Selective Extraction of Ni from Superalloy Scraps by Molten Mg-Zn
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
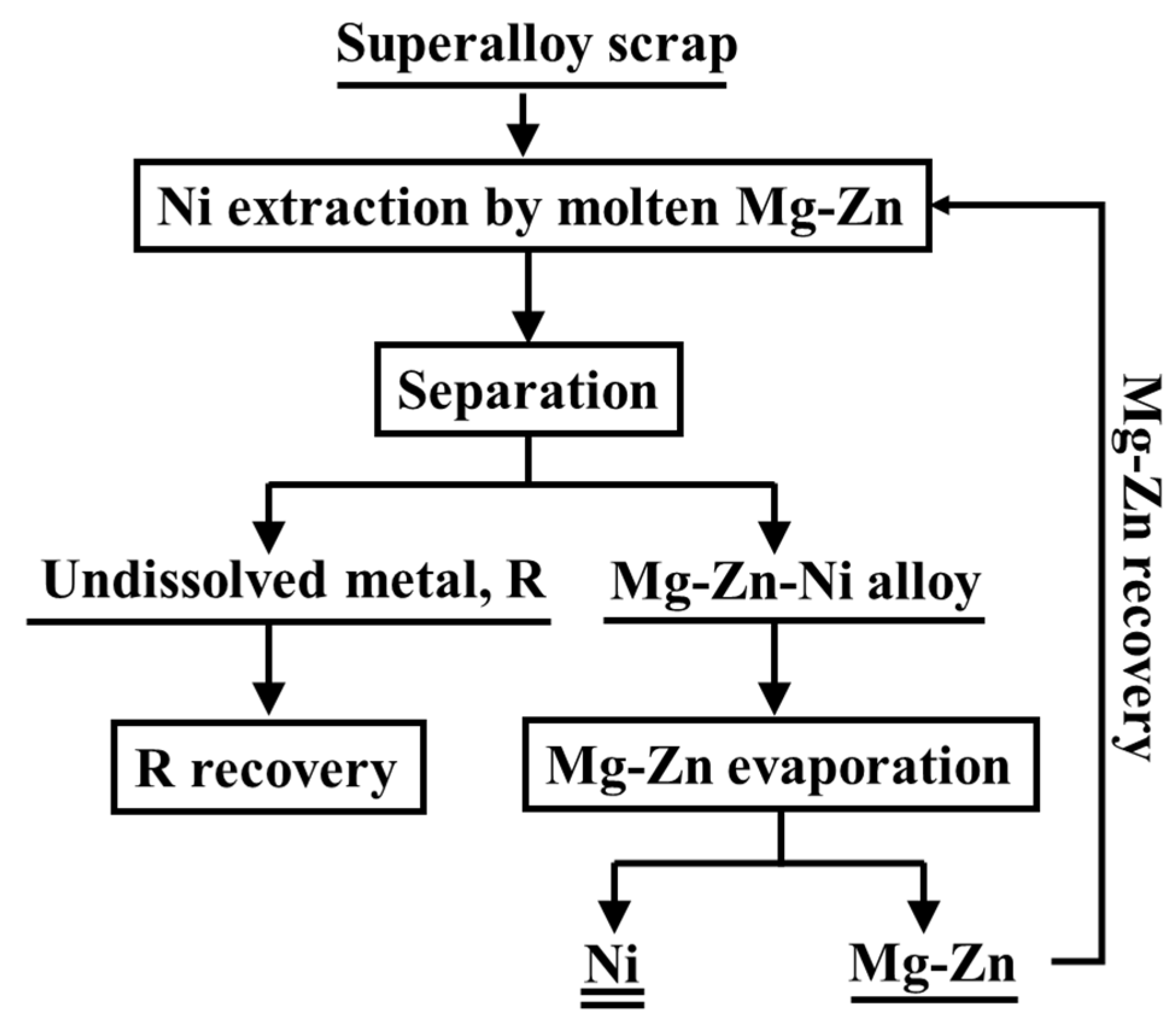
2.2. Methods
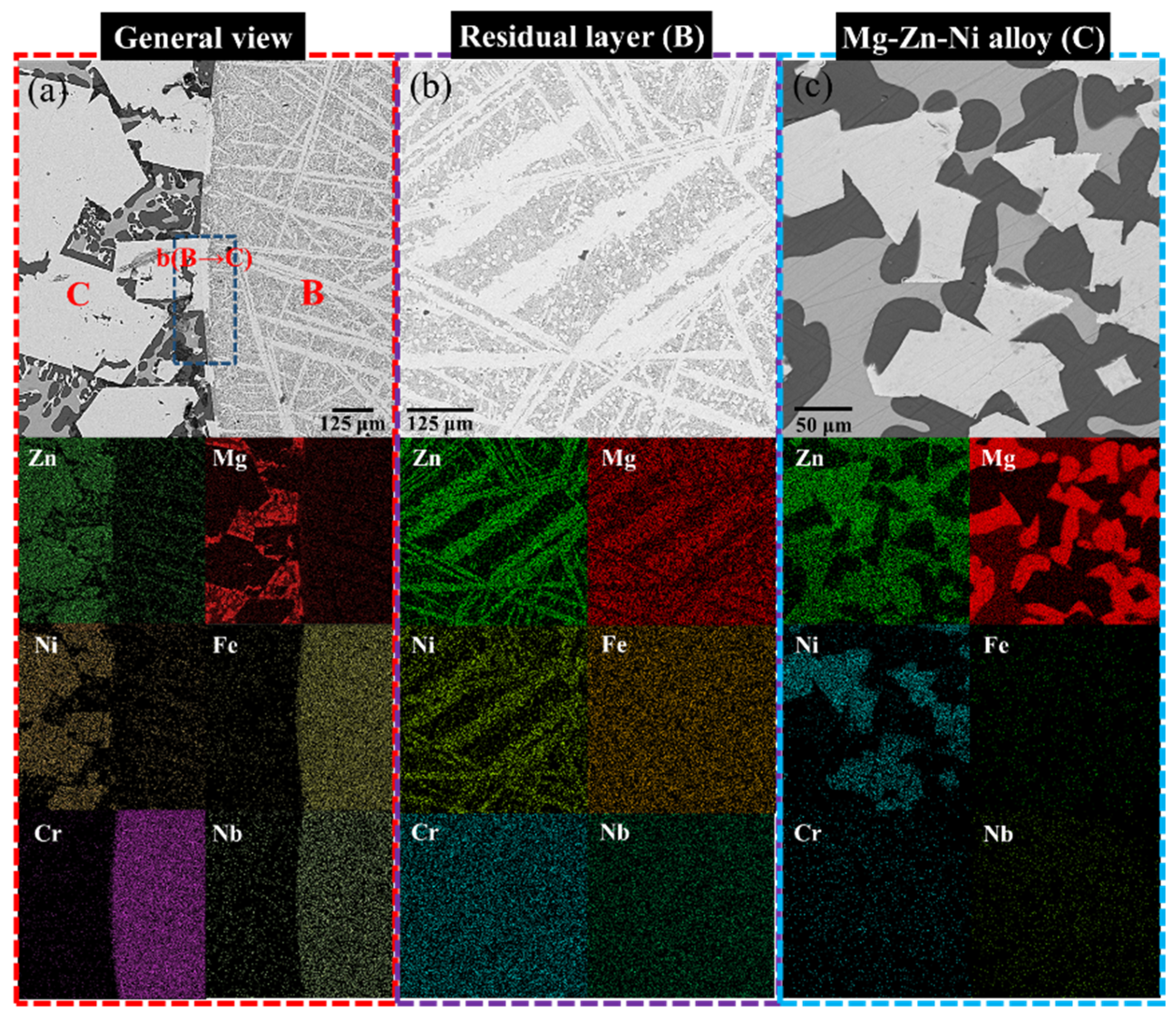
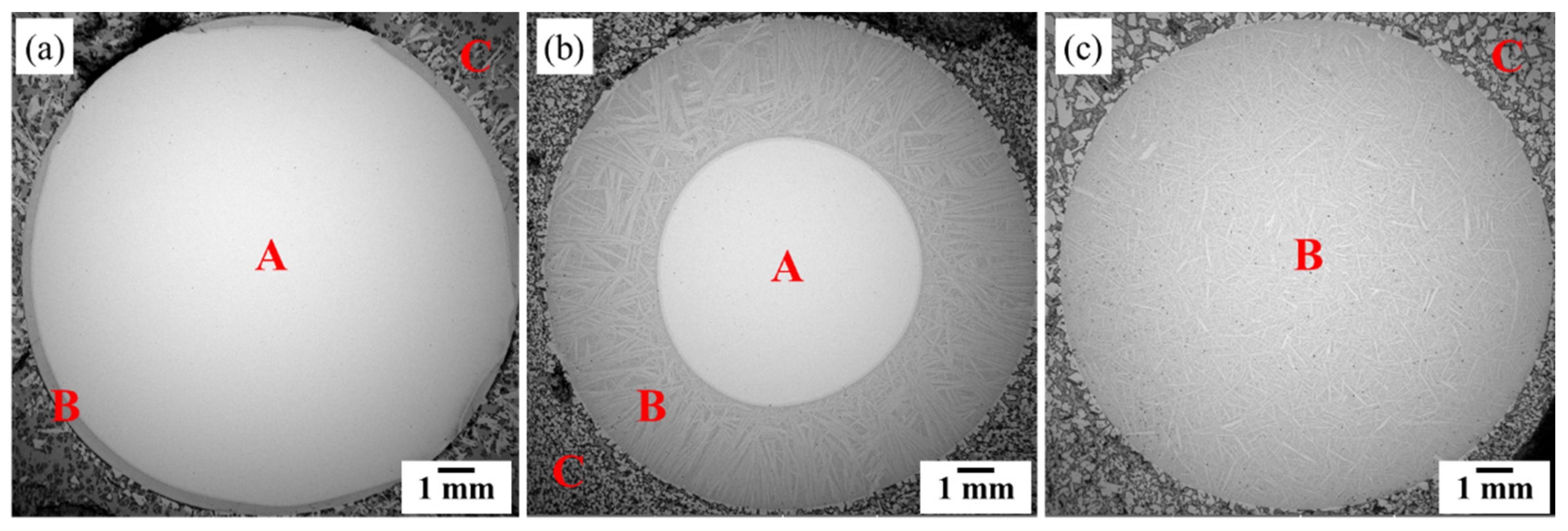
2.3. Characterization
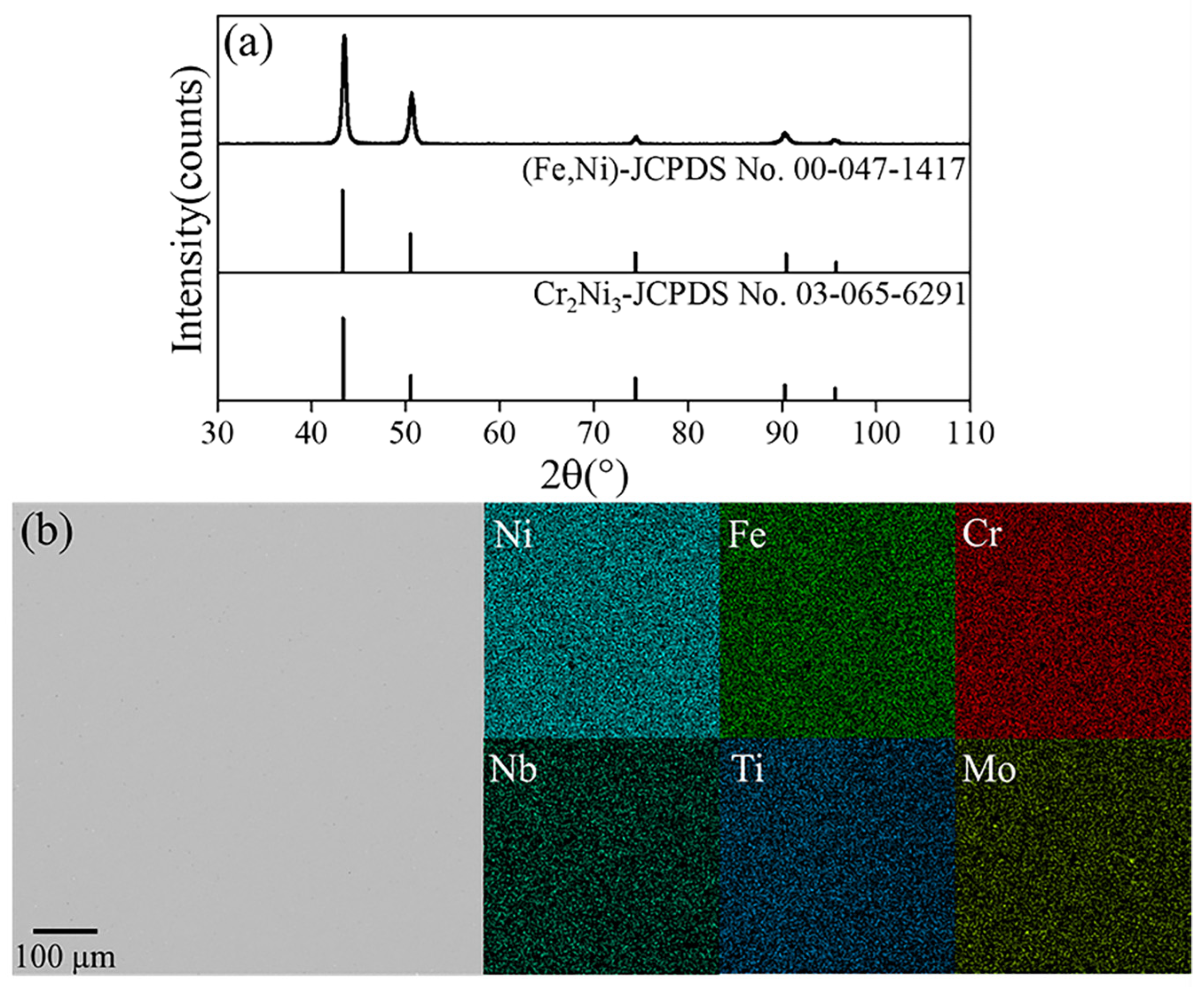
3. Results
3.1. Liquid Metal Extraction Process
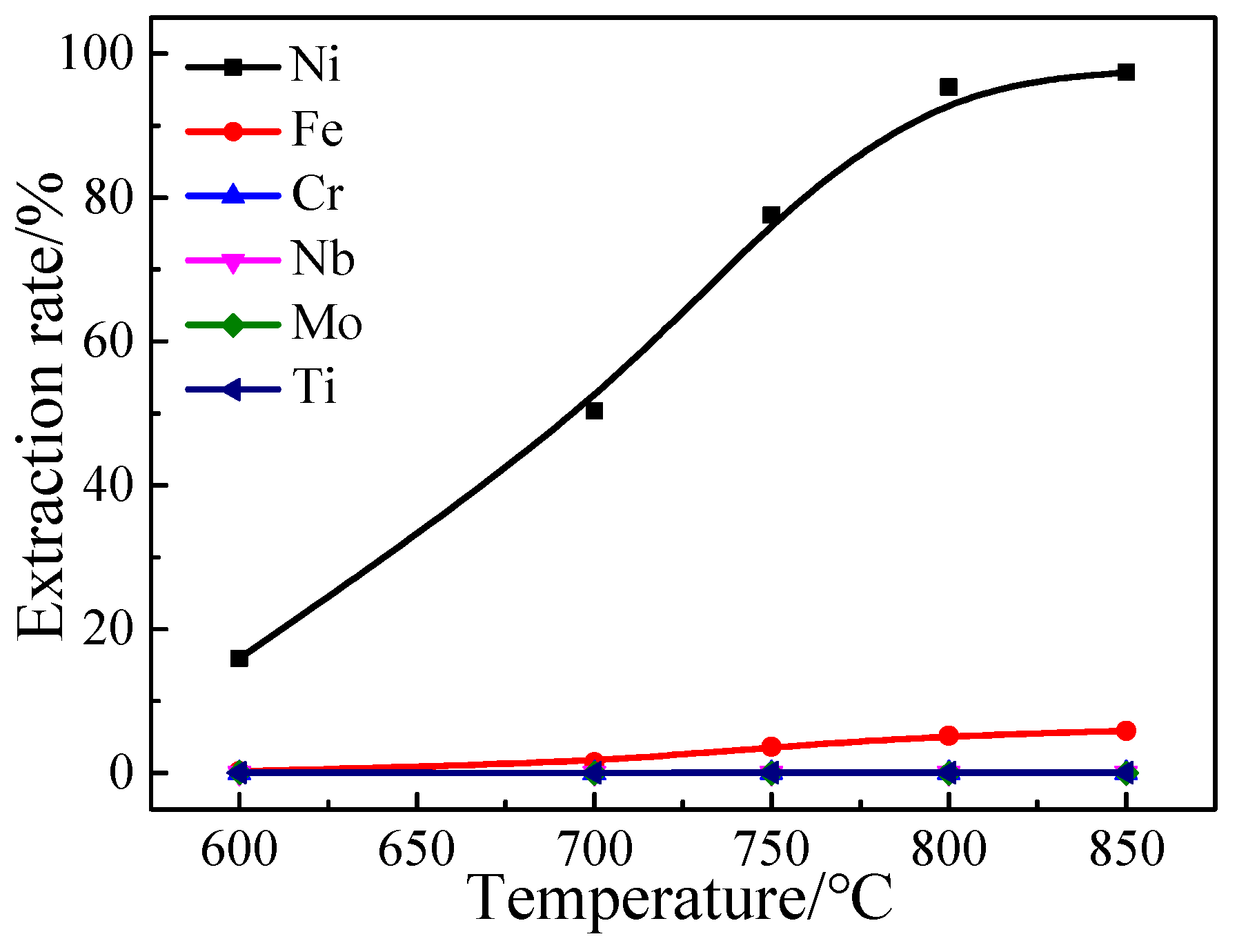
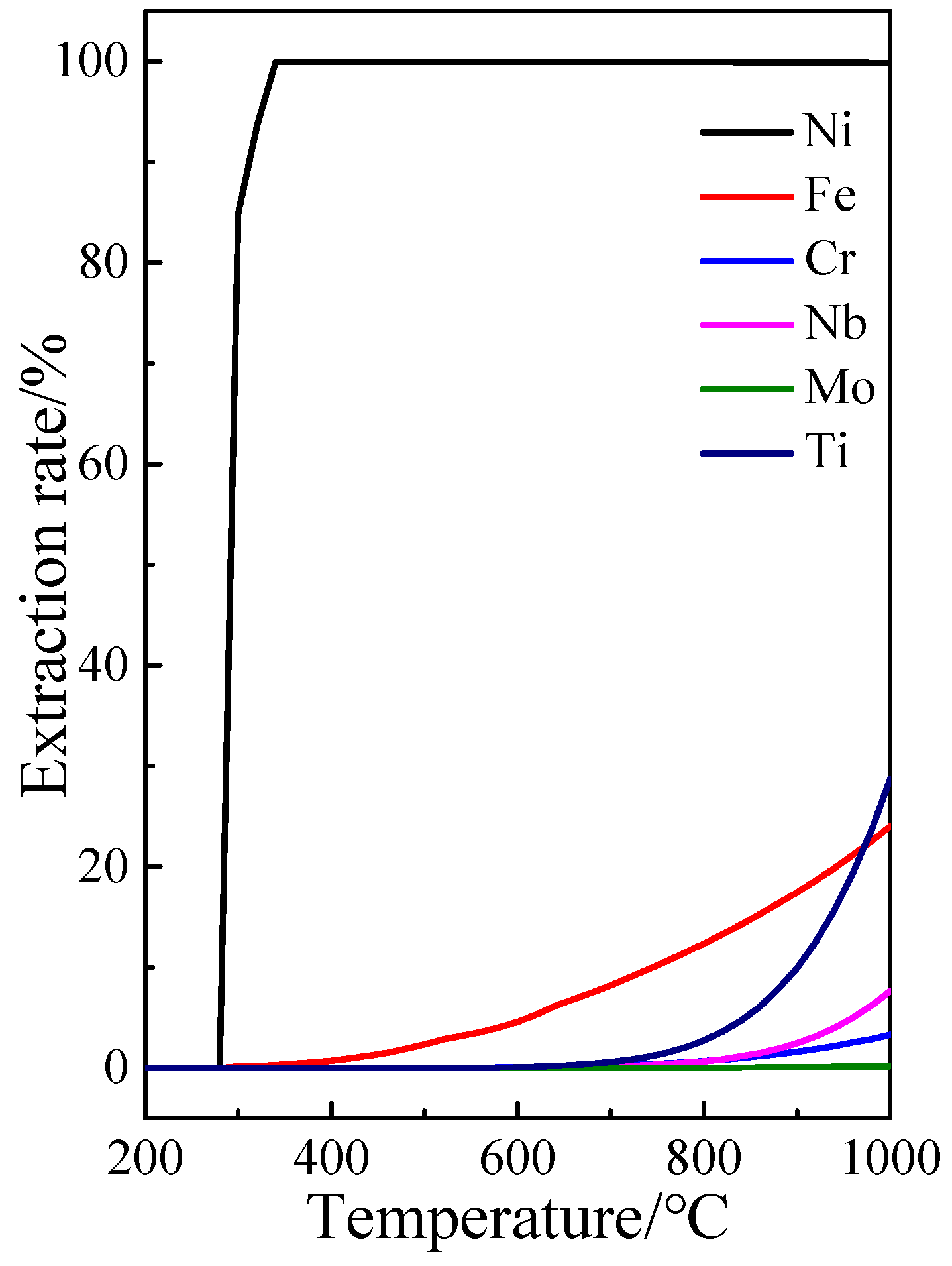
3.1.1. Effect of Heating Temperature
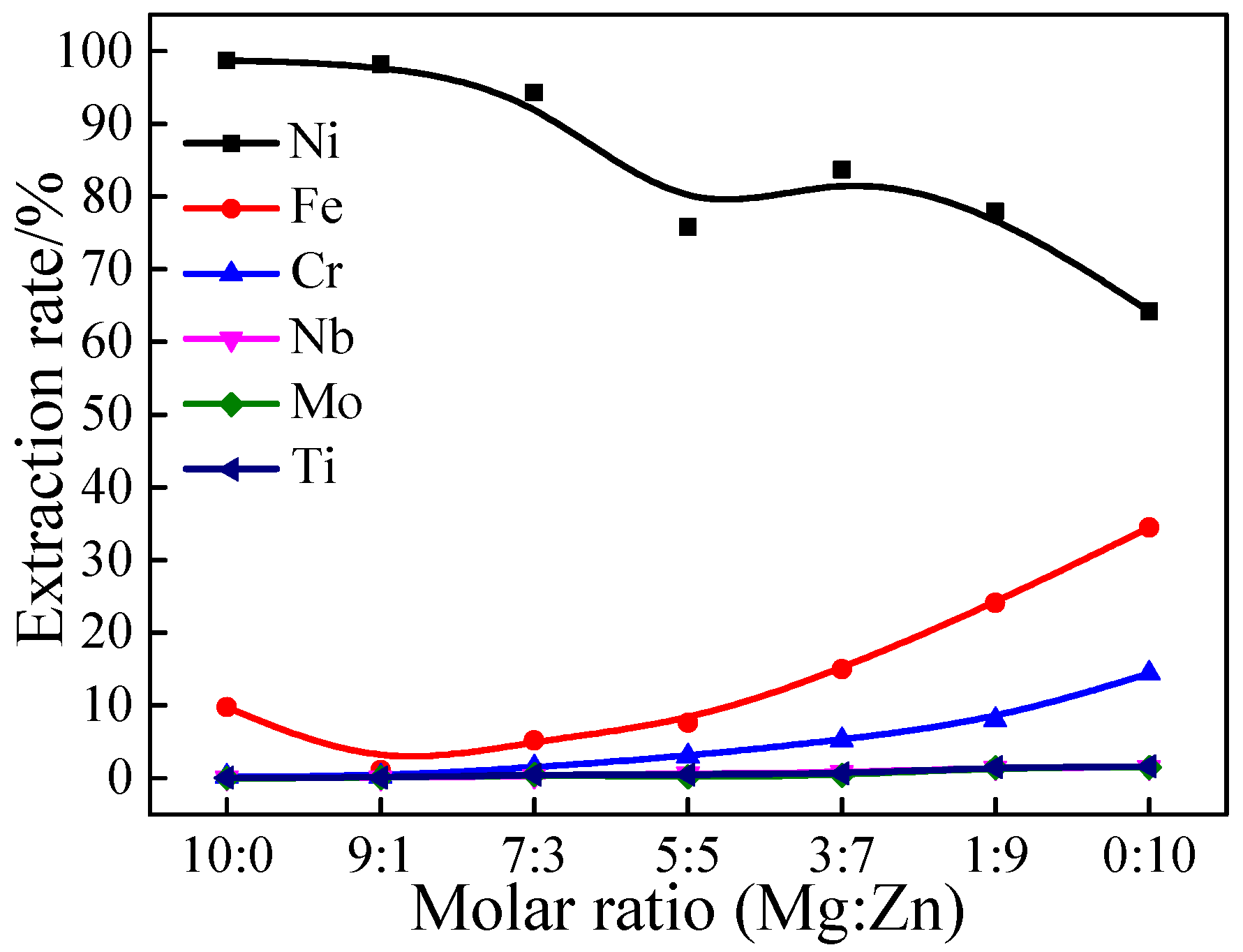
3.1.2. Effect of Molar Ratio of Mg to Zn
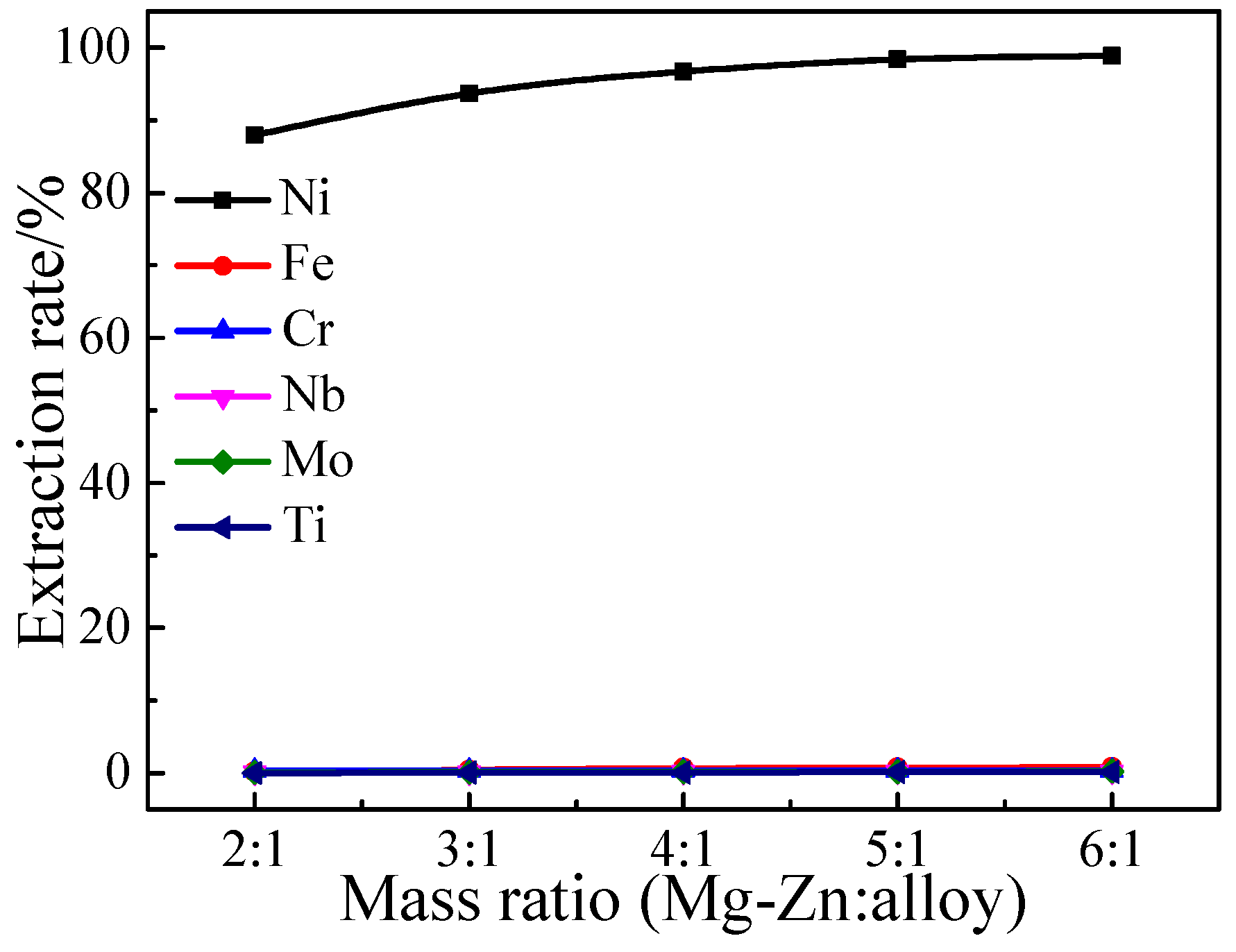
3.1.3. Effect of the Mass Ratio of Mg-Zn to Superalloy
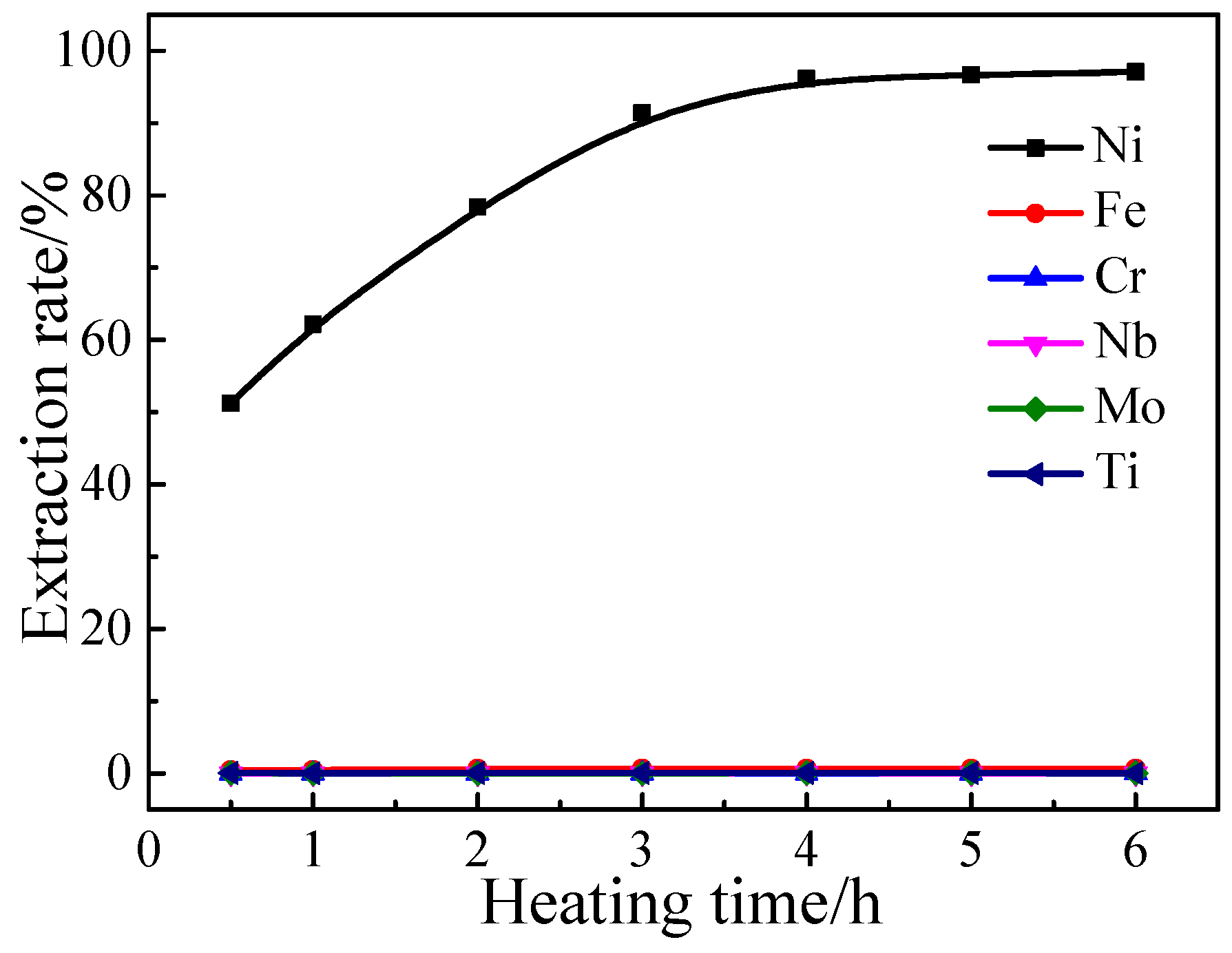
3.1.4. Effect of Heating Time
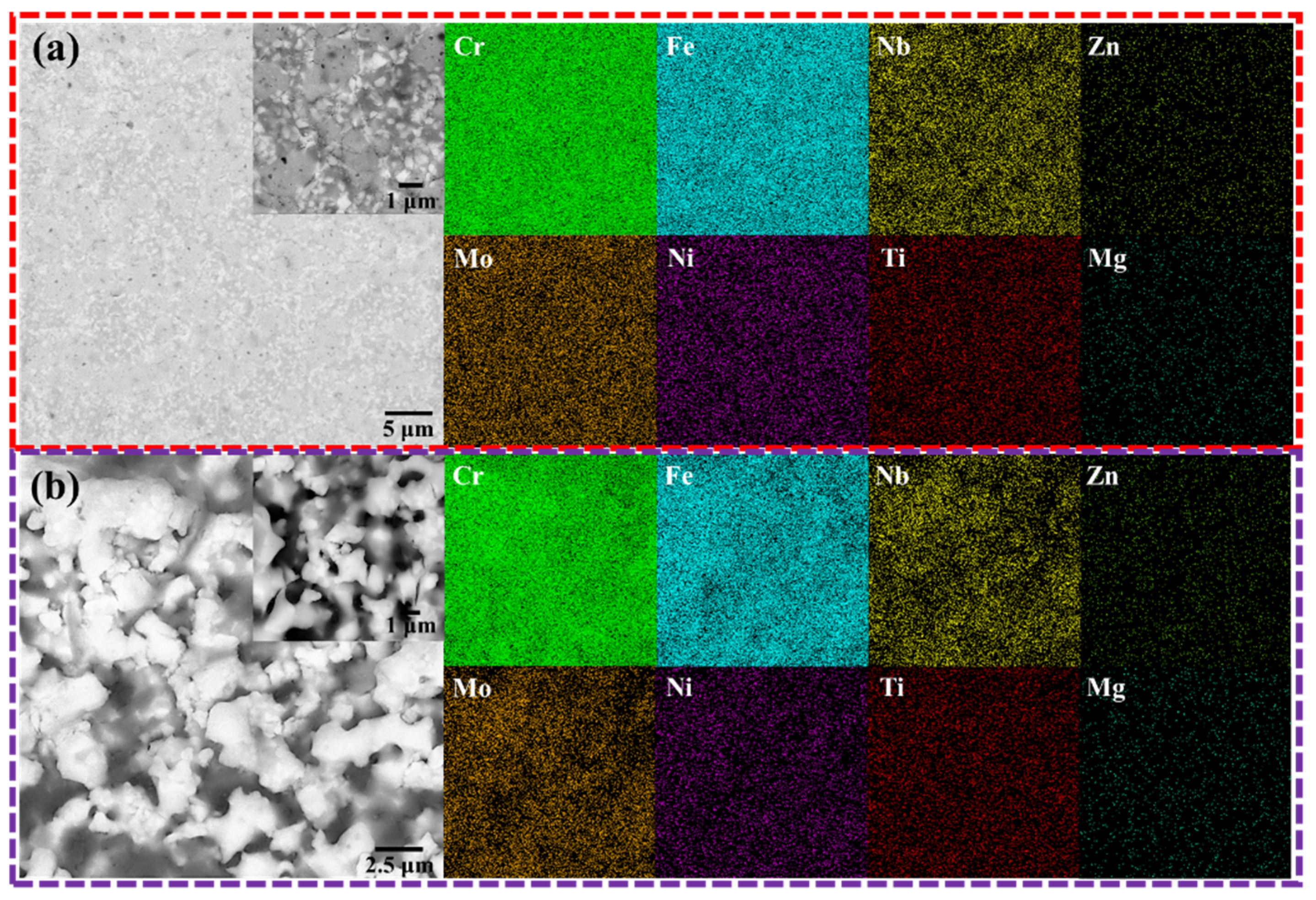
3.2. Separation of Mg-Zn Alloy by Vacuum Distillation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makuza, B.; Tian, Q.H.; Guo, X.Y.; Chattopadhyay, K.; Yu, D.W. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Zhu, H.J. Exploration laterite-nickel ore and analysis on utilization technology. World Nonferrous Metals 2007, 10, 7–10. [Google Scholar]
