Leaching Behavior of Lithium-Bearing Bauxite with High-Temperature Bayer Digestion Process in K2O-Al2O3-H2O System
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Procedure
2.3. Characterization
3. Results and Discussion
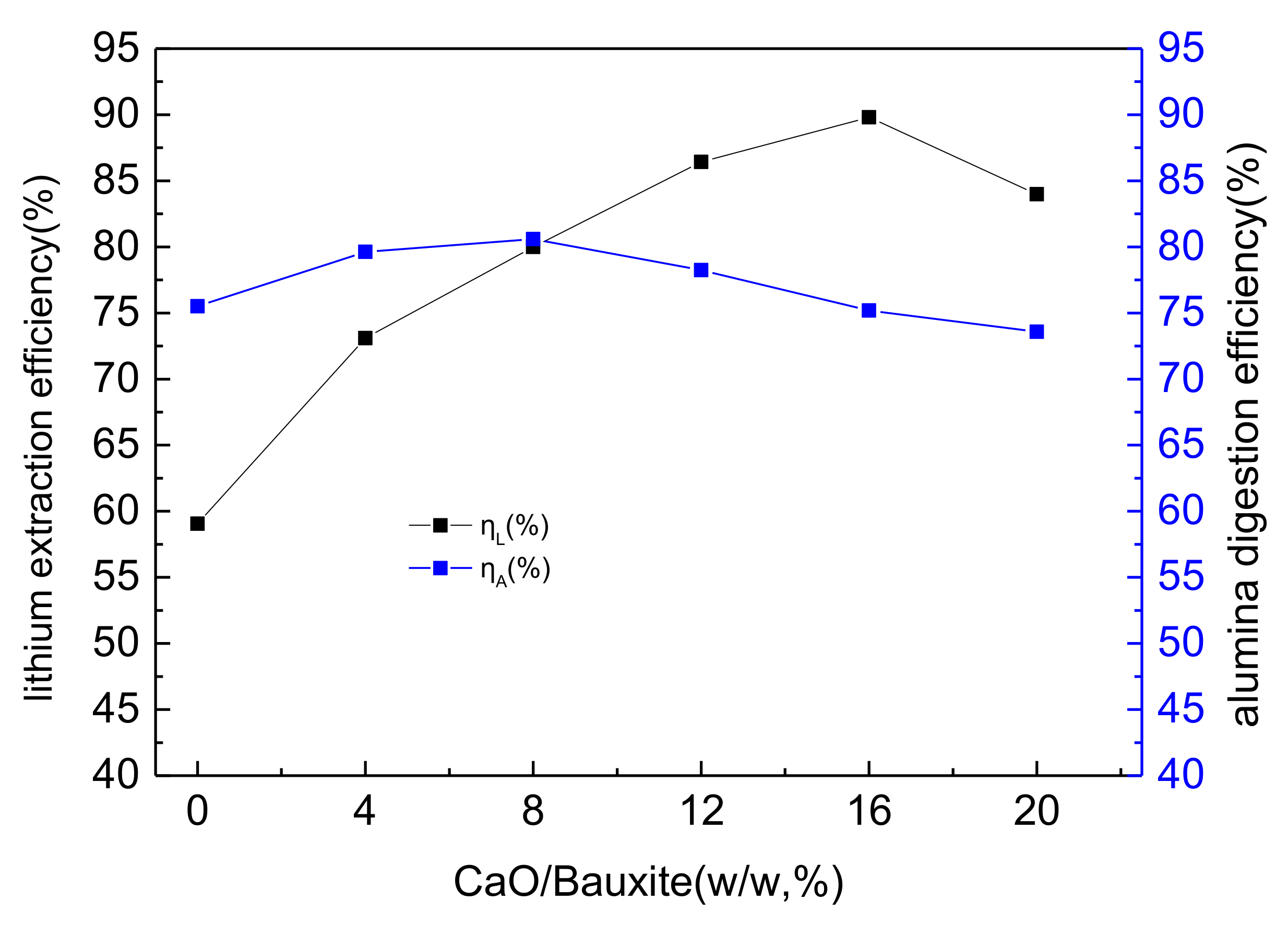
3.1. The Effects of Lime Dosage on Lithium and Alumina Extraction Efficiency
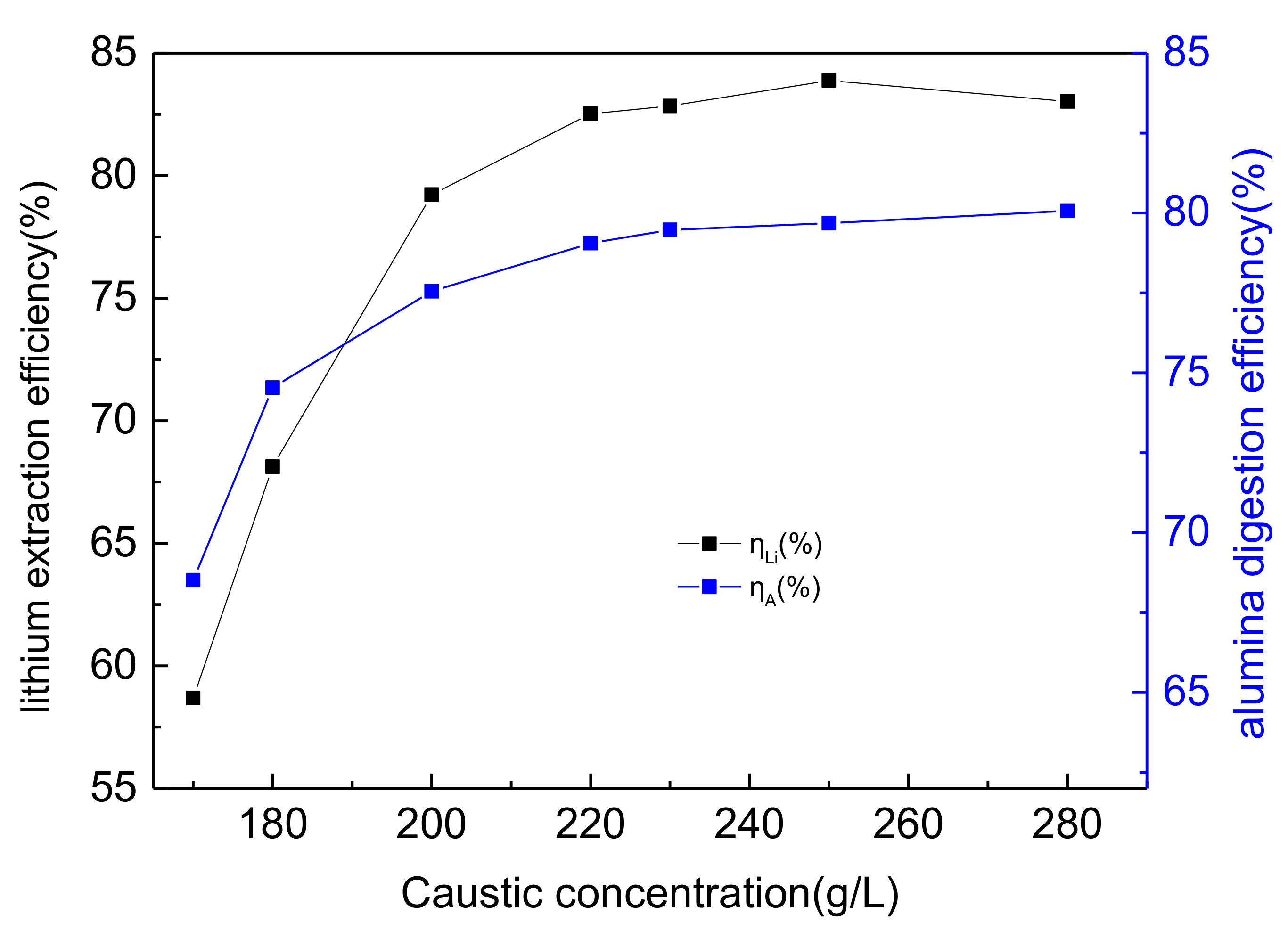
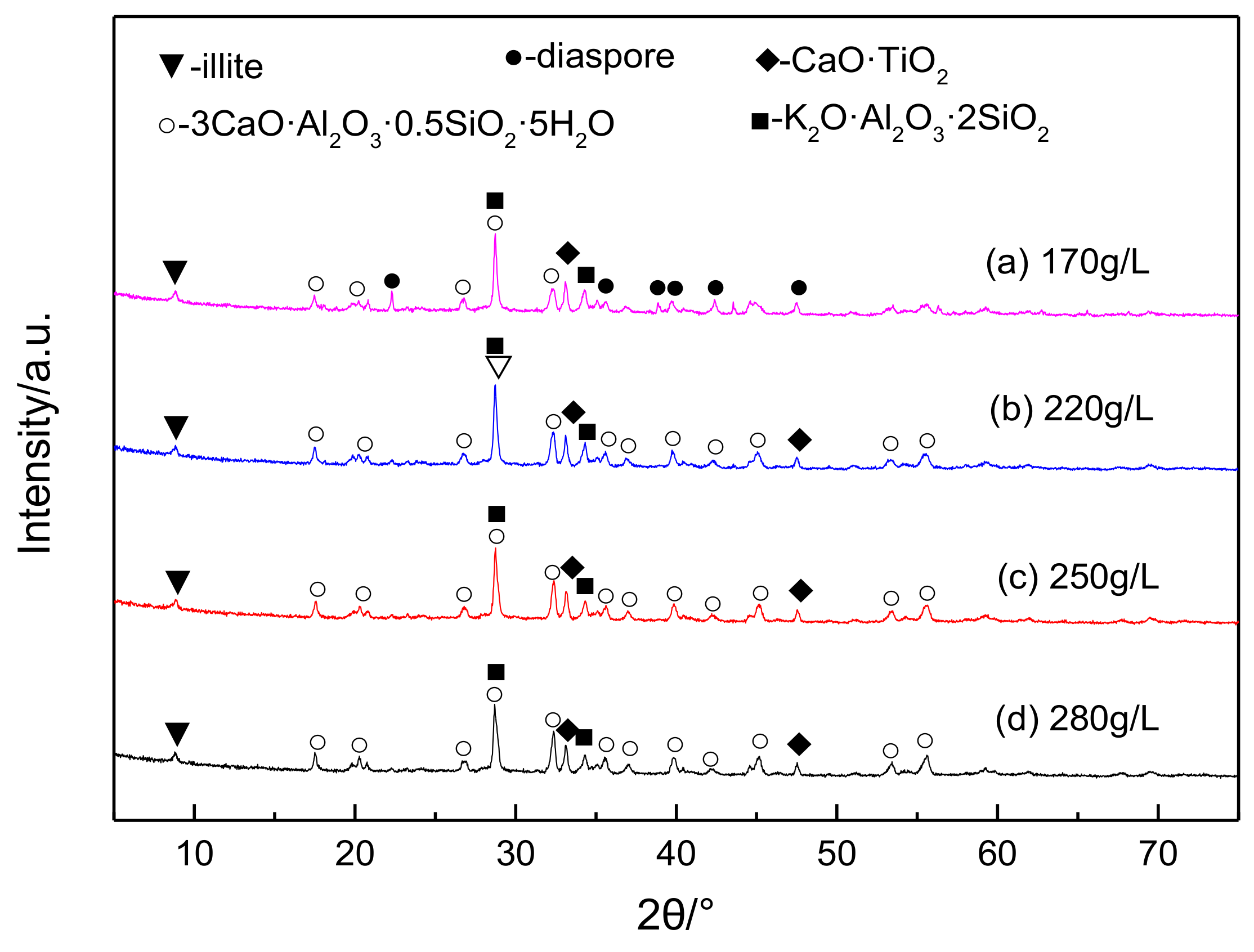
3.2. The Effects of Caustic Concentration on Lithium and Alumina Extraction Efficiency
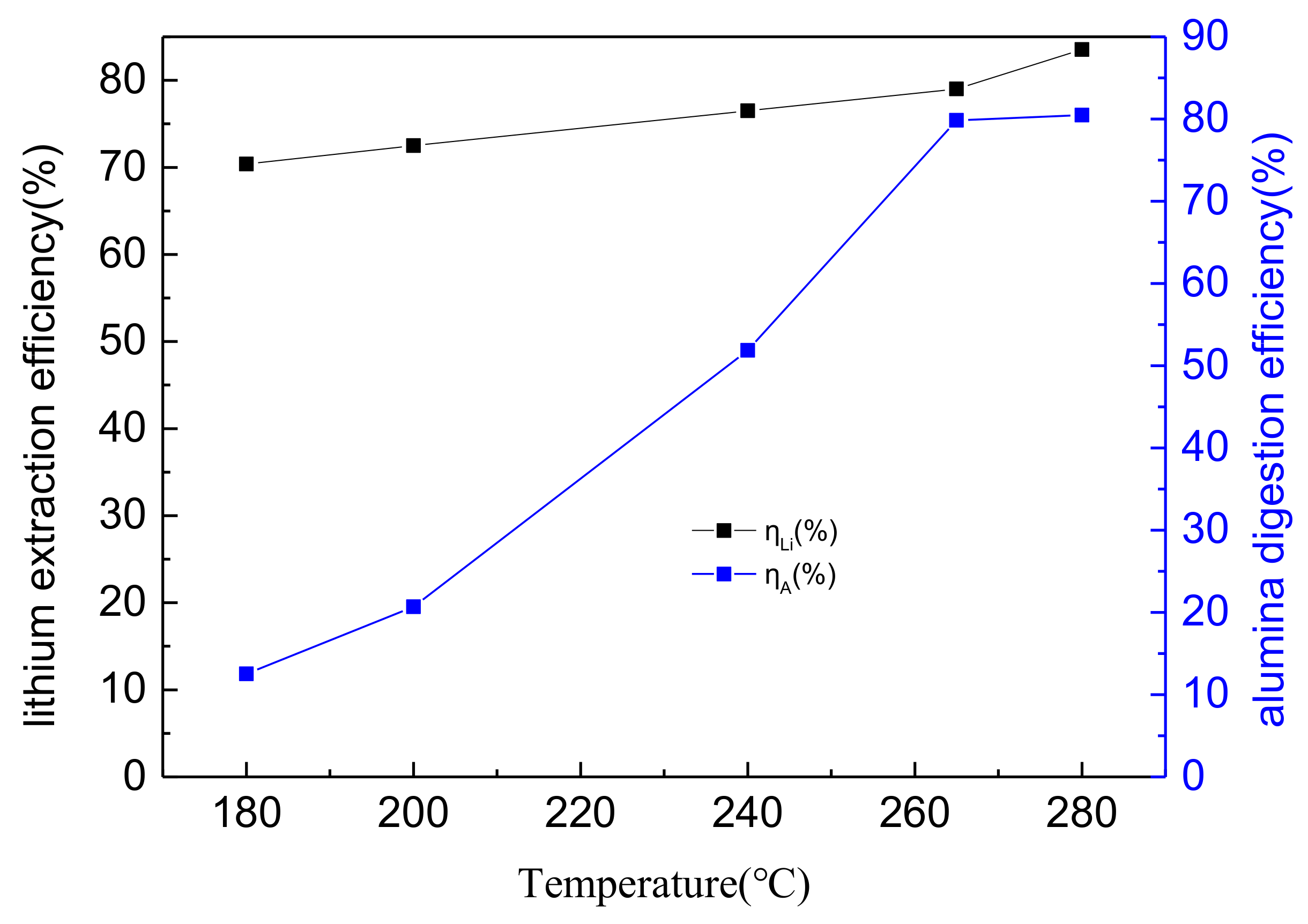
3.3. The Effects of Temperature on Lithium and Alumina Extraction Efficiency
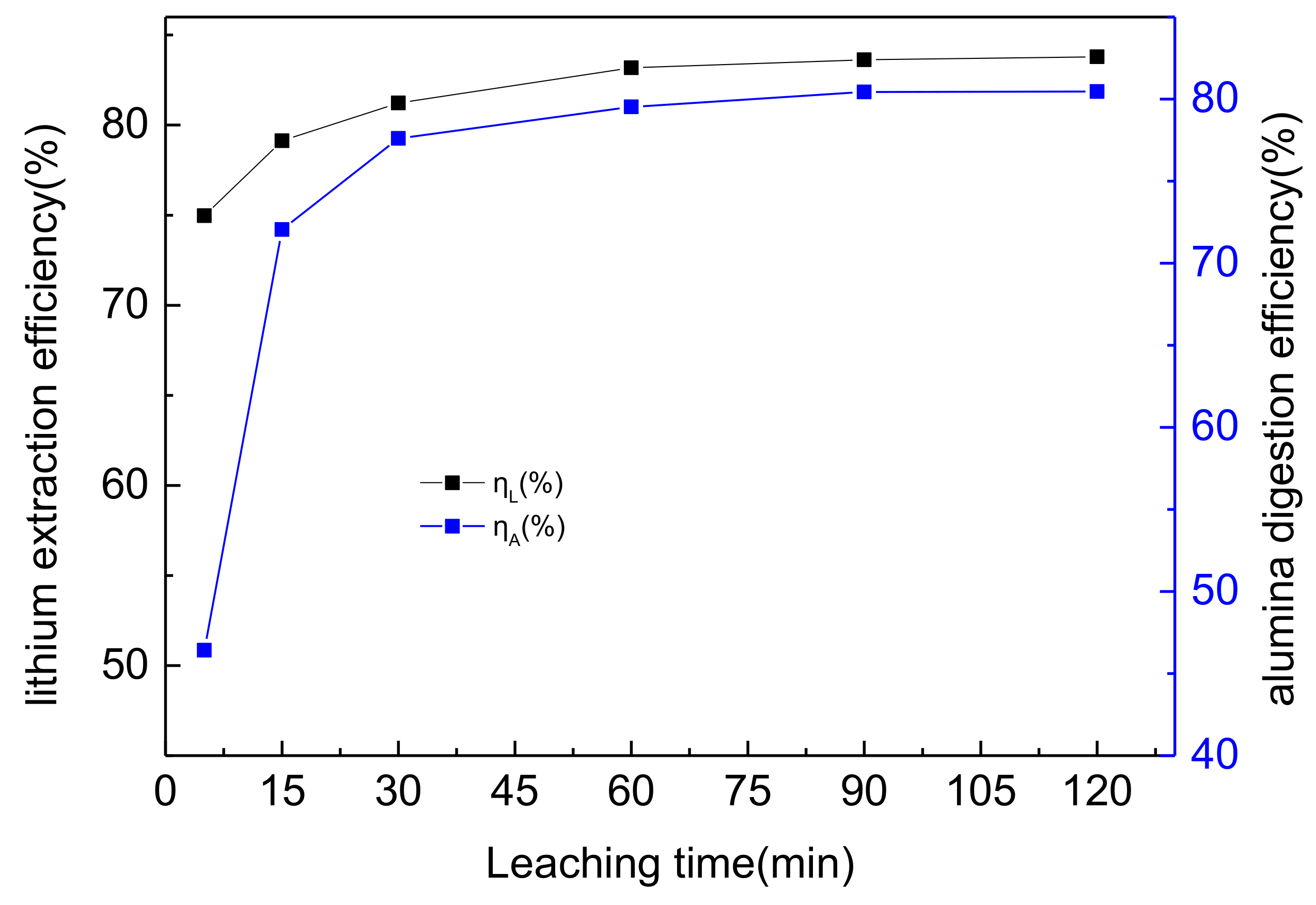
3.4. The Effects of Duration on Lithium and Alumina Extraction Efficiency
3.5. Orthogonal Experiment
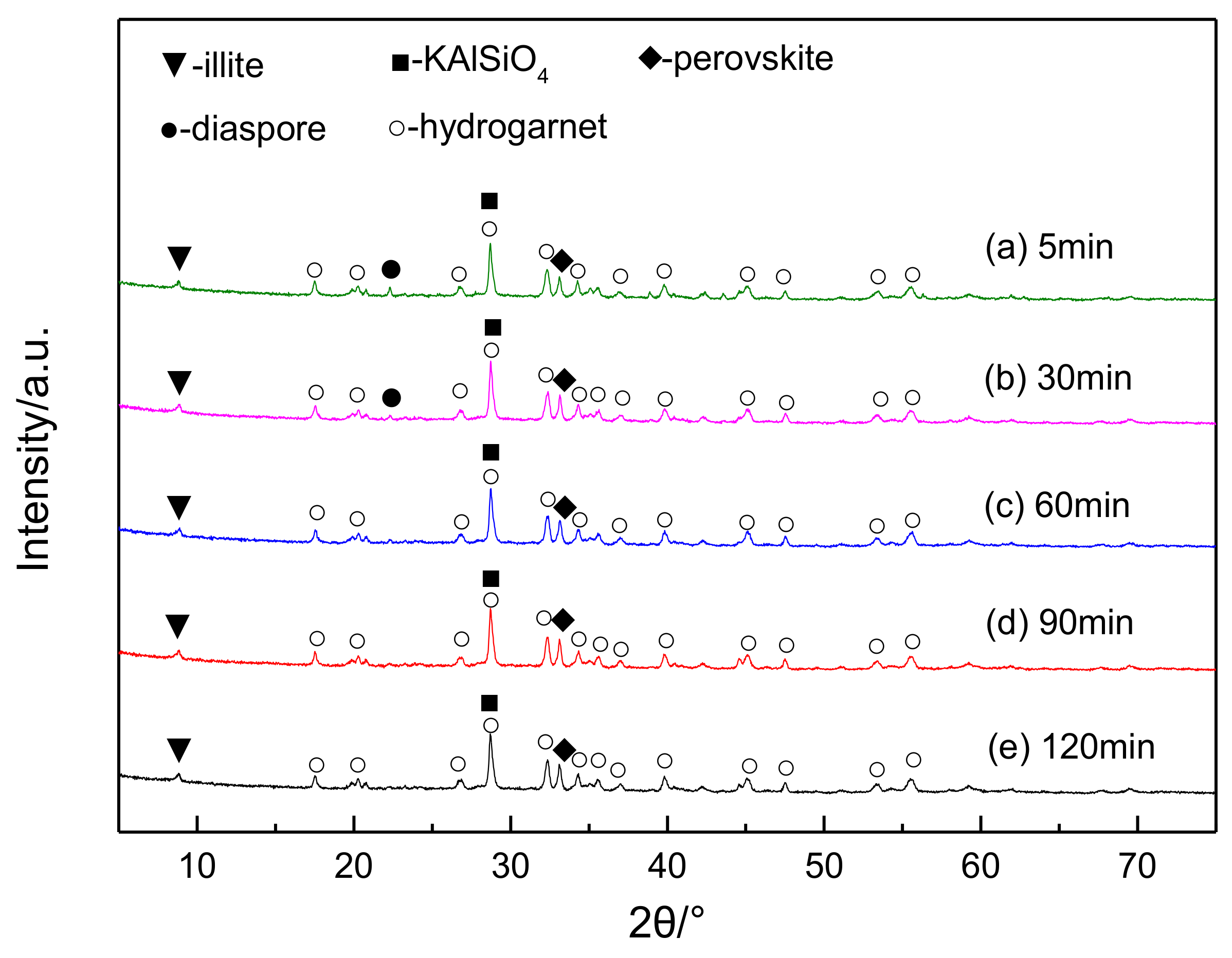
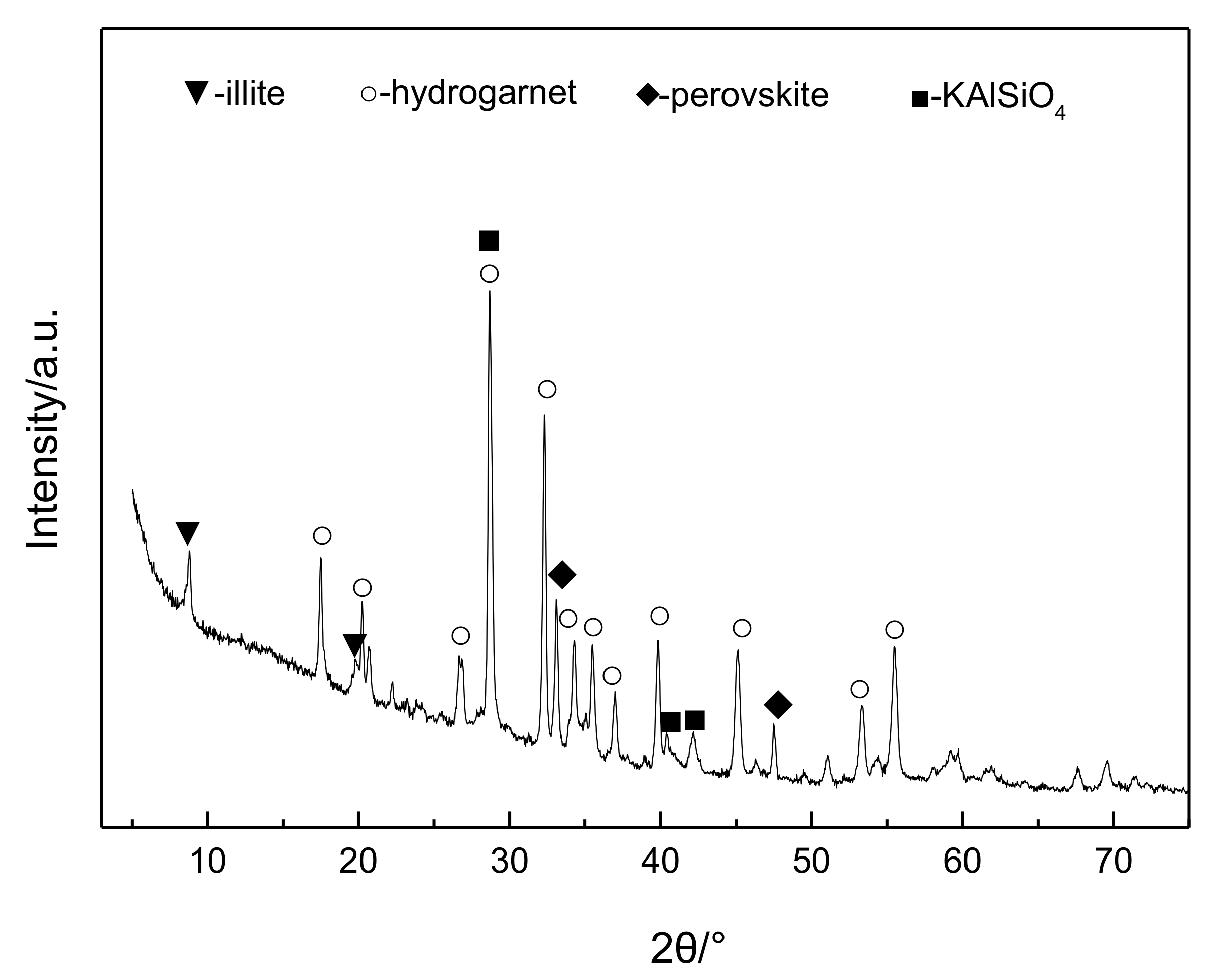
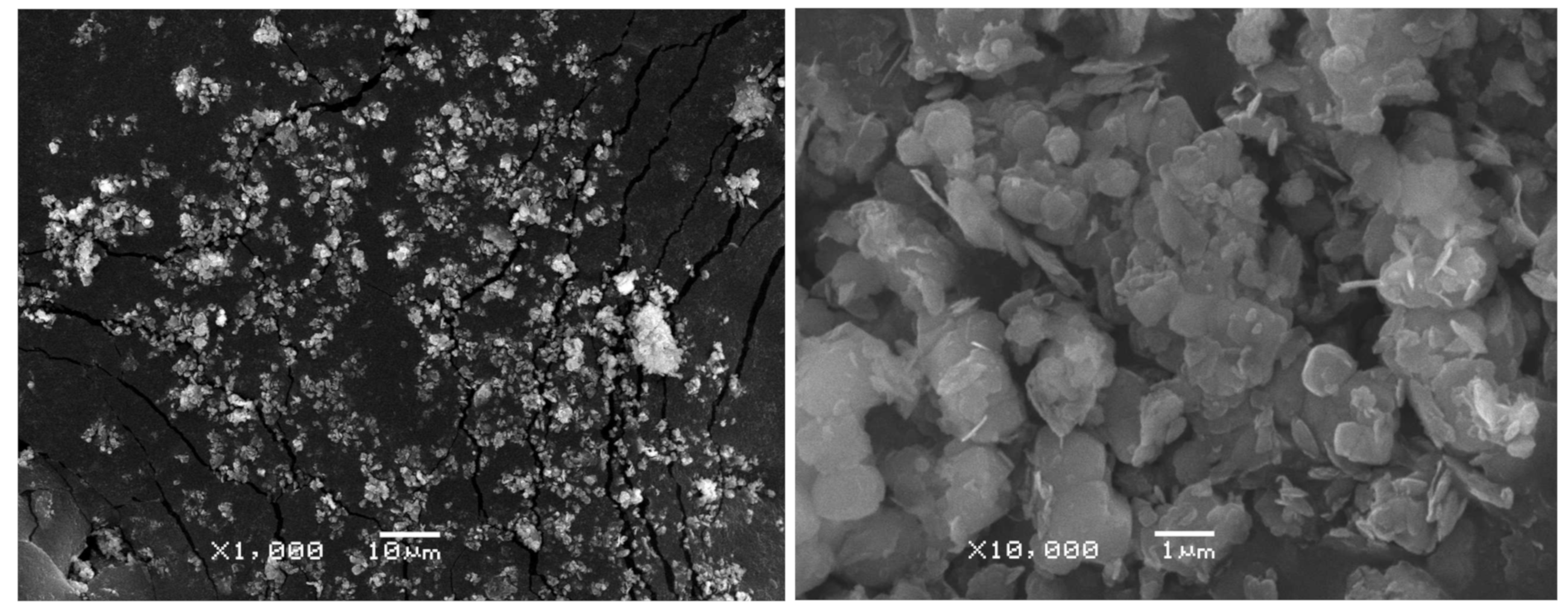
4. Behavior Mechanism of Lithium during Digestion Process
