Study on the Extraction and Separation of Zinc, Cobalt, and Nickel Using Ionquest 801, Cyanex 272, and Their Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solution Preparation
2.2. Metal Extraction pH Isotherms
3. Results and Discussion
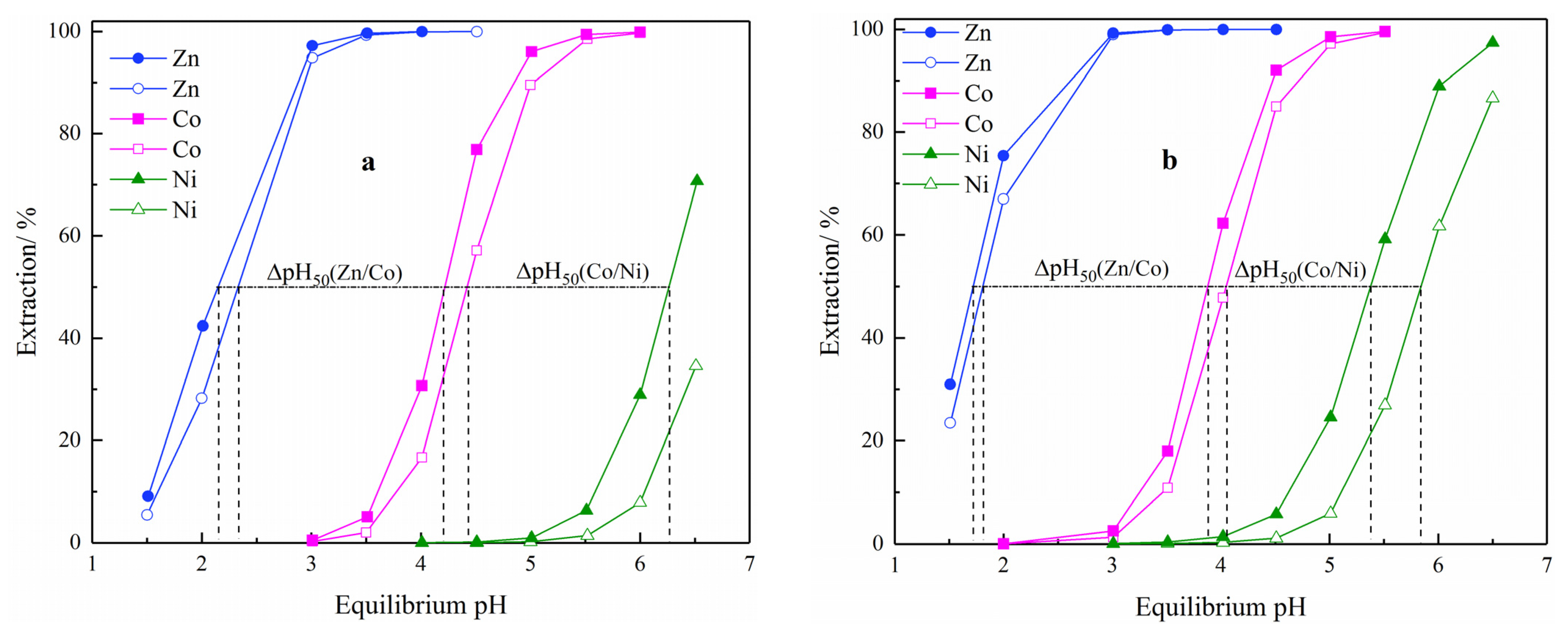
3.1. Metal Extraction pH Isotherms of Cyanex 272 and Ionquest 801