- Pollock, T.M.; Tin, S. Nickel-based superalloys for advanced turbine engines: Chemistry, microstructure, and properties. J. Propul. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Feng, R.S.; Xu, S.M. Research progress on recycling technology of indissoluble alloy with high melting point. Hydrometall. China 2012, 31, 338. (In Chinese) [Google Scholar]
- Donachie, M.J.; Donachie, S.J. Superalloys: A Technical Guide, 2nd ed.; ASM International: Materials Park, OH, USA, 2002. [Google Scholar]
- Greenfield, A.; Graedel, T.E. The omnivorous diet of modern technology. Resour. Conserv. Recy. 2013, 74, 1–7. [Google Scholar] [CrossRef]
- Liu, Z.G.; Sun, T.C.; Wang, X.P.; Gao, E.X. Generation process of FeS and its inhibition mechanism on iron mineral reduction in selective direct reduction of laterite nickel ore. Int. J. Min. Met. Mater. 2015, 22, 901–906. [Google Scholar] [CrossRef]
- Li, Y.J.; Yu, H.C.; Wang, D.Q.; Yin, W.X.; Bai, Y.S. The current status of laterite ore resources and its processing technology. Met. Mine 2010, 11, 5–9. [Google Scholar]
- Oxley, A.; Barcza, N. Hydro-pyro integration in the processing of nickel laterites. Miner. Eng. 2013, 54, 2–13. [Google Scholar] [CrossRef]
