Structure and Strength of Isothermally Heat-Treated Medium Carbon Ti-V Microalloyed Steel
Abstract
1. Introduction
2. Material and Methods
3. Results and Discussion
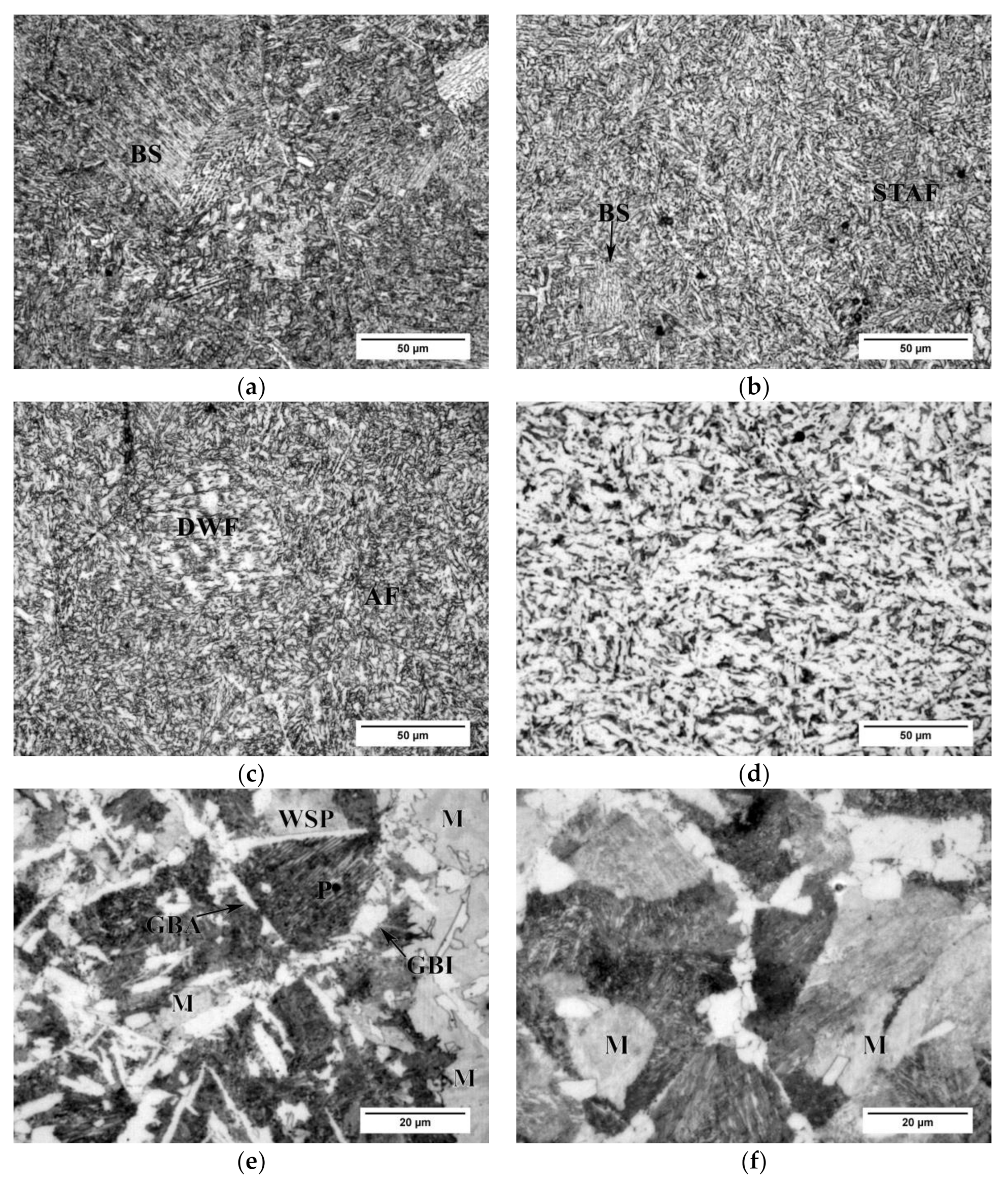
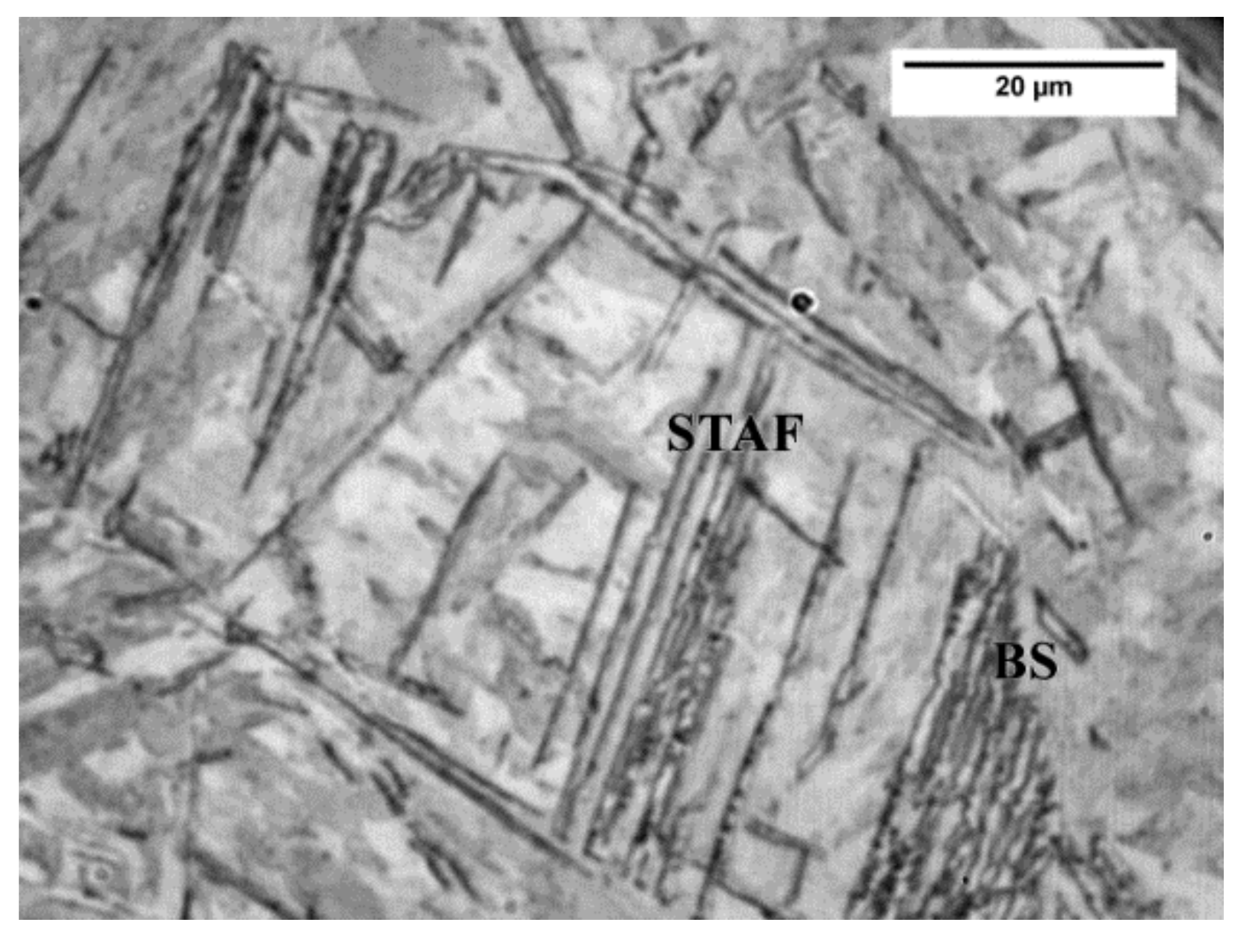
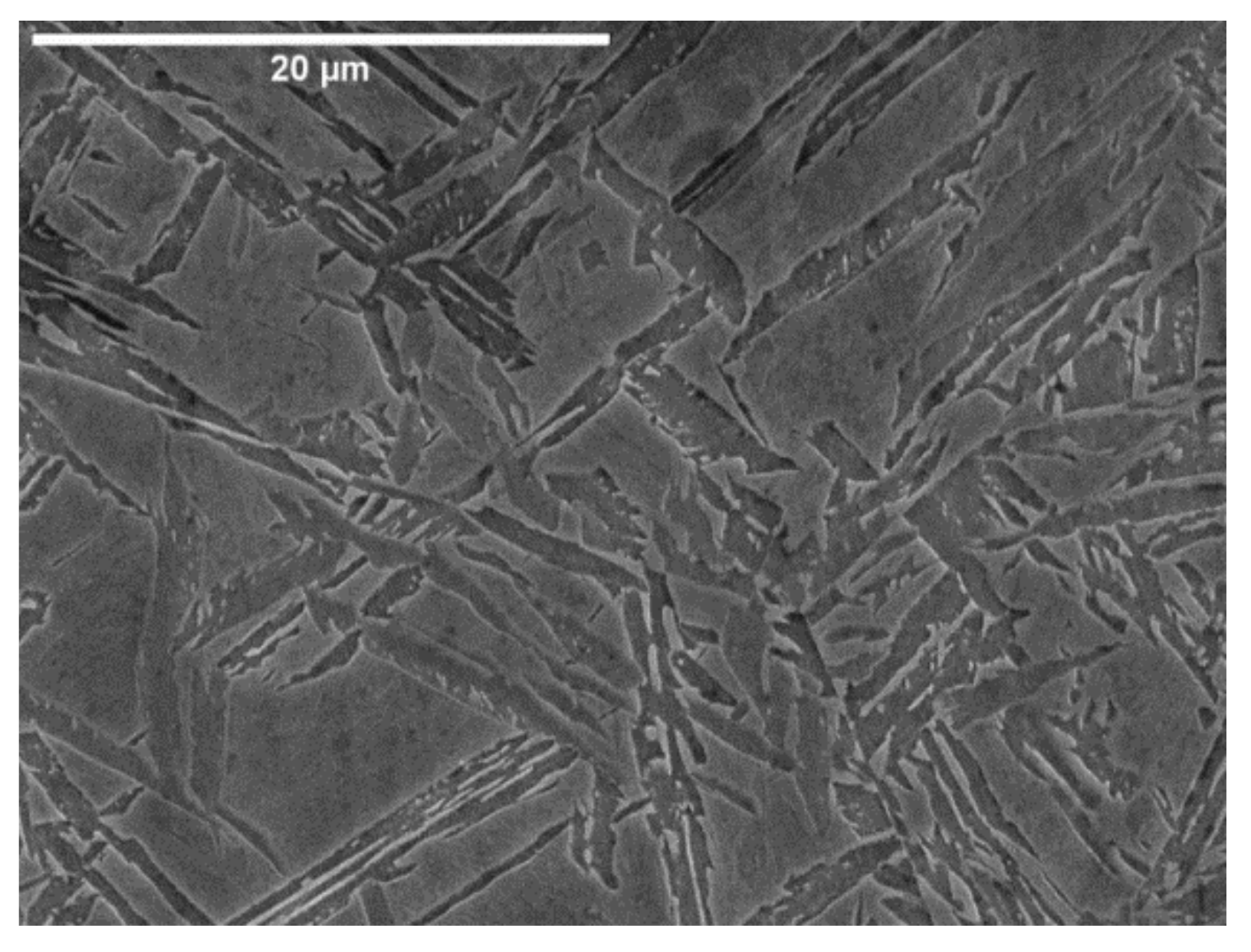
