In Vitro Biocompatibility Evaluation of a New Co-Cr-B Alloy with Potential Biomedical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Co-Cr-B Alloy Preparation
2.2. Metallurgical and Microstructural Characterization
2.2.1. Chemical Characterization
2.2.2. Hardness Test
2.3. Extraction of Mesenchymal Stem Cells from Dental Pulp (hDPSCs)
Cell Culture for Assays
2.4. Formation of Calcium Deposits by Alizarin Red Staining
2.5. Assay of Gene Expression Related to Osteodifferentiation
2.5.1. RNA Extraction and cDNA Synthesis
2.5.2. Real-Time PCR
2.6. Isolation of Human Peripheral Blood Mononuclear Cells (hPBMCs)
2.7. Viability Assay on hPBMCs
2.8. Inflammatory Response of PBMCs to Co-Cr-B Alloy Samples
2.9. Statistical Analysis
3. Results
3.1. Metallurgical and Microstructural Characterization
3.2. Chemical Characterization
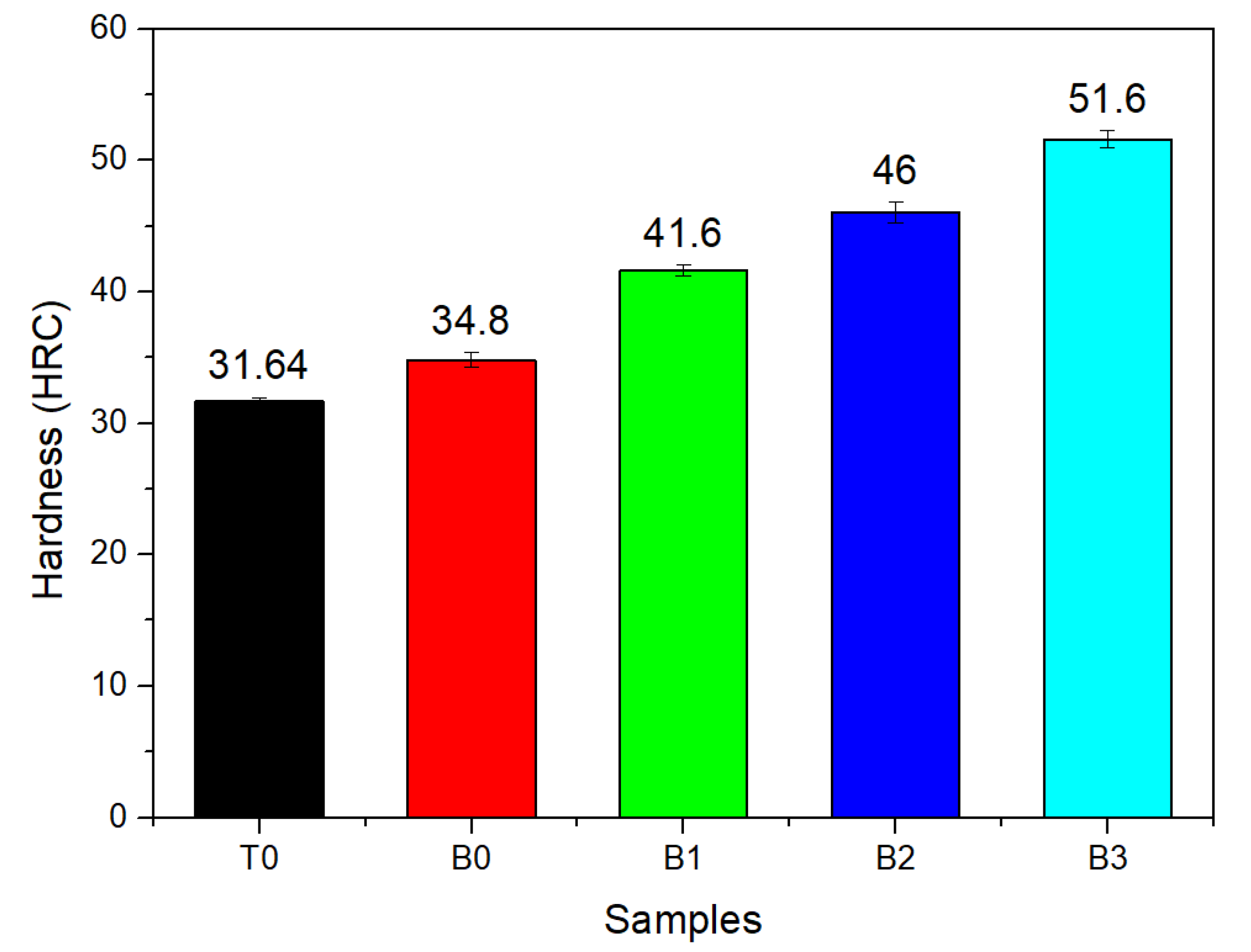
3.3. Hardness Test
3.4. Cytotoxicity on hPBMCs
3.5. Inflammatory Response of hPBMCs to Cr-Co-B Alloy
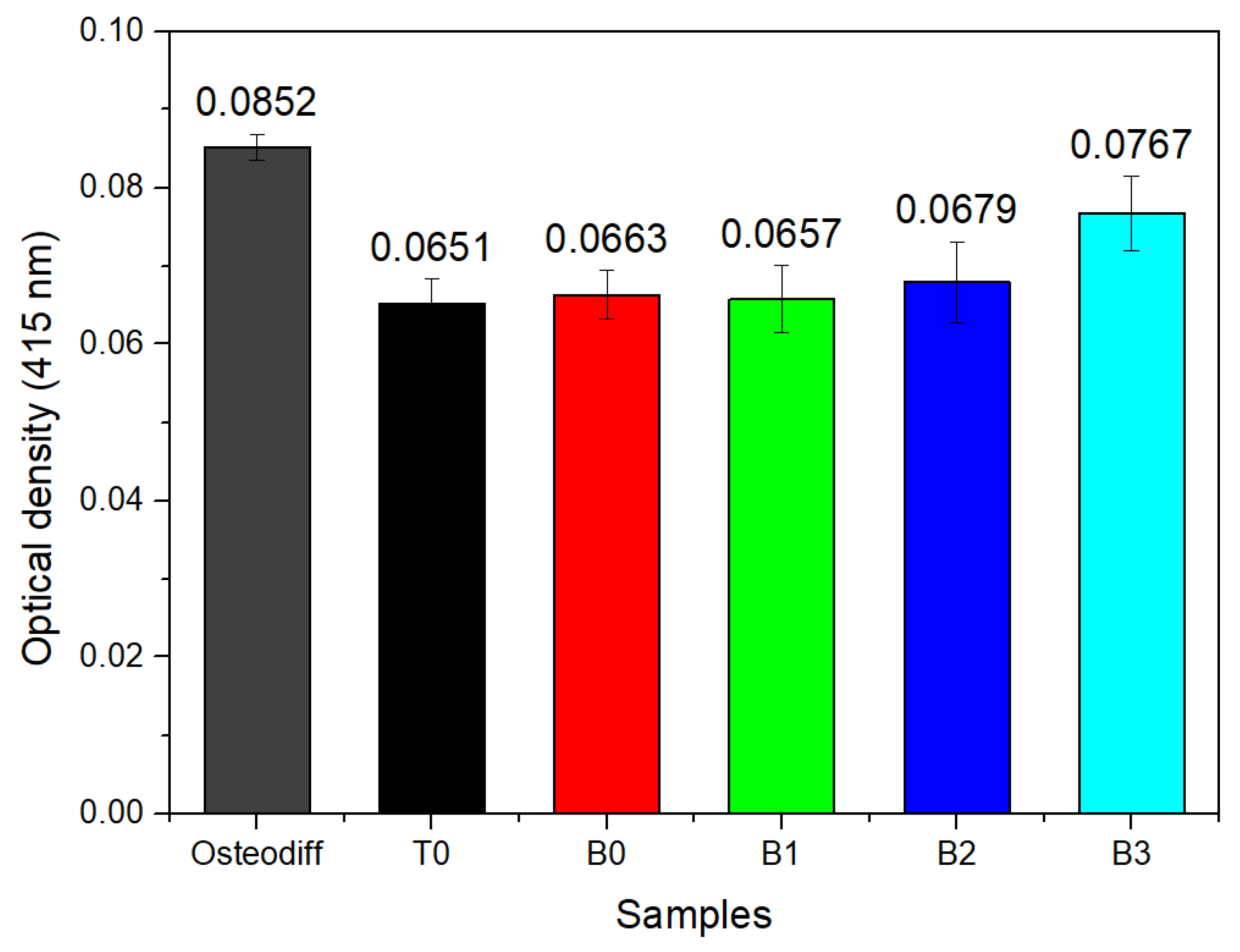
3.6. Formation of Extracellular Calcium Deposits in MSC Stimulated with the Co-Cr-B Alloy
3.7. The mRNA Expression of Mineralization-Related Genes on MSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saini, M. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Tribst, J.P.M.; de Oliveira Dal Piva, A.M.; Lo Giudice, R.; Borges, A.L.S.; Bottino, M.A.; Epifania, E.; Ausiello, P. The Influence of Custom-Milled Framework Design for an Implant-Supported Full-Arch Fixed Dental Prosthesis: 3D-FEA Study. Int. J. Environ. Res. Public Health 2020, 17, 4040. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, L. Metals Used in Maxillofacial Surgery. Oral Implant. 2016, 9, 107–111. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]