- Cui, F.H.; Mu, W.N.; Wang, S.; Xin, H.X.; Shen, H.T.; Xu, Q.; Zhai, Y.C.; Luo, S.H. Synchronous extractions of nickel, copper, and cobalt by selective chlorinating roasting and water leaching to low-grade nickel-copper matte. Sep. Purif. Technol. 2018, 195, 149–162. [Google Scholar] [CrossRef]
- Woulds, M.J. Recycling of Engine Serviced Superalloys. Superalloys 1980, 31–41. [Google Scholar] [CrossRef]
- deBarbadillo, J.J. Nickel-base superalloys; physical metallurgy of recycling. Metall. Trans. A 1983, 14, 329–341. [Google Scholar] [CrossRef]
- Schlatter, R. Melting and refining technology of high temperature steels and superalloys: A review of recent process developments. Superalloys 1972, A1–A40. [Google Scholar] [CrossRef]
- Fedika, A.A. Electro slag remelting. In Proceedings of the 2nd All Union Conference on Electro Slag Remelting, Metallurgiya, Moscow, Russia; 1969; p. 126. [Google Scholar]
- Kim, M.S.; Lee, J.C.; Kim, E.Y.; Yoo, Y.S. Leaching of CMSX-4 superalloy in hydrochloric acid solutions. J. Korean Inst. Resour. Recycl. 2010, 19, 25–30. [Google Scholar]
- Kim, M.S.; Lee, J.C.; Park, H.S.S.; Jun, M.J.; Kim, B.S. A multistep leaching of nickel-based superalloy scrap for selective dissolution of its constituent metals in hydrochloric acid solutions. Hydrometallurgy 2018, 176, 235–242. [Google Scholar] [CrossRef]