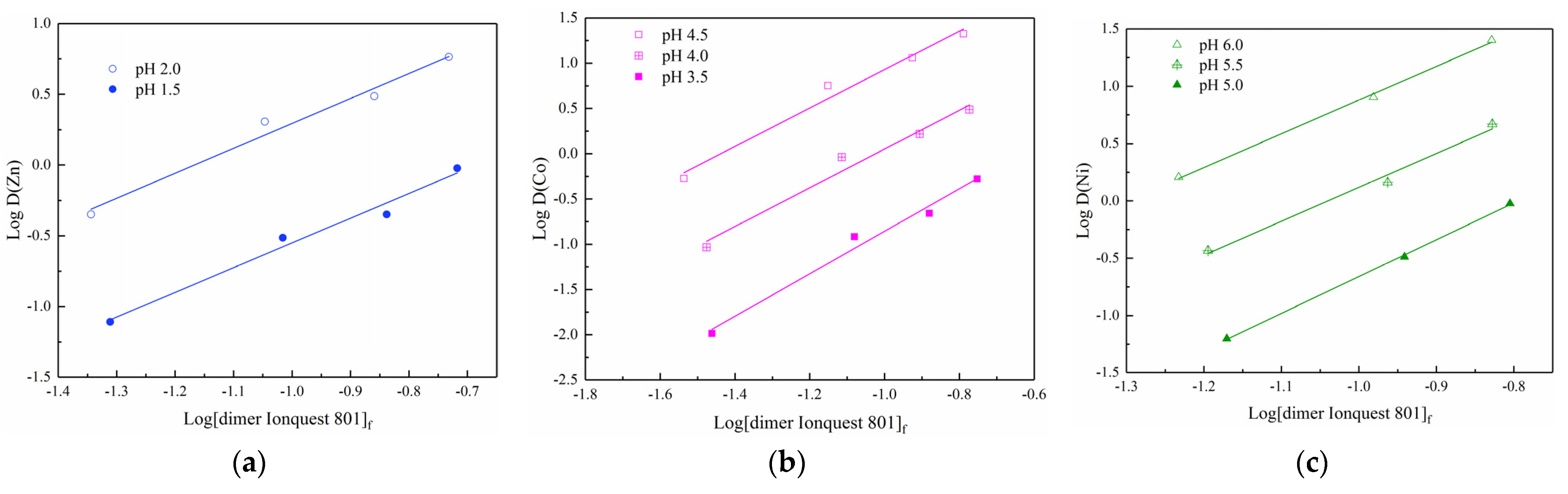
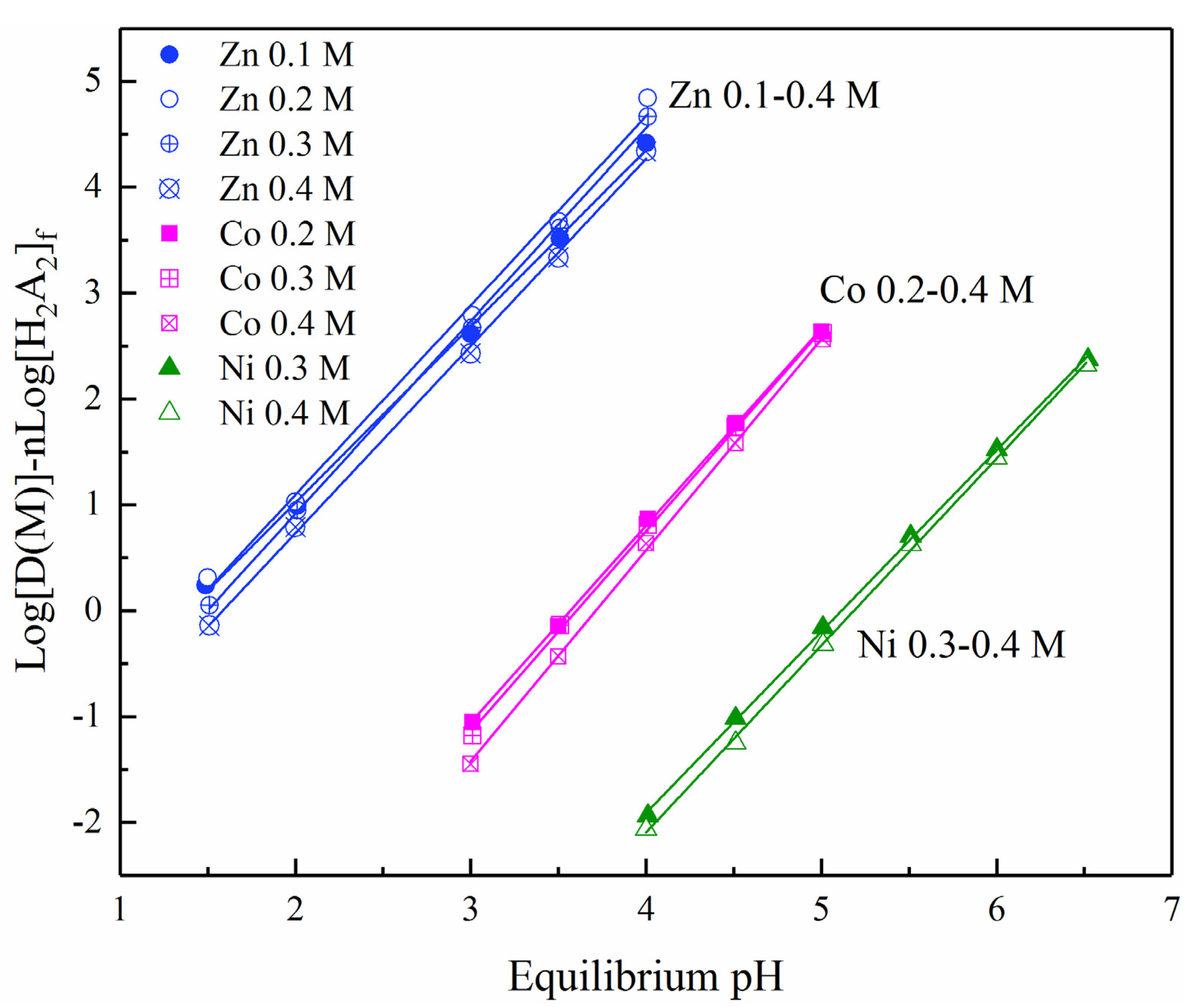
3.2. Metal Extraction Analysis
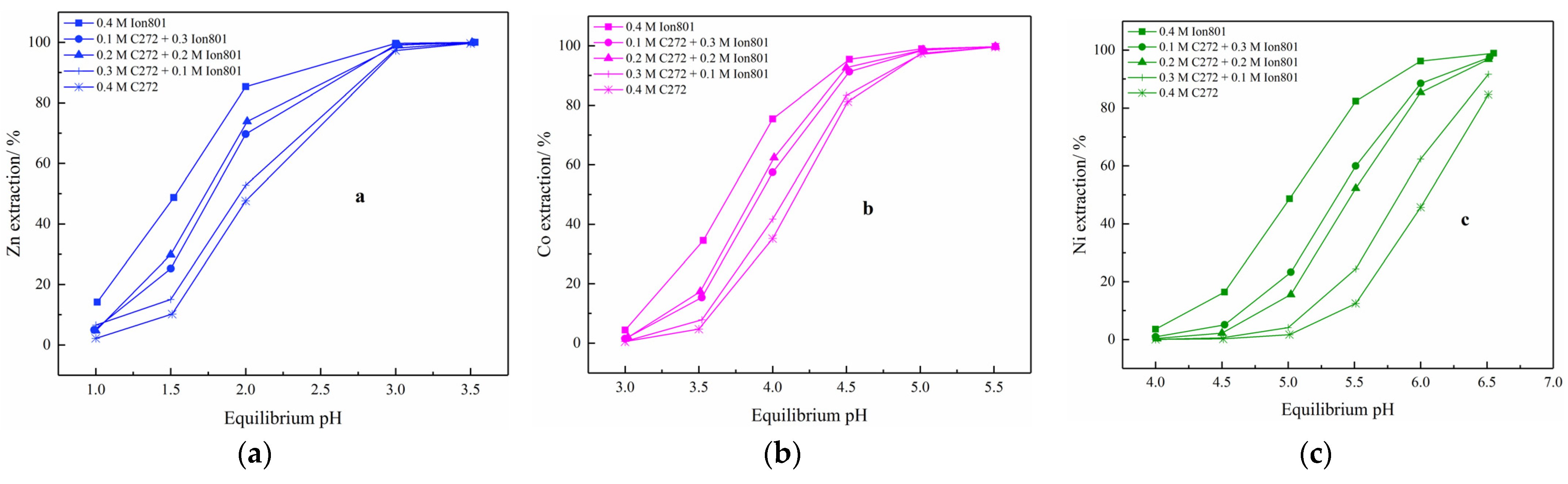
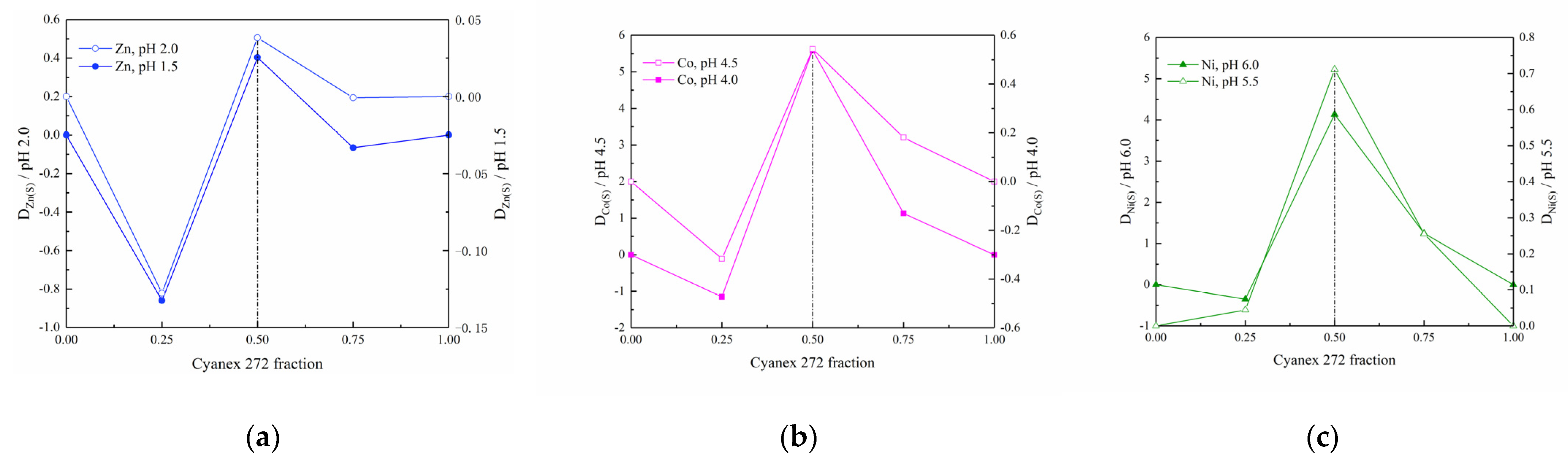
3.3. Synergistic Effect of Cyanex 272 and Ionquest 801
3.4. Discussion of Cyanex 272 and Ionquest 801 Application
- In terms of zinc extraction and separation from cobalt and nickel, Ionquest 801 performed similarly to or even better than Cyanex 272.
- Cyanex 272 is much superior to Ionquest 801 for cobalt and nickel separation, while usually having one magnitude order larger separation factors comparatively. Cyanex 272 would be a preferred selection for cobalt and nickel separation.
- Using low concentration of Ionqest 801 to reduce the free extractant availability during the metal extraction, very good separation of cobalt from nickel is also available with the separation factor over 1000, which is comparable to those using Cyanex 272 as discussed before. Therefore, more rigid concentration control is required if Ionquest 801 is used for cobalt and nickel separation by taking its advantage to lower the cost.
