Electrophoretic Deposition of a Hybrid Graphene Oxide/Biomolecule Coating Facilitating Controllable Drug Loading and Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
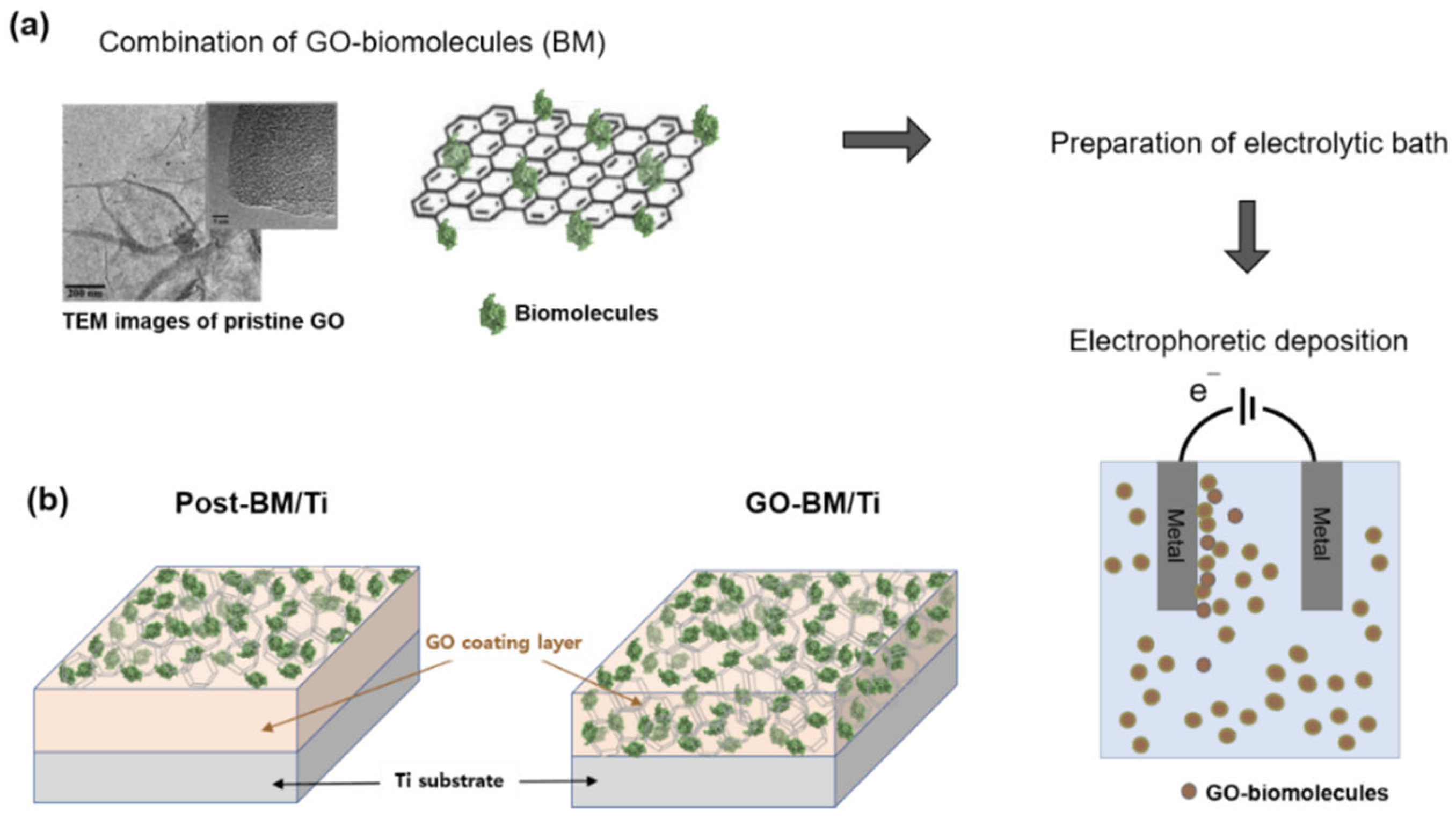
2.2. Electrophoretic GO Deposition
2.3. Characterization
2.4. BM Loading and Release
2.5. Cell Morphology
2.6. Alkaline Phosphatase (ALP) Activity
2.7. Alizarin Red S Staining to Detect Mineralization
2.8. Statistical Analysis
3. Results and Discussion
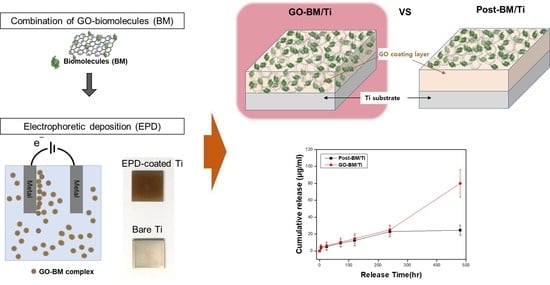
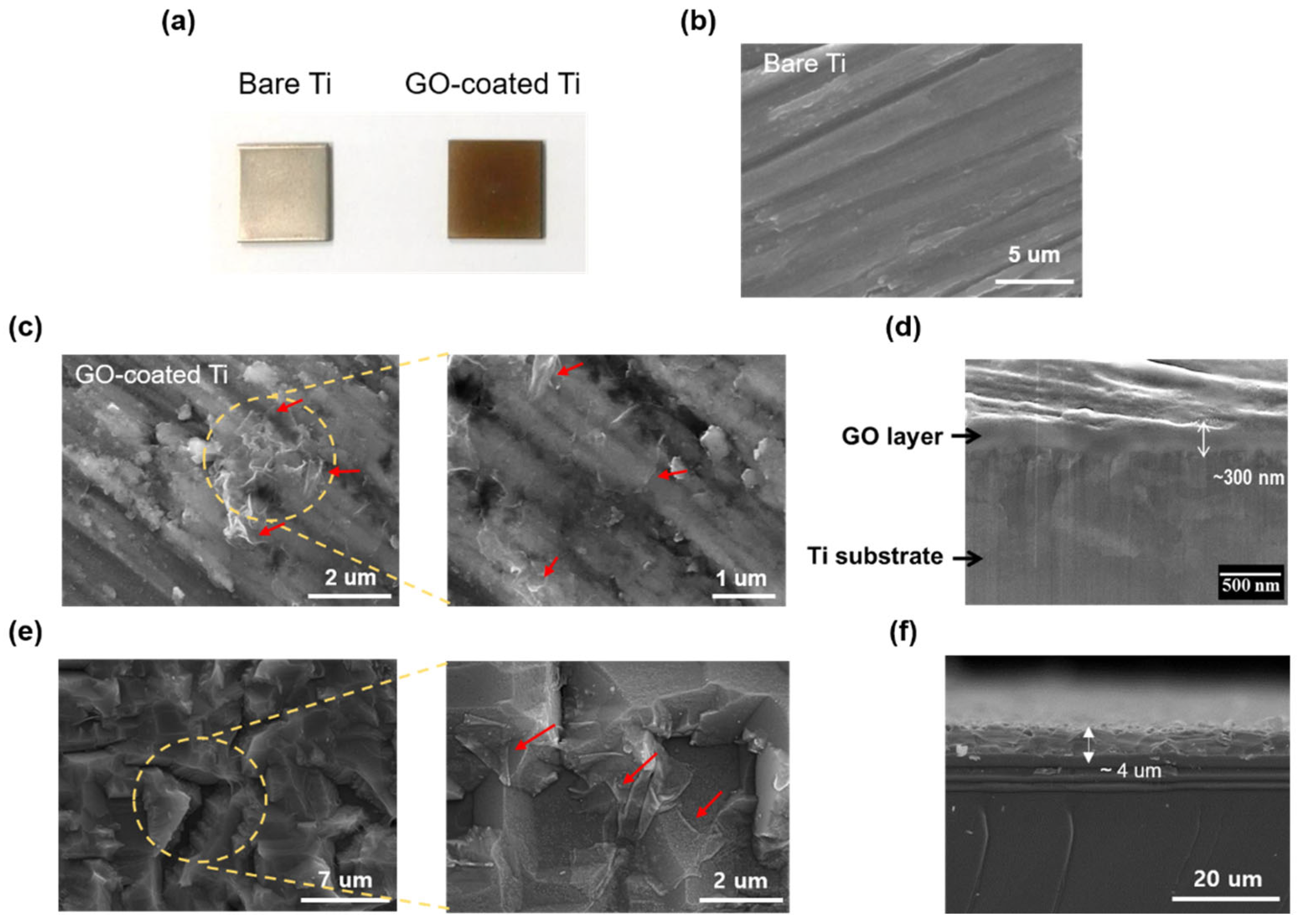
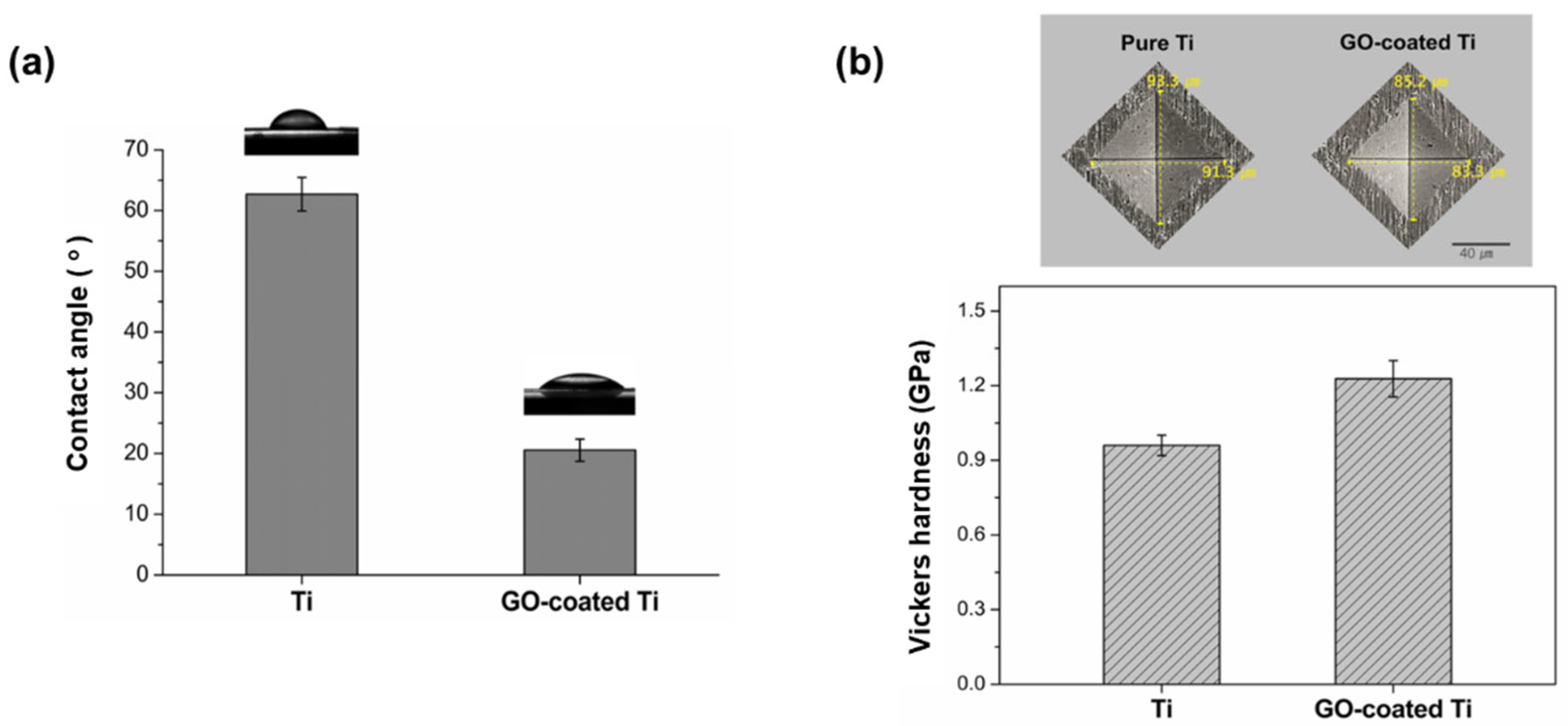
3.1. Preparation of GO-Coated Ti Plates
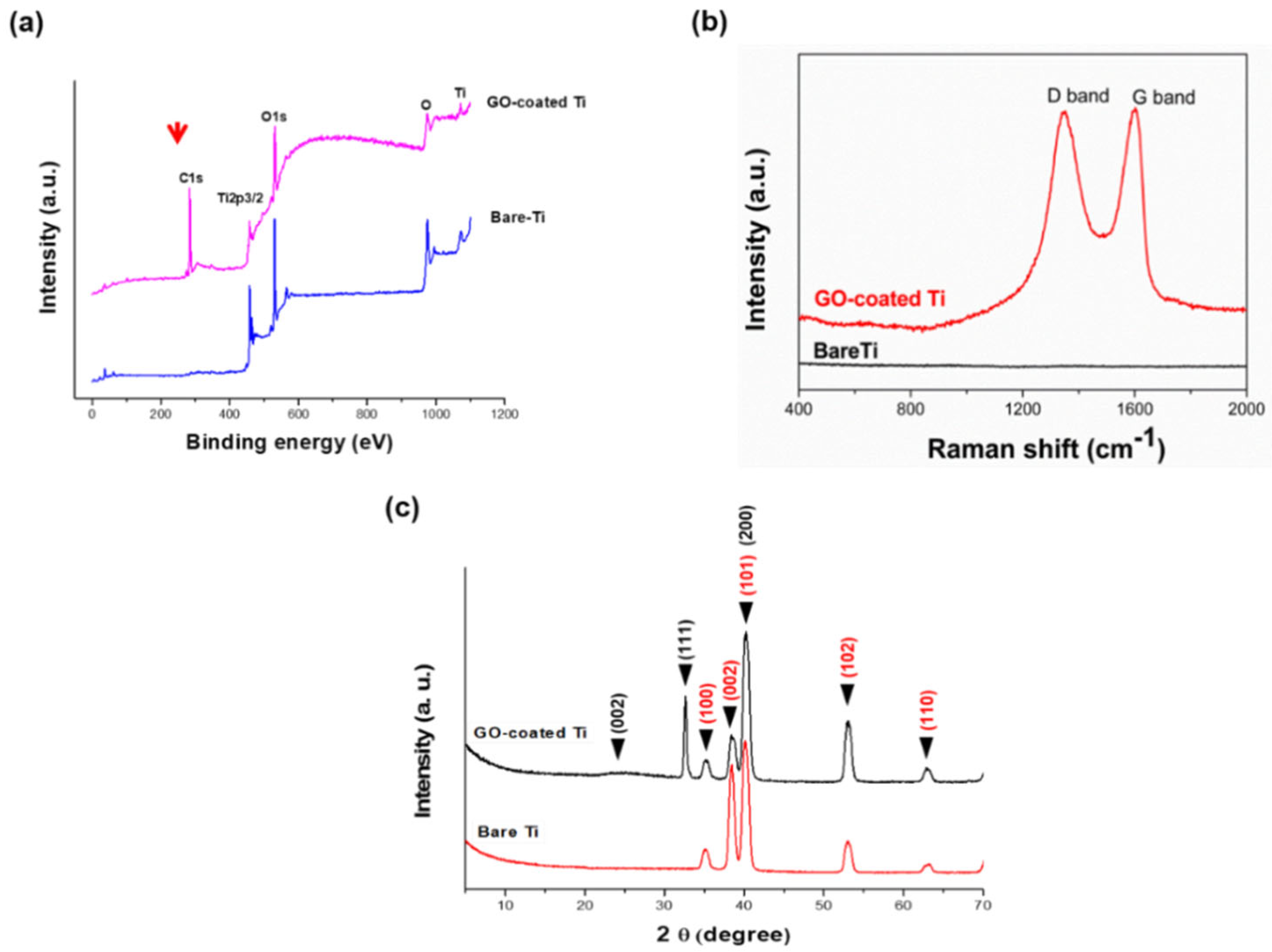
3.2. Characterization of GO-Coated Ti Plates
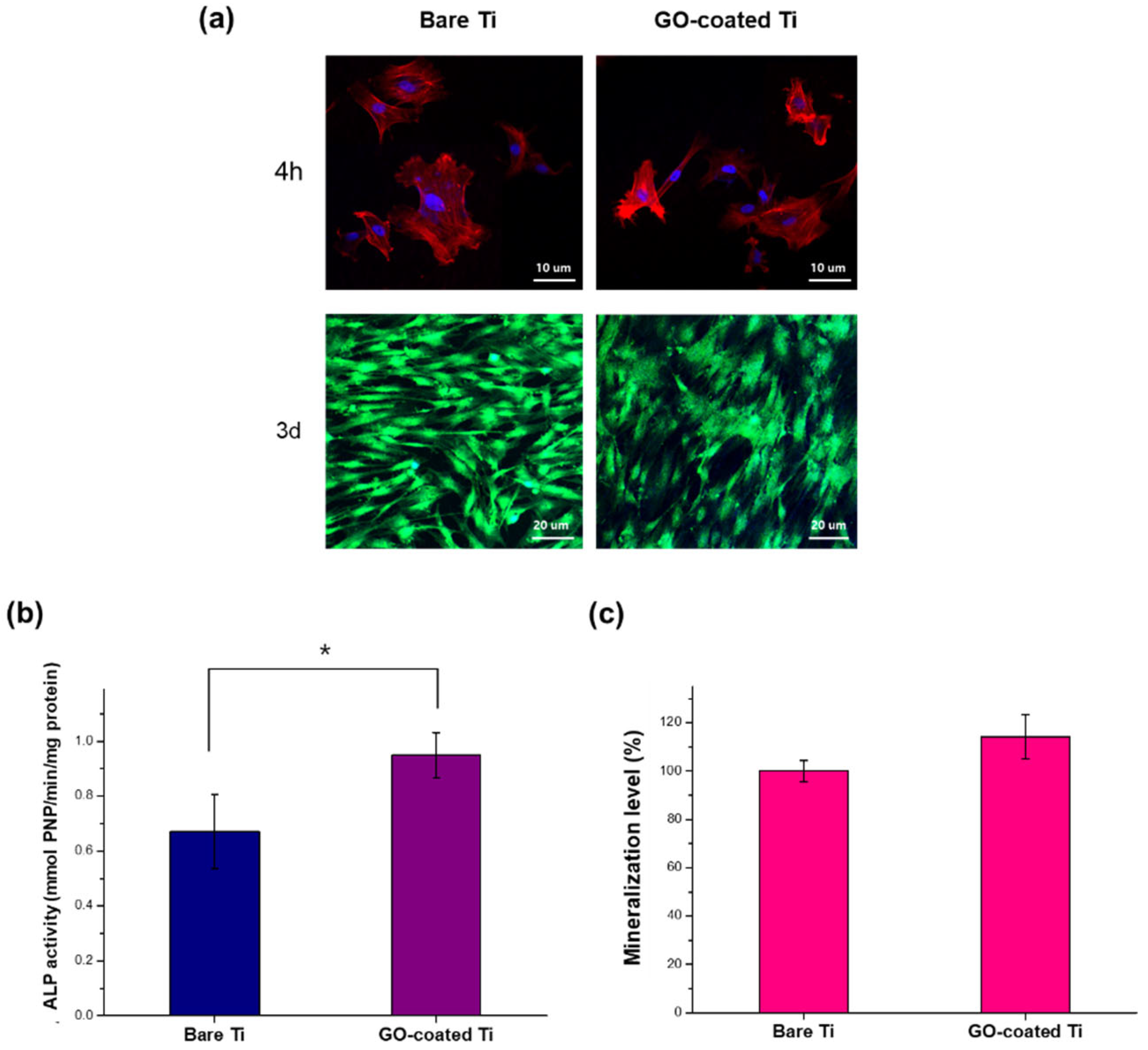
3.3. In Vitro Cellular Responses
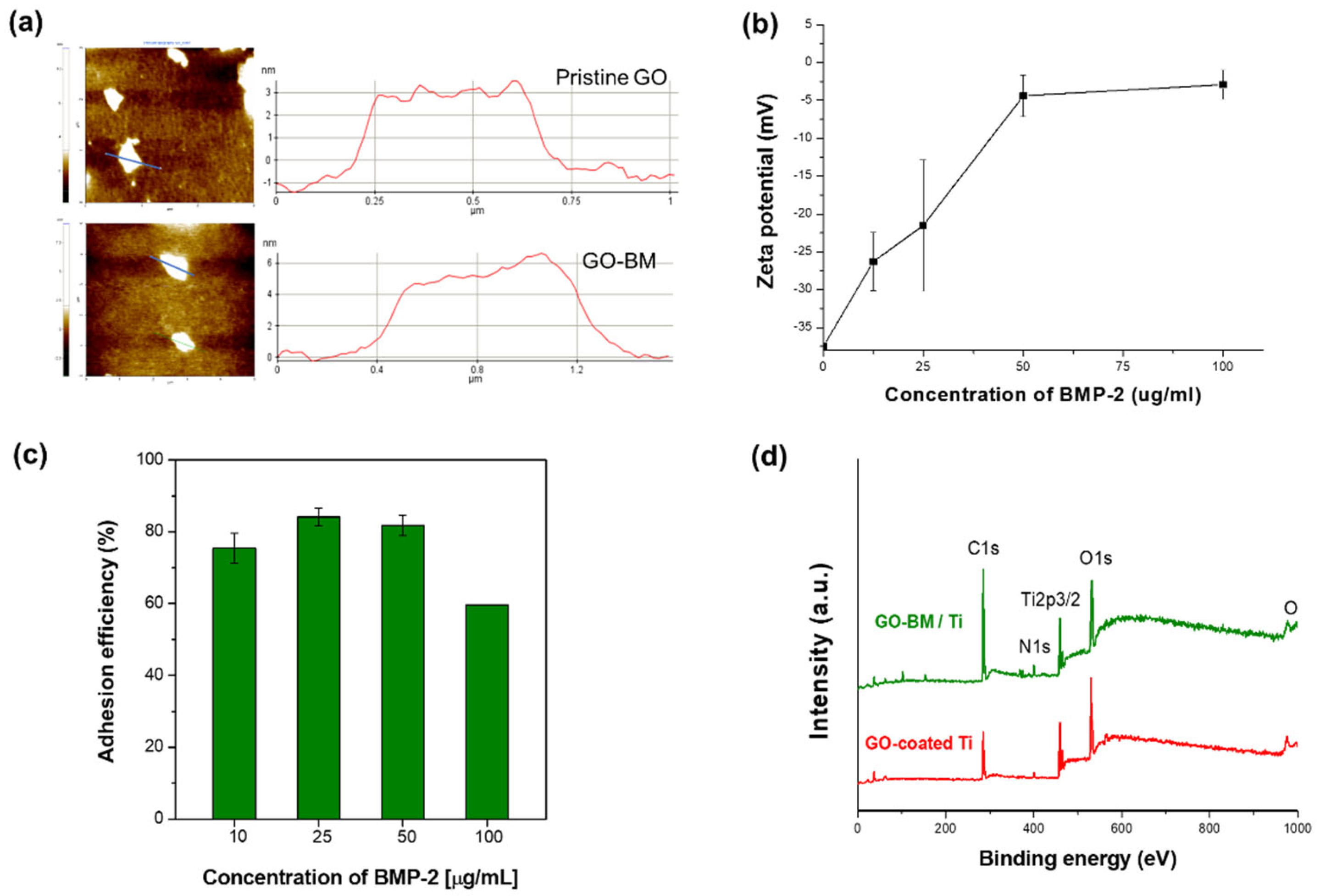
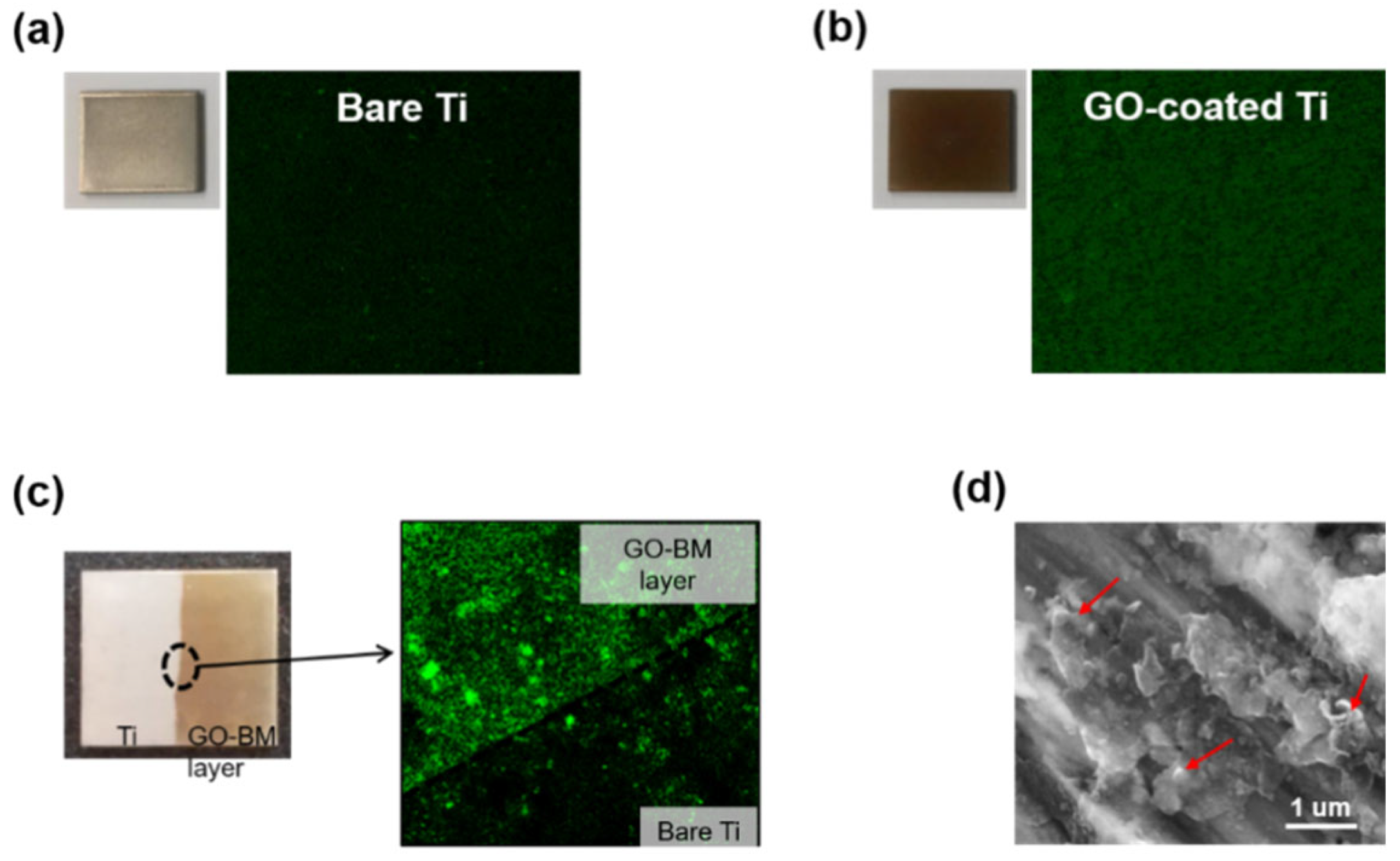
3.4. BM-Loading GO
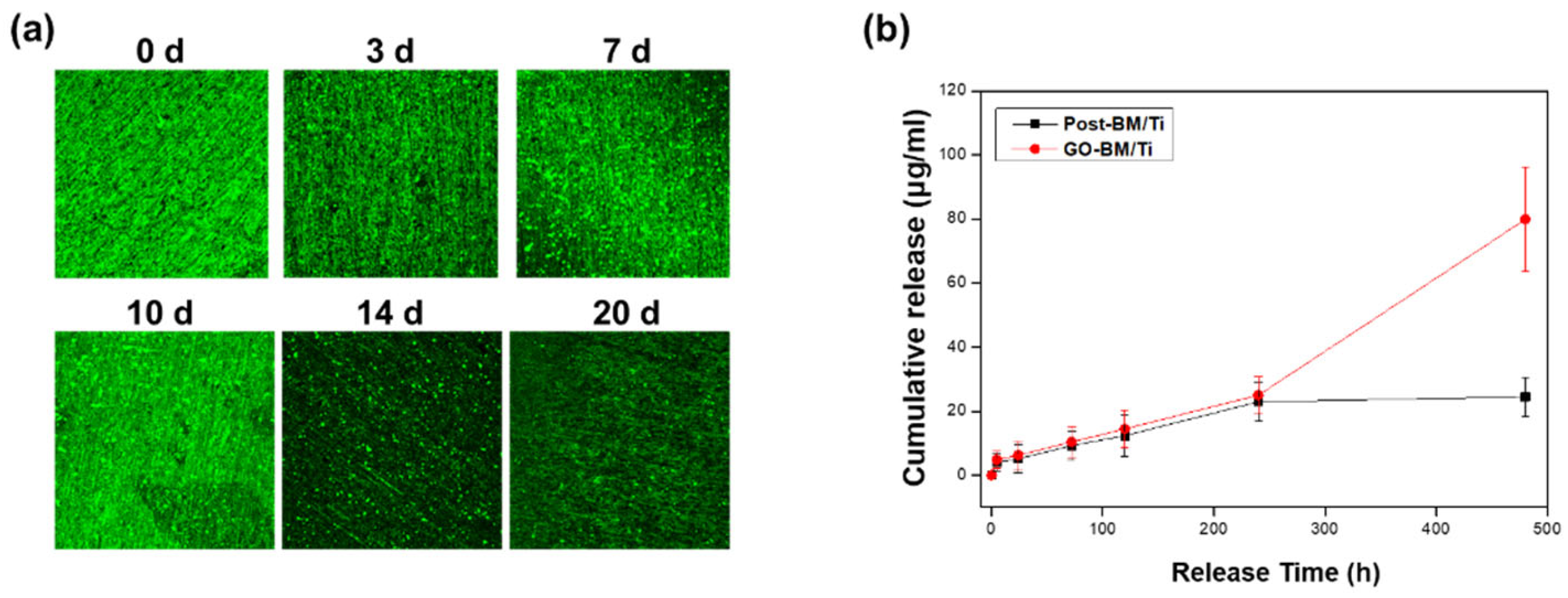
3.5. BM Release from GO-Coated Ti
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Pizzoferrato, A.; Cenni, E.; Ciapetti, G.; Granchi, D.; Savarino, L.; Stea, S. Inflammatory response to metals and ceramics. In Integrated Biomaterials Science; Barbucci, R., Ed.; Springer US: Boston, MA, USA, 2002; pp. 735–791. [Google Scholar]
- Shahali, H.; Jaggessar, A.; Yarlagadda, P.K.D.V. Recent advances in manufacturing and surface modification of titanium orthopaedic applications. Procedia Eng. 2017, 174, 1067–1076. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface modified techniques and emerging functional coating of dental implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Kim, Y.-W. Surface modification of ti dental implants by grit-blasting and micro-arc oxidation. Mater. Manuf. Process. 2010, 25, 307–310. [Google Scholar] [CrossRef]