- Fan, X.X.; Xing, W.D.; Dong, H.G.; Zhao, J.C.; Wu, Y.D.; Li, B.J.; Tong, W.F.; Wu, X.F. Factors research on the influence of leaching rate of nickel and cobalt from waste superalloys with sulfuric acid. IJNM 2013, 2, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.D. Method of Preparation of metal Salts. U.S. Patent 2,716,588, 30 August 1955. [Google Scholar]
- Ferron, C.G.; Seeley, L.E. Rhenium recovery. U.S. Patent 8,956,582, 24 March 2015. [Google Scholar]
- Mamo, S.K.; Elie, M.; Baron, M.G.; Simons, A.M.; Gonzalez-Rodriguez, J. Leaching kinetics, separation, and recovery of rhenium and component metals from CMSX-4 superalloys using hydrometallurgical processes. Separ. Purif. Technol. 2019, 212, 150–160. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Liu, L.; Zhou, W.Z.; Chang, Y.L. Adsorption of Re from Electrolyte of Superalloy by Using D296 Resin. Rare Metals 2017, 41, 679. (In Chinese) [Google Scholar]
- Armin, O. Recycling of Superalloys with the Aid of an Alkali Metal Salt Bath. U.S. Patent 0255372 A1, 15 October 2009. [Google Scholar]
- Heshmatpour, B.; Mcdonald, R. Recovery and refining of rhenium, tungsten and molybdenum from W-Re, Mo-Re and other alloy scraps. J. Less Common. Met. 1982, 86, 121–128. [Google Scholar] [CrossRef]