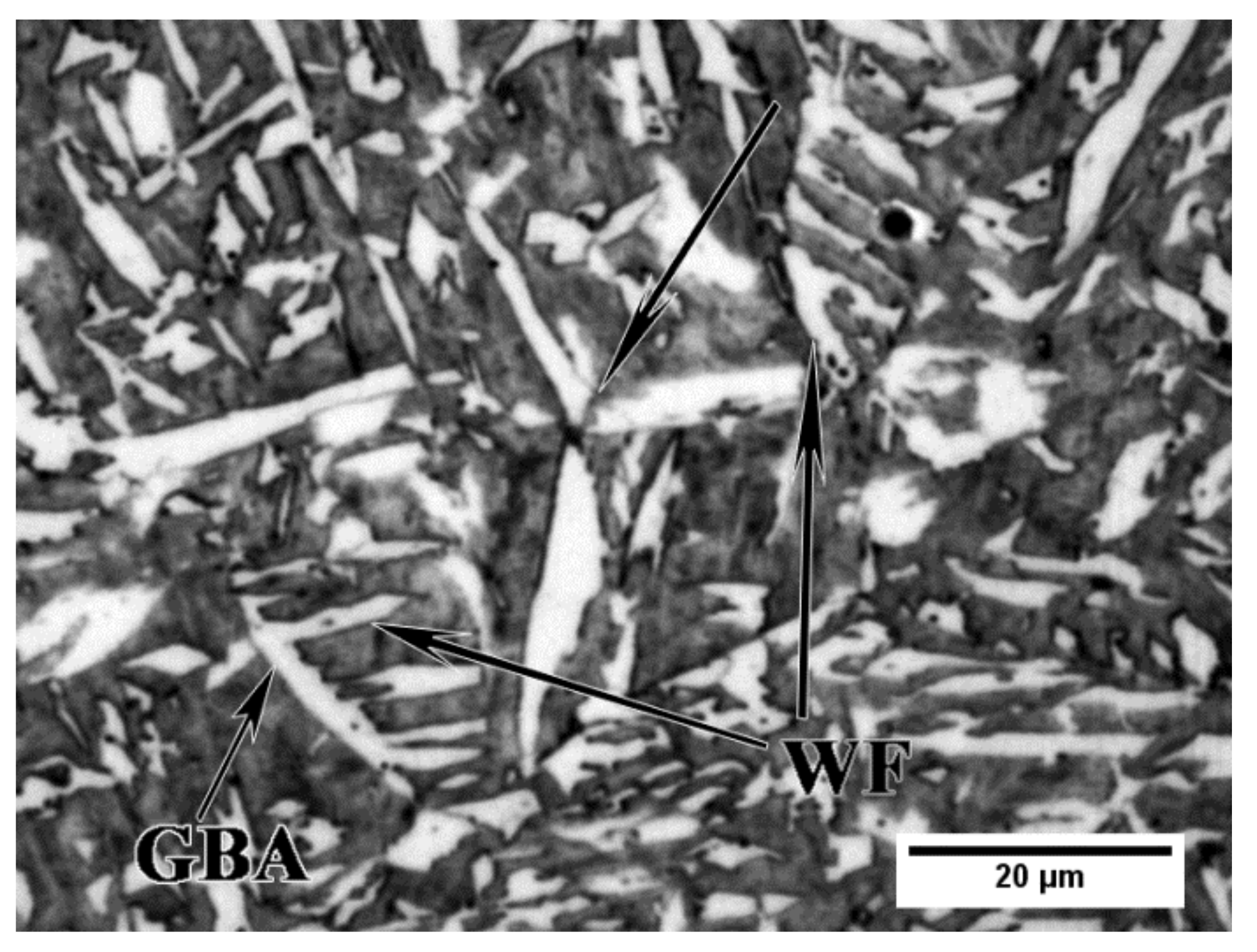
3.1. Microstructure
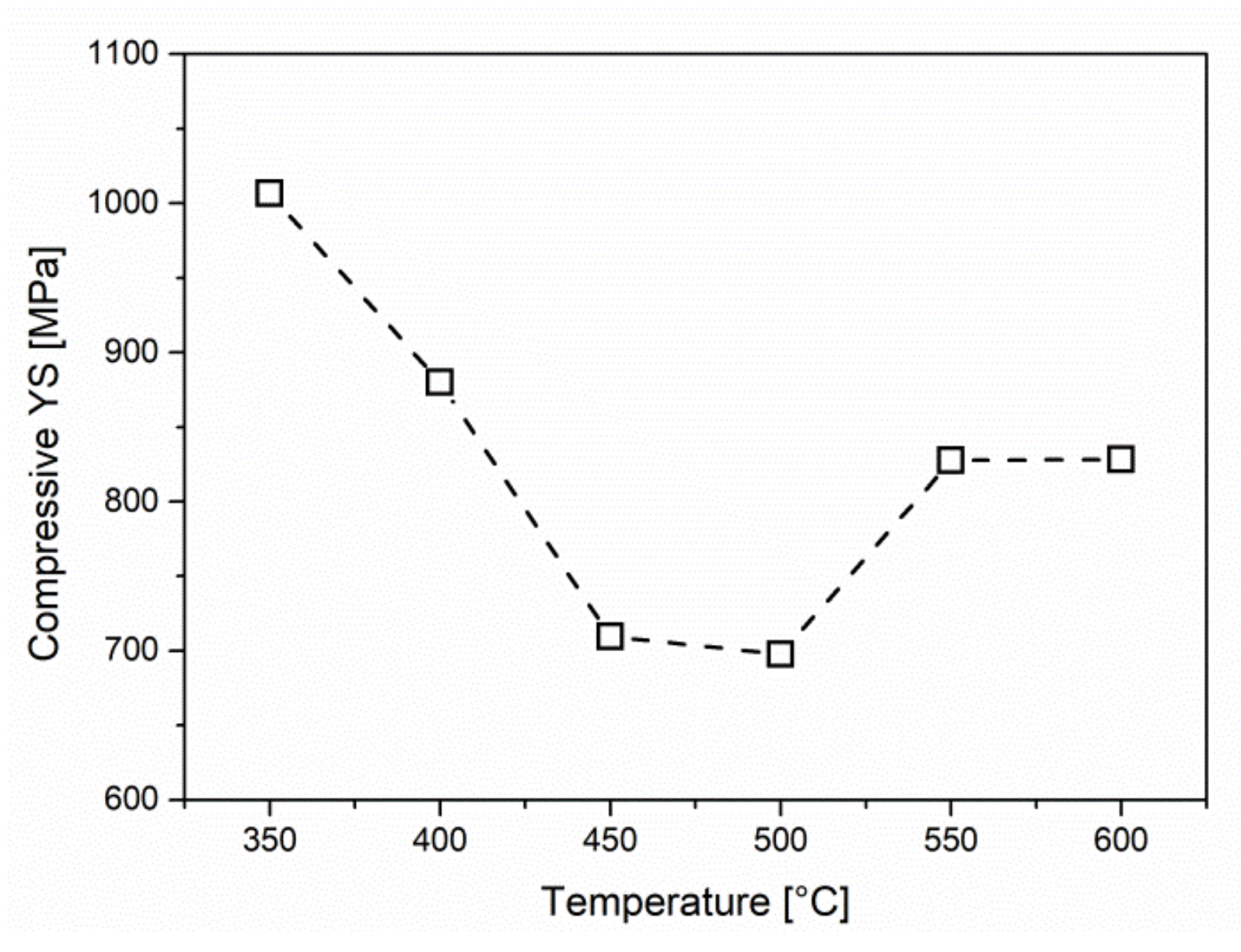
3.2. Compression Yield Strength
4. Conclusions
- Nucleation of bainite at grain boundaries of austenite at 350 °C shifts to the grain interiors, at complex particles with MnS core, and acicular ferrite becomes predominant at 400 °C and 450 °C, which is ascribed to the increased contribution of sympathetic nucleation, the presence of suitable second phase particles for intragranular nucleation, and the influence of V in solid solution.
- The high compressive YS of the samples isothermally treated at 350 °C is attributed to the incomplete reaction phenomenon and the consequent formation of the martensite upon the water quenching.
- Formation of acicular ferrite at 400 °C and 450 °C is accompanied by a noticeable decrease of compressive YS.
- Lowest compressive YS attained at 500 °C is related to the formation of coarse ferrite grains, formed by the nucleation of Widmanstätten ferrite plates and the process of their coalescence and further growth by diffusional mechanism at 500 °C.