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, A.; Li, C. Enrichment mechanism of lithium in Bayer process of alumina production. Nonferrous Met. (Extr. Metall.) 2017, 9, 23–26. [Google Scholar]
- Li, C.; Huang, J. The existing state of lithium in the process of alumina production. Light Met. 2005, 6, 17–19. (In Chinese) [Google Scholar]
- Wang, D.; Li, P.; Qu, W.; Yin, L.; Zhao, Z.; Lei, Z.; Wen, S. Discovery and preliminary study of the high tungsten and lithium contents in the Dazhuyuan bauxite deposit, Guizhou, China. Sci. China (Earth Sci.) 2013, 56, 145–152. (In Chinese) [Google Scholar] [CrossRef]
- Qing-Tao, H.U.; Liang, Y.D.; Wang, C.Z.; Xu-Jian, W.U.; Zhou, C.Q.; Niu, T.T. Effect of high lithium electrolyte on aluminum electrolysis production and improvement measures. Nonferrous Met. (Extr. Metall.) 2018, 43, 34–38. (In Chinese) [Google Scholar]
- Huang, W.; Liu, G.; Liu, P.; Qi, T.-G.; Li, X.-B.; Peng, Z.-H.; Zhou, Q.-S. Equilibrium concentration of lithium ion in sodium aluminate solution. J. Cent. South Univ. 2019, 26, 304–311. [Google Scholar] [CrossRef]
