Pressure Oxidation of Arsenic (III) Ions in the H3AsO3-Fe2+-Cu2+-H2SO4 System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Analysis
2.3. Experimental Procedure
3. Results and Discussion
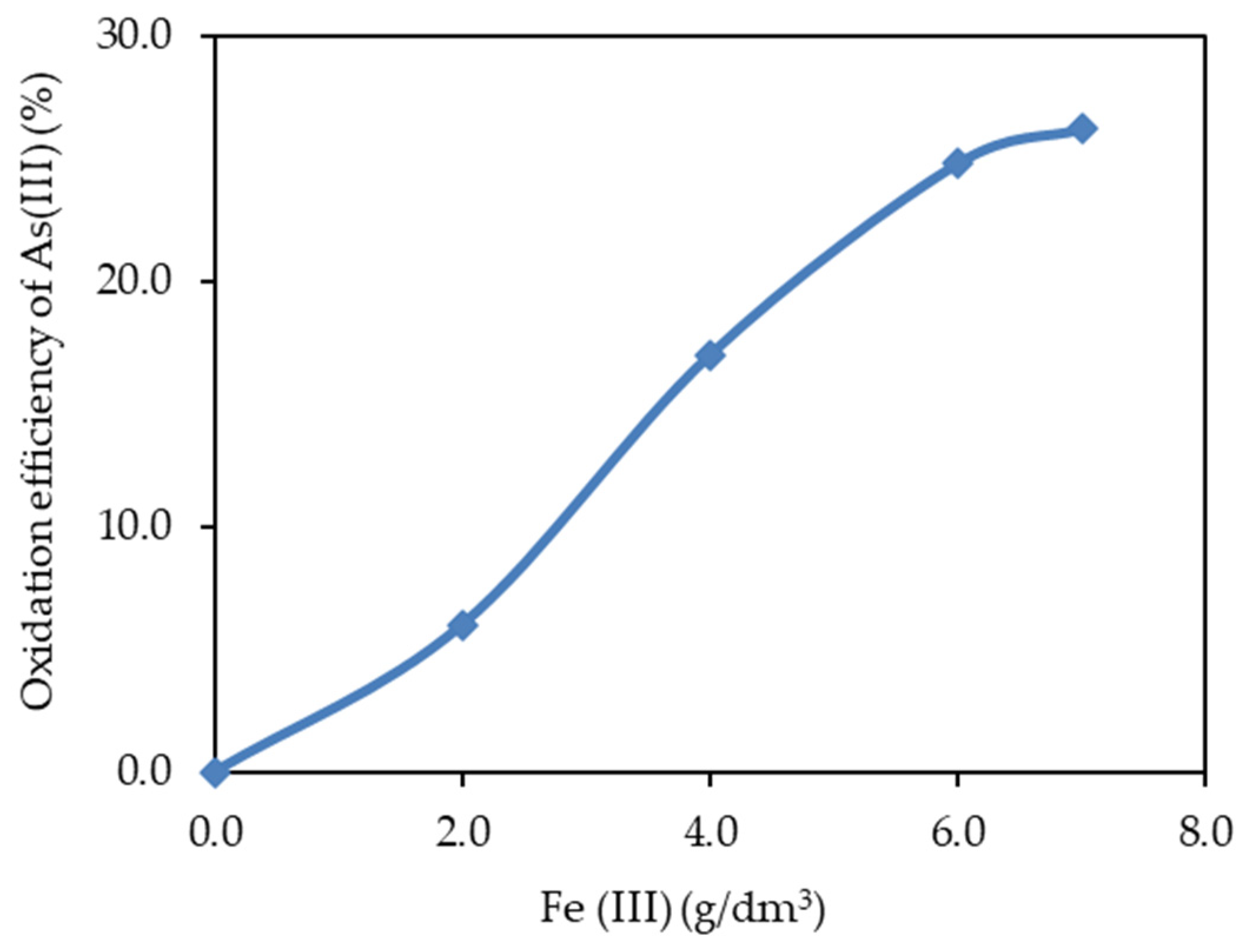
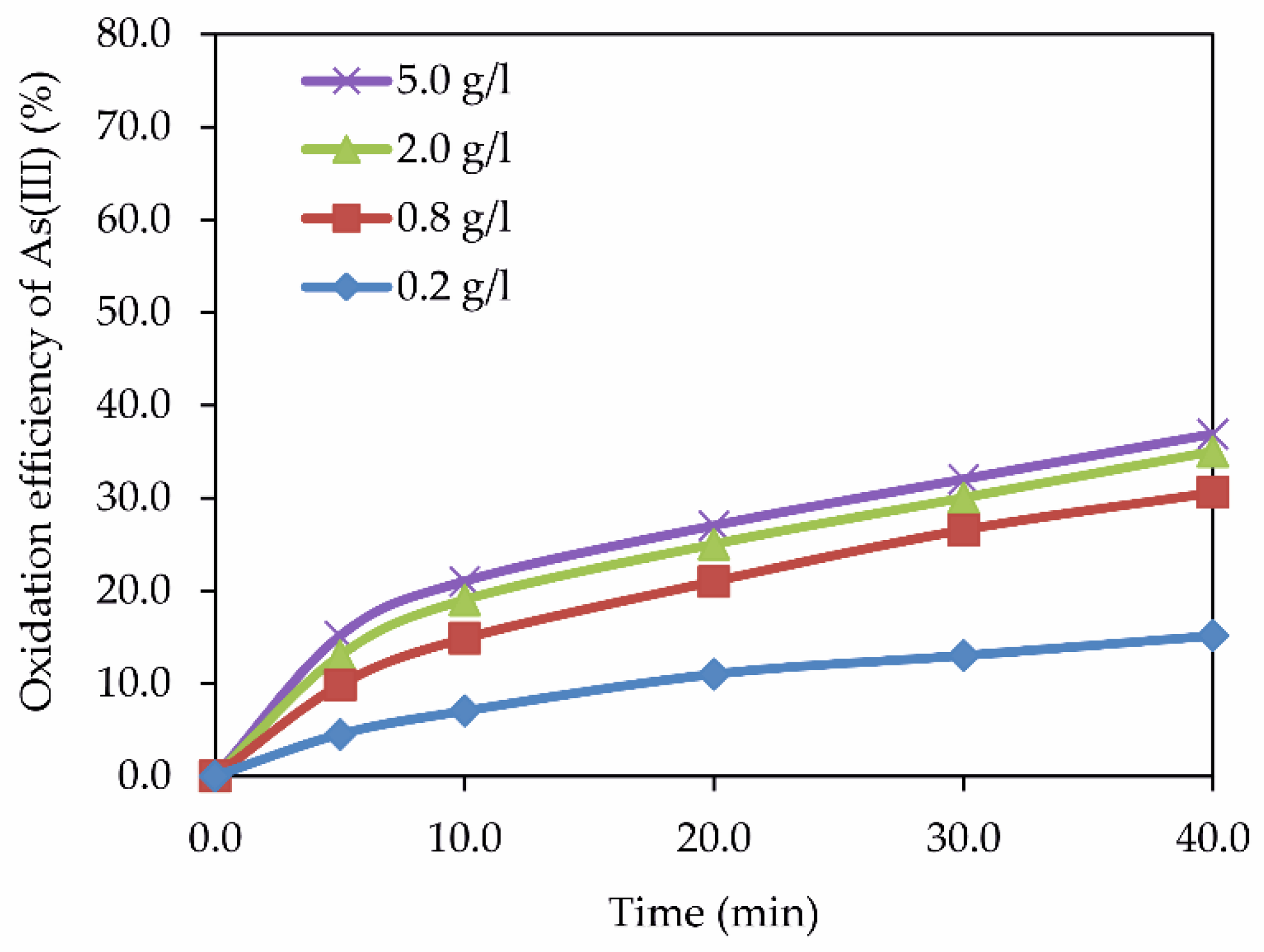
3.1. Influence of Iron Ions on Arsenic (III) Oxidation under Hydrothermal Conditions
3.1.1. Influence of Fe (II) and Fe (III) Ions
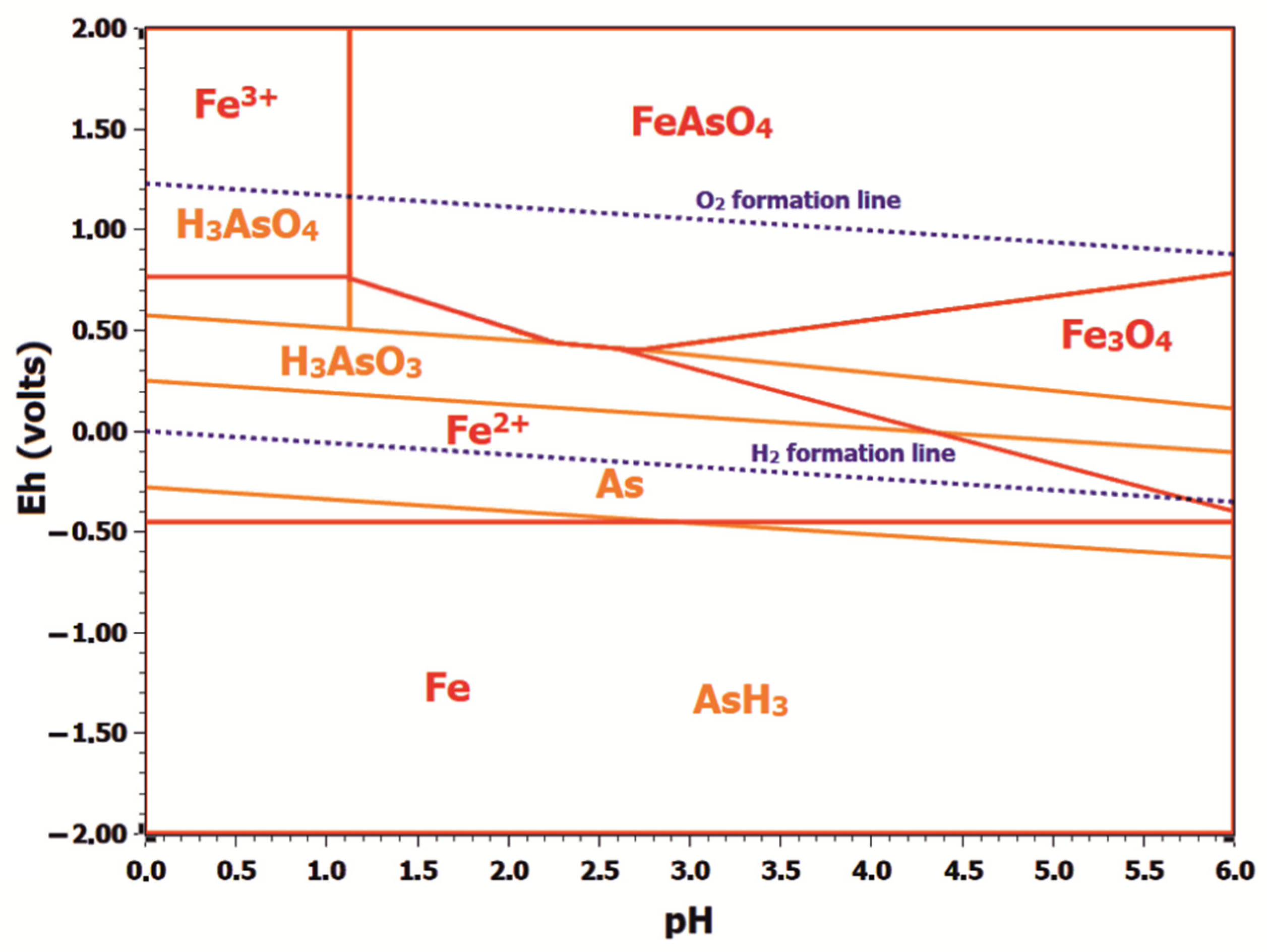
