Optimization on Temperature Strategy of BOF Vanadium Extraction to Enhance Vanadium Yield with Minimum Carbon Loss
Abstract
1. Introduction
2. Experimental Procedure
3. Results
4. Discussions
4.1. V andC Removal in Various Temperatures
4.2. Thermodynamic Analyses of Vanadium Extraction
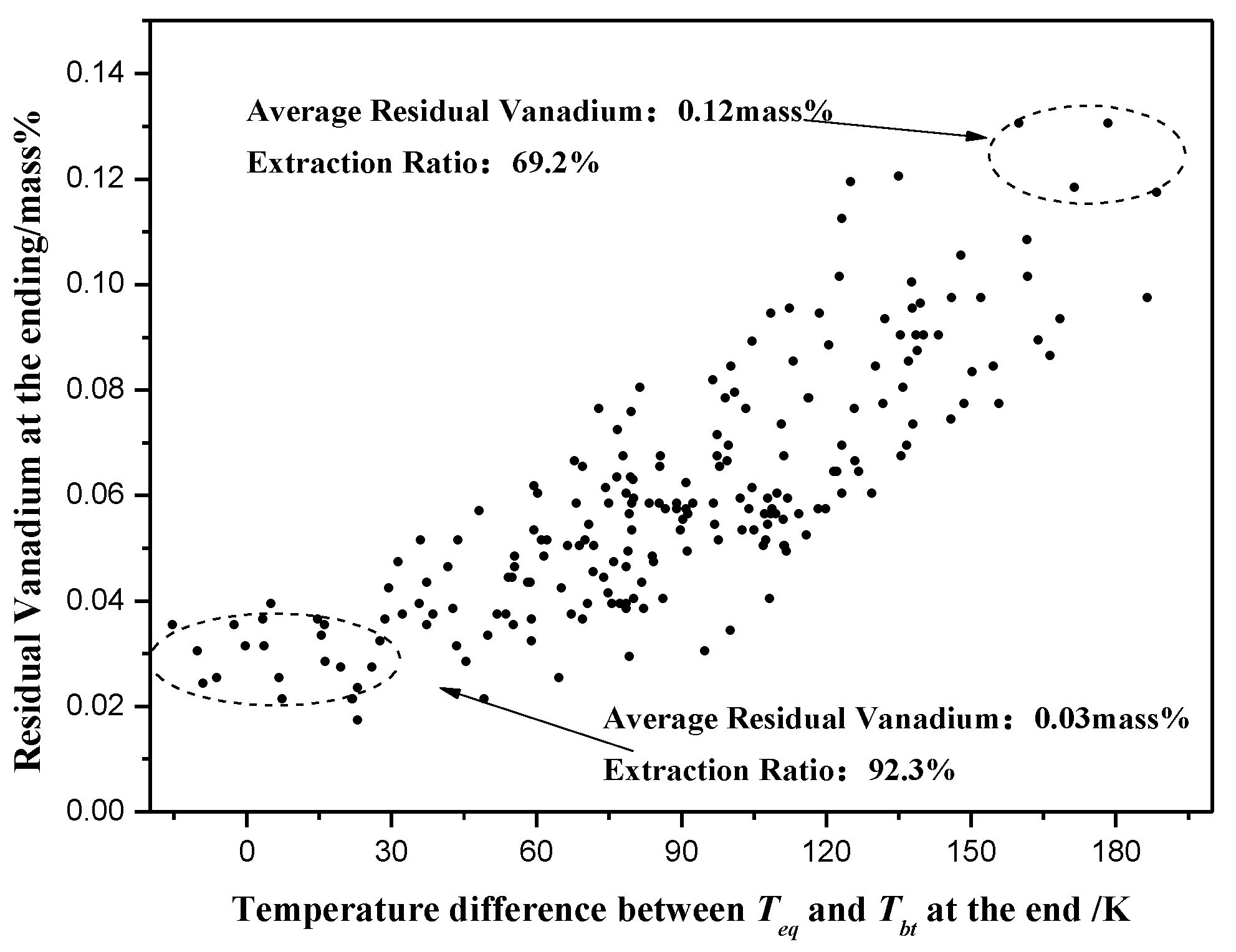
4.3. Applicability Analyses of Teq for the Final Temperature
4.3.1. Final Samples
4.3.2. Production Data
5. Conclusions
- (1)
- A temperature strategy of vanadium extraction process has been developed to consider the effects of Tsl in the earlier smelting and Teq for the high-limit temperature to ensure ‘enhance vanadium removal’ with ‘minimum carbon loss’.