- Atkinson, G.B.; Nicks, L.J. Leaching aluminum superalloy melts with hydrochloric and sulfuric acids. Resour. Conserv. Recy. 1986, 9, 197–209. [Google Scholar] [CrossRef]
- Hilliard, H.E. Recycling Superalloy Scrap by Vapor Phase Zinc Embrittlement. U.S. Patent 4718939-A, 12 January 1988. [Google Scholar]
- Iskander, A.A.; Bjorling, G.E.; Agden, J.S. Process for Recovery from Secondary Material of Such Metals as Nickel Cobalt Iron and Copper Said secondary MATERIAL Comprising in Addition One or More Metals Having a high MELTING Point. U.S. Patent 3649487, 14 March 1972. [Google Scholar]
- Guo, X.Y.; Zhang, C.X.; Tian, Q.H.; Yu, D.W. Liquid Metals Dealloying as a General Approach for the Selective Extraction of Metals and the Fabrication of Nanoporous Metals: A review. Mater. Today Commun. 2021, 26, 102007. [Google Scholar] [CrossRef]
- Yagi, R.; Okabe, T.H. Recovery of Nickel from Nickel-Based Superalloy Scraps by Utilizing Molten Zinc. Metall. Mater. Trans. B 2017, 48, 335–345. [Google Scholar] [CrossRef]
- Cui, F.H.; Wang, G.; Yu, D.W.; Gan, X.D.; Tian, Q.H.; Guo, X.Y. Towards “zero waste” extraction of nickel from scrap nickel-based superalloy using magnesium. J. Clean. Prod. 2020, 262, 121275. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, H.; Shin, S.; Wang, J.P. Nickel Recycling by Magnesium Reaction of Fe-Ni Alloy Scrap. Adv. Mater. Res. 2014, 1025, 519–524. [Google Scholar] [CrossRef]
- Lee, D.W.; Yu, J.H.; Jang, T.S.; Kim, B.K. Nanocrystalline iron particles synthesized by chemical vapor condensation without chilling. Mater. Lett. 2005, 59, 2124–2127. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Degterov, S.A.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.D.; Petersen, S. Factsage thermochenical software and databases. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Nayeb-Hashemi, A.A.; Clark, J.B.; Swartzendruber, L.J. The Fe-Mg (Iron-Magnesium) system. Bull. Alloy Phase Diagr. 1985, 6, 335–336. [Google Scholar] [CrossRef]
- Su, X.P.; Tang, N.Y.; Toguri, J.M. Thermodynamic evaluation of the Fe-Zn system. J. Alloys Compd. 2001, 325, 129–136. [Google Scholar] [CrossRef]
- Yu, D.W.; Gan, X.D.; Cui, F.H.; Guo, X.Y.; Tian, Q.H. Dissolution behavior of nickel-based superalloy in molten zinc: Its mechanism and kinetics. J. Alloys Compd. 2021, 878, 160338. [Google Scholar] [CrossRef]
- Bhan, S.; Jain, K.C.; Lal, A. The Mg-Ni-Zn System (Magnesium-Nickel-Zinc). J. Phase Equilib. 1997, 18, 305. [Google Scholar] [CrossRef]
