Design and Construction of a Laboratory-Scale Direct-Current Electric Arc Furnace for Metallurgical and High-Titanium Slag Smelting Studies
Abstract
:1. Introduction
2. Theoretical Design and Calculation
2.1. Electrical Conception
2.1.1. Selection of Power Supply
2.1.2. Electrode and Holder Design
2.1.3. Electrical Control Design
2.2. Mechanical Design
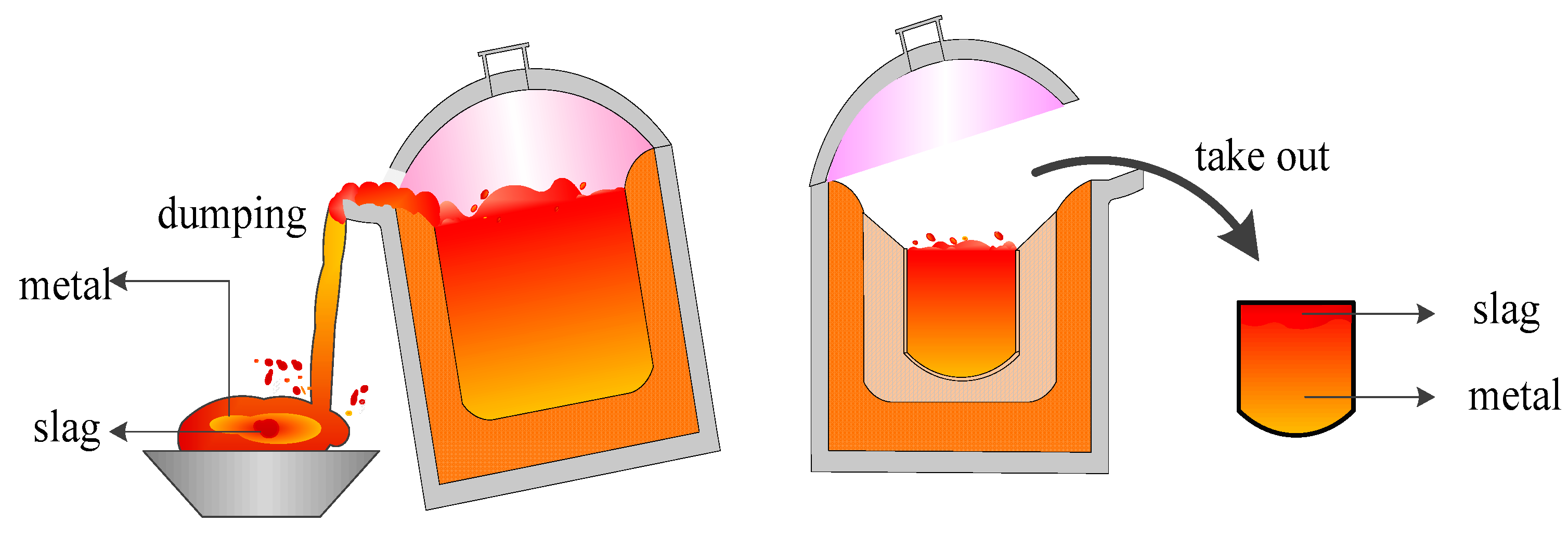
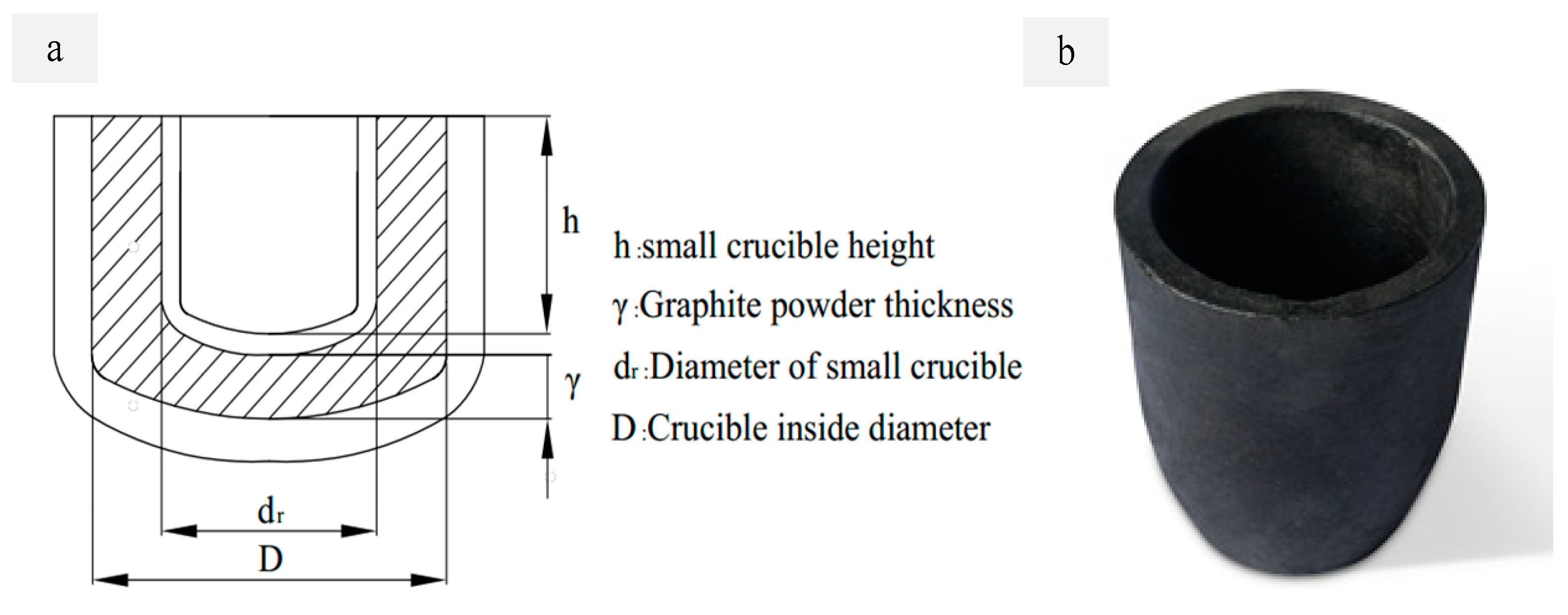
2.2.1. Crucible and Reaction Chamber Design
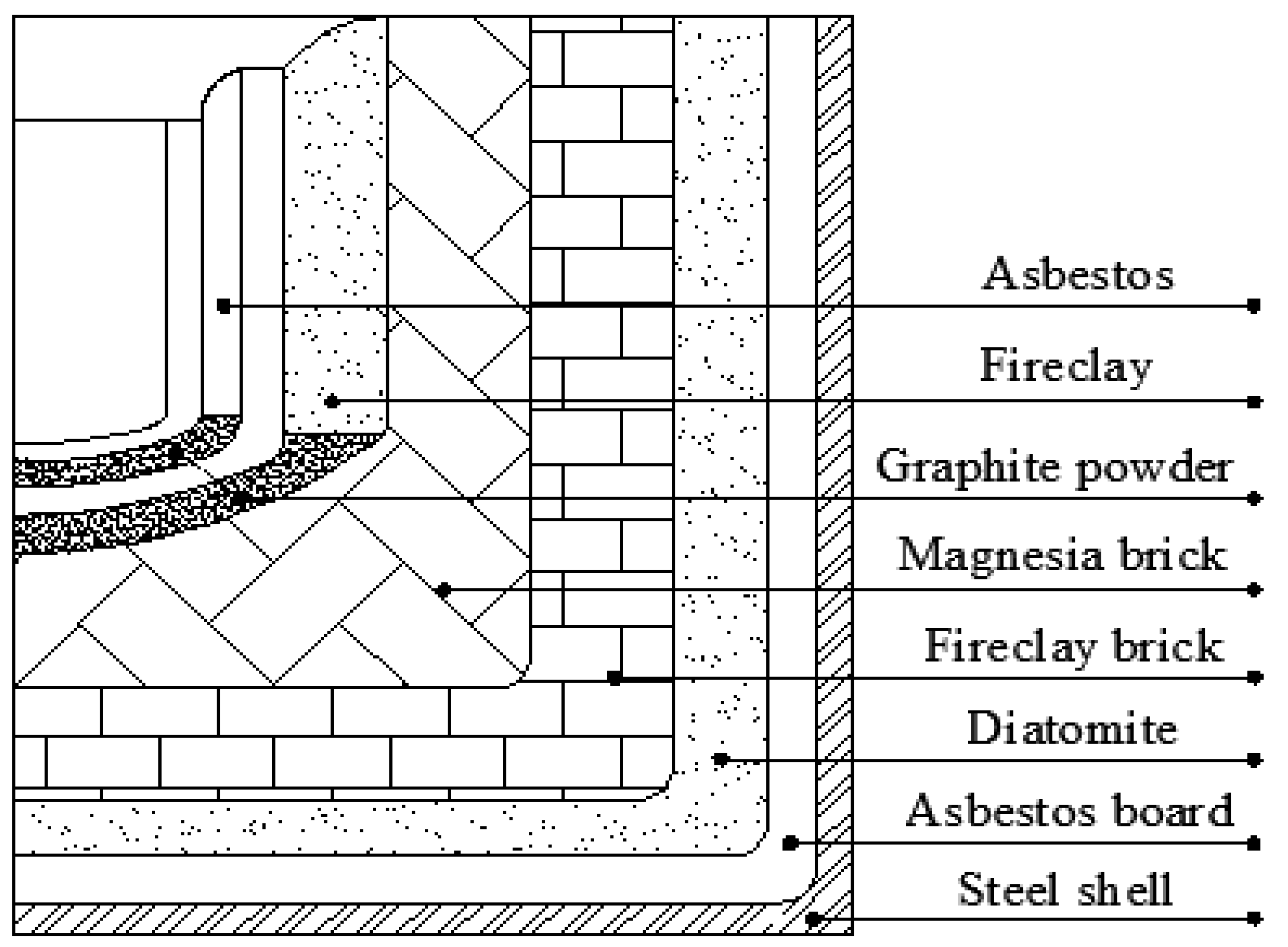
2.2.2. Furnace Lining Design
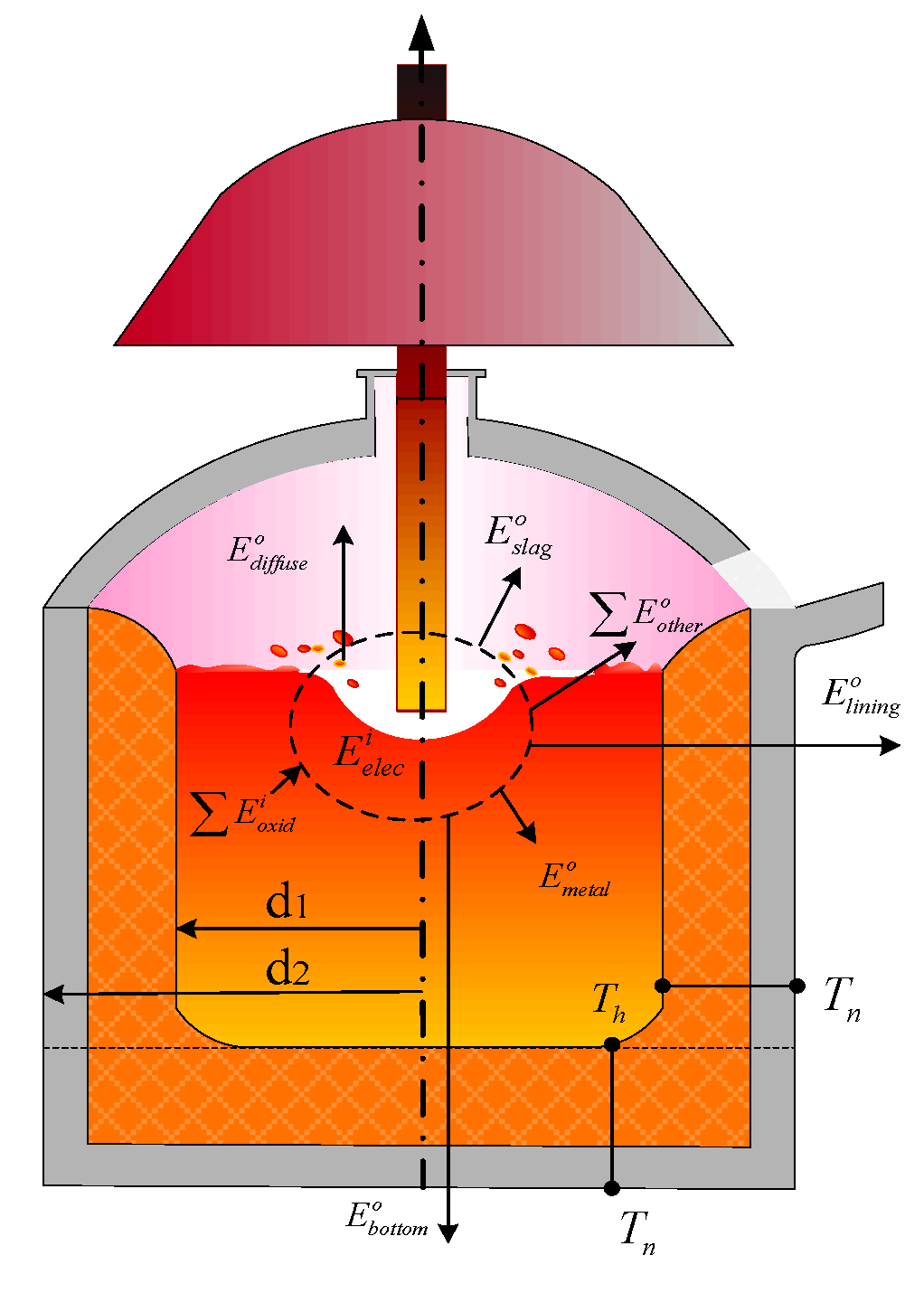
- : amount of electrical energy in an DC-EAF.