- Grischke, J.; Eberhard, J.; Stiesch, M. Antimicrobial dental implant functionalization strategies—A systematic review. Dent. Mater. J. 2016, 35, 545–558. [Google Scholar] [CrossRef] [Green Version]
- Raikar, S.; Talukdar, P.; Kumari, S.; Panda, S.K.; Oommen, V.M.; Prasad, A. Factors affecting the survival rate of dental implants: A retrospective study. J. Int. Soc. Prev. Community Dent. 2017, 7, 351–355. [Google Scholar] [CrossRef]
- Norowski, P.A.; Bumgardner, J.D. Biomaterial and antibiotic strategies for peri-implantitis: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 530–543. [Google Scholar] [CrossRef]
- Przekora, A. Current Trends in Fabrication of Biomaterials for Bone and Cartilage Regeneration: Materials Modifications and Biophysical Stimulations. Int. J. Mol. Sci. 2019, 20, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Vera, M.; Ortega, J.; Hernandez-Rodriguez, M.A.L. A study of the wear performance in a hip simulator of a metal–metal Co–Cr alloy with different boron additions. Wear 2013, 301, 175–181. [Google Scholar] [CrossRef]
- Alvarez-Vera, M.; Juarez-Hernandez, A.; Gonzalez-Rivera, C.; Mercado-Solis, R.D.; Hernandez-Rodriguez, M. Biotribological response of Co Cr alloy with added boron under ball-on-disc tests. Wear 2013, 301, 243–249. [Google Scholar] [CrossRef]
- Lu, Y.; Ren, L.; Wu, S.; Yang, C.; Lin, W.; Xiao, S.; Yang, Y.; Yang, K.; Lin, J. CoCrWCu alloy with antibacterial activity fabricated by selective laser melting: Densification, mechanical properties and microstructural analysis. Powder Technol. 2018, 325, 289–300. [Google Scholar] [CrossRef]
- Perez, R.; Kim, J.-H.; Buitrago, J.O.; Wall, I.B.; Kim, H.-W. Novel therapeutic core–shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater. 2015, 23, 295–308. [Google Scholar] [CrossRef]