- Transformation of the austenite to ferrite and pearlite at 550–600 °C is characterized by the delay of transformation finish, due to the influence of relatively high content of C and substitutional alloying elements, Cr and Ni, but in particular V on retardation of diffusion in austenite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krauss, G.; Banerji, S.K. Fundamentals of Microalloying Forging Steel. Metall. Soc. TMS 1987, 55. [Google Scholar] [CrossRef]
- Langeborg, R.; Hutchinson, B.; Siwecki, T.; Zajac, S. The Role of Vanadium in Microalloyed Steels. Scand. J. Metall. 1999, 28, 186–241. [Google Scholar]
- Fernández, J.; Illescas, S.; Guilemany, J.M. Effect of microalloying elements on the austenitic grain growth in a low carbon HSLA steel. Mater. Lett. 2007, 61, 2389–2392. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Capdevila, C.; Caballero, F.G.; Andrés, C.G. De Influence of V Precipitates on Acicular Ferrite Transformation Part 1: The Role of Nitrogen. ISIJ Int. 2008, 48, 1270–1275. [Google Scholar] [CrossRef]
- Gomez, M.; Rancel, L.; Escudero, E.; Medina, S.F. Phase Transformation under Continuous Cooling Conditions in Medium Carbon Microalloyed Steels. J. Mater. Sci. Technol. 2014, 30, 511–516. [Google Scholar] [CrossRef]
- Garcia de Andres, C.; Capdevila, C.; Caballero, F.G.; San Martin, D. Effect of molybdenum on continuous cooling transformations in two medium carbon forging steels. J. Mater. Sci. 2001, 36, 565–571. [Google Scholar] [CrossRef]
- Khodabandeh, A.R.; Jahazi, M.; Yue, S.; Bocher, P. Impact Toughness and Tensile Properties Improvement through Microstructure Control in Hot Forged Nb-V Microalloyed Steel. ISIJ Int. 2005, 45, 272–280. [Google Scholar] [CrossRef]
- Babu, S.S.; Bhadeshia, H.K.D.H. Mechanism of the Transition from Bainite to Acicular Ferrite. JIM 1991, 32, 679–688. [Google Scholar] [CrossRef]
- Glišić, D.; Radović, N.; Koprivica, A.; Fadel, A.; Drobnjak, D. Influence of Reheating Temperature and Vanadium Content on Transformation Behavior and Mechanical Properties of Medium Carbon Forging Steels. ISIJ Int. 2010, 50, 601–606. [Google Scholar] [CrossRef][Green Version]
- Madariaga, I.; Gutierrez, I.; Bhadeshia, H.K.D.H. Acicular Ferrite Morphologies in a Medium-Carbon Microalloyed Steel. Metall. Mater. Trans. A 2001, 32, 2187–2197. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels; IOM Communications Ltd.: London, UK, 2001; ISBN 1861251122. [Google Scholar]
- Ishikawa, F.; Takahashi, T.; Ochi, T. Intragranular Ferrite Nucleation in Medium-Carbon Vanadium Steels. Metall. Mater. Trans. A 1994, 25, 929–936. [Google Scholar] [CrossRef]
- Fadel, A. Austenite Decomposition in Medium Carbon Microalloyed Steels: Meshanism, Structure and Properties. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2013. [Google Scholar]
- Hu, J.; Du, L.X.; Xie, H.; Gao, X.H.; Misra, R.D.K. Microstructure and mechanical properties of TMCP heavy plate microalloyed steel. Mater. Sci. Eng. A 2014, 607, 122–131. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Cornide, J.; Capdevila, C.; Caballero, F.G.; Garcia de Andres, C. Influence of V Precipitates on Acicular Ferrite Transformation Part 2: Transformation Kinetics. ISIJ Int. 2008, 48, 1276–1279. [Google Scholar] [CrossRef]
- Siwecki, T.; Eliasson, J.; Langeborg, R.; Bevis, H. Vanadium Microalloyed Bainitic Hot Strip Steels. ISIJ Int. 2010, 50, 760–767. [Google Scholar] [CrossRef]
- Garcia de Andres, C.; Caballero, F.G.; Capdevila, C.; San Martin, D. Revealing austenite grain boundaries by thermal etching: Advantages and disadvantages. Mater. Charact. 2003, 49, 121–127. [Google Scholar] [CrossRef]