- : energy of slag before tapping.
- : chemical energy of the oxidation reactions.
- : enthalpy of liquid metal before tapping.
- : other energy lost (dust, spatter, gas).
- Electrical energy
- 2.
- Chemical energy of element reactions
- Energy of liquid metal before tapping
- 2.
- Energy of slag at tapping
- 3.
- Other energy losses
2.3. Overall Design of the Electric Furnace
3. Data Collection and Applications
3.1. Data Collection
3.2. Software Development
4. Results and Discussion
5. Conclusions
- The mechanical structure was designed, which included the power supply mode, electrode and electrode holder, electric circuit, crucible, and the reaction chamber structure. The embedded design of the crucible was used to separate the metal and the slag. In addition, the surface lining and bottom thicknesses were determined using the heat balance equations.
- The EAF smelting efficiency factors were analyzed. The voltage, current, smelting time, and temperature data of the experimental electrode were collected by PLC, and the electric parameter monitoring system of the DC-EAF was developed.
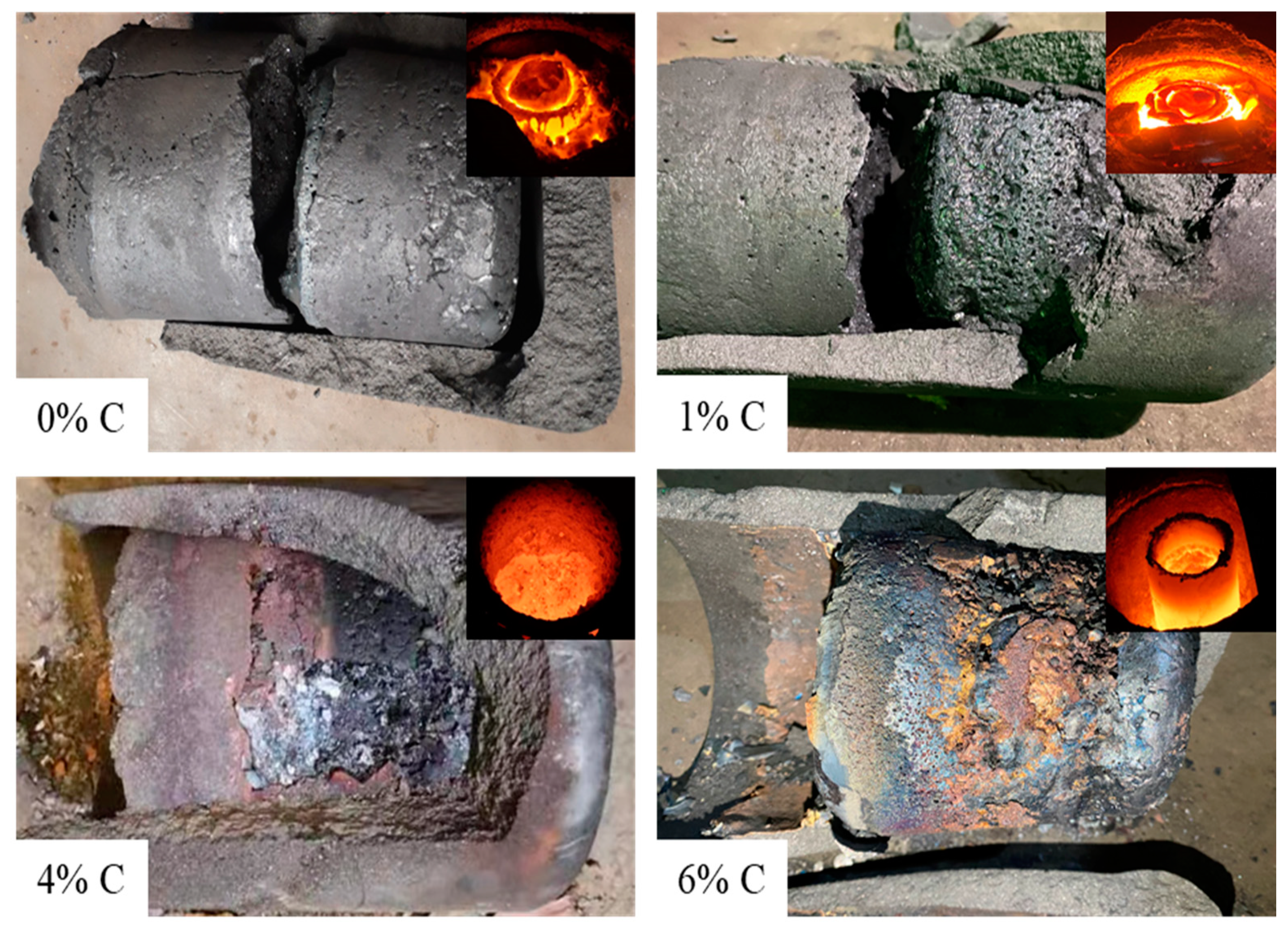
- In the performance evaluation test of the arc furnace, the optimal weight percentage of the reducing agent was investigated. The results show that for separating 12 kg of titanium-containing metallized pellets when the temperature (1607 °C) and smelting time (90 min) were constant, coke with a total weight of 4% coke was the most effective condition for facilitating the enrichment effect of TiO2 in high-titanium slag; consequently, the TiO2 content in slag reached 93.34%. This shows that the proposed DC-EAF meets the design requirements of lining thickness and achieves a good metal-slag separation.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madias, J. Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 271–300. [Google Scholar]
- Toulouevski, Y.N.; Zinurov, I.Y. Innovation in Electric Arc Furnaces Ⅱ: Scientific Basis for Selection; Toulouevski, Y.N., Zinurov, I.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–24. [Google Scholar]
- Ministry of Industry and Information Technology of the People’s Republic of China. The Ministry of Industry and Information Technology Issued a Notice on the Steel. Industry Adjustment and Upgrading Plan (2016–2020). Available online: https://www.miit.gov.cn/zwgk/zcwj/wjfb/zh/art/2020/art_4ec2537d42b947e3bbac6a70babbaa05.html (accessed on 14 November 2016).