3.1.2. Thermodynamics of As (III) Ions Oxidation in the Presence of Fe (III) Ions under Hydrothermal Conditions
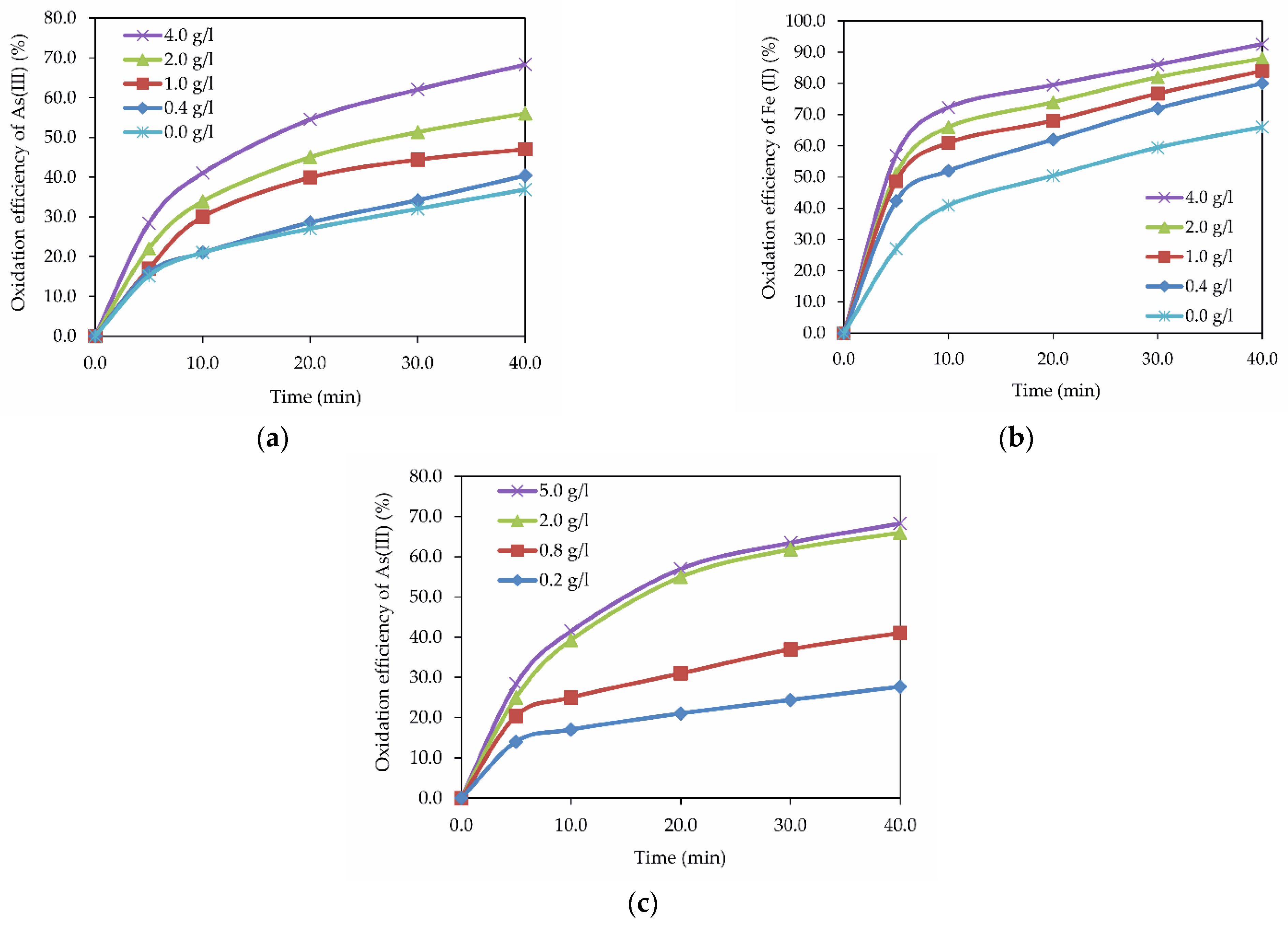
3.2. Influence of Cu (II) Ions on Fe (II) and As (III) Oxidation in Hydrothermal Conditions
3.3. Influence of Temperature on As (III) Ion Oxidation
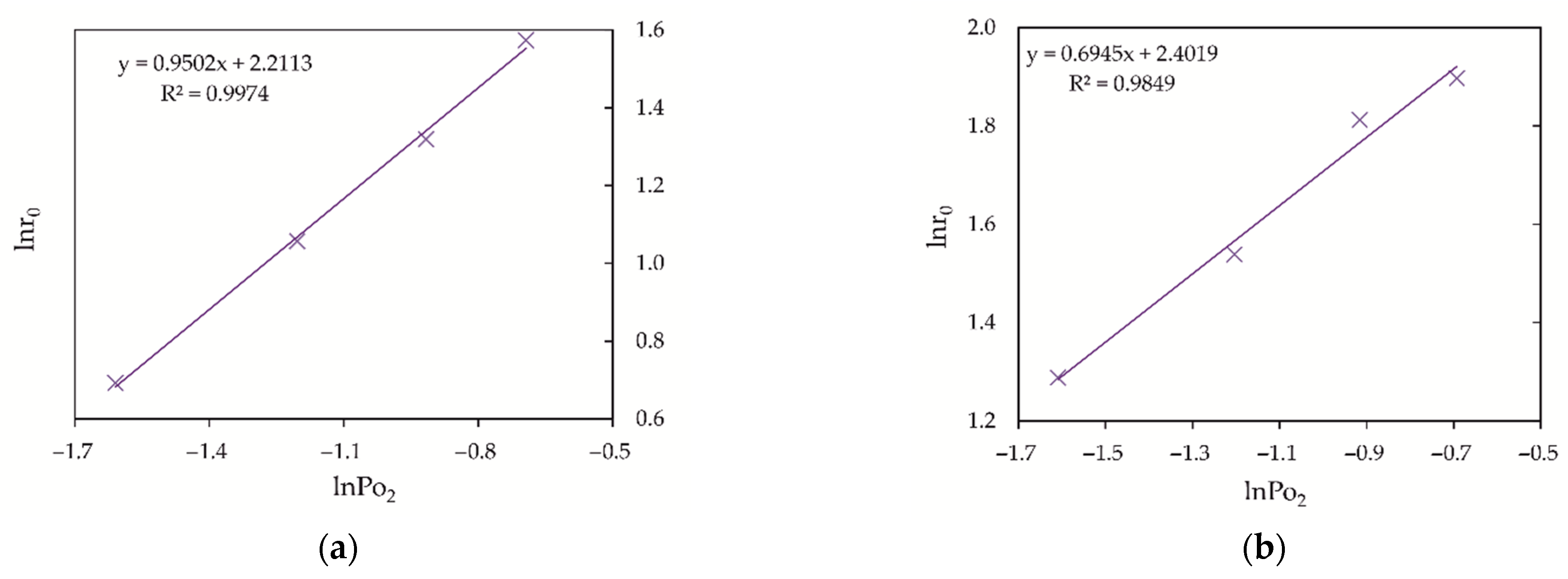
3.4. Influence of Oxygen Pressure on the Oxidation of Fe (II) and As (III) Ions
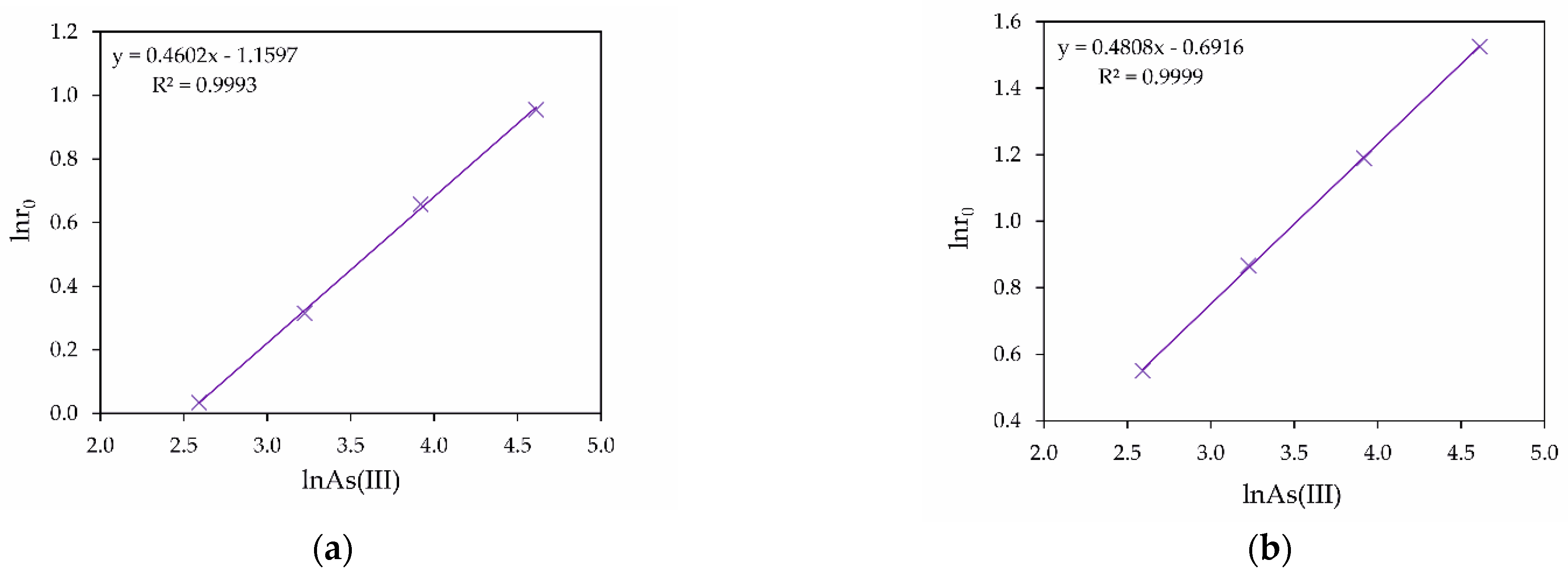
4. Kinetics Analysis
5. Conclusions
- Upon hydrothermal oxidation, Fe (II) ions are oxidized by oxygen to Fe (III) ions, which act as As (III) oxidizing agents. The influence of Fe (III) ions on arsenic oxidation can be associated with the formation of stable intermediate FeH2AsO42+ complexes in acidic media, which reduce the standard oxidation potential of As (III) to 0.40 V.
- It has been shown that the positive effect of copper ions on As (III) oxidation is associated with an increase in the oxygen oxidizing power in the presence of variable valency ions, prone to complex formation or valency changes. This explains the increase in the As (III) oxidation rate in the presence of Cu (II) ions. In addition, Cu (II) ions promote the oxidation of Fe (II) to Fe (III) ions.
- General kinetic equations for the As (III) oxidation rate in the H3AsO3-Fe2+-Cu2+-H2SO4 and H3AsO3-Fe2+-H2SO4 systems were determined. The experimental reaction orders relative to arsenic and iron concentrations and oxygen pressure were obtained.