- Wang, P.S.; Zhao, J.R.; Du, Y.; Xu, H.H.; Gang, T.; Fen, J.C.; Zhang, L.J.; He, C.Y.; Liu, S.H.; Ouyang, H.W. Experimental investigation and thermodynamic calculation of the Fe-Mg-Mn and Fe-Mg-Ni systems. Int. J. Mater. Res. 2011, 102, 6–16. [Google Scholar] [CrossRef]
- Xu, K.; Liu, S.H.; Du, Y.; Dreval, L.Y.; Cai, G.M.; Jin, Z.P. Thermodynamic investigation of the Mg-Ni-Zn system by experiments and calculations and its application. J. Alloys Compd. 2019, 784, 769–787. [Google Scholar] [CrossRef]
- Niu, H.Y.; Deng, K.K.; Nie, K.B.; Cao, F.F.; Zhang, X.C.; Li, W.G. Microstructure, mechanical properties and corrosion properties of Mg-4Zn-xNi alloys for degradable fracturing ball applications. J. Alloys Compd. 2019, 787, 1290–1300. [Google Scholar] [CrossRef]




| Superalloy | Element | Co | Cr | Al | Fe | Nb | Ni | Ti | Mo |
|---|---|---|---|---|---|---|---|---|---|
| Mass percent (wt%) | 0.40 | 19.70 | 0.40 | 17.90 | 4.80 | 52.60 | 0.60 | 3.60 | |
| Mg ingot | Element | Fe | Zn | Al | Ni | Ca | Cu | Mn | Mg |
| Mass percent (wt%) | 0.01 | 0.01 | 0.01 | 0.001 | 0.01 | 0.02 | 0.01 | 99.9 | |
| Zn shot | Element | Cu | Mg | Ni | Co | Al | Pb | Fe | Zn |