- Lupi, S. Fundamentals of Electroheat: Electrical Technologies for Process Heating; Lupi, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 83–205. [Google Scholar]
- Xu, X. Overview of the development of DC arc furnaces at home and abroad. Hum. Metall 1996, 2, 57–60. [Google Scholar]
- Yu, M.Q. Overview of the development of DC arc furnace technology at home and abroad. Shanghai Metall Inf. 1998, 4, 1–10. [Google Scholar]
- Oyawale, F.A.; Olawale, D.O. Design and prototype development of a mini-electric arc furnace. Pac. J. Sci. Technol. 2007, 8, 12–16. [Google Scholar]
- Yin, J.F. Experimental study on process granulated water quenching slag by dc electric arc furnace. Min. Metall 2011, 20, 82–85. [Google Scholar]
- Barbouche, M.; Hajji, M.; Ezzaouia, H. Electric arc furnace design and construction for metallurgical and semiconductor research. Int. J. Adv. Manuf. Technol. 2016, 82, 997–1006. [Google Scholar] [CrossRef]
- Sun, D.C.; Sun, Y.C. Current Situation and Development of DC Arc Furnace at Home and Abroad. In Proceedings of the 9th Academic Conference on Carbon Materials, Chinese Society of Metals, Shanghai, China, 1 April 1994. [Google Scholar]
- Schub, M.; Xiao, B. Economical and energy-saving unarc DC arc furnace. Energy Metall Ind. 1993, 12, 57–60. [Google Scholar]
- Gu, H.X. Direct current electric arc furnace. Spec. Steel. 1983, 4, 88–90. [Google Scholar]
- Zhang, H.S. Reconstruction of 5—ton arc furnace. Heavy Mach. 1989, 4, 47–48. [Google Scholar]
- Wang, J.Z.; Liu, X.W. Research and development of DC arc furnace in Chengdu Seamless Steel. Tube Company. Spec. Steel. Technol. 1995, 1, 23–24. [Google Scholar]
- Liu, X.R. Design and Operation of 10t Single Electrode DC Arc Furnace. Ind. Heat 1995, 2, 33–35. [Google Scholar]
- Sui, L.L. Theory and Technology of Green Utilization of Titanium Slag; Northeastern University: Boston, MA, USA, 2015. [Google Scholar]
- Ma, X.; Han, F.X.; Lei, T. Factor of reductant carbon during titanium slag smelting in closed direct current arc furnace. J. Kunming Univ. Technol. 2013, 38, 6–10. [Google Scholar]