- To separate zinc, cobalt, and nickel in an integral process, if Ionquest 801 is selected in the first solvent circuit to separate zinc and it is followed by Cyanex 272 to separate cobalt, leaving nickel in the raffinate. Ionquest 801 could contaminate Cyanex 272 by the phase carryover. However, as discussed previously, very good cobalt and nickel separation is still available when a small amount of Ionquest 801 mixed in the Cyanex 272 system.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Pranolo, Y.; Zhang, W.S.; Cheng, C.Y. Separation of cobalt and zinc from concentrated nickel sulphate solutions with Cyanex 272. J. Chem. Technol. Biotechnol. 2011, 86, 75–81. [Google Scholar] [CrossRef]
- Mishra, R.K.; Rout, P.C.; Sarangi, K.; Nathsrma, K.C. Solvent extraction of zinc, manganese, cobalt and nickel from nickel laterite bacterial leach liquor using sodium salts of TOPS-99 and Cyanex 272. Trans. Nonferrous Met. Soc. China 2016, 26, 301–309. [Google Scholar] [CrossRef]
- Senapati, D.; Chaudhury, G.R.; Sarma, P.V.R.B. Purification of nickel sulphate solutions containing iron, copper, cobalt, zinc and manganese. J. Chem. Technol. Biotechnol. 1994, 59, 335–339. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Ichlas, Z.T.; Kaya, M. Solvent extraction process for the recovery of nickel and cobalt from Caldag laterite leach solution: The first bench scale study. Hydrometallurgy 2017, 169, 135–141. [Google Scholar] [CrossRef]
- Ichlas, Z.T.; Ibana, D.C. Process development for the direct solvent extraction of nickel and cobalt from nitrate solution: Aluminum, cobalt, and nickel separation using Cyanex 272. Int. J. Miner. Metall. Mater. 2017, 24, 37–46. [Google Scholar] [CrossRef]
- Donegan, S. Direct solvent extraction of nickel at Bulong operations. Miner. Eng. 2006, 19, 1234–1245. [Google Scholar] [CrossRef]
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, G.; Davenport, W.G. Hydrometallurgical Production of High-Purity Nickel and Cobalt. In Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Elsevier: Radarweg, The Netherlands, 2011. [Google Scholar]
- Parhi, P.K.; Padhan, E.; Palai, A.K.; Sarangi, K.; Nathsarma, K.C.; Park, K.H. Separation of Co (II) and Ni (II) from the mixed sulphate/chloride solution using NaPC-88A. Desalination 2011, 267, 201–208. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.H.; Guo, S.H.; Zhang, L.B. Extraction and separation of cobalt from nickel by P507 microemulsion liquid membrane. Nonferrous Met. Eng. 2016, 6, 40–44. [Google Scholar]
- Liu, M.X.; Shen, Y.Y.; Shao, Q.T.; Wu, Z.M.; Zhong, S.H. Study on mixed phase disengagement of cobalt and nickel by extraction with P 507. Biol. Chem. Eng. 2016, 2, 1–3. [Google Scholar]
- Wang, J.K.; Gao, K.; Xu, W.X.; Wang, Z.J.; Guo, R.; Zhou, Y.; Li, J. Study on extraction and separation of nickel and cobalt with P 507. Nonferrous Met. 2018, 8, 19–22. [Google Scholar]
- Wu, J.H.; Dong, B.; Zhang, X.P.; Ye, F.C.; Wang, H.J.; Ji, H.W.; Guo, F.Y.; Qiu, S.W.; Liu, Z.D. Solvent extraction of Cu, Zn, Co from nickel sulphate solution applying P507. Nonferrous Met. Sci. Eng. 2018, 9, 19–24. [Google Scholar]