- (2)
- The vanadium removal rate was highly efficient accompanied by slight carbon removal when the molten bath temperature was lower than Tsl. The removal rate of vanadium decreased and carbon removal increased when the molten bath temperature exceeded Tsl.
- (3)
- Vanadium removal efficiency remained poor while significant carbon loss was expected when the melt temperature went over Teq, and Teq must be considered as the high-limit temperature in vanadium extraction.
- (4)
- With the optimized temperature strategy, vanadium removal increased from 69.2 wt% to 92.3 wt% with a promotion by 23 wt% by production data.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edward, J.; Maxim, I.; Kenneth, M. Reduction of Vanadium(V) by Iron(II)-Bearing Minerals. Minerals 2021, 11, 316. [Google Scholar] [CrossRef]
- Lidiane, M.A.; Heide, M.; Gaebler, D.J.; Silva, W.E.; Belian, M.F.; Lira, E.C.; Crans, D.C. Acute Toxicity Evaluation of Non-Innocent Oxidovanadium(V) Schiff Base Complex. Inorganics 2021, 9, 42. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, W.; Xue, Z.L. An Environment-Friendly Process Featuring Calcified Roasting and Precipitation Purification to Prepare Vanadium Pentoxide from the Converter Vanadium Slag. Metals 2019, 9, 21. [Google Scholar] [CrossRef]
- Huang, D.X. Vanadium Extracting and Steelmaking; Metallurgical Industry Press: Beijing, China, 2010; pp. 52–53. [Google Scholar]
- Hong, Y.; Peng, J.; Sun, Z.; Yu, Z.; Wang, A.; Wang, Y.; Liu, Y.-Y.; Xu, F.; Sun, L.-X. Transition Metal Oxodiperoxo Complex Modified Metal-Organic Frameworks as Catalysts for the Selective Oxidation of Cyclohexane. Materials 2020, 13, 829. [Google Scholar] [CrossRef] [PubMed]