- Edneral, F.P. Electrometallurgy of Steel and Ferro-Alloys; MIR Publishers: Moscow, Russia, 1979; pp. 1–87. [Google Scholar]
- Bowman, B. Performance Comparison Update-Ac vs Dc Furnaces. Iron Steel. Eng. 1995, 72, 26–29. [Google Scholar]
- Saevarsdottir, G. Handbook of Ferroalloys; Gasik, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2013; pp. 139–175. [Google Scholar]
- Zheng, J.H. Comparison and technology analysis of UHP electrode performance in AC and DC electric arc furnaces. Carbon Tech. 2002, 4, 28–32. [Google Scholar]
- Yan, L.Y.; Wu, Z.T.; Rui, S.S. Design and application of the bottom electrode of DC arc furnace. Metall. Equip. 1995, 05, 16–18. [Google Scholar]
- Mullinger, P.; Jenkins, B. Industrial and Process. Furnaces, 2nd ed.; Mullinger, P., Jenkins, B., Eds.; Butterworth-Heinemann: Oxford, UK, 2013; pp. 289–335. [Google Scholar]
- Dednev, A.A.; Elizariov, K.A.; Kissel’man, M.A.; Nekhamin, S.M. Control systems of melting electric furnaces in metallurgy and mechanical engineering. Russ. Metall 2013, 6, 442–449. [Google Scholar] [CrossRef]
- Shinya, O. Programmable Logic Controller. Progr Log. Control. 2012, 62, 1–22. [Google Scholar]
- Skripchenko, S.V.; Nikol’skii, L.E.; Pis’mennyi, V.A. Study of the efficiency of use of electric power in the production of steel in an arc furnace. Metall 1988, 32, 150–151. [Google Scholar] [CrossRef]
- Siemens. S7-200 Programming Manual. Available online: https://cache.industry.siemens.com/dl/files/582/1109582/att_22063/v1/s7200_system_manual_en-US.pdf (accessed on 10 February 2021).
- Postel, J. DOD standard transmission control protocol. ACM SIGCOMM Comput. Commun. Rev. 1980, 10, 52–132. [Google Scholar] [CrossRef]
- IEEE. National Electrical Safety Code (NESC) Handbook, 7th ed.; IEEE: Piscataway Township, NJ, USA, 2011; pp. 1–802. [Google Scholar]
- Allen, L. Clapp. Installation and Maintenance of Equipment. In NESC Handbook: A Discussion of the National Electrical Safety Code; IEEE: Piscataway Township, NJ, USA, 2011; pp. 148–176. [Google Scholar]