- López, V.; Sundaram, R.S.; Gómez-Navarro, C.; Olea, D.; Burghard, M.; Gómez-Herrero, J.; Zamora, F.; Kern, K. Chemical vapor deposition repair of graphene oxide: A route to highly-conductive graphene monolayers. Adv. Mater. 2009, 21, 4683–4686. [Google Scholar] [CrossRef]
- Vats, N.; Rauschenbach, S.; Sigle, W.; Sen, S.; Abb, S.; Portz, A.; Dürr, M.; Burghard, M.; van Aken, P.A.; Kern, K. Electron microscopy of polyoxometalate ions on graphene by electrospray ion beam deposition. Nanoscale 2018, 10, 4952–4961. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, T.-O.; Saiki, K. Growth of graphene on cu by plasma enhanced chemical vapor deposition. Carbon 2012, 50, 869–874. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, Q.; Sun, S.; Han, B. Templated patterning of graphene oxide using self-assembled monolayers. Carbon 2012, 50, 1083–1089. [Google Scholar] [CrossRef]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface 2010, 7, S93–S105. [Google Scholar] [CrossRef] [PubMed]
- Haimov, H.; Yosupov, N.; Pinchasov, G.; Juodzbalys, G. Bone morphogenetic protein coating on titanium implant surface: A systematic review. J. Oral. Maxillofac. Res. 2017, 8, e1. [Google Scholar] [CrossRef]
- Ajori, S.; Ameri, A.; Ansari, R. Adsorption analysis and mechanical characteristics of carbon nanotubes under physisorption of biological molecules in an aqueous environment using molecular dynamics simulations. Mol. Simul. 2020, 46, 388–397. [Google Scholar] [CrossRef]