- As (III) oxidation in the H3AsO-Fe2+-Cu2+-H2SO4 and H3AsO3-Fe2+-H2SO4 systems was controlled by a chemical reaction with the apparent activation energy (Ea (≈84.3–86.3 kJ/mol)) for the following reasons: the increase in the concentration of Fe (II) ions and addition of an external catalyst (Cu (II) ions) both have a positive effect on the process; at a reaction order of 0.43–0.20, Ea values are elevated. When Cu (II) ions are introduced into the solution, their catalytic effect is confirmed by a decrease in the specific orders of Fe (II) ions and the oxygen pressure. The revealed catalytic effect is associated with a positive effect of Cu (II) ions on the oxidation of Fe (II) to Fe (III) ions, which further participate in As (III) oxidation. This confirms that the formed Fe (III) ions act as oxidizing agents towards As (III).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azcue, J.M.; Mudroch, A.; Rosa, F.; Hall, G. Effects of abandoned gold mine tailings on the arsenic concentrations in water and sediments of jack of Clubs Lake, B.C. Environ. Technol. 1994, 15, 669–678. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H. Arsenic—A review. Part I: Occurrence, Toxicity, Speciation, Mobility. Acta Hydroch. Hydrob. 2003, 31, 9–18. [Google Scholar] [CrossRef]
- Shibayama, A.; Takasaki, Y.; William, T.; Yamatodani, A.; Higuchi, Y.; Sunagawa, S.; Ono, E. Treatment of smelting residue for arsenic removal and recovery of copper using pyro–hydrometallurgical process. J. Hazard. Mater. 2010, 181, 1016–1023. [Google Scholar] [CrossRef]
- Kovyazin, A.; Timofeev, K.; Krauhin, S. Copper smelting fine dust autoclave leaching. Mater. Sci. Forum 2019, 615–620. [Google Scholar] [CrossRef]
- Jarošíková, A.; Ettler, V.; Mihaljevič, M.; Penížek, V.; Matoušek, T.; Culka, A.; Drahota, P. Transformation of arsenic-rich copper smelter flue dust in contrasting soils: A 2-year field experiment. Environ. Pollut. 2018, 237, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Karimov, K.A.; Naboichenko, S.S. Sulfuric Acid Leaching of High-Arsenic Dust from Copper Smelting. Metallurgist 2016, 60, 456–459. [Google Scholar] [CrossRef]
- Montenegro, V.; Sano, H.; Fujisawa, T. Recirculation of high arsenic content copper smelting dust to smelting and converting processes. Miner. Eng. 2013, 49, 184–189. [Google Scholar] [CrossRef]