- Icten, O.; Hosmane, N.S.; Kose, D.A.; Zumreoglu-Karan, B. Production of Magnetic Nano-bioconjugates via Ball Milling of Commercial Boron Powder with Biomolecules. Z. Anorg. Allg. Chem. 2016, 642, 828–832. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [Green Version]
- Taşlı, P.N.; Doğan, A.; Demirci, S.; Şahin, F. Boron Enhances Odontogenic and Osteogenic Differentiation of Human Tooth Germ Stem Cells (hTGSCs) In Vitro. Biol. Trace Element Res. 2013, 153, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Papagelopoulos, P.J.; Babis, G.C. Osseointegration of Cobalt-Chrome Alloy Implants. J. Autom. Inf. Sci. 2011, 21, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Hakki, S.S.; Dundar, N.; Kayis, S.A.; Hakki, E.E.; Hamurcu, M.; Kerimoglu, U.; Baspinar, N.; Basoglu, A.; Nielsen, F.H. Boron enhances strength and alters mineral composition of bone in rabbits fed a high energy diet. J. Trace Elem. Med. Biol. 2013, 27, 148–153. [Google Scholar] [CrossRef]
- Hernandez-Rodriguez, M.A.; Laverde-Cataño, D.A.; Lozano, D.; Martinez-Cazares, G.; Bedolla-Gil, Y. Influence of Boron Addition on the Microstructure and the Corrosion Resistance of CoCrMo Alloy. Meterials 2019, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Vander Voort, G.F. Metallography, Principles and Practice; ASM International: Materials Park, OH, USA, 1999; p. 1821. [Google Scholar]
- Del Angel-Mosqueda, C.; Gutierrez-Puente, Y.; López-Lozano, A.P.; Romero-Zavaleta, R.E.; Mendiola-Jiménez, A.; La Garza, C.E.M.