- Ouyang, L.; Deng, Y.; Yang, L.; Shi, X.; Dong, T.; Tai, Y.; Yang, W.; Chen, Z.-G. Graphene-oxide-decorated microporous polyetheretherketone with superior antibacterial capability and in vitro osteogenesis for orthopedic implant. Macromol. Biosci. 2018, 18, 1800036. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, X.; Zanden, C.; Liu, J. The promising application of graphene oxide as coating materials in orthopedic implants: Preparation, characterization and cell behavior. Biomed. Mater. 2015, 10, 015019. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E.; Ménard-Moyon, C.; Venturelli, E.; Takita, H.; Watari, F.; Bianco, A.; Yokoyama, A. Carbon nanotubes functionalized with fibroblast growth factor accelerate proliferation of bone marrow-derived stromal cells and bone formation. Nanotechnology 2013, 24, 435101. [Google Scholar] [CrossRef] [PubMed]
- La, W.-G.; Park, S.; Yoon, H.-H.; Jeong, G.-J.; Lee, T.-J.; Bhang, S.H.; Han, J.Y.; Char, K.; Kim, B.-S. Delivery of a therapeutic protein for bone regeneration from a substrate coated with graphene oxide. Small 2013, 9, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-S.; Lee, E.-J. Enhanced effect of polyethyleneimine-modified graphene oxide and simvastatin on osteogenic differentiation of murine bone marrow-derived mesenchymal stem cells. Biomedicines 2021, 9, 501. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, A.; Liu, Y.; Jiao, D.; Zeng, D.; Wang, X.; Cao, L.; Jiang, X. Enhanced bone regeneration of the silk fibroin electrospun scaffolds through the modification of the graphene oxide functionalized by bmp-2 peptide. Int. J. Nanomed. 2019, 14, 733–751. [Google Scholar] [CrossRef]