- Lv, X.J.; Shuang, Y.J.; Jie, L. Physicochemical Properties of Industrial Aluminum Electrolytes Enriching Li and K: The liquidus temperature. Metall. Mater. Trans. B 2017, 48, 1315–1320. [Google Scholar] [CrossRef]
- Saitov, A.V.; Bazhin, V.Y.; Povarov, V.G. On the application of lithium additives in the electrolytic production of primary aluminum. Russ. Metall. (Metall.) 2017, 12, 1018–1024. [Google Scholar] [CrossRef]
- Morishige, T.; Haarberg, G.M.; Gudbrandsen, H.; Skybakmoen, E.; Solheim, A.; Takenaka, T. Effects of composition and temperature on current efficiency for aluminum electrolysis from cryolite-based molten alumina electrolytes. ECS Trans. 2017, 77, 997–1002. [Google Scholar] [CrossRef]
- Chen, F. Effect of lithium and potassium salts on the production of prebaked cell. J. Mater. Metall. 2010, 9, 148–149. [Google Scholar]
- Huang, W.; Liu, G.; Ju, J.; Li, X.; Zhou, Q.; Qi, T.; Peng, Z. Effect of lithium ion on seed precipitation from sodium aluminate solution. Trans. Nonferrous Met. Soc. China 2019, 29, 1323–1331. [Google Scholar] [CrossRef]