| Mass percent (wt%) | 0.01 | 0.01 | 0.001 | 0.01 | 0.01 | 0.01 | 0.01 | 99.9 |
| Sample | Concentration (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mg | Zn | Cr | Fe | Ni | Nb | Mo | Ti | |
| Mg-Zn alloy | 69.5 | 21.5 | 0.0006 | 0.02 | 8.92 | 0.0006 | 0.0005 | 0.0005 |
| Distillation product | 1.24 | 0.29 | 0.0088 | 0.12 | 98.3 | 0.0078 | 0.0034 | 0.0013 |
| Alloy residue | 0.29 | 0.02 | 38.5 | 40.99 | 2.86 | 10.31 | 5.67 | 1.36 |
| Obtained Mg-Zn alloy | 72.79 | 22.84 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Gan, X.; Cui, F.; Yu, D.; Guo, X. Selective Extraction of Ni from Superalloy Scraps by Molten Mg-Zn. Metals 2021, 11, 993. https://doi.org/10.3390/met11060993
Tian Q, Gan X, Cui F, Yu D, Guo X. Selective Extraction of Ni from Superalloy Scraps by Molten Mg-Zn. Metals. 2021; 11(6):993. https://doi.org/10.3390/met11060993
Chicago/Turabian StyleTian, Qinghua, Xiangdong Gan, Fuhui Cui, Dawei Yu, and Xueyi Guo. 2021. "Selective Extraction of Ni from Superalloy Scraps by Molten Mg-Zn" Metals 11, no. 6: 993. https://doi.org/10.3390/met11060993
APA StyleTian, Q., Gan, X., Cui, F., Yu, D., & Guo, X. (2021). Selective Extraction of Ni from Superalloy Scraps by Molten Mg-Zn. Metals, 11(6), 993. https://doi.org/10.3390/met11060993