- Persson, J.A.; Andrews, R.E.; Maola, M. Arc Furnace Electrode Control. US US4620308 A, 28 October 1986. Available online: https://www.freepatentsonline.com/4620308.pdf (accessed on 10 February 2021).
- Dong, Q.; Zhang, J. Simulation of superfluid and heat transfer in plasma arc region of AC electric arc furnace. In CFD Modeling and Simulation in Materials Processing; Nastac, L., Zhang, L., Thomas, B.G., Zhu, M., Ludwig, A., Sabau, A.S., Pericleous, K., Combeau, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 35–42. [Google Scholar]
- Reynolds, Q.G.; Jones, R.T.; Reddy, B.D. Mathematical and computational modelling of the dynamic behaviour of direct current plasma arcs. J. S. Afr. Inst. Min. Metall. 2010, 110, 733–742. [Google Scholar]
- Biswas, S.; Sarkar, D. Introduction to Refractories for Iron- and Steelmaking; Biswas, S., Sarkar, D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 249–267. [Google Scholar]
- Song, X.W. Technology of Refractory Materials; Beijing Chemical Industry Press: Beijing, China, 2008; pp. 63–105. [Google Scholar]
- Kazak, O. Modeling of vortex flows in direct current (DC) electric arc furnace with different bottom electrode positions. Metall Mater. Trans. B 2013, 44, 1243–1245. [Google Scholar] [CrossRef]

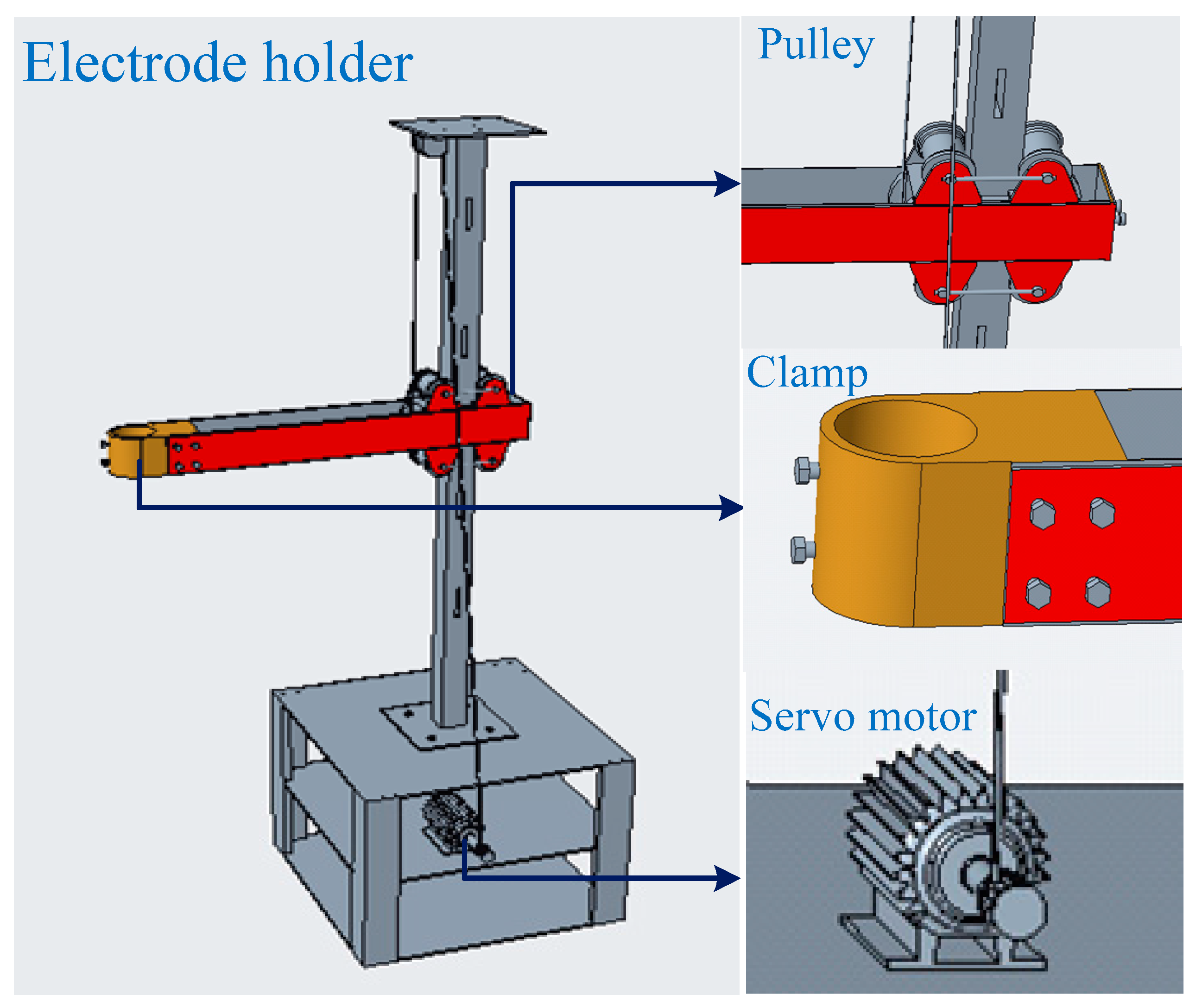
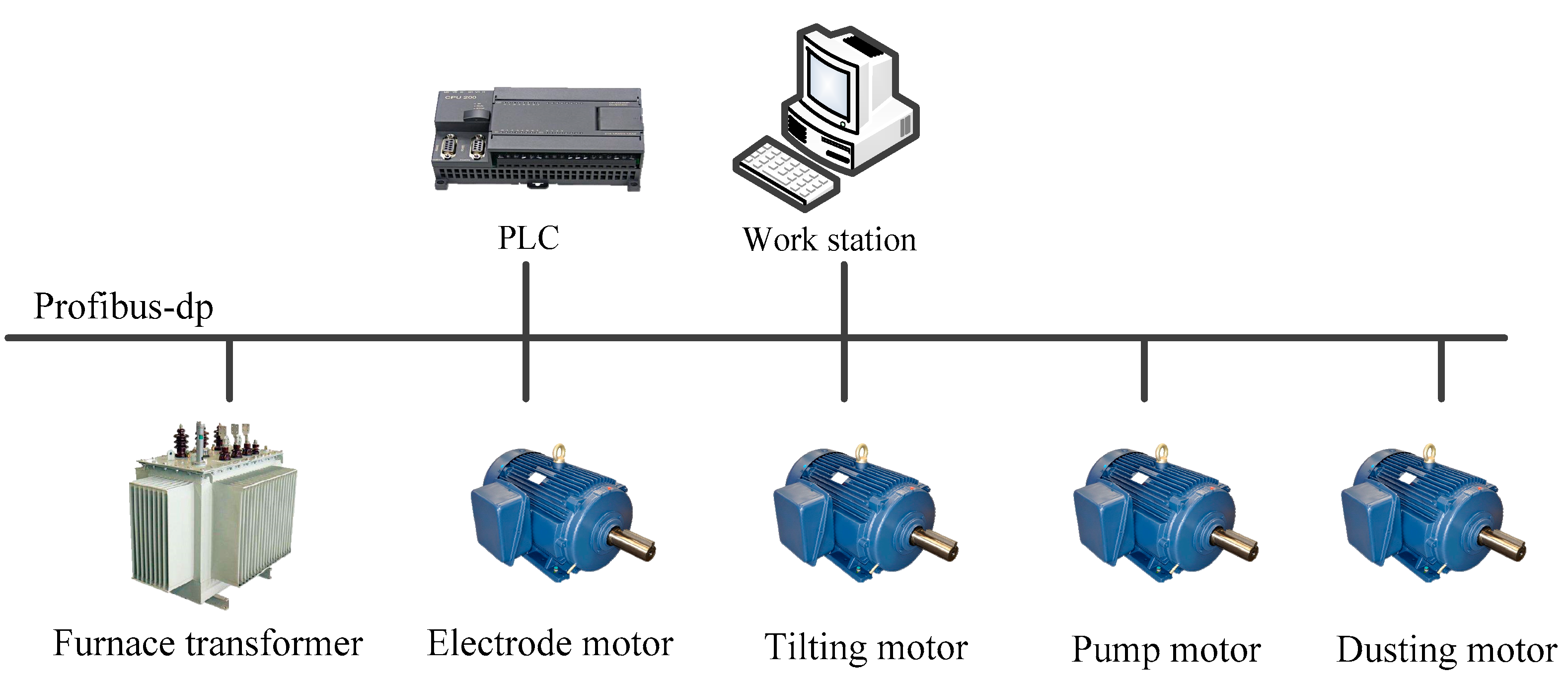
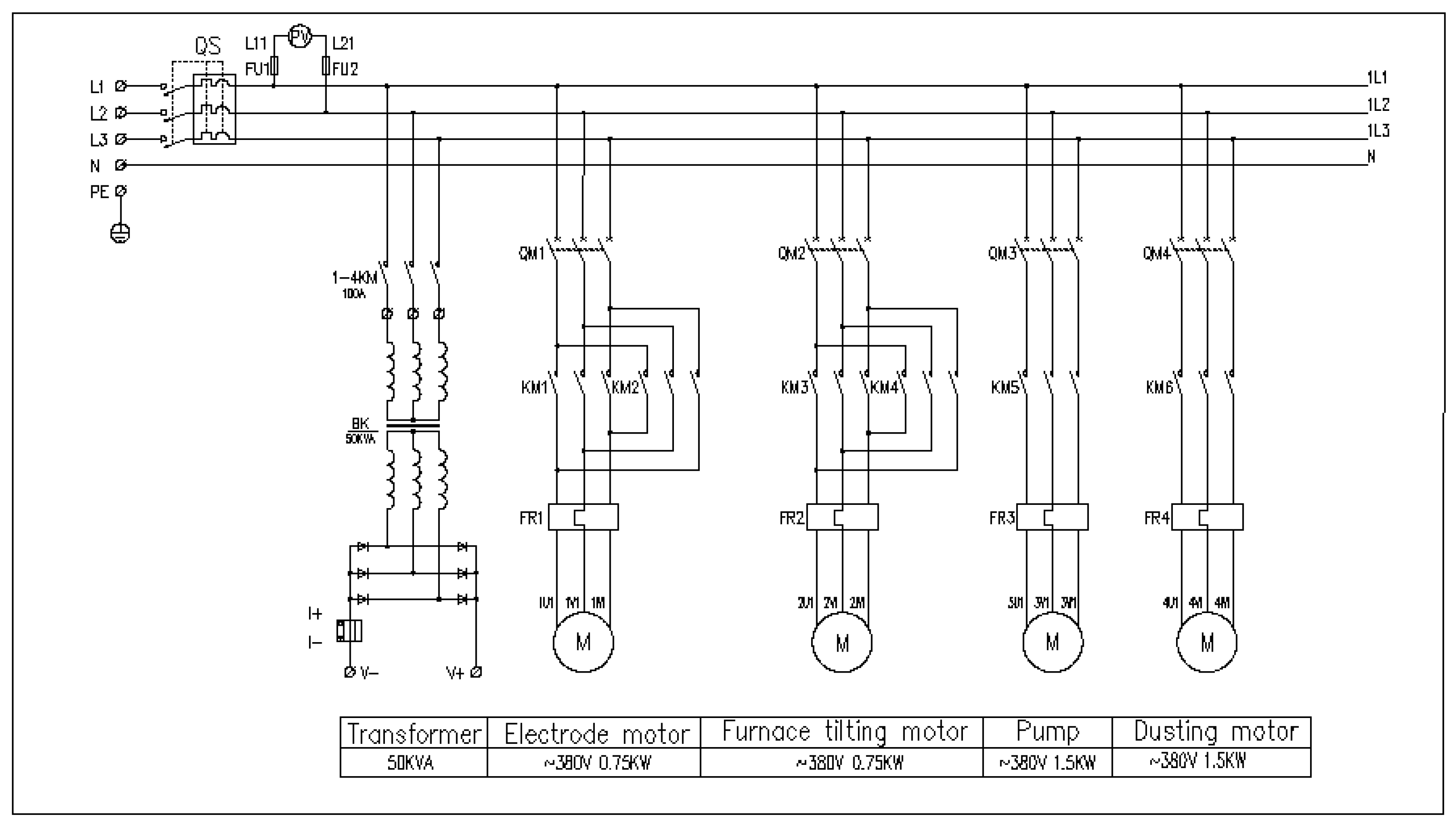
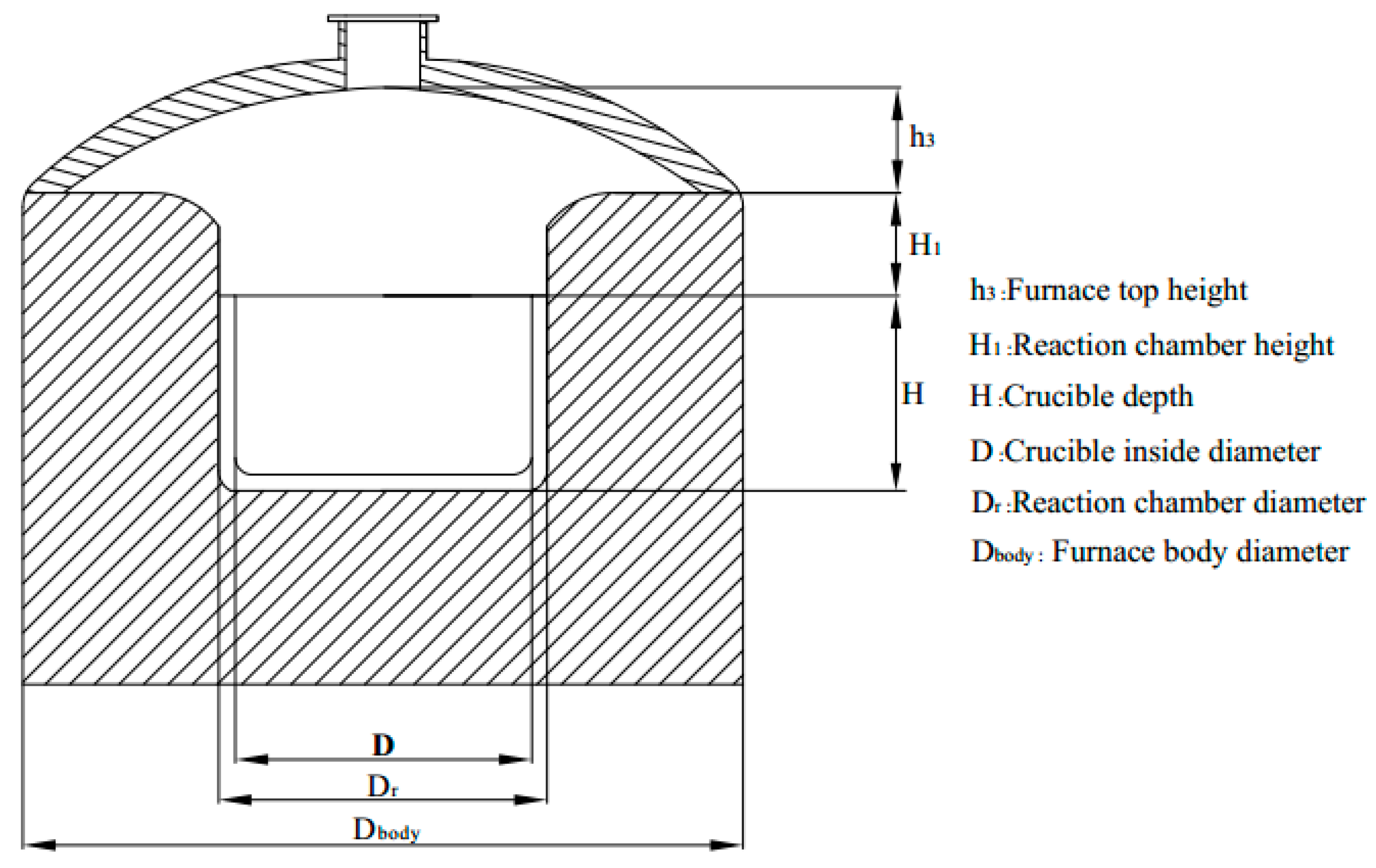
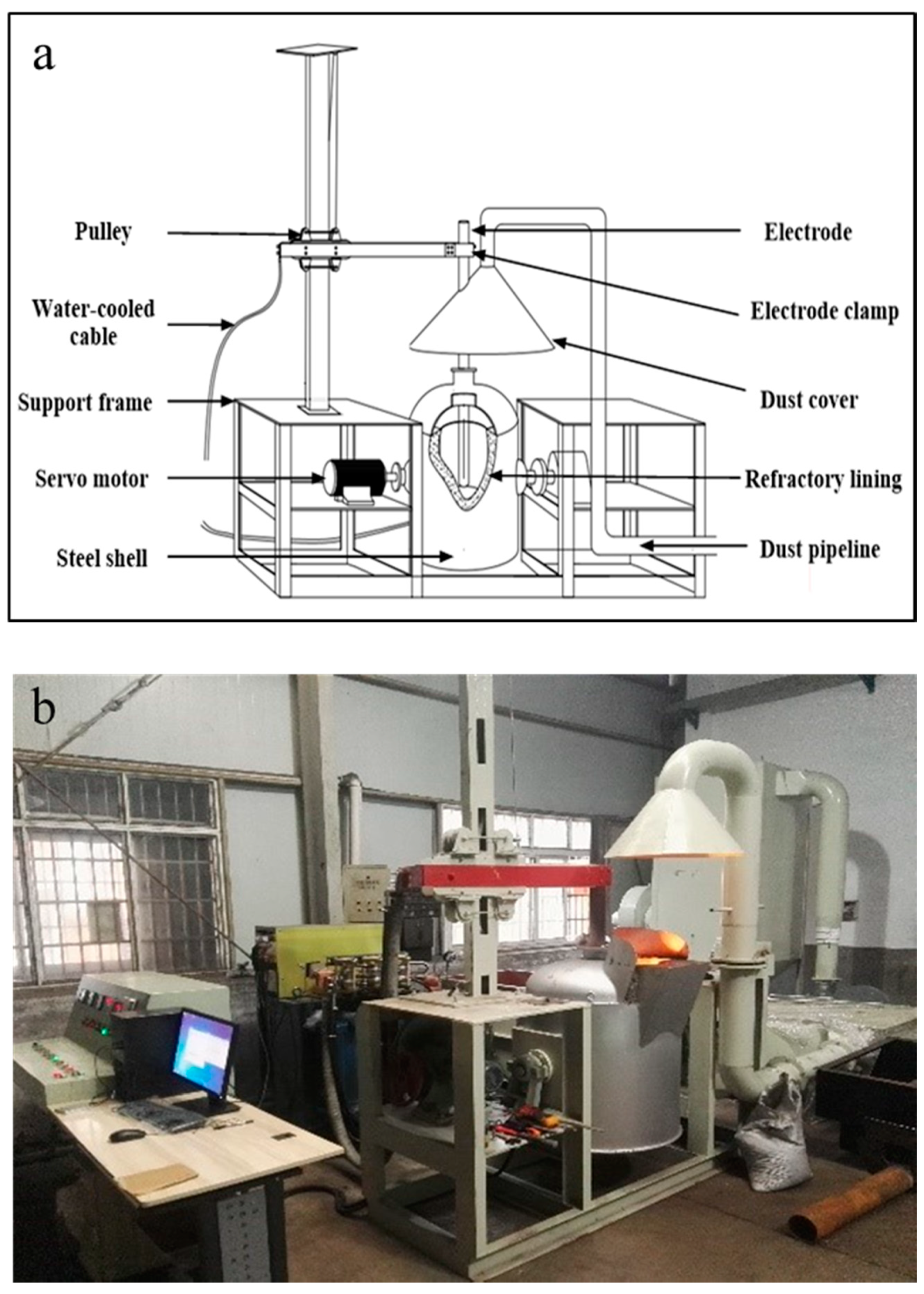
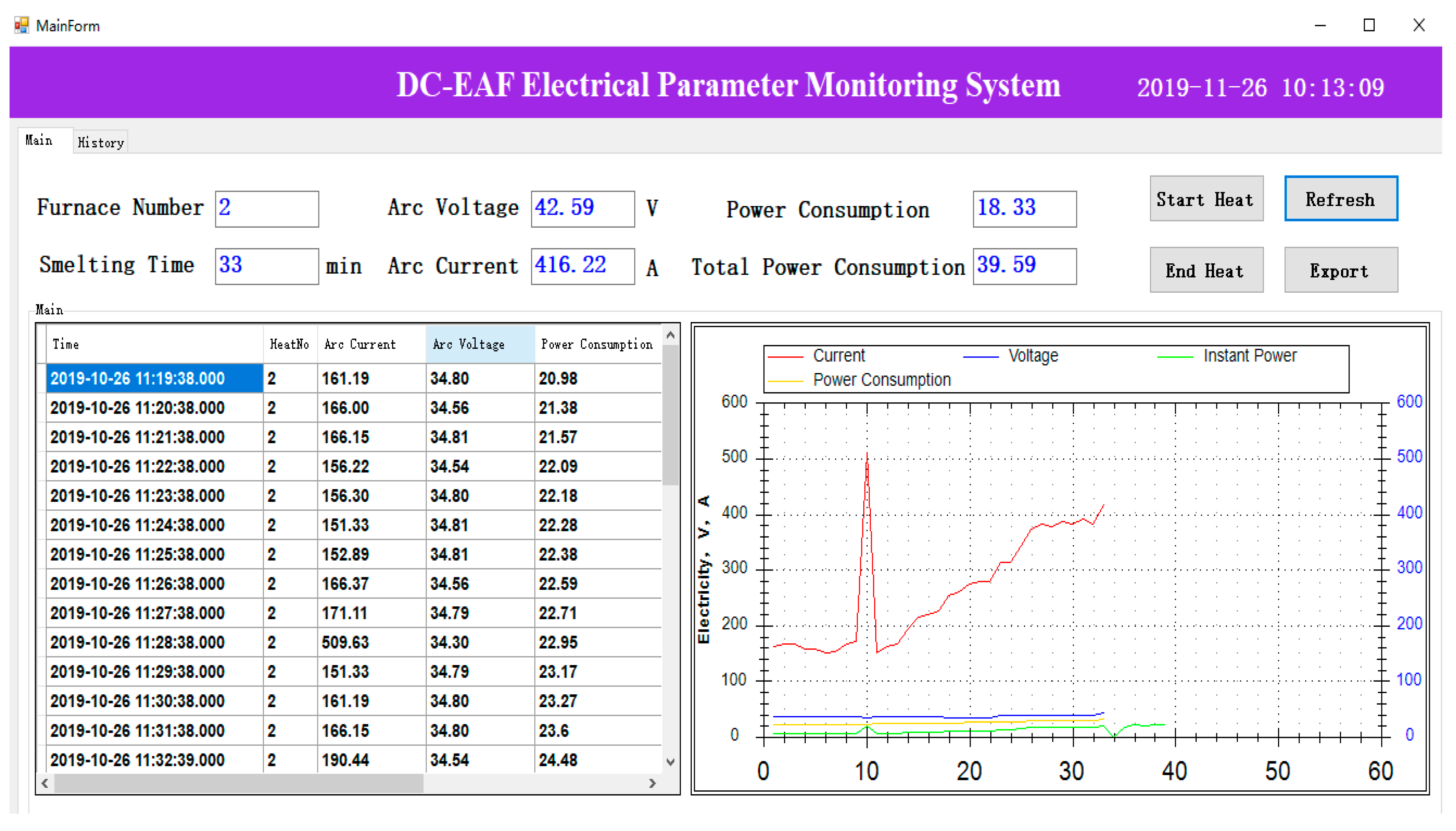
| Company | Year | Capacity | Note | References |
|---|---|---|---|---|
| ABB | 1972 | 7 t | \ | Sun et al. [10] |
| IRSID | 1979 | 6 t | \ | Xu [5] |
| MAN-GHH | 1982 | 12 t | Bottom tapping | Schub., M et al. [11] |
| SMS | 1983 | 12 t | Tilting tapping | Gu [12] |
| MAN-GHH | 1985 | 0.5 t | \ | Xu [5] |
| Taiyuan Heavy Machinery | 1989 | 5 t | Tilting tapping | Zhang [13] |
| Chengdu Seamless Steel Tube | 1991 | 5 t | Tilting tapping | Wang et al. [14] |
| Shanghai Fifth Steel Works | 1995 | 10 t | EBT | Liu [15] |
| \ | 2007 | 2 kg | Tilting tapping | Festus et al. [7] |