- Flett, D.S. Cobalt-Nickel separation in hydrometallurgy: A review. Chem. Sustain. Dev. 2004, 12, 81–91. [Google Scholar]
- Flett, D.S. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants. J. Organomet. Chem. 2005, 690, 2426–2438. [Google Scholar] [CrossRef]
- Thakur, N.V. Extraction studies of base metals (Mn, Cu, Co and Ni) using the extractant 2-ethylhexyl 2-ethylhexyl phosphonic acid, PC 88A. Hydrometallurgy 1998, 48, 125–131. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini-Leonardou, S. Simultaneous solvent extraction of cobalt and magnesium in the presence of nickel from sulfate solutions by Ionquest 801. J. Chem. Technol. Biotechnol. 2005, 80, 1236–1243. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Zhang, J.; Yi, A.F.; Su, H.; Wang, L.N.; Qi, T. Magnesium removal from concentrated nickel solution by solvent extraction using Cyanex 272. Int. J. Miner. Process. Ext. Metall. 2019, 4, 36–43. [Google Scholar]
- Preston, J.S.; du Preez, A.C. Solvent extraction of nickel from acidic solutions using synergistic mixtures containing pyridinecarboxylate esters, Part 1: Systems based on organophosphorus acids. J. Chem. Technol. Biotechnol. 1996, 66, 86–94. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Barnard, K.R.; Zhang, W.S.; Zhu, Z.W.; Pranolo, Y. Recovery of nickel, cobalt, copper and zinc in sulphate and chloride solutions using synergistic solvent extraction. Chin. J. Chem. Eng. 2016, 24, 237–248. [Google Scholar] [CrossRef]
- Sulaiman, R.N.R.; Othman, N. Synergistic green extraction of nickel ions from electroplating waste via mixtures of chelating and organophosphorus carrier. J. Hazard. Mater. 2017, 340, 77–84. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, L.M.; Huang, S.T.; Xu, Z.; Wang, W. Synergistic solvent extraction of nickel by 2-hydroxy-5-nonylacetophenone oxime mixed with neodecanoic acid and bis(2-ethylhexyl) phosphoric acid: Stoichiometry and structure investigation. Miner. Eng. 2019, 132, 284–292. [Google Scholar] [CrossRef]
- Zhao, J.M.; Shen, X.Y.; Deng, F.L.; Wang, F.C.; Wu, Y.; Liu, H.Z. Synergistic extraction and separation of valuable metals from waste cathodic material of lithium ion batteries using Cyanex272 and PC-88A. Sep. Purif. Technol. 2011, 78, 345–351. [Google Scholar] [CrossRef]
- Liu, M.R.; Zhou, G.Y.; Wen, J.K. Separation of divalent cobalt and nickel ions using a synergistic solvent extraction system with P507 and Cyanex272. Chin. J. Process Eng. 2012, 12, 415–419. [Google Scholar]
- Liu, T.C.; Chen, J.; Li, H.L.; Li, K.; Li, D.Q. Further improvement for separation of heavy rare earths by mixtures of acidic organophosphorus extractants. Hydrometallurgy 2019, 188, 73–80. [Google Scholar] [CrossRef]
- Quinn, J.E.; Soldenhoff, K.H.; Stevens, G.W.; Lengkeek, N.A. Solvent extraction of rare earth elements using phosphonic/phosphinic acid mixtures. Hydrometallurgy 2015, 157, 298–305. [Google Scholar] [CrossRef]
- Dreisinger, D.B.; Cooper, W.C. The solvent extraction separation of cobalt and nickel using 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester. Hydrometallurgy 1984, 12, 1–20. [Google Scholar] [CrossRef]