- Saladino, M.L.; Markowska, M.; Carmone, C.; Cancemi, P.; Alduina, R.; Presentato, A.; Scaffaro, R.; Biały, D.; Hasiak, M.; Hreniak, D.; et al. Graphene oxide carboxymethylcellulose nanocomposite for dressing materials. Materials 2020, 13, 1980. [Google Scholar] [CrossRef]
- Li, H.; Gao, C.; Tang, L.; Wang, C.; Chen, Q.; Zheng, Q.; Yang, S.; Sheng, S.; Zan, X. Lysozyme (lys), tannic acid (ta), and graphene oxide (go) thin coating for antibacterial and enhanced osteogenesis. ACS Appl. Bio Mater. 2020, 3, 673–684. [Google Scholar] [CrossRef]
- Han, L.; Sun, H.; Tang, P.; Li, P.; Xie, C.; Wang, M.; Wang, K.; Weng, J.; Tan, H.; Ren, F.; et al. Mussel-inspired graphene oxide nanosheet-enwrapped ti scaffolds with drug-encapsulated gelatin microspheres for bone regeneration. Biomater. Sci. 2018, 6, 538–549. [Google Scholar] [CrossRef]
- Oliver, J.-A.N.; Su, Y.; Lu, X.; Kuo, P.-H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.E.; Tran, N.L.; Nguyen, T.A.; Nguyen, T.T.; Tran, P.A. 8—surface modification of medical devices at nanoscale—recent development and translational perspectives. In Biomaterials in Translational Medicine; Yang, L., Bhaduri, S.B., Webster, T.J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 163–189. [Google Scholar]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (epd). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Ma, Y.; Han, J.; Wang, M.; Chen, X.; Jia, S. Electrophoretic deposition of graphene-based materials: A review of materials and their applications. J. Mater. 2018, 4, 108–120. [Google Scholar] [CrossRef]