- Condruz, M.R.; Matache, G.; Paraschiv, A.; Badea, T.; Badilita, V. High Temperature Oxidation Behavior of Selective Laser Melting Manufactured IN 625. Metals 2020, 10, 668. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Xin, W.A.N.G.; Pei, G.S.; Huang, Q.Y.; Lü, X.W. Recovery of vanadium from vanadium slag by composite roasting with CaO/MgO and leaching. Trans. Nonferrous Metals Soc. China 2020, 30, 3114. [Google Scholar] [CrossRef]
- Penz, F.M.; Schenk, J.; Ammer, R.; Klösch, G.; Pastucha, K.; Reischl, M. Diffusive Steel Scrap Melting in Carbon-Saturated Hot Metal-Phenomenological Investigation at the Solid-Liquid Interface. Materials 2019, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Q.; Xie, B.; Wang, Y.; Guan, T.; Cao, H.L.; Zeng, X.L. Reaction of FeOV2O5 System at High Temperature. J. Iron Steel Res. Int. 2012, 19, 33. [Google Scholar] [CrossRef]
- Chen, S.K.; Cai, Z.J. An Improved Dynamic Model of the Vanadium Extraction Process in Steelmaking Converters. Appl. Sci. 2020, 10, 111. [Google Scholar] [CrossRef]
- Zhou, C.G.; Li, J.; Wu, H. Dependence of Temperature and Slag Composition on Dephosphorization at the First Deslagging in BOF Steelmaking Process. High Temp. Mater. Process. 2014, 49, 24. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, H.J. Converter practice in China with respect to steelmaking and ferroalloys. Miner. Process. Extr. Metall. 2019, 128, 46. [Google Scholar] [CrossRef]
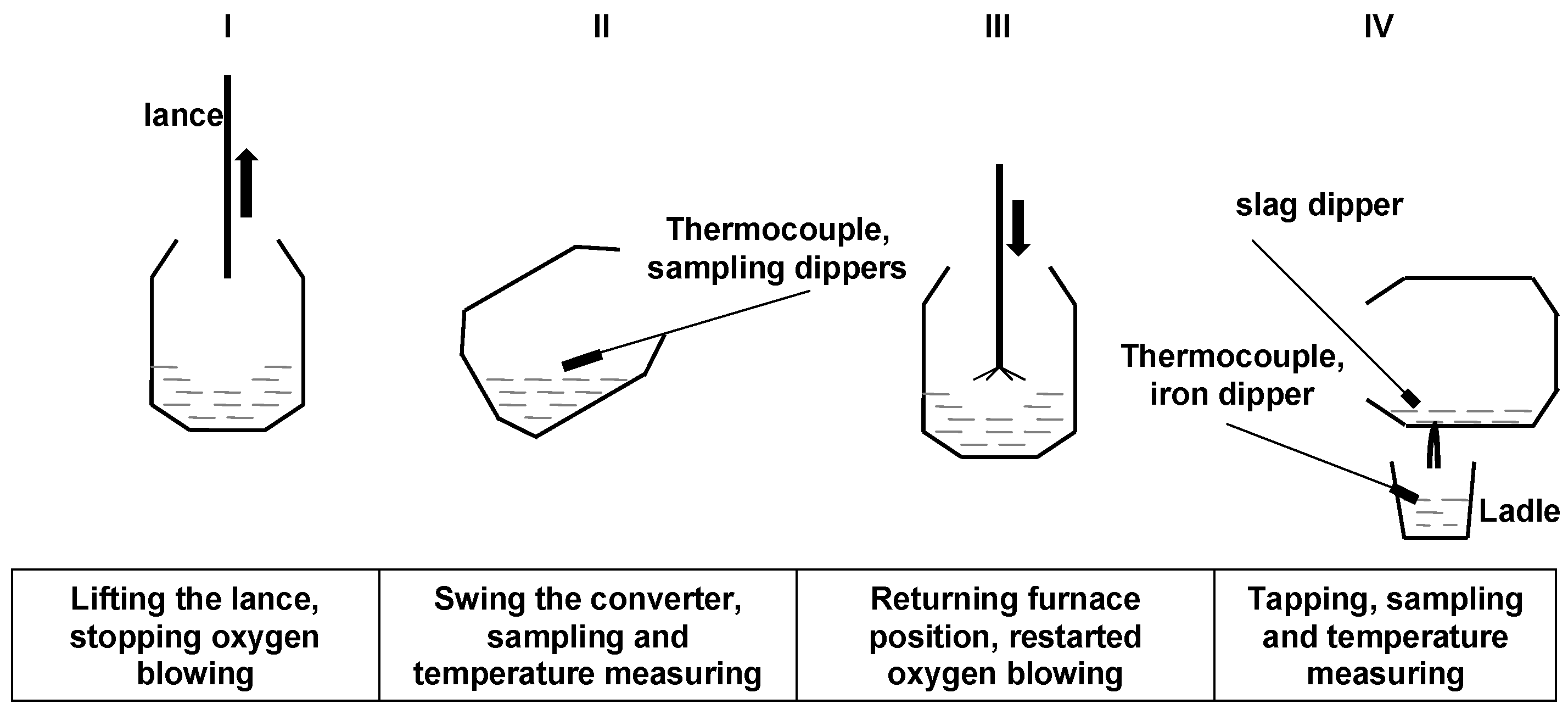



| Temperature/°C | Composition (wt%) | ||||
|---|---|---|---|---|---|
| C | V | Si | Mn | Ti | |
| 1294 ± 6 | 4.35 ± 0.05 | 0.39 ± 0.03 | 0.11 ± 0.02 | 0.22 ± 0.02 | 0.12 ± 0.02 |
| Furnace Capacity/t | Lance Height/m | Top Flow Rate (Nm3/h) | Bottom Flow Rate (Nm3/h) | Number of Nozzle | Ma | Blowing Time/s | Coolant (Kg/t) |
|---|---|---|---|---|---|---|---|
| 210 | 1.6~1.9 | 24,000 | 1000 | 4 | 1.99 | ~360 | 41.6 ± 0.6 |