| \ | 2011 | 250 kg | Tilting tapping | Yin et al. [8] |
| \ | 2015 | \ | \ | Barbouche et al. [9] |
| Input Energy | kWh (10 kg)−1 | % | |
| 1. Electric energy | 15 | 96.22 | |
| 2. Chemical energy of oxidation reactions | 0.59 | 3.78 | |
| energy of element oxidation | 0.44 | 2.82 | |
| other small energy sources | 0.15 | 0.96 | |
| - | - | Ʃ 15.59 | 100 |
| Output energy | kWh (10 kg)−1 | % | |
| 1. Energy of liquid metal before tapping | 3.82 | 24.50 | |
| 2. Energy of slag before tapping | 0.22 | 1.41 | |
| 3. Other energy losses | 0.14 | 0.90 | |
| energy of the splash | 0.03 | 0.19 | |
| energy carried away by the iron beads | 0.01 | 0.07 | |
| energy carried away by the dust | 0.07 | 0.45 | |
| energy taken away by the gas | 0.03 | 0.19 | |
| 4. Energy diffused at the bottom | 1.28 | 8.21 | |
| 5. Energy diffused in the furnace | 4.5 | 28.87 | |
| 6. Loss of energy from furnace walls | 5.63 | 36.11 | |
| - | - | Ʃ 15.59 | 100 |
| Materials | Temperature T/°C | Density ρ/(kg m−3) | Thermal Conductivity λ/(W m−1 °C−1) |
|---|---|---|---|
| Asbestos board | 600–700 | 80–140 | 0.10–0.26 |
| Diatomite | 900 | 440–500 | 0.0395 + 0.00019 T |
| Fireclay | 1100–1300 | 800–1300 | [0.29–0.41] + 0.00026 T |
| Fireclay brick | 1350–1450 | 1800–2040 | [0.7–0.84] + 0.00058 T |
| Magnesia brick | 600–1700 | 2300–2600 | 2.1 + 0.00019 T |
| Items | Parameters | Items | Parameters |
|---|---|---|---|
| Connection Mode | Triangle/Star | Rectifier Mode | Bridge Rectifier |
| Input voltage | 380 V | Direct voltage output | 35/40/45/50 V |
| Input current | 75 A | Direct current output | ≤1250 A |
| Items | Parameters | Items | Parameters |
|---|---|---|---|
| Electrode Diameter | 40 mm | Stroke of Electrode | 1000 mm |
| Reaction chamber height (H1) | 90 mm | Small crucible thickness | 20 mm |
| Small crucible diameter (dr) | 165 mm | Large crucible thickness | 25 mm |
| Large crucible diameter (Dr) | 255 mm | Small crucible height (h) | 50 mm |
| Furnace top height (h3) | 130 mm | Large crucible height (H) | 70 mm |
| Furnace bottom thickness | 330 mm | Furnace lining thickness | 265 mm |
