, 551. [Google Scholar] [CrossRef] [Green Version]
- Merson, D.; Vasilev, E.; Markushev, M.; Vinogradov, A. On the corrosion of ZK60 magnesium alloy after severe plastic deformation. Lett. Mater. 2017, 7, 421–427. [Google Scholar] [CrossRef]
- Aghion, E.; Levy, G.; Ovadia, S. In-vivo behavior of biodegradable Mg-Nd-Y-Zr-Ca alloy. J. Mater. Sci. Mater. Med. 2012, 23, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Burwinkel, M.; Palissa, C.; Ephraim, E.; Schmidt, M.F. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Vet. Microbiol. 2012, 160, 468–472. [Google Scholar] [CrossRef]
- Li, H.; Pang, S.; Liu, Y.; Sun, L.; Liaw, P.K.; Zhang, T. Biodegradable Mg–Zn–Ca–Sr bulk metallic glasses with enhanced corrosion performance for biomedical applications. Mater. Des. 2015, 67, 9–19. [Google Scholar] [CrossRef]
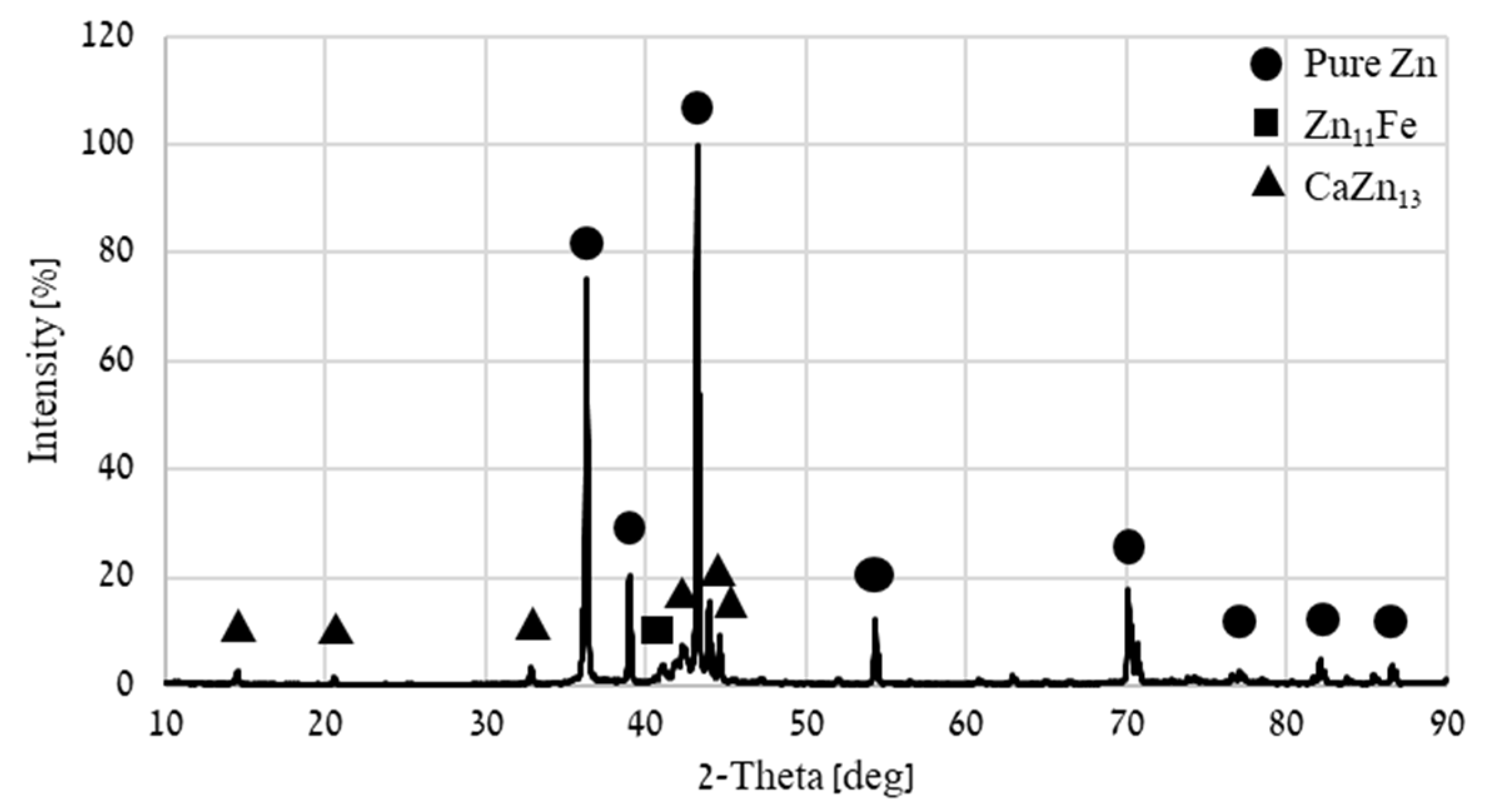
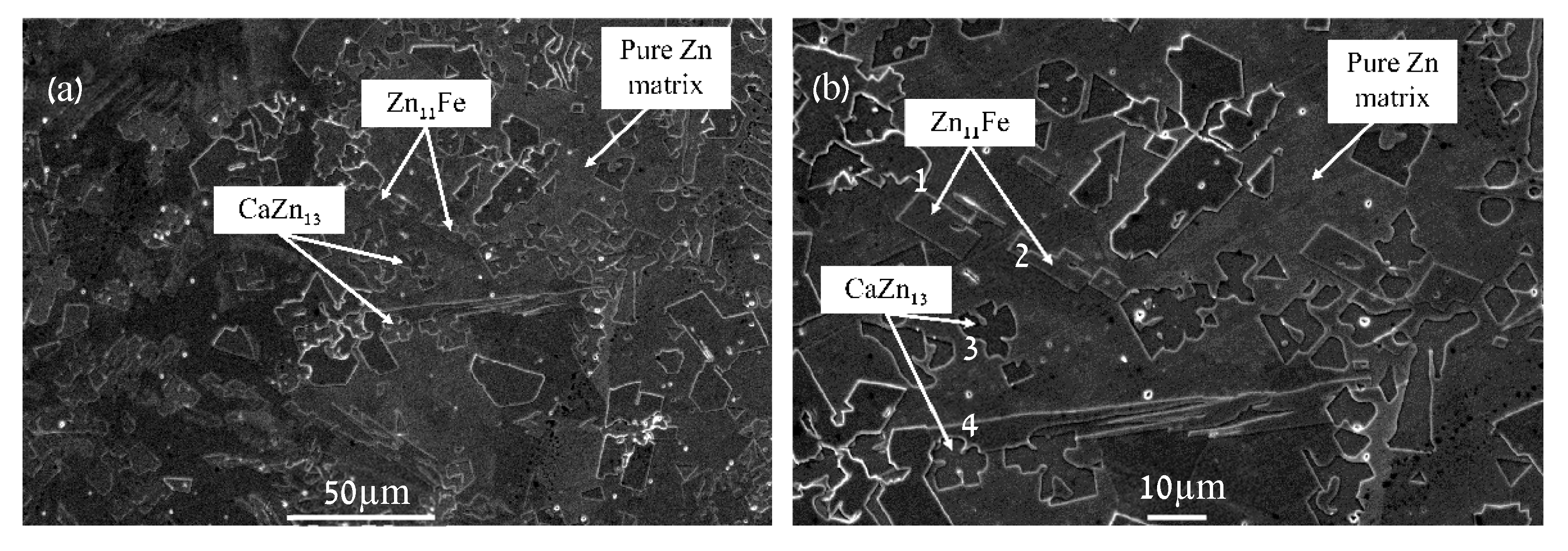
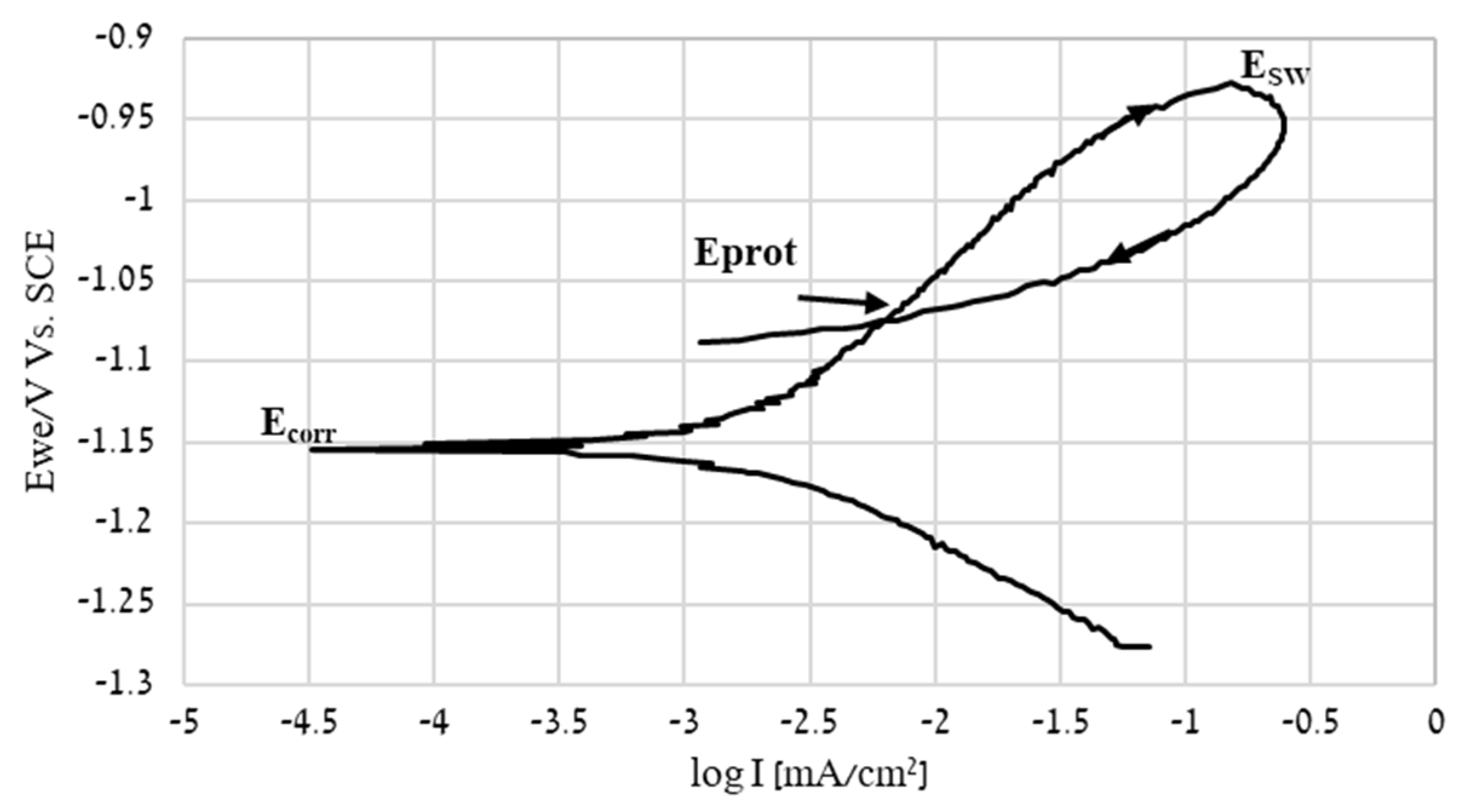
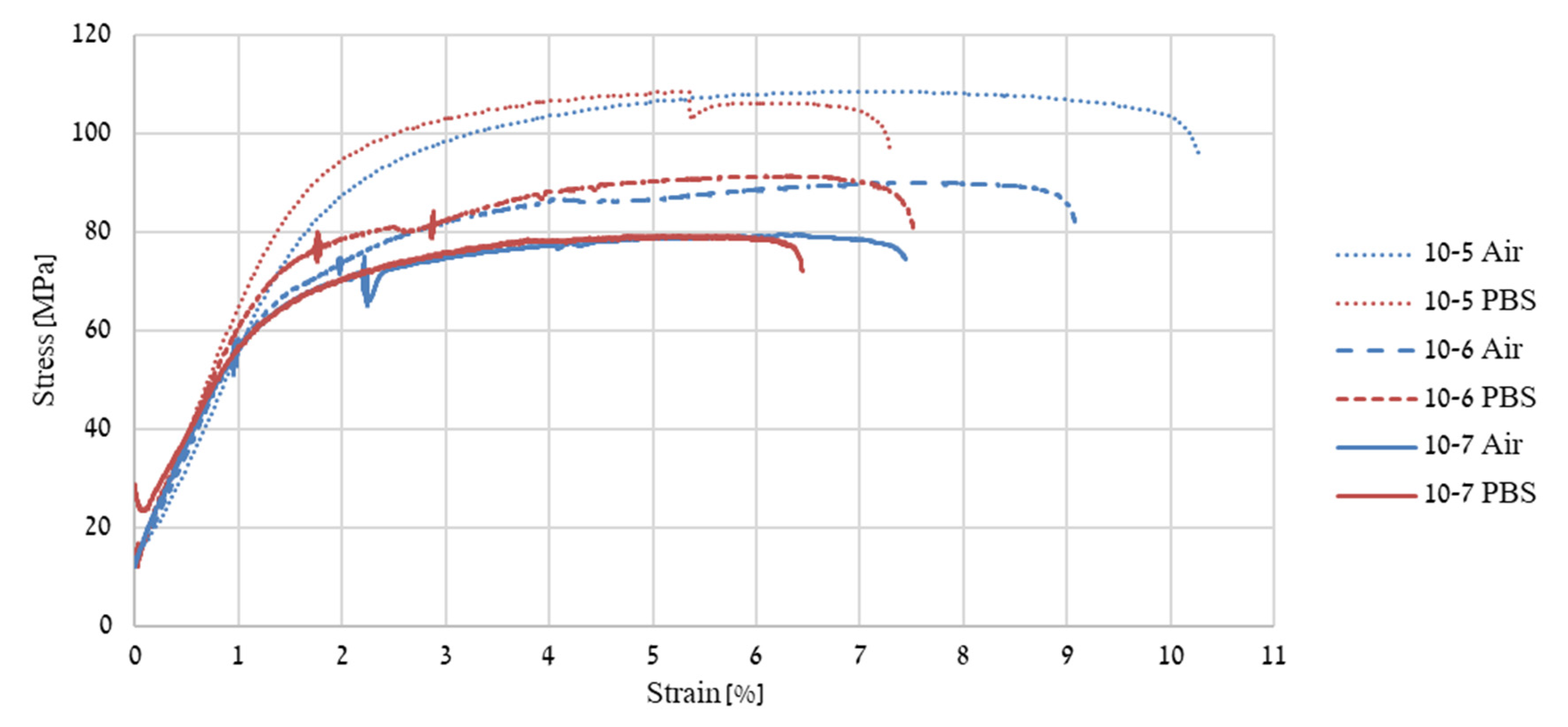
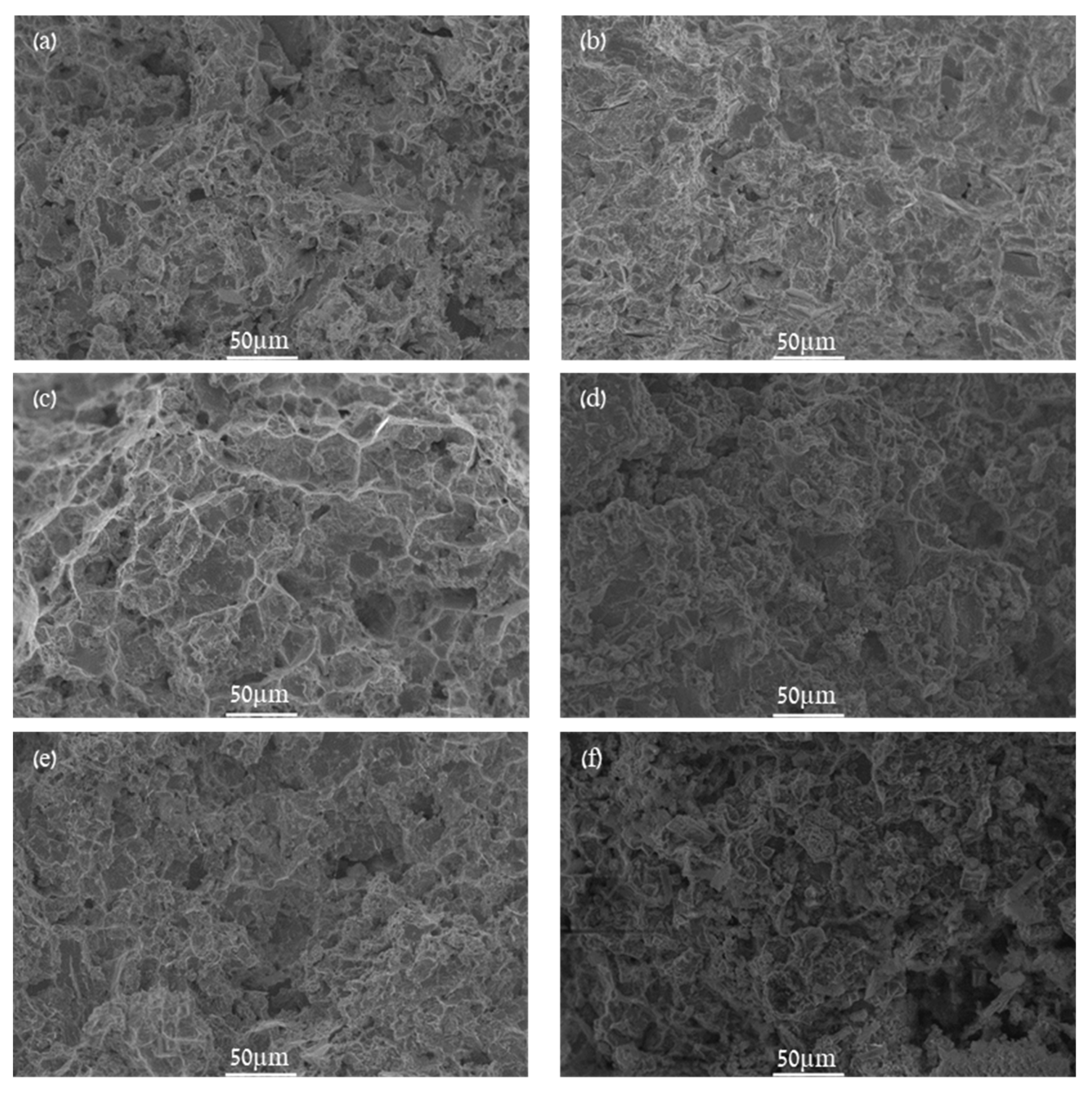