-D.; Marquez-M, M.; De La Garza-Ramos, M.A. Epidermal growth factor enhances osteogenic differentiation of dental pulp stem cells in vitro. Head Face Med. 2015, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Curran, J.; Pu, F.; Zhuola, Z.; Bayon, Y.; Hunt, J.A. In Vitro Response of Human Peripheral Blood Mononuclear Cells (PBMC) to Collagen Films Treated with Cold Plasma. Polymers 2017, 9, 254. [Google Scholar] [CrossRef] [Green Version]
- Fuss, I.J.; Kanof, M.E.; Smith, P.D.; Zola, H. Isolation of Whole Mononuclear Cells from Peripheral Blood and Cord Blood. Curr. Protoc. Immunol. 2009, 85, 7.1.1–7.1.8. [Google Scholar] [CrossRef]
- Szuhanek, C.A.; Watz, C.G.; Avram, Ș.; Moacă, E.-A.; Mihali, C.V.; Popa, A.; Campan, A.A.; Nicolov, M.; Dehelean, C.A. Comparative Toxicological In Vitro and In Ovo Screening of Different Orthodontic Implants Currently Used in Dentistry. Materials 2020, 13, 5690. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, V.; Wolf, W.; Zepon, G.; Coury, F.; Kaufman, M.; Bolfarini, C.; Kiminami, C.; Botta, W. Effect of boron addition on the solidification sequence and microstructure of AlCoCrFeNi alloys. J. Alloys Compd. 2019, 775, 1235–1243. [Google Scholar] [CrossRef]
- Bermingham, M.J.; McDonald, S.; StJohn, D.; Dargusch, M. The effect of boron on the refinement of microstructure in cast cobalt alloys. J. Mater. Res. 2011, 26, 951–956. [Google Scholar] [CrossRef]
- Rogl, P.; Korniyenko, K.; Velikanova, T. Boron-Carbon-Molybdenum. Landolt Börnstein 2009, 11, 422. [Google Scholar]
- Deng, G.; Zhao, X.; Su, L.; Wei, P.; Zhang, L.; Zhan, L.; Chong, Y.; Zhu, H.; Tsuji, N. Effect of high pressure torsion process on the microhardness, microstructure and tribological property of Ti6Al4V alloy. J. Mater. Sci. Technol. 2021, 94, 183–195. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, B.S.; Hakki, E.E. Boron regulates mineralized tissue-associated proteins in osteoblasts (MC3T3-E1). J. Trace Elem. Med. Biol. 2010, 24, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Cheng, S.; Wang, W.; Lin, Z.; Chen, Q.; Zhang, W.; Kou, D.; Shen, Y.; Cheng, X.; Rompis, F.A.; et al. Effect of Boron on Osteogenic Differentiation of Human Bone Marrow Stromal Cells. Biol. Trace Elem. Res. 2011, 144, 306–315. [Google Scholar] [CrossRef]
- Najafabadi, B.-A.-H.M.; Abnosi, M.H. Boron Induces Early Matrix Mineralization via Calcium Deposition and Elevation of Alkaline Phosphatase Activity in Differentiated Rat Bone Marrow Mesenchymal Stem Cells. Cell J. 2016, 18, 62–73. [Google Scholar]
- Witek, L.; Tovar, N.; Lopez, C.; Morcos, J.; Bowers, M.; Petrova, R.; Coelho, P. Assessing osseointegration of metallic implants with boronized surface treatment. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e311–e317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Miron, R.; Zhao, Y.; Zhang, Q.; Wu, C.; Geng, S. In vivo experimental study on bone regeneration in critical bone defects using PIB nanogels/boron-containing mesoporous bioactive glass composite scaffold. Int. J. Nanomed. 2015, 10, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Routray, I.; Ali, S. Boron Induces Lymphocyte Proliferation and Modulates the Priming Effects of Lipopolysaccharide on Macrophages. PLoS ONE 2016, 11, e0150607. [Google Scholar] [CrossRef] [Green Version]
- Sabaliauskas, V.; Juciute, R.; Bukelskiene, V.; Rutkunas, V.; Trumpaite-Vanagiene, R.; Puriene, A. In vitro evaluation of cytotoxicity of permanent prosthetic materials. Stomatologija 2011, 13, 75–80. [Google Scholar] [PubMed]
- Messer, R.L.; Lucas, L.C. Cytotoxicity of nickel-chromium alloys: Bulk alloys compared to multiple ion salt solutions. Dent. Mater. 2000, 16, 207–212. [Google Scholar] [CrossRef]