- Devi, N.B.; Nathsarma, K.C.; Chakravortty, V. Separation and recovery of cobalt (II) and nickel (II) from sulphate solutions using sodium salts of D2EHPA, PC 88A and Cyanex 272. Hydrometallurgy 1998, 49, 47–61. [Google Scholar] [CrossRef]
- Tait, B.K. Cobalt-nickel separation: The extraction of cobalt (II) and nickel (II) by Cyanex 301, Cyanex302 and Cyanex 272. Hydrometallurgy 1993, 32, 365–372. [Google Scholar] [CrossRef]
- Sarangi, K.; Reddy, B.R.; Das, R.P. Extraction studies of cobalt (II) and nickel (II) from chloride solutions using Na-Cyanex 272: Separation of Co(II)/Ni(II) by the sodium salts of D2EHPA, PC88A and Cyanex 272 and their mixtures. Hydrometallurgy 1999, 52, 253–265. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Rashchi, F.; Ghafarizadeh, B.; Mostoufi, N. Synergistic effect of D2EHPA and Cyanex 272 on separation of zinc and manganese by solvent extraction. Sep. Sci. Technol. 2011, 46, 2305–2312. [Google Scholar] [CrossRef]
- Begum, N.; Bari, F.; Jamaludin, S.B.; Hussin, K. Solvent extraction of copper, nickel and zinc by Cyanex 272. Int. J. Phys. Sci. 2012, 7, 2905–2910. [Google Scholar]
- Innocenzi, V.; Veglio, F. Separation of manganese, zinc and nickel from leaching solution of nickel-metal hydride spent batteries by solvent extraction. Hydrometallurgy 2012, 50, 50–58. [Google Scholar] [CrossRef]
- Devi, N.B.; Nathsarma, K.C.; Chakravortty, V. Sodium salts of D2EHPA, PC-88A and Cyanex-272 and their mixtures as extractants for cobalt (II). Hydrometallurgy 1994, 34, 331–342. [Google Scholar] [CrossRef]
- Darvishia, D.; Haghshenas, D.F.; Alamdari, E.K.; Sadrnezhaad, S.K.; Halali, M. Synergistic effect of Cyanex 272 and Cyanex 302 on separation of cobalt and nickel by D2EHPA. Hydrometallurgy 2005, 77, 227–238. [Google Scholar] [CrossRef]
- Renny, J.S.; Tomasevich, L.L.; Tallmadge, E.H.; Collom, D.B. Method of Continuous Variations: Applications of Job Plots to the Study of Molecular Associations in Organometallic Chemistry. Angew. Chem. Int. Ed. 2013, 52, 11998–12013. [Google Scholar] [CrossRef] [PubMed]


| Cyanex 272 Concentration (M) | pH50 | ∆pH50 | |||
|---|---|---|---|---|---|
| Zn | Co | Ni | Co-Zn | Ni-Co | |
| 0.1 | 2.64 | 5.10 | >6.5 | 2.46 | - |
| 0.2 | 2.32 | 4.42 | >6.5 | 2.10 | - |
| 0.3 | 2.15 | 4.21 | 6.28 | 2.06 | 2.07 |
| 0.4 | 2.05 | 4.15 | 6.06 | 2.10 | 1.91 |
| Ionquest 801 Concentration (M) | pH50 | ∆pH50 | |||
| Zn | Co | Ni | Co-Zn | Ni-Co | |
| 0.1 | 2.30 | 4.78 | >6.5 | 2.48 | - |
| 0.2 | 1.80 | 4.05 | 5.85 | 2.25 | 1.80 |
| 0.3 | 1.71 | 3.89 | 5.40 | 2.18 | 1.51 |
| 0.4 | 1.55 | 3.70 | 2.15 | 1.35 | |
| Cyanex 272 Concentration (M) | Co Extraction (%) | SFCo/Ni | Metal Loaded Organic (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 5.0 | pH 5.5 | pH 6.0 | pH 5.0 | pH 5.5 | pH 6.0 | pH 5.0 | pH 5.5 | pH 6.0 | |
| 0.1 | 37.6 | 69.4 | 88.6 | 2973 | 6640 | 7163 | 42.9 | 53.3 | 59.3 |
| 0.2 | 89.5 | 98.6 | 99.7 | 4341 | 4725 | 4149 | 29.9 | 31.9 | 33.0 |
| 0.3 | 96.0 | 99.4 | 99.9 | 2514 | 2479 | 1888 | 20.4 | 21.3 | 23.8 |
| 0.4 | 97.5 | 99.6 | 99.9 | 2314 | 1630 | 1126 | 17.1 | 18.0 | 21.0 |
| Ionquest 801 Concentration (M) | Co-Extraction (%) | SFCo/Ni | Metal Loaded Organic (%) | ||||||