| Point | Ca [wt.%] | Fe [wt.%] | Zn [wt.%] |
|---|---|---|---|
| 1 | 0.00 ± 0.00 | 6.36 ± 0.38 | 93.64 ± 1.79 |
| 2 | 0.00 ± 0.07 | 5.60 ± 0.37 | 94.39 ± 1.74 |
| 3 | 4.20 ± 0.14 | 0.36 ± 0.15 | 95.45 ± 1.78 |
| 4 | 4.25 ± 0.14 | 0.11 ± 0.15 | 95.65 ± 1.78 |
| Corrosion Parameter | ECORR [V] | ICORR [µA/cm2] | Corrosion Rate [mmpy] |
|---|---|---|---|
| −1.1575 ± 0.0064 | 0.332 ± 0.065 | 0.0049 ± 0.0010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avior, O.; Ben Ghedalia-Peled, N.; Ron, T.; Goldman, J.; Vago, R.; Aghion, E. Stress Corrosion Analysis and Direct Cell Viability of Biodegradable Zn-Fe-Ca Alloy in In-Vitro Conditions. Metals 2022, 12, 76. https://doi.org/10.3390/met12010076
Avior O, Ben Ghedalia-Peled N, Ron T, Goldman J, Vago R, Aghion E. Stress Corrosion Analysis and Direct Cell Viability of Biodegradable Zn-Fe-Ca Alloy in In-Vitro Conditions. Metals. 2022; 12(1):76. https://doi.org/10.3390/met12010076
Chicago/Turabian StyleAvior, Orit, Noa Ben Ghedalia-Peled, Tomer Ron, Jeremy Goldman, Razi Vago, and Eli Aghion. 2022. "Stress Corrosion Analysis and Direct Cell Viability of Biodegradable Zn-Fe-Ca Alloy in In-Vitro Conditions" Metals 12, no. 1: 76. https://doi.org/10.3390/met12010076
APA StyleAvior, O., Ben Ghedalia-Peled, N., Ron, T., Goldman, J., Vago, R., & Aghion, E. (2022). Stress Corrosion Analysis and Direct Cell Viability of Biodegradable Zn-Fe-Ca Alloy in In-Vitro Conditions. Metals, 12(1), 76. https://doi.org/10.3390/met12010076