- Wataha, J.C.; Ratanasathien, S.; Hanks, C.T.; Sun, Z. In vitro IL-1β and TNF-α release from THP-1 monocytes in response to metal ions. Dent. Mater. 1996, 12, 322–327. [Google Scholar] [CrossRef]
- Stio, M.; Martinesi, M.; Treves, C.; Borgioli, F. In vitro response of human peripheral blood mononuclear cells to AISI 316L austenitic stainless steel subjected to nitriding and collagen coating treatments. J. Mater. Sci. Mater. Electron. 2015, 26, 100. [Google Scholar] [CrossRef] [PubMed]
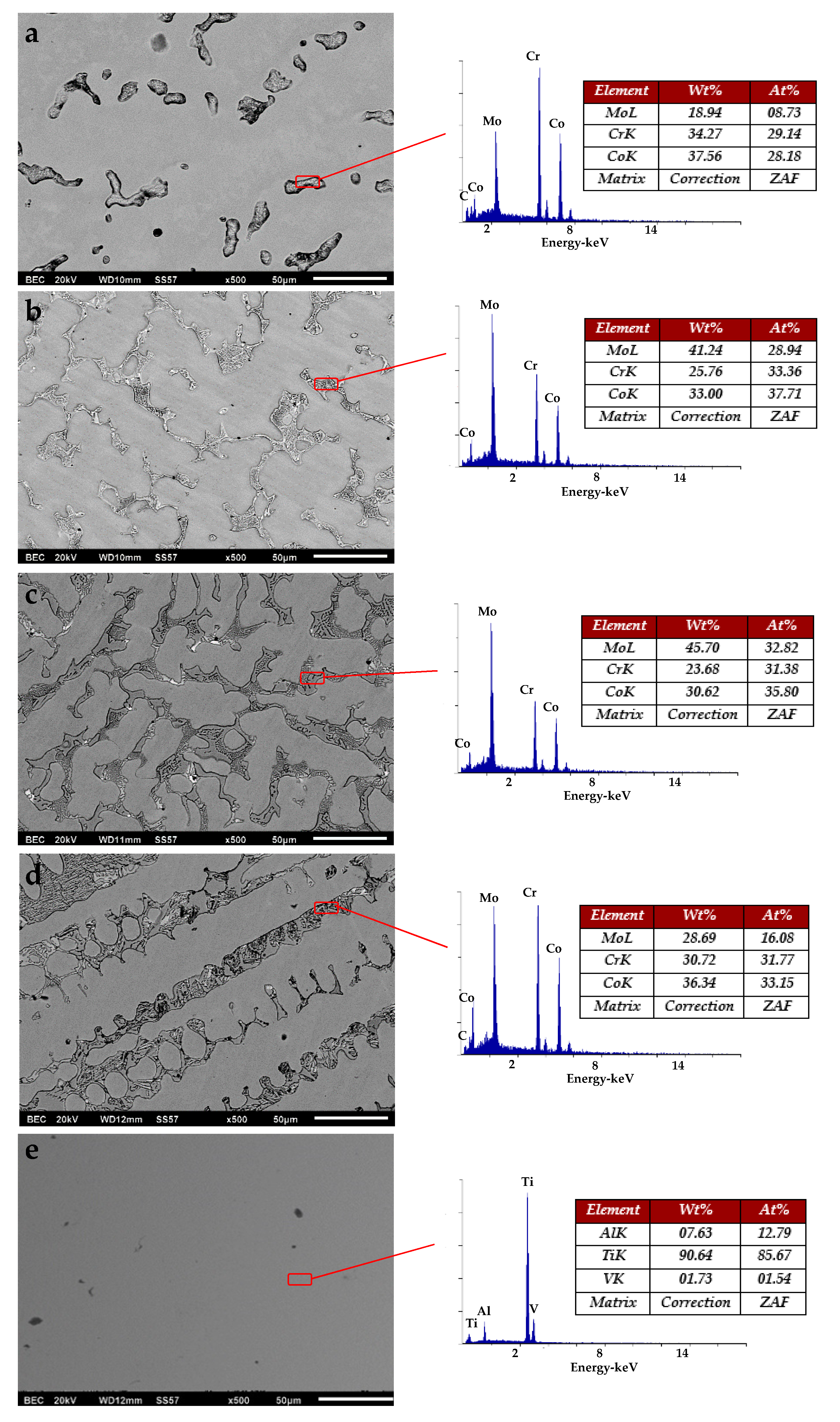
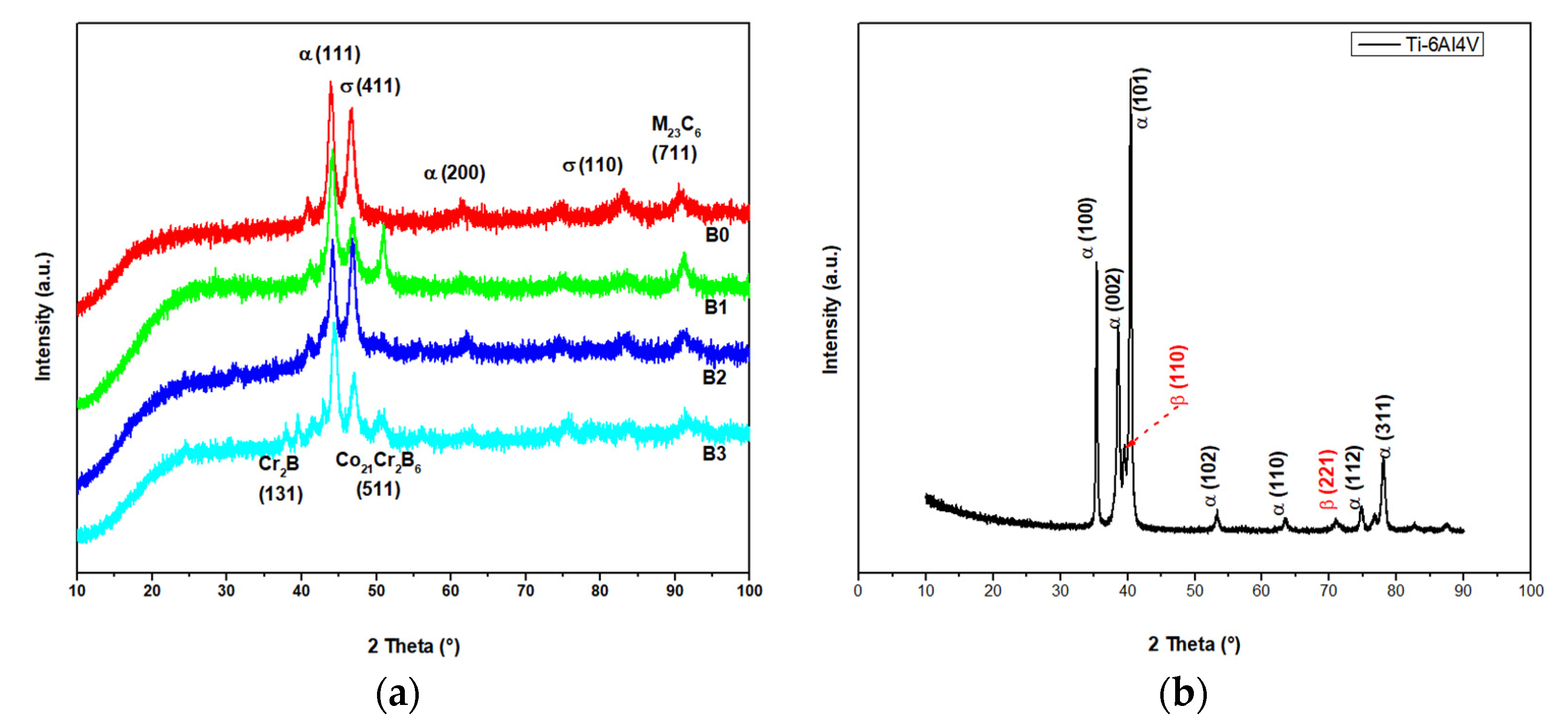

| Sample Name | Description |
|---|---|
| B0 | ASTM F75 cobalt alloy without boron |
| B1 | ASTM F75 cobalt alloy added with 0.3% boron |
| B2 | ASTM F75 cobalt alloy added with 0.6% boron |
| B3 | ASTM F75 cobalt alloy added with 1.0% boron |
| T0 | Ti-6Al-4V alloy |
| Treatment Groups | Description |
|---|---|
| Positive control | Cells treated with Osteodiff® |
| Negative control | Cells treated with DMEM |
| Treatment 1 | Titanium alloy |
| Treatment 2 | (Co-Cr) alloy without boron |
| Treatment 3 | (Co-Cr-B) with 0.3% of boron |
| Treatment 4 | (Co-Cr-B) with 0.6% of boron |
| Treatment 5 | (Co-Cr-B) with 1.0% of boron |
| Element | B0 | B1 | B2 | B3 |
|---|---|---|---|---|
| Co | Bal. | Bal. | Bal. | Bal. |
| Cr | 29.32 | 28.94 | 27.58 | 28.33 |
| Mo | 5.91 | 6.49 | 6.24 | 5.35 |
| Ni | 0.192 | 0.225 | 0.184 | 0.238 |
| Fe | 0.238 | 0.135 | 0.292 | 0.184 |
| C | 0.23 | 0.21 | 0.24 | 0.26 |
| Si | 0.28 | 0.58 | 0.43 | 0.63 |
| Mn | 0.43 | 0.14 | 0.23 | 0.46 |
| W | 0.15 | 0.09 | 0.11 | 0.13 |
| N | 0.09 | 0.12 | 0.18 | 0.16 |
| Al | 0.08 | 0.07 | 0.06 | 0.03 |