- Montenegro, V.; Sano, H.; Fujisawa, T. Recirculation of chilean copper smelting dust with high arsenic content to the smelting process. Mater. Trans. 2008, 49, 2112–2118. [Google Scholar] [CrossRef]
- Xue, J.; Long, D.; Zhong, H.; Wang, S.; Liu, L. Comprehensive recovery of arsenic and antimony from arsenic-rich copper smelter dust. J. Hazard. Mater. 2021, 413, 125365. [Google Scholar] [CrossRef]
- Karimov, K.A.; Naboichenko, S.S.; Kritskii, A.V.; Tret’yak, M.A.; Kovyazin, A.A. Oxidation Sulfuric Acid Autoclave Leaching of Copper Smelting Production Fine Dust. Metallurgist 2019, 62, 1244–1249. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Moats, M.S.; Miller, J.D. Recent trends in the processing of enargite concentrates. Miner. Process. Extr. Metall. Rev. 2014, 35, 283–367. [Google Scholar] [CrossRef]
- Rogozhnikov, D.A.; Shoppert, A.A.; Dizer, O.A.; Karimov, K.A.; Rusalev, R.E. Leaching Kinetics of Sulfides from Refractory Gold Concentrates by Nitric Acid. Metals 2019, 9, 465. [Google Scholar] [CrossRef]
- Fujita, T.; Fujieda, S.; Shinoda, K.; Suzuki, S. Environmental leaching characteristics of scorodite synthesized with Fe (II) ions. Hydrometallurgy 2012, 111–112, 87–102. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef]
- Otgon, N.; Zhang, G.; Zhang, K.; Yang, C. Removal and fixation of arsenic by forming a complex precipitate containing scorodite and ferrihydrite. Hydrometallurgy 2019, 186, 58–65. [Google Scholar] [CrossRef]
- Gomez, M.A.; Becze, L.; Celikin, M.; Demopoulos, G.P. The effect of copper on the precipitation of scorodite (FeAsO4·2H2O) under hydrothermal conditions: Evidence for a hydrated copper containing ferric arsenate sulfate-short lived intermediate. J. Colloid Interface Sci. 2004, 360, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Monhemius, A.J.; Swash, P.M. Removing and stabilizing As from copper refining circuits by hydrothermal processing. J. Miner. Met. Mater. Soc. 1999, 51, 30–33. [Google Scholar] [CrossRef]
- Krause, E.; Ettel, V.A. Solubilities and stabilities of ferric arsenate compounds. Hydrometallurgy 1989, 122, 311–337. [Google Scholar] [CrossRef]
- Demopoulos, G.P.; Droppert, D.J.; Van Weert, G. Precipitation of crystalline scorodite (FeAsO4·2H2O) from chloride solutions. Hydrometallurgy 1995, 38, 245–261. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Jambor, J.L. The synthesis of crystalline scorodite, FeAsO4·2H2O. Hydrometallurgy 1988, 19, 377–384. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, H.; Liu, F.; Wang, C.; Zhang, Z.; Zhang, J. Hydrothermal treatment of arsenic sulfide slag to immobilize arsenic into scorodite and recycle sulfur. J. Hazard. Mater. 2021, 406, 124735. [Google Scholar] [CrossRef]