- Prestidge, C.A.; Ametov, I. Cation effects during aggregation and agglomeration of gibbsite particles under synthetic Bayer crystallization conditions. J. Cryst. Growth 2000, 209, 924–933. [Google Scholar] [CrossRef]
- Van Straten, H.A.; Schoonen, M.A.A.; De Bruyn, P.L. Precipitation from supersaturated aluminate solutions. III. Influence of alkali ions with special reference to Li+. J. Colloid Interface Sci. 1985, 103, 493–507. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Fan, M.; Lv, Z.; Tang, Y.; Sun, Y.; Chen, Y.; Wan, P. Lithium adsorption performance of a three-dimensional porous H2TiO3-type lithium ion-sieve in strong alkaline Bayer liquor. RSC Adv. 2017, 7, 18883–18891. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Peng, Z.; Liu, G.; Zhou, Q.; Qi, T.; Li, X. Lithium removal from sodium aluminate solution and its reaction mechanism. J. Cent. South Univ. (Sci. Technol.) 2020, 51, 19–26. [Google Scholar]
- Yin, Z. Research on the Behavior of Diasporic Bauxite in the Bayer Preheating and Digestion Process. Ph.D. Thesis, Northeastern University, Shenyang, China, 2005. (In Chinese). [Google Scholar]
- Gu, S.; Yin, Z.; Zhou, H. Behavior of illite in the Bayer digestion process of diasporic bauxite. Light Met. 1992, 109–112. [Google Scholar]
- Yin, Z.; Gu, S.; Huang, H. The influence of K2O in spent liquor on Bayer process of diasporic bauxite. Light Met. 2003, 173–176. [Google Scholar]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Ma, S.H.; Wen, Z.G.; Chen, J.N.; Zheng, S.L. An environmentally friendly design for low-grade diasporic-bauxite processing. Miner. Eng. 2009, 22, 793–798. [Google Scholar] [CrossRef]
- Malts, N.S. Efficiency of lime use in Bayer alumina production. Light Met. 1992, 1337–1342. [Google Scholar]
- Smith, P. Reactions of lime under high temperature Bayer digestion conditions. Hydrometaurgy 2017, 170, 16–23. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.A.; Lv, G.; Zhang, W.; Zhu, X.; Xie, L. Reaction behaviors and amorphization effects of titanate species in pure substance systems relating to Bayer digestion. Hydrometallurgy 2017, 171, 86–94. [Google Scholar] [CrossRef]