| Ti | 0.01 | 0.06 | 0.02 | 0.03 |
| B | 0.003 | 0.28 | 0.61 | 0.98 |
| Treatment | Relative Expression | Standard Error | 95% C.I. | p Value | Result |
|---|---|---|---|---|---|
| IL-1β | |||||
| Calibrator normalized | 1.0 | - | - | - | - |
| LPS | 3.706 | 2.342–5.799 | 1.965–7.134 | 0.004 | UP |
| Co-Cr-B alloy | 0.58 | 0.359–0.882 | 0.309–1.132 | 0.15 | No change |
| TNF-α | |||||
| Calibrator normalized | 1.0 | - | - | - | - |
| LPS | 2.255 | 1.451–3.366 | 1.265–4.983 | 0.003 | UP |
| Co-Cr-B alloy | 0.822 | 0.586–1.357 | 0.518–1.536 | 0.473 | No change |
| IL-6 | |||||
| Calibrator normalized | 1.0 | - | - | - | - |
| LPS | 74.543 | 60.055–105.514 | 57.452–112.396 | 0.001 | UP |
| Co-Cr-B alloy | 2 | 1.587–2.845 | 1.485–3.079 | 0.065 | No change |
| IL-8 | |||||
| Calibrator normalized | 1.0 | - | - | - | - |
| LPS | 4.297 | 2.688–9.130 | 2.312–10.388 | 0.001 | UP |
| Co-Cr-B alloy | 1.526 | 0.966–3.134 | 0.873–3.631 | 0.332 | No change |
| Treatment | Relative Expression | Standard Error | p-Value | Result |
|---|---|---|---|---|
| Type 1 collagen | ||||
| Calibrator normalized | 1.0 | - | - | - |
| Osteodiff | 26.4 | 24.613–27.331 | 0.001 | UP |
| T0 | 2.1 | 1.980–2.341 | 0.330 | No regulation |
| B0 | 0.842 | 0.745–0.941 | 0.064 | No regulation |
| B1 | 232.862 | 120.53–245.170 | 0.026 | UP |
| B2 | 0.956 | 0.032–0.054 | 0.945 | No regulation |
| B3 | 0.875 | 0.454–0.966 | 0.854 | No regulation |
| Alkaline phosphatase | ||||
| Calibrator normalized | 1.0 | - | - | - |
| Osteodiff | 28.4 | 27.820–30.493 | 0.001 | UP |
| T0 | 0.0001 | 0.000–0.000 | 0.018 | Down |
| B0 | 0.0001 | 0.000–0.000 | 0.000 | Down |
| B1 | 1.101 | 0.0452–1.241 | 0.529 | No regulation |
| B2 | 17.877 | 11.570–34.224 | 0.009 | UP |