- Pozo, G.; Van Houtven, D.; Fransaer, J.; Dominguez-Benetton, X. Arsenic immobilization as crystalline scorodite by gas-diffusion electrocrystallization. React. Chem. Eng. 2009, 5, 1118–1128. [Google Scholar] [CrossRef]
- Gomez, M.A.; Becze, L.; Cutler, J.N.; Demopoulos, G.P. Hydrothermal reaction chemistry and characterization of ferric arsenate phases precipitated from Fe2(SO4)3-As2O5-H2SO4 solutions. Hydrometallurgy 2011, 107, 74–90. [Google Scholar] [CrossRef]
- Filippou, D.; Demopoulos, G.P. Arsenic immobilization by controlled scorodite precipitation. J. Miner. Met. Mater. Soc. 1997, 49, 52–55. [Google Scholar] [CrossRef]
- Gomez, M.A.; Becze, L.; Bluteau, M.C.; Le Berre, J.F.; Cutler, J.N.; Demopoulos, G.P. Autoclave precipitation and characterization of Fe(III)-AsO4-SO4 phases. In Proceedings of the Hydrometallurgy 2008: Proceedings of the 6th International Symposium, Phoenix, AZ, USA, 17–21 August 2008; pp. 1078–1086. [Google Scholar]
- Guo, F.; Wang, Q.; Demopoulos, G.P. Kinetics of iron (III)-catalyzed oxidation of arsenic (III) in acidic solutions with SO2/O2 gas mixture using different iron sources. Hydrometallurgy 2019, 189, 105130. [Google Scholar] [CrossRef]
- Song, K.Z.; Ke, P.C.; Liu, Z.Y.; Liu, Z.H. Co-oxidation of arsenic (III) and iron (II) ions by pressurized oxygen in acidic solutions. Int. J. Miner. Metall. Mater. 2020, 27, 181–189. [Google Scholar] [CrossRef]
- Marini, L.; Accornero, M. Prediction of the thermodynamic properties of metal-arsenate and metal-arsenite aqueous complexes to high temperatures and pressures and some geological consequences. Environ. Geol. 2007, 52, 1343–1364. [Google Scholar] [CrossRef]
- Lu, P.; Zhu, C. Arsenic Eh–pH diagrams at 25 °C and 1 bar. Environ. Earth Sci. 2011, 62, 1673–1683. [Google Scholar] [CrossRef]
- Zhang, P.; Li, C.; Wei, C.; Chu, M.; Zhang, J.; Deng, Z.; Li, M.; Li, X. Effects of zinc and copper ions on ferric arsenate precipitation in hydrothermal scorodite. J. Cent. South Univ. Sci. Technol. 2019, 50, 2645–2655. [Google Scholar] [CrossRef]