- Kirkaldy, J.S. Prediction of Microstructure and Hardenability in Low Alloy Steels. In: Phase Transformations in Ferrous Alloys. In Prediction of Microstructure and Hardenability in Low Alloy Steels, Proceedings of the Phase Transformations in Ferrous Alloys, Philadelphia, PA, USA, 4–6 October 1983; AIME: Warrendale, PA, USA, 1983; pp. 125–148. [Google Scholar]
- Diaz-Fuentes, M.; Gutiérrez, I. Analysis of different acicular ferrite microstructures generated in a medium-carbon molybdenum steel. Mater. Sci. Eng. A 2003, 363, 316–324. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Spanos, G.; Masamura, R.A.; Vardiman, R.G.; Moon, D.W.; Menon, E.S.K.; Hall, M.G. Sympathetic nucleation: An overview. Mater. Sci. Eng. B 1995, 32, 107–123. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Reynolds, W.T.; Purdy, G.R. The Incomplete Transformation Phenomenon in Steel. Metall. Mater. Trans. A 2006, 37, 1731–1745. [Google Scholar] [CrossRef]
- Wu, K.M.; Enomoto, M. Three-dimensional morphology of degenerate ferrite in an Fe-C-Mo alloy. Scr. Mater. 2002, 46, 569–574. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels Theory and Practice, 3rd ed.; Maney Publishing: London, UK, 2015; ISBN 9781909662742. [Google Scholar]
- Okaguchi, S.; Ohtani, H.; Ohmori, Y. Morphology of Widmanstatten Bainitic Ferrites. Mater. Trans. 1991, 32, 697–704. [Google Scholar] [CrossRef]
- Ishikawa, F.; Takahashi, T. The Formation ot Intragranular Ferrite Steels for Hot-torging and Its Effect on Plates in Medium-carbon the Toughness. ISIJ Int. 1995, 35, 1128–1133. [Google Scholar] [CrossRef]
- Shim, J.; Oh, Y.; Suh, J.; Cho, Y.W.; Shim, J.; Byun, J. Ferrite Nucleation Potency of Non-Metallic Inclusions in Medium Carbon Steels. Acta Mater. 2001, 49, 2115–2122. [Google Scholar] [CrossRef]
- Jin, H.H.; Shim, J.H.; Cho, Y.W.; Lee, H.C. Formation of Intragranular Acicular Ferrite Grains in a Ti-containing Low Carbon Steel. ISIJ Int. 2003, 43, 1111–1113. [Google Scholar] [CrossRef]
- Zhang, S.; Hattori, N.; Enomot, M.; Taru, T. Ferrite Nucleation at Ceramic/Austenite Interface. ISIJ Int. 1996, 36, 1301–1309. [Google Scholar] [CrossRef]
- Sarma, D.S.; Karasev, A.V.; Jönsson, P.G. On the Role of Non-metallic Inclusions in the Nucleation of Acicular Ferrite in Steels. ISIJ Int. 2009, 49, 1063–1074. [Google Scholar] [CrossRef]
- Madariaga, I.; Gutierrez, I. Role of the Particle–Matrix Interface on the Nucleation of Acicular Ferrite in a Medium Carbon Microalloyed Steel. Acta Mater. 1999, 47, 951–960. [Google Scholar] [CrossRef]
- Madariaga, I.; Romero, J.L.; Gutie, I. Upper Acicular Ferrite Formation in a Medium-Carbon Microalloyed Steel by Isothermal Transformation: Nucleation Enhancement by CuS. Metall. Mater. Trans. A 1998, 29, 1003–1015. [Google Scholar] [CrossRef]
- He, K.; Edmonds, D.V. Formation of Acicular Ferrite and Influence of Vanadium Alloying. Mater. Sci. Technol. 2002, 18, 289–296. [Google Scholar] [CrossRef]
- Adrian, H. A mechanism for effect of vanadium on hardenability of medium carbon manganese steel. Mater. Sci. Technol. 1999, 15, 366–378. [Google Scholar] [CrossRef]
- Garcia de Andres, C.; Capdevila, C.; Caballero, F.G.; San Martin, D. Effect of the microalloying elements on nucleation of allotriomorphic ferrite in medium carbon-manganese steels. J. Mater. Sci. 2001, 20, 1135–1137. [Google Scholar] [CrossRef]
- Capdevila, C.; Ferrer, J.P.; Garcia-Mateo, C.; Caballero, F.G.; Lopez, V.; Garcia de Andres, C. Influence of Deformation and Molybdenum Content on Acicular Ferrite Formation in Medium Carbon Steels Ferrite Formation in Medium Carbon Steels. ISIJ 2006, 46, 1093–1100. [Google Scholar] [CrossRef][Green Version]