| Furnace body height | 750 mm | Furnace body diameter | 800 mm |
| Composition | Fe | CaO | SiO2 | MgO | Al2O3 | K2O | Na2O | Cr2O3 | TiO2 | V2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 65.66 | 0.39 | 2.19 | 0.64 | 1.59 | 0.073 | 0.17 | 0.58 | 26.10 | 0.41 |
| Composition | C | O | Al | Si | S | Fe | Ca | Mg | Ti | Cr |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 89.10 | 5.45 | 1.67 | 1.64 | 1.12 | 0.32 | 0.23 | 0.16 | 0.05 | <0.01 |
| NO. | Pellet Weight (kg) | Coke Weight (kg) | Time (min) | Temperature (°C) | Content in Slag after Smelting | |||
|---|---|---|---|---|---|---|---|---|
| FeO (%) | TiO2 (%) | Cr2O3 (%) | V2O5 (%) | |||||
| 1 | 6 | 0 | 90 | 1841 | 19.35 | 59.44 | 1.29 | 1.14 |
| 2 | 12 | 0.12 | 90 | 1620 | 14.54 | 65.45 | 0.56 | 1.57 |
| 3 | 12 | 0.48 | 90 | 1607 | 3.04 | 93.34 | 0.31 | 0.47 |
| 4 | 12 | 0.72 | 90 | 1594 | 11.88 | 86.83 | 0.25 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, B.; Yang, L.; Guo, Y.; Chen, F.; Wang, S.; Zheng, F.; Yang, Z. Design and Construction of a Laboratory-Scale Direct-Current Electric Arc Furnace for Metallurgical and High-Titanium Slag Smelting Studies. Metals 2021, 11, 732. https://doi.org/10.3390/met11050732
Xue B, Yang L, Guo Y, Chen F, Wang S, Zheng F, Yang Z. Design and Construction of a Laboratory-Scale Direct-Current Electric Arc Furnace for Metallurgical and High-Titanium Slag Smelting Studies. Metals. 2021; 11(5):732. https://doi.org/10.3390/met11050732
Chicago/Turabian StyleXue, Botao, Lingzhi Yang, Yufeng Guo, Feng Chen, Shuai Wang, Fuqiang Zheng, and Zeshi Yang. 2021. "Design and Construction of a Laboratory-Scale Direct-Current Electric Arc Furnace for Metallurgical and High-Titanium Slag Smelting Studies" Metals 11, no. 5: 732. https://doi.org/10.3390/met11050732
APA StyleXue, B., Yang, L., Guo, Y., Chen, F., Wang, S., Zheng, F., & Yang, Z. (2021). Design and Construction of a Laboratory-Scale Direct-Current Electric Arc Furnace for Metallurgical and High-Titanium Slag Smelting Studies. Metals, 11(5), 732. https://doi.org/10.3390/met11050732