- Mallick, M.; Arunachalam, N. Electrophoretic deposited graphene based functional coatings for biocompatibility improvement of nitinol. Thin Solid Films 2019, 692, 137616. [Google Scholar] [CrossRef]
- Eshghinejad, P.; Farnoush, H.; Bahrami, M.S.; Bakhsheshi-Rad, H.R.; Karamian, E.; Chen, X.B. Electrophoretic deposition of bioglass/graphene oxide composite on ti-alloy implants for improved antibacterial and cytocompatible properties. Mater. Technol. 2020, 35, 69–74. [Google Scholar] [CrossRef]
- Mehrali, M.; Akhiani, A.R.; Talebian, S.; Mehrali, M.; Latibari, S.T.; Dolatshahi-Pirouz, A.; Metselaar, H.S.C. Electrophoretic deposition of calcium silicate–reduced graphene oxide composites on titanium substrate. J. Eur. Ceram. Soc. 2016, 36, 319–332. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Li, M.; Liu, Q.; Jia, Z.J.; Xu, X.C.; Cheng, Y.; Zheng, Y.F. Electrophoretic deposition of graphene oxide reinforced chitosan–hydroxyapatite nanocomposite coatings on ti substrate. J. Mater. Sci. Mater. Med. 2016, 27, 48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mondal, S. Functionalized graphene oxide for chemotherapeutic drug delivery and cancer treatment: A promising material in nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Rykowska, I.; Nowak, I.; Nowak, R. Drug-eluting stents and balloons-materials, structure designs, and coating techniques: A review. Molecules 2020, 25, 4624. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate chemical analysis of oxygenated graphene-based materials using x-ray photoelectron spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef]