- Garcia de Andres, C.; Bartolome, M.J.; Capdevila, C.; San Martın, D.; Caballero, F.G.; Lopez, F.G. Metallographic techniques for the determination of the austenite grain size in medium-carbon microalloyed steels. Mater. Charact. 2001, 46, 389–398. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Physical Metallurgy of Steels, 4th ed.; Elsevier: Amsterdam, Netherlands, 2014; Volume 1, ISBN 9780444537713. [Google Scholar]
- Bhadeshia, H.K.D.H.; Honeycombe, R.W.K. Steels Microstructure and Properties; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; ISBN 9780750680844. [Google Scholar]
- Kuhn, H.; Medlin, D. Mechanical Testing and Evaluation; ASM International: Material Park, OH, USA, 2000. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Christian, J.W. Bainite in Steels. Metall. Trans. A 1990, 21, 767–797. [Google Scholar] [CrossRef]
- Pereloma, E.; Edmonds, D. V Phase Transformations in Steels, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1, ISBN 978-1-84569-970-3. [Google Scholar]
- Miyamoto, G.; Hori, R.; Poorganji, B.; Furuhara, T. Interphase Precipitation of VC and Resultant Hardening in V-added Medium Carbon Steels. ISIJ Int. 2011, 51, 1733–1739. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Yang, Z.; Li, C. Microstructure, Precipitation, and Mechanical Properties of V-N-Alloyed Steel After Different Cooling Processes. Metall. Mater. Trans. A 2016, 47, 6621–6631. [Google Scholar] [CrossRef]
- Kestenbach, H.; Gallego, J. On Dispersion Hardening of Microalloyed Hot Strip Steels by Carbonitride Precipitation in Austenite. Scr. Mater. 2001, 44, 791–796. [Google Scholar] [CrossRef]
- Kestenbach, H.; Campos, S.S.; Morales, E.V. Role of interphase precipitation in microalloyed hot strip steels. Mater. Sci. Technol. 2006, 22, 615–626. [Google Scholar] [CrossRef]
- Chen, J.; Lv, M.; Tang, S.; Liu, Z.; Wang, G. Influence of cooling paths on microstructural characteristics and precipitation behaviors in a low carbon V—Ti microalloyed steel. Mater. Sci. Eng. A 2014, 594, 389–393. [Google Scholar] [CrossRef]
- Funakawa, Y.; Shiozaki, T.; Tomita, K.; Yamamoto, T.; Maeda, E. Development of High Strength Hot-rolled Sheet Steel Consisting of Ferrite and Nanometer-sized Carbides. ISIJ Int. 2004, 44, 1945–1951. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. The Bainite Transformation in a Silicon Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1979, 10, 895–907. [Google Scholar] [CrossRef]



| C | Si | Mn | P | S | Cr | Ni | Cu | Al | Mo | Ti | V | Nb | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.309 | 0.485 | 1.531 | 0.0077 | 0.0101 | 0.265 | 0.20 | 0.232 | 0.017 | 0.041 | 0.011 | 0.123 | 0.003 | 0.0221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikić, S.; Glišić, D.; Fadel, A.; Jovanović, G.; Radović, N. Structure and Strength of Isothermally Heat-Treated Medium Carbon Ti-V Microalloyed Steel. Metals 2021, 11, 1011. https://doi.org/10.3390/met11071011
Dikić S, Glišić D, Fadel A, Jovanović G, Radović N. Structure and Strength of Isothermally Heat-Treated Medium Carbon Ti-V Microalloyed Steel. Metals. 2021; 11(7):1011. https://doi.org/10.3390/met11071011
Chicago/Turabian StyleDikić, Stefan, Dragomir Glišić, Abdunnaser Fadel, Gvozden Jovanović, and Nenad Radović. 2021. "Structure and Strength of Isothermally Heat-Treated Medium Carbon Ti-V Microalloyed Steel" Metals 11, no. 7: 1011. https://doi.org/10.3390/met11071011
APA StyleDikić, S., Glišić, D., Fadel, A., Jovanović, G., & Radović, N. (2021). Structure and Strength of Isothermally Heat-Treated Medium Carbon Ti-V Microalloyed Steel. Metals, 11(7), 1011. https://doi.org/10.3390/met11071011