- Hidalgo, T.; Kuhar, L.; Beinlich, A.; Putnis, A. Kinetic study of chalcopyrite dissolution with iron (III) chloride in methanesulfonic acid. Miner. Eng. 2018, 125, 66–74. [Google Scholar] [CrossRef]
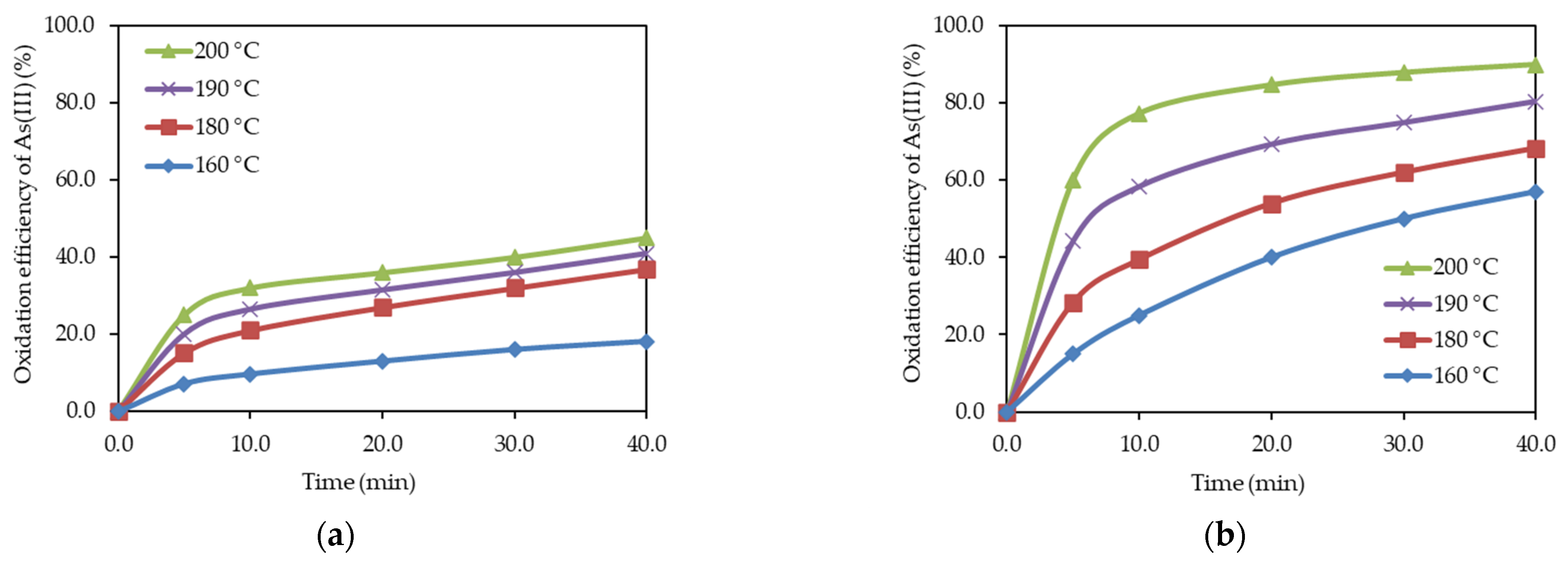

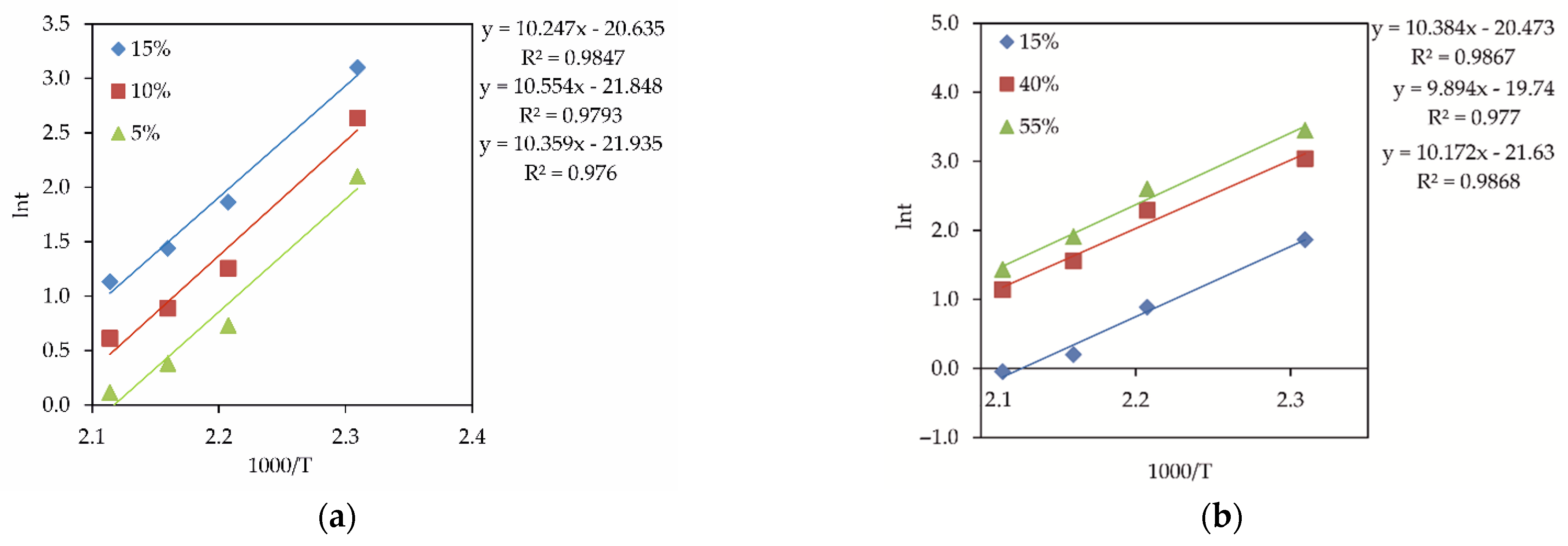
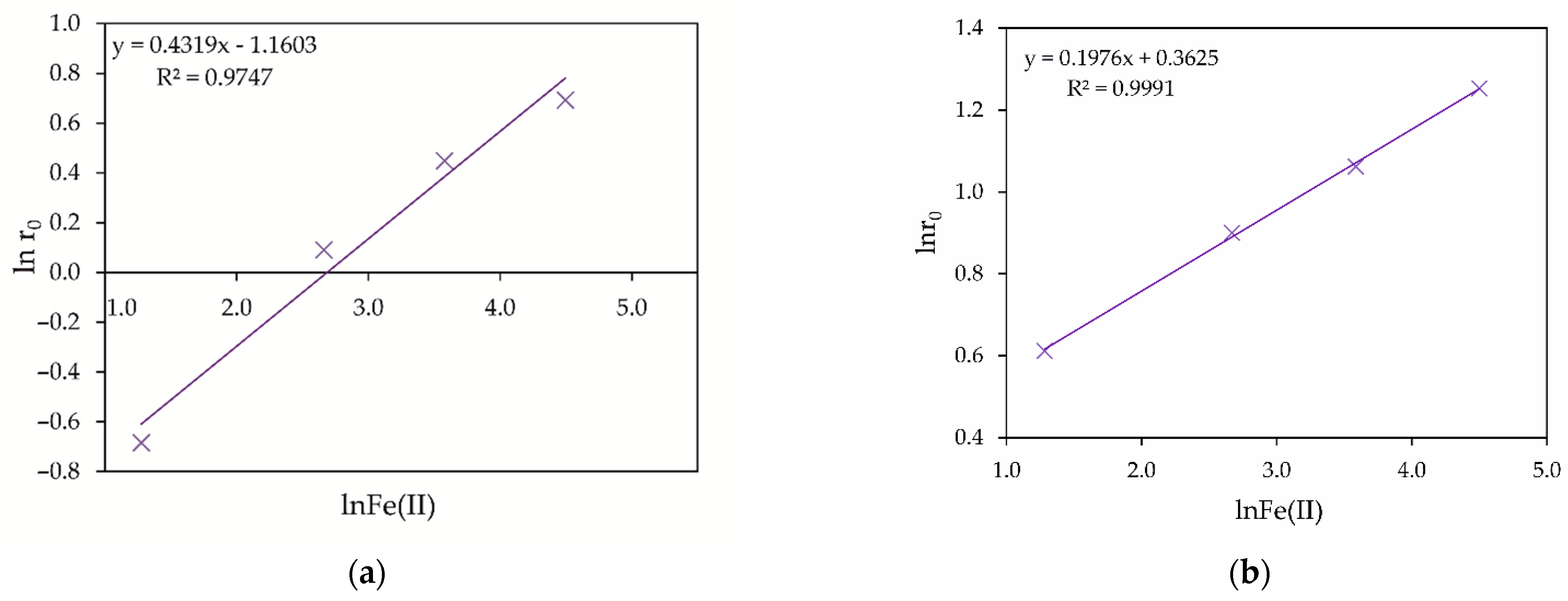
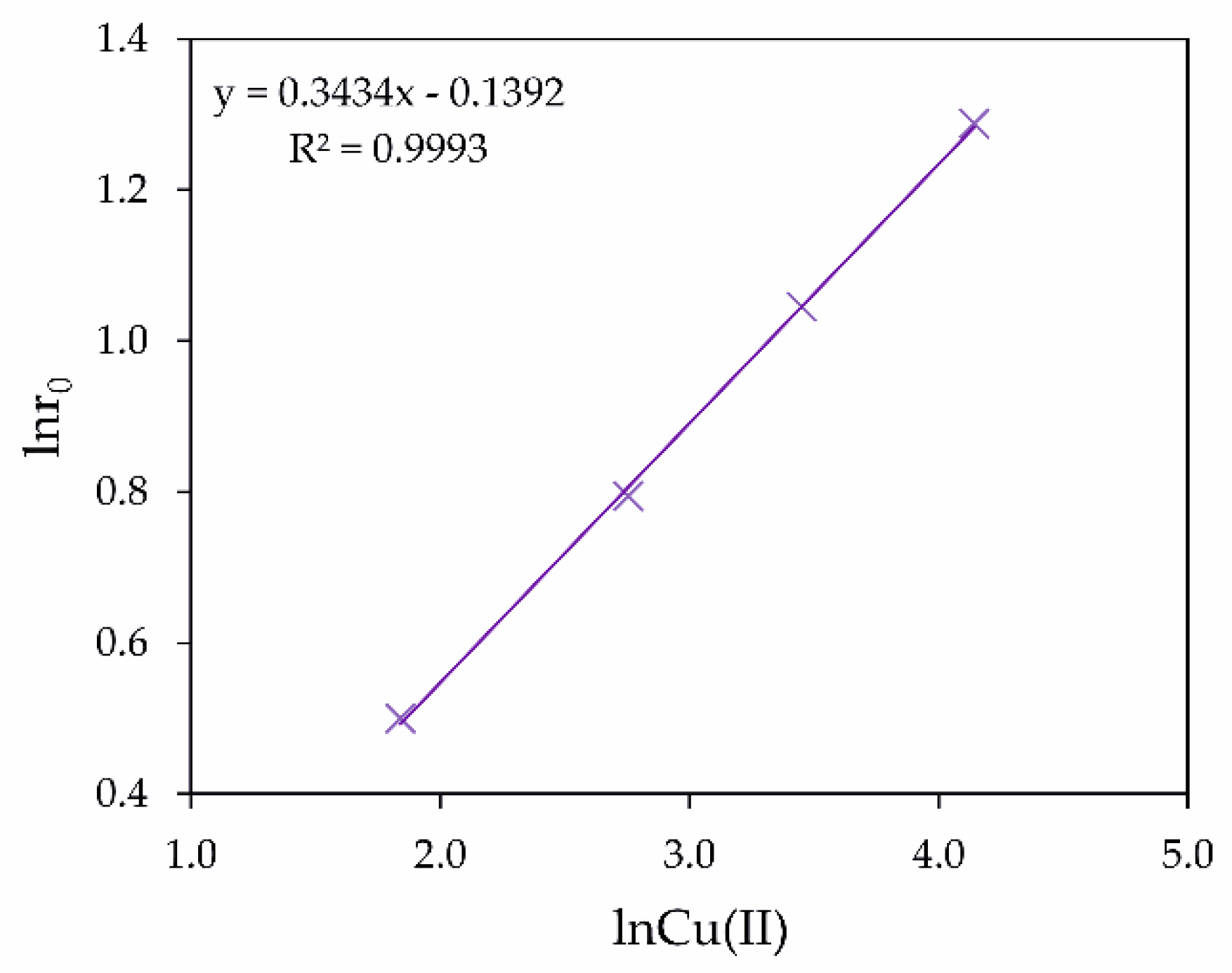

| No. | t (°C) | O2 (MPa) | Cu (g/dm3) | H2SO4 (g/dm3) | Oxidation Efficiency of As (III) |
|---|---|---|---|---|---|
| 1 | 150 | 0.5 | 0 | 4 | 2.1 |
| 2 | 180 | 0.5 | 0 | 4 | 5.5 |
| 3 | 200 | 0.5 | 0 | 4 | 7.2 |
| 4 | 150 | 1 | 0 | 20 | 3.4 |
| 5 | 180 | 1 | 0 | 20 | 6.2 |
| 6 | 200 | 1 | 0 | 20 | 9.1 |
| 7 | 150 | 1 | 4 | 10 | 3.8 |
| 8 | 180 | 1 | 4 | 10 | 6.9 |
| 9 | 200 | 1 | 4 | 10 | 8.9 |
| No. | Po2 (MPa) | Fe (II) (mmole/dm3) | t (K) | Cu (II) (mmole/dm3) | −dAs (III)/dτ |
|---|---|---|---|---|---|
| 1 | 0.2 | 90.01 | 433 | 0.00 | 0.14 |
| 2 | 0.2 | 90.01 | 463 | 0.00 | 1.92 |
| 3 | 0.2 | 3.58 | 453 | 0.00 | 0.12 |
| 4 | 0.2 | 35.81 | 453 | 0.00 | 1.02 |
| 5 | 0.3 | 90.01 | 453 | 0.00 | 2.16 |
| 6 | 0.4 | 90.01 | 453 | 0.00 | 2.23 |
| 7 | 0.2 | 90.01 | 453 | 6.29 | 1.30 |
| 8 | 0.2 | 90.01 | 453 | 31.47 | 2.25 |
| 9 | 0.2 | 90.01 | 433 | 62.95 | 1.24 |
| 10 | 0.2 | 90.01 | 463 | 62.95 | 4.91 |
| 11 | 0.2 | 3.58 | 453 | 62.95 | 0.98 |
| 12 | 0.2 | 35.81 | 453 | 62.95 | 2.58 |
| 13 | 0.3 | 90.01 | 453 | 62.95 | 3.98 |
| 14 | 0.4 | 90.01 | 453 | 62.95 | 5.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimov, K.; Rogozhnikov, D.; Dizer, O.; Tretiak, M.; Mamyachenkov, S.; Naboichenko, S. Pressure Oxidation of Arsenic (III) Ions in the H3AsO3-Fe2+-Cu2+-H2SO4 System. Metals 2021, 11, 975. https://doi.org/10.3390/met11060975
Karimov K, Rogozhnikov D, Dizer O, Tretiak M, Mamyachenkov S, Naboichenko S. Pressure Oxidation of Arsenic (III) Ions in the H3AsO3-Fe2+-Cu2+-H2SO4 System. Metals. 2021; 11(6):975. https://doi.org/10.3390/met11060975
Chicago/Turabian StyleKarimov, Kirill, Denis Rogozhnikov, Oleg Dizer, Maksim Tretiak, Sergey Mamyachenkov, and Stanislav Naboichenko. 2021. "Pressure Oxidation of Arsenic (III) Ions in the H3AsO3-Fe2+-Cu2+-H2SO4 System" Metals 11, no. 6: 975. https://doi.org/10.3390/met11060975
APA StyleKarimov, K., Rogozhnikov, D., Dizer, O., Tretiak, M., Mamyachenkov, S., & Naboichenko, S. (2021). Pressure Oxidation of Arsenic (III) Ions in the H3AsO3-Fe2+-Cu2+-H2SO4 System. Metals, 11(6), 975. https://doi.org/10.3390/met11060975