- Qin, H.; Gong, T.; Cho, Y.; Lee, C.; Kim, T. A conductive copolymer of graphene oxide/poly(1-(3-aminopropyl)pyrrole) and the adsorption of metal ions. Polym. Chem. 2014, 5, 4466–4473. [Google Scholar] [CrossRef]
- Karthik, P.; Vinoth, R.; Zhang, P.; Choi, W.; Balaraman, E.; Neppolian, B. Π–π interaction between metal–organic framework and reduced graphene oxide for visible-light photocatalytic h2 production. ACS Appl. Energy Mater. 2018, 1, 1913–1923. [Google Scholar] [CrossRef]
- Xu, X.; Li, W.; Wang, Y.; Dong, G.; Jing, S.; Wang, Q.; Feng, Y.; Fan, X.; Ding, H. Study of the preparation of cu-tic composites by reaction of soluble ti and ball-milled carbon coating tic. Results Phys. 2018, 9, 486–492. [Google Scholar] [CrossRef]
- Hwang, M.-J.; Kim, M.-G.; Kim, S.; Kim, Y.C.; Seo, H.W.; Cho, J.K.; Park, I.-K.; Suhr, J.; Moon, H.; Koo, J.C.; et al. Cathodic electrophoretic deposition (epd) of phenylenediamine-modified graphene oxide (go) for anti-corrosion protection of metal surfaces. Carbon 2019, 142, 68–77. [Google Scholar] [CrossRef]
- Zielinski, A.; Bartmanski, M. Electrodeposited biocoatings, their properties and fabrication technologies: A review. Coatings 2020, 10, 782. [Google Scholar] [CrossRef]
- Nemee, P.; Jaitanong, N.; Narksitipan, S. Surface modification of low carbon steel by using electrophoretic deposition technique with graphene oxide powder. Solid State Phenom. 2020, 302, 1–7. [Google Scholar] [CrossRef]