| NO. | Time/s | C | V | Si | Mn | Ti | Tbt/(°C) |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 4.31 | 0.39 | 0.11 | 0.22 | 0.12 | 1294 |
| 1#-1 | 210 | 4.07 | 0.168 | 0.044 | 0.096 | 0.011 | 1318 |
| 1#-2 | 240 | 4.0 | 0.137 | 0.030 | 0.060 | 0.008 | 1320 |
| 1#-3 | 252 | 3.82 | 0.115 | 0.019 | 0.054 | 0.006 | 1337 |
| 1#-4 | 282 | 3.60 | 0.087 | 0.018 | 0.046 | 0.006 | 1342 |
| 1#-5 | 282 | 3.66 | 0.094 | 0.022 | 0.049 | 0.004 | 1348 |
| 1#-6 | 306 | 3.59 | 0.078 | 0.010 | 0.034 | 0.003 | 1349 |
| 2#-1 * | 366 | 3.54 | 0.051 | 0.006 | 0.026 | 0.001 | 1376 |
| 2#-2 * | 366 | 3.45 | 0.042 | 0.009 | 0.023 | 0.001 | 1382 |
| 2#-3 * | 366 | 3.51 | 0.06 | 0.010 | 0.030 | 0.002 | 1378 |
| 2#-4 * | 372 | 3.61 | 0.053 | 0.007 | 0.027 | 0.001 | 1394 |
| 2#-5 * | 378 | 3.45 | 0.054 | 0.005 | 0.026 | 0.001 | 1382 |
| 2#-6 * | 390 | 3.49 | 0.06 | 0.006 | 0.024 | 0.001 | 1379 |
| NO. | Time/s | V2O3 | FeO | SiO2 | MnO | TiO2 | CaO | MgO |
|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 1.6 | 90.0 | 2 | 2 | 2 | 1.5 | 1.5 |
| 1#-1 | 210 | 8.2 | 70.6 | 4.4 | 4.4 | 5.8 | 1.6 | 1.4 |
| 1#-2 | 240 | 10.3 | 65.5 | 5.6 | 5.9 | 7.8 | 1.9 | 1.6 |
| 1#-3 | 252 | 10.7 | 62.5 | 5.9 | 6.7 | 8.6 | 1.9 | 1.5 |
| 1#-4 | 282 | 11.1 | 56.5 | 7.2 | 8 | 9.6 | 1.7 | 1.5 |
| 1#-5 | 282 | 11.5 | 54.4 | 8.4 | 8 | 9.2 | 2.0 | 1.7 |
| 1#-6 | 306 | 14.0 | 48.6 | 9.2 | 8.5 | 10.0 | 1.8 | 1.9 |
| 2#-1 * | 366 | 16.2 | 32.4 | 10.2 | 9.8 | 10.5 | 2.0 | 2.0 |
| 2#-2 * | 366 | 17.3 | 34.5 | 10.4 | 10.2 | 11.2 | 2.4 | 1.8 |
| 2#-3 * | 366 | 16.5 | 33.4 | 9.8 | 10.4 | 10.0 | 1.8 | 2.2 |
| 2#-4 * | 372 | 15.7 | 35.4 | 10.2 | 10.2 | 11.4 | 2.0 | 1.8 |
| 2#-5 * | 378 | 16.5 | 30.9 | 10.6 | 11.0 | 10.8 | 2.2 | 2.4 |
| 2#-6 * | 390 | 16.5 | 32.6 | 10.2 | 10.6 | 10.6 | 2.4 | 2.0 |
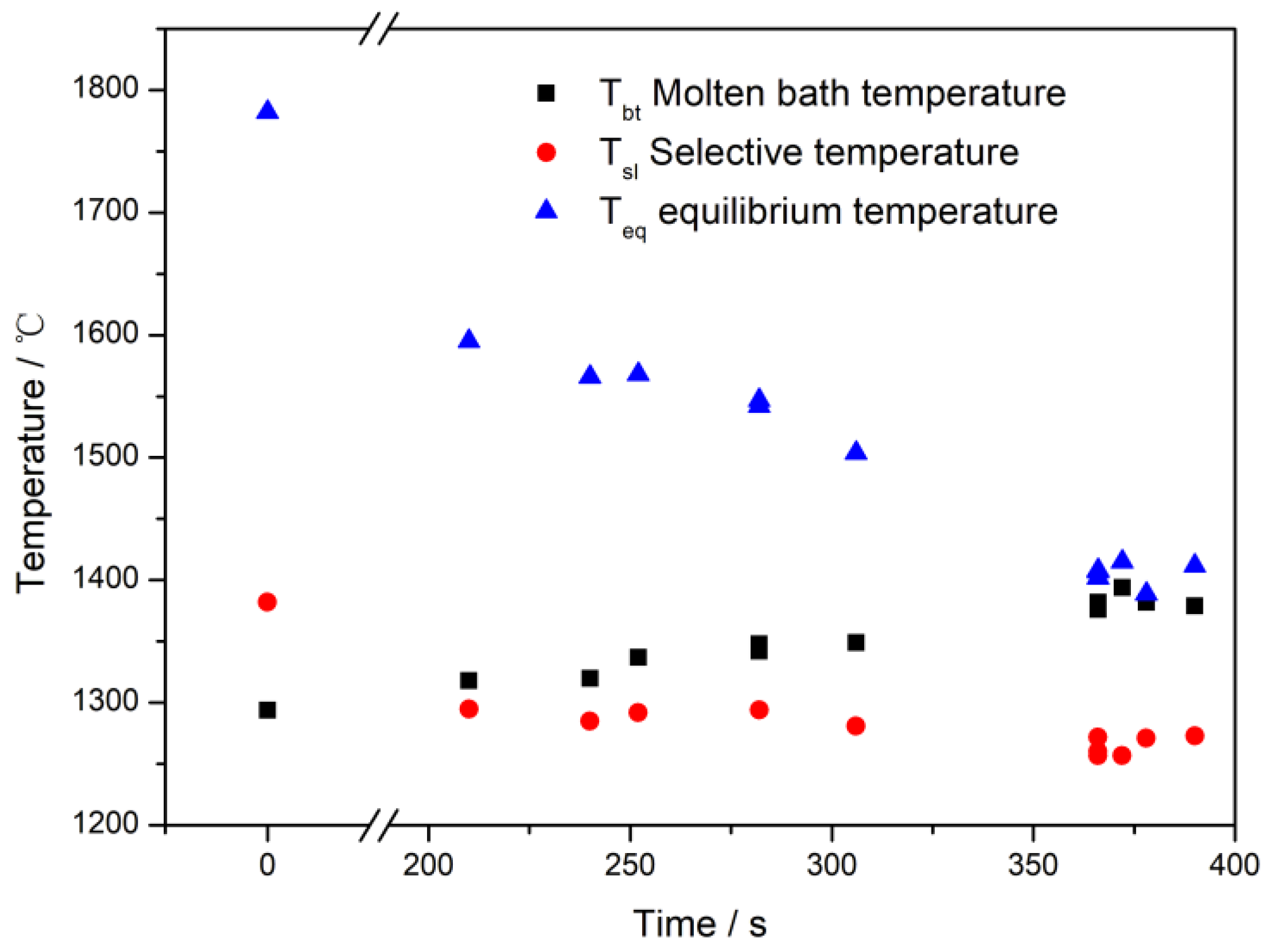