| B3 | 0.057 | 0.038–0.107 | 0.000 | Down |
| Runx2 | ||||
| Calibrator normalized | 1.0 | - | - | - |
| Osteodiff | 34.3 | 29.430–35.534 | 0.001 | UP |
| T0 | 1.02 | 0.976–1.120 | 0.081 | No regulation |
| B0 | 0.003 | 0.001–0.010 | 0.032 | Down |
| B1 | 0.802 | 0.032–1.101 | 0.344 | No regulation |
| B2 | 9.624 | 3.383–33.344 | 0.000 | UP |
| B3 | 0.943 | 0.701–1.196 | 0.732 | No regulation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Mendez, M.C.; Urrutia-Baca, V.H.; Cuao-Moreu, C.A.; Lorenzo-Bonet, E.; Alvarez-Vera, M.; Ortiz-Martinez, D.M.; de la Garza-Ramos, M.A. In Vitro Biocompatibility Evaluation of a New Co-Cr-B Alloy with Potential Biomedical Application. Metals 2021, 11, 1267. https://doi.org/10.3390/met11081267
Garcia-Mendez MC, Urrutia-Baca VH, Cuao-Moreu CA, Lorenzo-Bonet E, Alvarez-Vera M, Ortiz-Martinez DM, de la Garza-Ramos MA. In Vitro Biocompatibility Evaluation of a New Co-Cr-B Alloy with Potential Biomedical Application. Metals. 2021; 11(8):1267. https://doi.org/10.3390/met11081267
Chicago/Turabian StyleGarcia-Mendez, María Cristina, Victor Hugo Urrutia-Baca, Carlos A. Cuao-Moreu, Ernesto Lorenzo-Bonet, Melvyn Alvarez-Vera, David Mizael Ortiz-Martinez, and Myriam Angelica de la Garza-Ramos. 2021. "In Vitro Biocompatibility Evaluation of a New Co-Cr-B Alloy with Potential Biomedical Application" Metals 11, no. 8: 1267. https://doi.org/10.3390/met11081267
APA StyleGarcia-Mendez, M. C., Urrutia-Baca, V. H., Cuao-Moreu, C. A., Lorenzo-Bonet, E., Alvarez-Vera, M., Ortiz-Martinez, D. M., & de la Garza-Ramos, M. A. (2021). In Vitro Biocompatibility Evaluation of a New Co-Cr-B Alloy with Potential Biomedical Application. Metals, 11(8), 1267. https://doi.org/10.3390/met11081267