- Shayesteh Moghaddam, N.; Taheri Andani, M.; Amerinatanzi, A.; Haberland, C.; Huff, S.; Miller, M.; Elahinia, M.; Dean, D. Metals for bone implants: Safety, design, and efficacy. Biomanuf. Rev. 2016, 1, 1. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Fotovvati, B. Coating techniques for functional enhancement of metal implants for bone replacement: A review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef]
- Su, J.; Du, Z.; Xiao, L.; Wei, F.; Yang, Y.; Li, M.; Qiu, Y.; Liu, J.; Chen, J.; Xiao, Y. Graphene oxide coated titanium surfaces with osteoimmunomodulatory role to enhance osteogenesis. Mater. Sci. Eng. C 2020, 113, 110983. [Google Scholar] [CrossRef]
- Qiu, J.; Geng, H.; Wang, D.; Qian, S.; Zhu, H.; Qiao, Y.; Qian, W.; Liu, X. Layer-number dependent antibacterial and osteogenic behaviors of graphene oxide electrophoretic deposited on titanium. ACS Appl. Mater. Interfaces 2017, 9, 12253–12263. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Chen, M.; Lin, C.; Lu, L.; Yuan, Z.; Liu, J.; Liao, Q.; Xia, Z.; Peng, Z.; Cai, K. Zn-incorporation with graphene oxide on ti substrates surface to improve osteogenic activity and inhibit bacterial adhesion. J. Biomed. Mater. Res. A 2019, 107, 2310–2326. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Song, M.-J.; Amirian, J.; Linh, N.T.B.; Lee, B.-T. Bone morphogenetic protein-2 immobilization on porous pcl-bcp-col composite scaffolds for bone tissue engineering. J. Appl. Polym. Sci. 2017, 134, 45186. [Google Scholar] [CrossRef]