| NO. | Time/s | V | V Removal Ratio/wt% | C | C Removal Ratio/wt% | Tbt/(°C) | Tsl/(°C) | Teq/(°C) |
|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0.39 | - | 4.31 | 0.0 | 1294 | 1382 | 1782 |
| 1#-1 | 210 | 0.168 | 56.9 | 4.07 | 5.6 | 1318 | 1295 | 1595 |
| 1#-2 | 240 | 0.137 | 64.9 | 4.0 | 7.2 | 1320 | 1285 | 1566 |
| 1#-3 | 252 | 0.115 | 70.5 | 3.82 | 11.4 | 1337 | 1292 | 1568 |
| 1#-4 | 282 | 0.087 | 77.7 | 3.60 | 16.5 | 1342 | 1294 | 1547 |
| 1#-5 | 282 | 0.094 | 75.9 | 3.66 | 15.1 | 1348 | 1294 | 1542 |
| 1#-6 | 306 | 0.078 | 80.0 | 3.59 | 16.7 | 1349 | 1281 | 1504 |
| 2#-1 * | 366 | 0.051 | 86.9 | 3.54 | 17.9 | 1376 | 1260 | 1407 |
| 2#-2 * | 366 | 0.042 | 89.2 | 3.45 | 20.0 | 1382 | 1257 | 1402 |
| 2#-3 * | 366 | 0.06 | 84.6 | 3.51 | 18.6 | 1378 | 1272 | 1408 |
| 2#-4 * | 372 | 0.053 | 86.4 | 3.61 | 16.2 | 1394 | 1257 | 1415 |
| 2#-5 * | 378 | 0.054 | 86.2 | 3.45 | 20.0 | 1382 | 1271 | 1389 |
| 2#-6 * | 390 | 0.06 | 84.6 | 3.49 | 19.0 | 1379 | 1273 | 1412 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.-Y.; Tang, P. Optimization on Temperature Strategy of BOF Vanadium Extraction to Enhance Vanadium Yield with Minimum Carbon Loss. Metals 2021, 11, 906. https://doi.org/10.3390/met11060906
Zhou Z-Y, Tang P. Optimization on Temperature Strategy of BOF Vanadium Extraction to Enhance Vanadium Yield with Minimum Carbon Loss. Metals. 2021; 11(6):906. https://doi.org/10.3390/met11060906
Chicago/Turabian StyleZhou, Zhen-Yu, and Ping Tang. 2021. "Optimization on Temperature Strategy of BOF Vanadium Extraction to Enhance Vanadium Yield with Minimum Carbon Loss" Metals 11, no. 6: 906. https://doi.org/10.3390/met11060906
APA StyleZhou, Z.-Y., & Tang, P. (2021). Optimization on Temperature Strategy of BOF Vanadium Extraction to Enhance Vanadium Yield with Minimum Carbon Loss. Metals, 11(6), 906. https://doi.org/10.3390/met11060906