- Bi, S. Alumina Production Process; Chemical Industry Press: Beijing, China, 2010; pp. 8–9. [Google Scholar]
- Wu, H.F.; Chen, C.Y.; Li, J.Q.; Lan, Y.P.; Wang, L.Z.; Quan, B.L.; Jin, H.X. Digestion mechanism and crystal simulation of roasted low-grade high-sulfur bauxite. Trans. Nonferrous Met. Soc. China 2020, 30, 1662–1673. [Google Scholar] [CrossRef]
- Ma, Q.X.; Zhang, Y.F.; Cao, S.T.; Zhang, Y. Leaching behavior of diaspore bauxite in KOH sub-molten salt. Chin. J. Process Eng. 2013, 13, 391–396. (In Chinese) [Google Scholar]
- Zheng, H.; Bailey, S.W. Refinement of the cookeite “r” structure. Am. Mineral. 1997, 82, 1007–1013. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, C.; Ma, H.; Wang, L.; Li, J.; Liu, Y. Digestion of potassium feldspar ore through high pressure hydrochemical process. China’s Manganese Ind. 2002, 20, 27–29, (In Chinese with English Abstract). [Google Scholar]




| +250 μm | 150–250 μm | 90–150 μm | 45–90 μm | –45 μm |
|---|---|---|---|---|
| 22.3 | 28.4 | 11.1 | 7.60 | 30.6 |
| Al | Si | Fe | Ti | K | Na | Ca | Mg | Li | A/S |
|---|---|---|---|---|---|---|---|---|---|
| 34.4 | 6.04 | 2.06 | 1.49 | 0.61 | 0.032 | 0.12 | 0.13 | 0.07 | 5.01 |
| Diaspore | Kaolinite | Hematite | Illite | Quartz | Anatase | Rutile |
|---|---|---|---|---|---|---|
| 62.5 | 24.5 | 1.7 | 7.0 | 0.5 | 2.0 | 0.5 |
| NO. | Lime Dosage (%) | Temperature (°C) | K2O (g/L) |
|---|---|---|---|
| 1 | 0 | 240 | 200 |
| 2 | 0 | 260 | 240 |
| 3 | 0 | 280 | 280 |
| 4 | 8 | 240 | 240 |
| 5 | 8 | 260 | 280 |
| 6 | 8 | 280 | 200 |
| 7 | 16 | 240 | 280 |
| 8 | 16 | 260 | 200 |
| 9 | 16 | 280 | 240 |
| NO. | A | B | C | ηL (%) | ηA (%) |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 70.00 | 31.46 |
| 2 | 1 | 2 | 2 | 69.95 | 70.28 |
| 3 | 1 | 3 | 3 | 75.40 | 81.00 |
| 4 | 2 | 1 | 2 | 79.48 | 70.29 |
| 5 | 2 | 2 | 3 | 83.34 | 79.08 |
| 6 | 2 | 3 | 1 | 66.83 | 78.08 |
| 7 | 3 | 1 | 3 | 83.89 | 71.55 |