- Pugalenthi, R.; Arunachalam, P.; Amalraj, S.; Rathinavel, J.; Subramaniyan, D.S.; Bhagavathsingh, J.; Samuel, V. Covalent grafting of n-containing compound with graphene oxide: Efficient electrode material for supercapacitor. Chem. Sci. Eng. Res. 2019, 1. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, J.; Shi, Y.; Guo, J.; Huang, G.; Hong, C.; Zhao, Y. Hydrothermal synthesis of highly nitrogen-doped few-layer graphene via solid–gas reaction. Mater. Res. Bull. 2015, 61, 252–258. [Google Scholar] [CrossRef]
| Amount (At. %) | Bare Ti | GO | GO-BM |
|---|---|---|---|
| Ti | 13.45 | 9.88 | 5.1 |
| C | 44.57 | 51.3 | 64.5 |
| O | 39.79 | 35.69 | 26.59 |
| N | 2.17 | 3.13 | 3.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-S.; Jang, J.-H.; Lee, E.-J. Electrophoretic Deposition of a Hybrid Graphene Oxide/Biomolecule Coating Facilitating Controllable Drug Loading and Release. Metals 2021, 11, 899. https://doi.org/10.3390/met11060899
Oh J-S, Jang J-H, Lee E-J. Electrophoretic Deposition of a Hybrid Graphene Oxide/Biomolecule Coating Facilitating Controllable Drug Loading and Release. Metals. 2021; 11(6):899. https://doi.org/10.3390/met11060899
Chicago/Turabian StyleOh, Jun-Sung, Jun-Hwee Jang, and Eun-Jung Lee. 2021. "Electrophoretic Deposition of a Hybrid Graphene Oxide/Biomolecule Coating Facilitating Controllable Drug Loading and Release" Metals 11, no. 6: 899. https://doi.org/10.3390/met11060899
APA StyleOh, J.-S., Jang, J.-H., & Lee, E.-J. (2021). Electrophoretic Deposition of a Hybrid Graphene Oxide/Biomolecule Coating Facilitating Controllable Drug Loading and Release. Metals, 11(6), 899. https://doi.org/10.3390/met11060899