| pH 5.0 | pH 5.5 | pH 6.0 | pH 5.0 | pH 5.5 | pH 6.0 | pH 5.0 | pH 5.5 | pH 6.0 | |
| 0.1 | 64.1 | 85.3 | 96.4 | 4938 | 7313 | 6103 | 51.9 | 59.0 | 62.8 |
| 0.2 | 97.2 | 99.4 | 99.9 | 549 | 459 | 279 | 32.5 | 36.2 | 41.5 |
| 0.3 | 98.5 | 99.6 | >99.9 | 207 | 179 | 98 | 23.6 | 27.3 | 30.5 |
| 0.4 | 99.0 | 99.8 | >99.9 | 104 | 87 | 48 | 21.0 | 24.5 | 25.1 |
| Metal | pH | n Value | |
|---|---|---|---|
| Cyanex 272 | Ionquest 801 | ||
| Zn | 3.0 | 1.79 | - |
| 2.0 | 1.71 | 1.76 | |
| 1.5 | 1.75 | ||
| Co | 5.0 | 2.29 | |
| 4.5 | 2.10 | 2.12 | |
| 4.0 | - | 2.14 | |
| 3.5 | - | 2.35 | |
| Ni | 6.5 | 2.71 | - |
| 6.0 | 2.74 | 2.94 | |
| 5.5 | - | 2.96 | |
| 5.0 | - | 3.21 | |
| Metal | Cyanex 272 Concentration (M) | Ionquest 801 Concentration (M) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | 0.1 | 0.2 | 0.3 | 0.4 | |
| Zn | 1.70 | 1.79 | 1.82 | 1.77 | 1.70 | 1.70 | 1.67 | - |
| Co | - | 1.78 | 1.86 | 1.99 | - | 1.69 | 1.63 | 1.58 |
| Ni | - | 1.68 | 1.69 | 1.77 | - | 1.54 | 1.49 | 1.51 |
| Organic Concentration (M) | pH50 | ∆pH50 | ||||
|---|---|---|---|---|---|---|
| Cyanex 272 | Ionquest 801 | Zn | Co | Ni | Co-Zn | Ni-Co |
| 0.4 | 0 | 2.05 | 4.15 | 6.06 | 2.10 | 1.91 |
| 0.3 | 0.1 | 1.96 | 4.10 | 5.84 | 2.14 | 1.74 |
| 0.2 | 0.2 | 1.74 | 3.89 | 5.48 | 2.15 | 1.59 |
| 0.1 | 0.3 | 1.79 | 3.92 | 5.38 | 2.13 | 1.46 |
| 0 | 0.4 | 1.55 | 3.70 | 5.05 | 2.15 | 1.35 |
| Organic Concentration (M) | SFCo/Ni | ||||
|---|---|---|---|---|---|
| Cyanex 272 | Ionquest 801 | pH 4.0 | pH 4.5 | pH 5.0 | pH 5.5 |
| 0.4 | 0 | 1421 | 2000 | 2314 | 1630 |
| 0.3 | 0.1 | 696 | 884 | 776 | 759 |
| 0.2 | 0.2 | 446 | 555 | 398 | 315 |
| 0.1 | 0.3 | 143 | 196 | 217 | 266 |
| 0 | 0.4 | 83 | 107 | 104 | 87 |
| Organic Concentration (M) | SCZn | SCCo | SCNi | ||||
|---|---|---|---|---|---|---|---|
| Cyanex 272 | Ionquest 801 | pH = 1.5 | pH = 2.0 | pH = 4.5 | pH = 5.5 | pH = 5.5 | 6.0 |
| 0.4 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 0.3 | 0.1 | 1.00 | 0.94 | 1.34 | 1.29 | 4.74 | 4.03 |
| 0.2 | 0.2 | 1.10 | 1.17 | 1.49 | 1.80 | 2.86 | 3.44 |
| 0.1 | 0.3 | 0.73 | 0.73 | 0.81 | 0.90 | 1.03 | 0.96 |
| 0 | 0.4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhang, J.; Xu, Z.; Liang, J.; Zhu, Z. Study on the Extraction and Separation of Zinc, Cobalt, and Nickel Using Ionquest 801, Cyanex 272, and Their Mixtures. Metals 2021, 11, 401. https://doi.org/10.3390/met11030401
Liu W, Zhang J, Xu Z, Liang J, Zhu Z. Study on the Extraction and Separation of Zinc, Cobalt, and Nickel Using Ionquest 801, Cyanex 272, and Their Mixtures. Metals. 2021; 11(3):401. https://doi.org/10.3390/met11030401
Chicago/Turabian StyleLiu, Wensen, Jian Zhang, Zhenya Xu, Jie Liang, and Zhaowu Zhu. 2021. "Study on the Extraction and Separation of Zinc, Cobalt, and Nickel Using Ionquest 801, Cyanex 272, and Their Mixtures" Metals 11, no. 3: 401. https://doi.org/10.3390/met11030401
APA StyleLiu, W., Zhang, J., Xu, Z., Liang, J., & Zhu, Z. (2021). Study on the Extraction and Separation of Zinc, Cobalt, and Nickel Using Ionquest 801, Cyanex 272, and Their Mixtures. Metals, 11(3), 401. https://doi.org/10.3390/met11030401