| 8 | 3 | 2 | 1 | 85.26 | 73.90 |
| 9 | 3 | 3 | 2 | 86.34 | 76.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Peng, Z.; Song, E.; Shen, L. Leaching Behavior of Lithium-Bearing Bauxite with High-Temperature Bayer Digestion Process in K2O-Al2O3-H2O System. Metals 2021, 11, 1148. https://doi.org/10.3390/met11071148
Han D, Peng Z, Song E, Shen L. Leaching Behavior of Lithium-Bearing Bauxite with High-Temperature Bayer Digestion Process in K2O-Al2O3-H2O System. Metals. 2021; 11(7):1148. https://doi.org/10.3390/met11071148
Chicago/Turabian StyleHan, Dongzhan, Zhihong Peng, Erwei Song, and Leiting Shen. 2021. "Leaching Behavior of Lithium-Bearing Bauxite with High-Temperature Bayer Digestion Process in K2O-Al2O3-H2O System" Metals 11, no. 7: 1148. https://doi.org/10.3390/met11071148
APA StyleHan, D., Peng, Z., Song, E., & Shen, L. (2021). Leaching Behavior of Lithium-Bearing Bauxite with High-Temperature Bayer Digestion Process in K2O-Al2O3-H2O System. Metals, 11(7), 1148. https://doi.org/10.3390/met11071148







